Abstract
The main route of mother-to-child transmission (MTCT) of human T cell leukemia virus type 1 is vertical transmission via breastfeeding. Although the most reliable method for preventing MCTC is exclusive formula feeding (ExFF), short-term breastfeeding (STBF) or frozen–thawed breast milk feeding (FTBMF) has been offered as an alternative method if breastfeeding is strongly desired. The aim of this review was to clarify the pooled risk ratio of MCTC of STBF and FTBMF compared with ExFF. This study was registered with PROSPERO (number 42018087317). A literature search of PubMed, CINAHL, the Cochrane Database, EMBASE, and Japanese databases through September 2018 identified 1979 articles, 10 of which met the inclusion criteria. Finally, 11 articles, including these 10 studies and the report of a recent Japanese national cohort study, were included in the meta-analysis. The pooled relative risks of STBF ≤3 months, STBF ≤6 months, and FTBMF compared with ExFF were 0.72 (95% confidence interval (CI): 0.30–1.77; p = 0.48), 2.91 (95% CI: 1.69–5.03; p = 0.0001), and 1.14 (95% CI: 0.20–6.50; p = 0.88), respectively. This meta-analysis showed no statistical difference in the risk of MTCT between STBF ≤3 months and ExFF, but the risk of MTCT significantly increased in STBF ≤6 months.
Keywords: early postnatal nutrition, human T cell leukemia virus, mother-to-child transmission
1. Introduction
Human T cell leukemia virus type 1 (HTLV-1) is the first pathogenic retrovirus found in humans. After infection with human T lymphocytes (CD4+), it synthesizes DNA by the action of reverse transcriptase, and exists as a provirus that integrates into the chromosomal DNA of host cells. HTLV-1 carriers are usually asymptomatic, but after a long incubation period, approximately 2% to 7% develop adult T cell leukemia (ATL) [1], and 0.25% to 3.8% develop HTLV-1-associated myelopathy (HAM)/tropical spastic paraparesis (TSP) [2]. A recent meta-analysis shows that HTLV-1 infection is associated with the development of various diseases, other than ATL and HAM/TSP, and people with HTLV-1 infection have a higher risk of death due to any cause than individuals with such an infection [3]. The number of HTLV-1 carriers is estimated to be 5 to 10 million worldwide, and they are unevenly distributed in specific endemic areas, such as Japan (mainly Kyushu and Okinawa regions), West and Central Africa, the Caribbean, and South America [4]. Japan has the largest number of carriers among developed countries, exceeding 1 million [5].
HTLV-1 has weak infectivity, and infection is transmitted by contact between cells via infected lymphocytes. The main transmission routes include mother-to-child transmission (MTCT), sexually transmitted infections, and blood transfusions. In Japan, there is no infection by blood transfusion, because screening tests are conducted, and it is believed that male-to-female transmission by sexual activity accounts for 20% and MTCT accounts for ≥60%, according to a report published in the 1990s [6]. ATL is rarely caused by infection in adulthood, and most ATL cases are derived from MTCT [7]; therefore, the prevention of MTCT is essential.
In recent years, HTLV-1 infection has been spreading to non-endemic areas, such as Tokyo and Osaka. To eradicate HTLV-1-related diseases, such as ATL, the Ministry of Health, Labour, and Welfare of Japan decided to conduct a nationwide HTLV-1 antibody screening of all pregnant women for HTLV-1 antibody by the 30th week of gestation since 2010 [8].
In the 1980s, Japanese research groups reported the presence of HTLV-1-infected cells in the breast milk of carrier mothers [9,10], and subsequent animal studies [11] and epidemiological studies [12,13] have demonstrated that breastfeeding is the main route of MTCT. To prevent transmission via breast milk, the most reliable method is not to feed breast milk containing infected cells, that is, to provide exclusive formula feeding (ExFF). However, ExFF cannot give the mothers and infants the advantage of breastfeeding, such as prevention from other infectious diseases, nutritional effects, economic efficiency, and formation of a good mother–child relationship. Many HTLV-1 carrier mothers are afraid that they cannot form a mother–child attachment because they cannot breastfeed their babies [14]. In recent years, breastfeeding has been actively promoted in Japan. According to a 2015 survey by the Ministry of Health, Labour, and Welfare in Japan, the exclusive breastfeeding rate was 51.3%, and the mixed nutrition rate was 96.5% at the first month of life [15].
As in other countries, the current Japanese manual for the prevention of MTCT by the Health, Labor, and Welfare Science Research Group recommends ExFF for infants born to HTLV-1 carrier mothers [8,16]. If the HTLV-1 carrier mothers strongly desire to breastfeed their babies, short-term breastfeeding (STBF) or frozen–thawed breast milk feeding (FTBMF) has been offered as alternative methods other than ExFF. In the Kagoshima prefecture, which is another endemic area in Japan, >60% of HTLV-1 carrier mothers choose STBF ≤3 months; thus, it is clear that there are many HTLV-1 carrier mothers who desire to breastfeed their babies [17]. However, the evidence for the efficacy of STBF and FTBMF is insufficient, because this is based on small observational studies only.
From April 2012 to December 2015, we prospectively recruited a cohort of HTLV-1 carrier mother at 92 facilities in Japan and calculated the MTCT rates of each feeding option chosen by HTLV-1 carrier mothers [18]. Among the 313 HTLV-1 carrier mothers, 55.0%, 35.1%, 6.1%, and 3.8% selected STBF ≤3 months, ExFF, FTBMF, and long-term breastfeeding, respectively. The MTCT rates of STBF ≤3 months, ExFF, FTBMF, and long-term breastfeeding were 2.3%, 6.4%, 5.3%, and 16.7%, respectively. The risk ratio for STBM compared with ExFF was not statistically different (0.364; 95% confidence interval (CI): 0.116–1.145). Because of the small population, the MTCT rate of FTBMF was not statically reliable.
The aim of this study was to conduct a meta-analysis that combines the recent cohort study we conducted and previous studies to clarify the pooled risk ratio of MTCT of STBF and FTBMF compared with ExFF.
2. Material and Methods
This study was registered with PROSPERO (number 42018087317).
2.1. Search Strategies
We searched for published studies related to infant feeding and MTCT of HTLV-1 in the following databases from their inception to September 2018: PubMed (from 1949), CINAHL (1981), Cochrane Databases (from 1939), and EMBASE (from 1947). The search strategy included the terms below: (“HTLV” or “human T-lymphotropic” or “human T cell leukemia”) and ((“mother” and “child”) or (“milk” or “vertical”)) and (“transmission” or “infection).
We also searched the following Japanese databases: ICHUSHI (from 1983), CiNii (from 1881), KAKEN, and the Database of Health Labour Science Research Grant. ICHUSHI contains bibliographic citations and abstracts from biomedical journals and other serial publications published in Japan. CiNii contains information on academic articles published in academic society journals, university research bulletins, or articles included in the National Diet Library’s Japanese Periodical Index Database. KAKEN is a public database that includes information on adopted projects, assessment, and research achievements from the Grants-in-Aid for Scientific Research (KAKENHI) Program. Since these four databases are electronic databases in Japanese, we used comparable Japanese terms. We also examined the list of references in the included studies.
2.2. Definition of Terms
The definition of STBF varies among articles. In this study, we defined STBF as breastfeeding within three months of age (STBF ≤3 months) or within six months of age (STBF ≤6 months). MTCT was confirmed by the detection of the HTLV-1 antibody in infants ≥12 months.
2.3. Inclusion Criteria
We included studies if they met the following criteria: the mothers were found to be an HTLV-1 carrier by an antibody test; the HTLV-1 antibody tests were performed on their children aged >12 months to <15 years; and the MTCT rate of the intervention group (STBF or FTBMF) was compared with that of the ExFF group (control).
2.4. Exclusion Criteria
We excluded studies if the study population included children aged <12 months or >15 years, who had a history of blood transfusion, had married, or had sexual intercourse.
2.5. Extraction of Data
The titles and abstracts of the studies were retrieved using the search strategy, and those from additional sources were screened independently by two of the four reviewers (T.M., H.Y., M.M., and M.S.) to identify studies that potentially met the inclusion criteria. Second, the full text of these potentially eligible studies was retrieved and independently assessed for eligibility by two reviewers. Any disagreement between the two reviewers regarding the eligibility of a particular study was resolved through discussion with a third reviewer.
2.6. Quality Assessment
To assess the quality of the included studies, the Newcastle–Ottawa Scale (NOS) was applied in this review [19]. According to this scale, each study is evaluated according to eight items, categorized into three sections: selection, comparability, and exposure. The total score ranges from 0 to 9, and studies with scores ≥5 are generally considered as having a high enough quality to be included in the meta-analysis. Each study was independently assessed by two authors (T.M. and K.I.). If the evaluation of the two did not match, the third author was involved with the final decision.
2.7. Data Synthesis and Statistical Analysis
Our primary outcome was MTCT transmission rate by feeding strategy. We provided a synthesis of the findings from the included studies, structured around the type of intervention, study population characteristics, and type of outcome. In addition, we provided summaries of the intervention effects for each study by calculating the risk ratio.
The meta-analysis was carried out using Review Manager version 5.3. (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark, 2014). The pooled relative risks (RRs) and 95% CIs were calculated using the Mantel–Haenszel random effect model. Heterogeneity across the studies was assessed using the I2 statistic, and the I2 values were considered low, moderate, and high, with upper limits of 25%, 50%, and 75%, respectively [20]. Publication bias was assessed using a funnel plot. Statistical significance was set at p < 0.05.
3. Results
3.1. Search and Article Selection
The search strategy identified 1797 records for potential inclusion in the study: PubMed (n = 330), CINAHL (n = 18), EMBASE (n = 589), ICHUSHI (n = 788), CiNii (n = 28), KAKEN (n = 1), and the Database of Health Labour Sciences Research Grant (n = 43) (Figure 1). After reviewing the title and abstract of 1798 articles, including the report of our recent cohort study [18], the full text of 211 articles was obtained for further assessment of their eligibility. Assessment of the full-text articles yielded 11 articles, and these 11 articles were included in the meta-analysis. The characteristics of the included studies that compared the MTCT rates between ExFF and STBF ≤3 months, STBF ≤6 months, and FTBMF are shown in Table 1, Table 2 and Table 3, respectively. According to the NOS, seven of the 11 articles achieved a score ≥5 (see Table 4). Figure 2 shows the funnel plot of the adopted articles in this meta-analysis. Since the number of articles included in each comparison is small, it is difficult to evaluate publication bias, although the distribution seems to be symmetric.
Figure 1.
Flow diagram of the study selection. STBF, short-term breastfeeding; FTBMF, frozen–thawed breast milk feeding.
Table 1.
Characteristics of the included studies comparing the MTCT rates between ExFF and STBF ≤3 months.
| Author(s), Year | Study Area | Study Period |
Study Population | ExFF | STBF | Timing of Antibody Test of Children | Study Design |
Reference Number | |
|---|---|---|---|---|---|---|---|---|---|
| Seroconversion n/N (%) | Definition | Seroconversion n/N (%) | |||||||
| Uemura et al., 1989 | Okayama, Japan | NA | Children born to HTLV-1 carrier mothers, including older siblings | 0/8 (0%) | Breastfeeding <3 months | 0/3 (0%) | ≥12 months | Retrospective | [21] |
| Ureta-Vidal et al., 1999 | French Guyana | 1989 t–NA | Children born to HTLV-1 carrier mothers, including older siblings | 0/23 (0%) | Breastfeeding ≤3 months | 1/12 (8.3%) | 18 months to 12 years | Retrospective | [22] |
| Takezaki, 2009 | Kagoshima, Japan | 1986–2006 | Children born to HTLV-1 carrier mothers | 16/331 (4.8%) | Breastfeeding <3 months | 2/126 (1.6%) | ≥18 months | Retrospective | [23] |
| Masuzaki et al., 2013 | Nagasaki, Japan | 1998–2008 | Children born to HTLV-1 carrier mothers | 8/218 (3.7%) | Breastfeeding <3 months | 1/36 (3.7%) | Three years | Retrospective | [24] |
| Moriuchi et al., 2017 | Nagasaki, Japan | 2011–2017 | Children born to HTLV-1 carrier mothers | 4/91 (4.4%) | Breastfeeding ≤90 days | 3/35 (8.5%) | ≥36 months | Retrospective | [25] |
| Itabashi et al., 2020 | Japan (national survey) | 2012–2015 | Children born to HTLV-1 carrier mothers | 7/110 (6.4%) | Breastfeeding ≤3 months | 4/172 (2.3%) | Three years | Prospective | [18] |
| Total | 35/781 (4.5%) | 11/384 (2.9%) | |||||||
MTCT, mother-to-child transmission; ExFF, exclusive formula feeding; STBF, short-term breastfeeding; NA, not assessed; HTLV-1, human T cell leukemia virus type 1.
Table 2.
Characteristics of included studies comparing MTCT rates between ExFF and STBF ≤6 months.
| Author(s), Year | Study Area | Study Period | Study Population | ExFF | STBF | Timing of Antibody Test of Children | Study Design | Reference Number | |
|---|---|---|---|---|---|---|---|---|---|
| Seroconversion n/N (%) | Definition | Seroconversion n/N (%) | |||||||
| Nakayama et al., 1992 | Kagoshima, Japan (single-center survey) | 1986–1990 | ExFF: children born to HTLV-1 carrier mothers STBF: older siblings | 1/53 (1.9%) | Breastfeeding ≤6 months | 4/41 (9.8%) | 1–5 years | Retrospective | [26] |
| Oki et al., 1992 | Kagoshima and Miyazaki, Japan | 1986–1991 | Children born to HTLV-1 carrier mothers | 10/177 (5.6%) | Breastfeeding ≤6 months | 1/26 (3.8%) | 1–3 years | Prospective | [27] |
| Takezaki et al., 1997 | Tsusima and Kamigoto, Nagasaki, Japan | 1985–1991 | Children born to HTLV-1 carrier mothers | 4/162 (2.5%) | Breastfeeding ≤6 months | 2/51 (3.9%) | ≥30 months | Retrospective | [28] |
| Ureta-Vidal et al., 1999 | French Guyana | 1989–NA | Children born to HTLV-1 carrier mothers, including older siblings | 0/23 (0%) | Breastfeeding ≤6 months | 2/29 (6.9%) | 18 months to 12 years | Retrospective | [22] |
| Masuzaki et al., 2013 | Nagasaki, Japan | 1987–1997 | Children born to HTLV-1 carrier mothers | 23/962 (2.4%) | Breastfeeding <6 months | 14/169 (8.3%) | Three years | Retrospective | [24] |
| Total | 38/1377 (2.8%) | 23/316 (7.3%) | |||||||
MTCT, mother-to-child transmission; ExFF, exclusive formula feeding; STBF, short-term breastfeeding; NA, not assessed; HTLV-1, human T cell leukemia virus type 1.
Table 3.
Characteristics of included studies comparing MTCT rates between ExFF and FTBMT.
| Author, Year | Study Area | Study Period | Study Population | ExFF | FTBMF | Timing of Antibody Test of Children | Study Design | Reference Number | |
|---|---|---|---|---|---|---|---|---|---|
| Seroconversion n/N (%) | Definition | Seroconversion n/N (%) | |||||||
| Maehama et al., 1992 | Okinawa, Japan | 1986–1989 | Children born to HTLV-1 carrier mothers | 0/46 (0%) | 12 h of freezing at a home freezer followed by natural thawing | 2/26 (7.7%) | 1–3 years | Prospective | [29] |
| Ekuni, 1997 | Okinawa, Japan | 1983–1984 | Children born to HTLV-1 carrier mothers | 5/108 (4.6%) | 12 h of freezing at −20 °C followed by natural thawing | 0/33 (0%) | 24 months | Prospective | [30] |
| Itabashi et al., 2020 | Japan (national survey) | 2012–2015 | Children born to HTLV-1 carrier mothers | 7/110 (6.4%) | 24 h of freezing in a home freezer followed by natural thawing | 1/19 (5.3%) | Three years | Prospective | [18] |
| Total | 12/264 (4.5%) | 3/78 (3.8%) | |||||||
MTCT, mother-to-child transmission; ExFF, exclusive formula feeding; FTBMF, frozen–thawed breast milk feeding; STBF, short-term breastfeeding; HTLV-1, human T cell leukemia virus type 1.
Table 4.
Quality assessment of the studies by the NOS.
| Author, Year | Selection | Comparability | Outcome | Total Quality Score | Reference Number | Language | Types of Article | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Representativeness of Exposed Cohort | Selection of the Non-Exposed Cohort | Ascertainment of Exposure | Demonstration That Outcome of Interest was not Present at Start of Study | Comparability of Cohorts on the Basis of the Design or Analysis | Assessment of Outcome | Follow-Up Length for Outcomes to Occur | Adequacy of Follow-Up | |||||
| Uemura et al., 1989 | ★ | ★ | ★ | ★ | 4 | [21] | Japanese | Meeting abstract | ||||
| Nakayama et al., 1992 | ★ | ★ | ★ | 3 | [26] | Japanese | Original article | |||||
| Oki et al., 1992 | ★ | ★ | ★ | ★ | ★ | 5 | [28] | English | Original article | |||
| Maehama et al., 1992 | ★ | ★ | ★ | ★ | 4 | [29] | Japanese | Original article | ||||
| Takezaki et al., 1997 | ★ | ★ | ★ | ★ | ★ | ★ | 6 | [27] | English | Original article | ||
| Ekuni, 1997 | ★ | ★ | ★ | ★ | 4 | [30] | Japanese | Original article | ||||
| Ureta-Vidal et al., 1999 | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 8 | [22] | English | Original article | |
| Takezaki, 2009 | ★ | ★ | ★ | ★ | ★ | 5 | [23] | Japanese | Public research report | |||
| Masuzaki et al., 2013 | ★ | ★ | ★ | ★ | ★ | 5 | [24] | Japanese | Public research report | |||
| Moriuchi et al., 2017 | ★ | ★ | ★ | ★ | ★ | 5 | [25] | Japanese | Public research report | |||
| Itabashi et al., 2020 | ★ | ★ | ★ | ★ | ★ | ★ | 6 | [18] | English | Original article | ||
NOS, Newcastle–Ottawa Scale.
Figure 2.
Funnel plots of the included studies. Blue indicates the STBF ≤3 months compared with ExFF, green indicates the STBF ≤6 months compared with ExFF, and red indicates the FTBMF compared with ExFF.
Selection (a maximum of one star can be given for each numbered item)
-
Representativeness of the exposed cohort
Truly representative of the average HTLV-1 carrier mothers and their children in the community ★
Somewhat representative of the average HTLV-1 carrier mothers and their children in the community ★
Selected group of users e.g., nurses, volunteers
No description of the derivation of the cohort
-
Selection of the non-exposed cohort
Drawn from the same community as the exposed cohort ★
Drawn from a different source
No description of the derivation of the non-exposed cohort
-
Ascertainment of exposure
Secure record (e.g., surgical records) ★
Structured interview ★
Written self-report
No description
-
Demonstration that outcome of interest was not present at start of study
Yes ★
No
Comparability (a maximum of two stars can be given)
-
Comparability of cohorts on the basis of the design or analysis
Study controls for maternal anti-HTLV-1 antibody titer and/or maternal HTLV-1 pro-viral load ★
Study controls for any additional factor ★
Outcome (a maximum of one star can be given for each numbered item)
-
Assessment of outcome
Independent blind assessment ★
Record linkage ★
Self-report
No description
-
Was follow-up long enough for outcomes to occur
Yes ★
No
-
Adequacy of follow-up of cohorts
Complete follow-up ★
Subjects lost to follow-up unlikely introduce bias, with follow-up >70% or description provided of those lost ★
Follow-up rate <70% and no description of those lost
No statement
3.2. Comparison between STBF ≤3 Months and ExFF
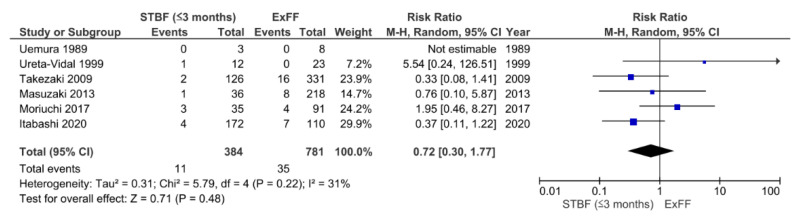
We identified a total of five retrospective studies [21,22,23,24,25] and one prospective [18] study that were eligible for the comparison of STBF ≤3 months and ExFF (Figure 3). There were 11 cases of HTLV-1 positivity among 384 children in the STBF ≤3 months group compared with 35 cases among 781 children in the ExFF group (pooled RR: 0.72; 95% CI: 0.30–1.77; p = 0.48). There was moderate heterogeneity between the studies (I2 = 31%, p = 0.22).
Figure 3.
Forest plots of the RRs of mother-to-child transmission of human T cell leukemia virus type 1 in the STBF ≤3 months group compared with the ExFF group. STBF, short-term breastfeeding; ExFF, exclusive formula feeding; M-H, Mantel–Haenszel; CI, confidence interval; RR, relative risk.
3.3. Comparison between STBF ≤6 Months and ExFF
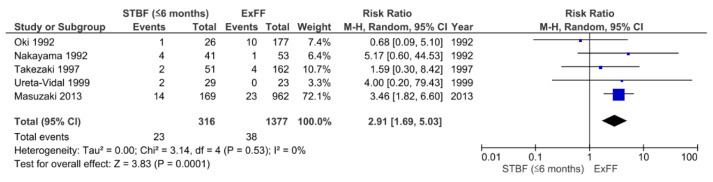
We identified a total of four retrospective studies [22,24,26,27] and one prospective study [28] that were eligible for the comparison of STBF ≤6 months and ExFF (Figure 4). There were 23 cases of HTLV-1 positivity among 316 children in the STBF ≤6 months group compared with 38 cases among 1377 participants in the ExFF group (pooled RR: 2.91; 95% CI: 1.69–5.03; p = 0.0001). There was low heterogeneity between the studies (I2 = 0%, p = 0.53).
Figure 4.
Forest plots of the RRs of mother-to-child transmission of human T cell leukemia virus type 1 in the STBF ≤6 months group compared with the ExFF group. STBF, short-term breastfeeding; ExFF, exclusive formula feeding; M-H, Mantel–Haenszel; CI, confidence interval; RR, relative risk.
3.4. Comparison between FTBMF and ExFF
We identified only three prospective studies [18,29,30] that were eligible for the comparison of FTBMF and ExFF (Figure 5). There were three cases of HTLV-1 positivity among 78 children in the FTBMF group compared with 12 cases among 264 children in the ExFF group (pooled RRL: 1.14; 95% CI: 0.20–6.50; p = 0.88). In the study of Maehama et al. [29], the duration of FTBMF was limited to one month, followed by formula feeding. The study of Ekuni [30] did not describe the duration of FTBMF, but the follow-up study by the same authors [31] stated that the mean duration of FTBMF was two months (varying from two weeks to six months). There was moderate heterogeneity between the studies (I2 = 27%, p = 0.26).
Figure 5.
Forest plots of the RRs of mother-to-child transmission of human T cell leukemia virus type 1 in the FTBMF group compared with the ExFF group. FTBMF, frozen–thawed breast milk feeding; ExFF, exclusive formula feeding; M-H, Mantel–Haenszel; CI, confidence interval; RR, risk ratio.
4. Discussion
In the recent technical report on HTLV-1, the World Health Organization recommends that “available data should be further analyzed to better define the risk of HTLV-1 transmission associated with specific durations of breastfeeding, balanced with the risks of other adverse health outcomes that may result from reduced breastfeeding” [32]. This meta-analysis combined the data of a recent cohort study we conducted and those of previous studies to clarify the pooled risk ratio of MTCT of STBF and FTBMF compared with ExFF, and the results showed that the risk of MTCT between STBF ≤3 months and ExFF had no statistical difference, but it significantly increased at STBF ≤6 months.
The preventive effect of short-term breastfeeding on MTCT is assumed to be due to the suppression of infection by the neutralizing antibody that is transplacentally transferred from the mother in utero [33]. STBF <3 or <6 months has been reported to have protective effects in endemic areas in Japan, but these are based on small studies, and no evidence has been established at this time.
The duration of breastfeeding is considered to be one of the factors affecting the MTCT rate, but there are no studies directly comparing the MTCT rate with breastfeeding periods <3 and <6 months. As a result of this meta-analysis, there was no statistical difference in the risk ratio for MTCT of STBF ≤3 months compared with ExFF; however, the number of subjects and events was small. Meanwhile, the risk of MTCT for STBF ≤6 months was 2.91 times higher than that for ExFF. This result suggests that STBF ≤3 months may be as effective as ExFF in preventing MTCT, but continued breastfeeding >3 months may increase the risk of MTCT.
In our recent cohort study, approximately 8% of the mothers who selected STBF ≤3 months were unable to wean breastfeeding by six months of life [18]. If the mother selects STBF, it is necessary to explain in advance that it is not easy to stop breastfeeding by three months and to support weaning from approximately two months of life.
High maternal HTLV-1 pro-viral load (PVL) has been reported to be a risk factor of MTCT [22,34,35]. Among the studies examined in this meta-analysis, only the study of Ureta-Vidal et al. [22] showed that high PVL increases the risk of MTCT by logistic analysis adjusted for breastfeeding duration. Further studies are needed to determine whether STBF >3 months increases the risk of MTCT, even when maternal PVL is low.
It is well-known that antiretroviral therapy (ART) can reduce the risk of MTCT in patients with human immunodeficiency virus, and similar effects are expected [36]. Clinical studies are needed on the efficacy and safety of ART on breastfeeding HTLV-1 mothers, as it will allow mothers to be offered with alternative feeding options.
A meta-analysis published in 2018 by Boostani et al. [37] showed that STBF ≤6 months does not increase the risk of MTCT compared with ExFF, which is different from our analysis.
The reason for this difference is unknown, but while Boostani et al. [37] extracted only articles written in English, we included articles written in Japanese, large public research reports that have not been published, and the results of the latest nationwide cohort study in Japan. Some of the articles included by Boostani et al. [37] overlap with the Japanese articles and public reports; thus, we adopted the one with the highest cumulative number of cases.
FTBMF has been reported to be effective in preventing MTCT, because infected T lymphocytes are destroyed by freezing and thawing treatment, and the infectivity is inactivated [30]. In this meta-analysis, there were no differences in the MTCT rate between FTBMF and ExFF, but the number of subjects analyzed was small, and evidence on its preventive effect was insufficient. Moreover, due to the short duration of FTBMF in the adopted studies, the effect may be due to STBF. In fact, FTBMF is a time-consuming activity, and it might be difficult for mothers to continue FTBMF in the long term.
This study has several limitations. First, most of the studies included in the meta-analysis are retrospective observational studies, and there are no randomized controlled trials. These retrospective studies have low follow-up rates, and the details of the nutritional methods may be inaccurate. In addition, almost none of the studies described the proportion of mixed nutrition or the actual period of breastfeeding in the STBF group. Second, there is an inconsistent timing of antibody testing in children. Seroconversion of the HTLV-1 antibody is thought to occur by the age of three years [38,39]. The Japanese manual recommends antibody testing for children aged three years [16]. In most of the studies adopted in this meta-analysis, the antibody tests were performed at the age of one year to two years; thus, the MTCT rate may have been underestimated. Third, all of the studies adopted in the meta-analysis were conducted in Japan, except for a report from French Guyana; thus, it is unclear whether our results are applicable to individuals from countries other than Japan. Further research is needed in various countries with different settings.
5. Conclusions
This meta-analysis showed that there was no statistical difference in the risk of MTCT between STBF ≤3 months and ExFF, but the risk of MTCT significantly increased in STBF ≤6 months. Although the superiority of ExFF remains unchanged in the prevention of MTCT, it is necessary to understand the risks of prolonged breastfeeding when STBF was selected and to establish a system to support mothers and children.
Author Contributions
Statistical analysis, review of published studies for eligibility, quality assessment, and review of the first draft, T.M.; review of published studies for eligibility, Y.H., M.M., and M.S.; statistical analysis and quality assessment, K.I.; search of electric databases and statistical analysis, N.Y. All authors have contributed to manuscript revisions and have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Health, Labour and Welfare (grant number H29-Sukoyaka-Shitei3 and 20DA1007).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used in this study can be found in the full-text articles used in this systematic review.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Iwanaga M., Watanabe T., Yamaguchi K. Adult T cell leukemia: A review of epidemiological evidence. Front. Microbiol. 2012;3:322. doi: 10.3389/fmicb.2012.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamano Y., Sato T. Clinical pathophysiology of human T-lymphotropic virus-type 1-associated myelopathy/tropical spastic paraparesis. Front. Microbiol. 2012;3:389. doi: 10.3389/fmicb.2012.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schierhout G., McGregor S., Gessain A., Einsiedel L., Martinello M., Kaldor J. Association between HTLV-1 infection and adverse health outcomes: A systematic review and meta-analysis of epidemiological studies. Lancet Infect. Dis. 2020;20:133–143. doi: 10.1016/S1473-3099(19)30402-5. [DOI] [PubMed] [Google Scholar]
- 4.Gessain A., Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front. Microbiol. 2012;3:388. doi: 10.3389/fmicb.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satake M., Yamaguchi K., Tadokoro K. Current prevalence of HTLV-1 in Japan as determined by screening of blood donors. J. Med. Virol. 2012;84:327–335. doi: 10.1002/jmv.23181. [DOI] [PubMed] [Google Scholar]
- 6.Take H., Umemoto M., Kusuhara K., Kuraya K. Transmission routes of HTLV-1: An analysis of 66 families. Jpn. J. Cancer Res. 1993;84:1265–1267. doi: 10.1111/j.1349-7006.1993.tb02832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy E.L., Hanchard B., Figueroa J.P., Gibbs W.N., Lofters W.S., Campbell M., Goedert J.J., Blattner W.A. Modelling the risk of adult T cell leukemia/lymphoma in persons infected with human T-lymphotropic virus type I. Int. J. Cancer. 1989;43:250–253. doi: 10.1002/ijc.2910430214. [DOI] [PubMed] [Google Scholar]
- 8.Itabashi K., Miyazawa T., Sekizawa A., Tokita A., Saito S., Moriuchi H., Nerome Y., Uchimaru K., Watanabe T. A nationwide antenatal human T cell leukemia virus type-1 antibody screening in Japan. Front. Microbiol. 2020;11:595. doi: 10.3389/fmicb.2020.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinoshita K., Hino S., Amagaski T., Ikeda S., Yamada Y., Suzuyama J., Momita S., Toriya K., Kamihira S., Ichimaru M. Demonstration of adult T cell leukemia virus antigen in milk from three sero-positive mothers. Gan. 1984;75:103–105. [PubMed] [Google Scholar]
- 10.Nakano S., Ando Y., Ichijo M., Moriyama I., Saito S., Sugamura K., Hinuma Y. Search for possible routes of vertical and horizontal transmission of adult T cell leukemia virus. Gan. 1984;75:1044–1045. [PubMed] [Google Scholar]
- 11.Kinoshita K., Yamanouchi K., Ikeda S., Momita S., Amagasaki T., Soda H., Ichimaru R., Katamine S., Miyamoto T. Oral infection of a common marmoset with human T cell leukemia virus type-1 (HTLV-1) by inoculating fresh human milk of HTLV-1 carrier mothers. Jpn. J. Cancer Res. 1985;76:1147–1153. [PubMed] [Google Scholar]
- 12.Ando Y., Nakano S., Saito K., Shimamoto I., Ichijo M., Toyama T., Hinuma Y. Transmission of adult T cell leukemia retrovirus (HTLV-I) from mother to child: Comparison of bottle- with breast-fed babies. Jpn. J. Cancer Res. 1987;78:322–324. [PubMed] [Google Scholar]
- 13.Hino S., Sugiyama H., Doi H., Ishimaru T., Yamabe T., Tsuji Y., Miyamoto T. Breaking the Cycle of HTLV-1 Transmission via Carrier Mother’s Milk. Lancet. 1987;18:158–159. doi: 10.1016/S0140-6736(87)92358-0. [DOI] [PubMed] [Google Scholar]
- 14.Carroll C., Booth A., Campbell F., Relton C. Qualitative evidence synthesis of values and preferences to inform infant feeding in the context of non-HIV transmission risk. PLoS ONE. 2020;15:e0242669. doi: 10.1371/journal.pone.0242669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ministry of Health, Labour and Welfare Nyuyoji Eiyouchousa Kekka No Gaiyou. [(accessed on 29 January 2021)]; Available online: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000134208.html. (In Japanese)
- 16.Itabashi K. HTLV-1 Boshikansen Yobou Taisaku Manyuaru. [(accessed on 29 January 2021)]; Available online: https://www.mhlw.go.jp/bunya/kodomo/boshi-hoken16/dl/06.pdf. (In Japanese)
- 17.Nerome Y., Kojyo K., Ninomiya Y., Ishikawa T., Ogiso A., Takei S., Kawano Y., Douchi T., Takezaki T., Owaki T. Current Human T cell lymphotropic virus type 1 mother-to-child transmission prevention status in Kagoshima. Pediatr. Int. 2014;56:640–643. doi: 10.1111/ped.12385. [DOI] [PubMed] [Google Scholar]
- 18.Itabashi K., Miyazawa T., Nerome Y., Sekizawa A., Moriuchi H., Saito S., Yonemoto N. Issues of infant feeding for postnatal prevention of HTLV-1 mother-to-child transmission. Pediatr. Int. 2020;63:284–289. doi: 10.1111/ped.14356. [DOI] [PubMed] [Google Scholar]
- 19.Wells G.A., Shea B., O’Connell D., Peterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. [(accessed on 30 January 2021)]; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 20.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uemura S., Yonezawa Y., Hiramatsu Y., Eguchi K., Sekiba K. Okayama-Ken Ni Okeru HTLV-1 Boshikansen Chousaseiseki. Okayama Ken Bosei Eisei. 1989;6:51. (In Japanese) [Google Scholar]
- 22.Ureta-Vidal A., Angelin-Duclos C., Tortevoye P., Murphy E., Lepère J.F., Buigues R.P., Jolly N., Joubert M., Carles G., Pouliquen J.F., et al. Mother-to-child transmission of human T cell-leukemia/lymphoma virus type I: Implication of high antiviral antibody titer and high proviral load in carrier mothers. Int. J. Cancer. 1999;82:832–836. doi: 10.1002/(SICI)1097-0215(19990909)82:6<832::AID-IJC11>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 23.Takezaki T. Kagoshima-Ken Ni Okeru HTLV-1 Kyaria Haha Karano Shusseiji Ni Okeru Tsuiseki Kenkyuu. [(accessed on 30 January 2021)];Health Lab. Sci. Res. Grant. 2009 59–61 Available online: https://www.mhlw.go.jp/bunya/kodomo/boshi-hoken16/dl/02_4.pdf. (In Japanese) [Google Scholar]
- 24.Masuzaki H., Moriuchi H., Miura K., Kamihira K.S. 25nenkann Keizoku Shita HTLV-1 Koutai Kensa Kara Erareta Boshikansen Yobou Kouka No Kenshou Oyobi Kouseido Sukuriiningu Jigyou Seika No Kenshou. [(accessed on 30 January 2021)];Health Lab. Sci. Res. Grant. 2013 :19–25. Available online: https://mhlw-grants.niph.go.jp/niph/search/NIDD00.do?resrchNum=201318019A. (In Japanese) [Google Scholar]
- 25.Moriuchi H., Takeda K., Nakajima Y. Kyaria Botai Kara Umareta Ji No Suiseki Chousa. [(accessed on 30 January 2021)];Health Lab. Sci. Res. Grant. 2017 :92–94. Available online: https://mhlw-grants.niph.go.jp/niph/search/NIDD00.do?resrchNum=201606014B. (In Japanese) [Google Scholar]
- 26.Nakayama H., Take H., Umemoto M., Seki S., Kuraya K. Mother-to-child transmission of HTLV-1-Comparison between breast- and bottled-fed children- J. Jpn. Pediatr. Soc. 1992;96:2092–2096. (In Japanese) [Google Scholar]
- 27.Oki T., Yoshinaga M., Otsuka H., Miyata K., Sonoda S., Nagata Y. A sero-epidemiological study on mother-to-child transmission of HTLV-I in southern Kyushu, Japan. Asia Ocean. J. Obstet. Gynaecol. 1992;18:371–377. doi: 10.1111/j.1447-0756.1992.tb00333.x. [DOI] [PubMed] [Google Scholar]
- 28.Takezaki T., Tajima K., Ito M., Ito S., Kinoshita K., Tachibana K., Matsunaga Y. The Tsushima ATL Study Group. Short-term breast-feeding may reduce the risk of vertical transmission of HTLV-1. Leukemia. 1997;11:60–62. [PubMed] [Google Scholar]
- 29.Maehama T., Nakayama M., Nagamine M., Nakashima Y., Takei H., Nakachi H. Studies on factor affecting mother-to-child HTLV-I transmission. Acta Obstet. Gynaecol. Jpn. 1992;44:215–222. (In Japanese) [PubMed] [Google Scholar]
- 30.Ekuni Y. Prevention of HTLV-I vertical transmission-usefulness of frozen–thawed breast milk. Adv. Obstet. Gynecol. 1997;49:171–179. (In Japanese) [Google Scholar]
- 31.Ando Y., Ekuni Y., Matsumoto Y., Nakano S., Saito K., Kakimoto K., Tanigawa T., Kawa M., Toyama T. Long-term serological outcome of infants who received frozen-thawed milk from human T-lymphotropic virus type-1 positive mothers. J. Obstet. Gynaecol. Res. 2004;30:436–438. doi: 10.1111/j.1447-0756.2004.00227.x. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization . Human T-lymphotropic Virus Type 1: Technical Report. World Health Organization; Geneva, Switzerland: 2021. [(accessed on 27 April 2021)]. Available online: https://www.who.int/publications/i/item/9789240020221. [Google Scholar]
- 33.Takahashi K., Takezaki T., Oki T., Kawakami K., Yashiki S., Fujiyoshi T., Usuku K., Mueller N., Osame M., Miyata K. Inhibitory effect of maternal antibody on mother-to-child transmission of human T-lymphotropic virus type I. The Mother-to-Child Transmission Study Group. Int. J. Cancer. 1991;49:673–677. doi: 10.1002/ijc.2910490508. [DOI] [PubMed] [Google Scholar]
- 34.Hisada M., Maloney E.M., Sawada T., Miley W.J., Palmer P., Hanchard B., Goedert J.J., Manns A. Virus markers associated with vertical transmission of human T lymphotropic virus type 1 in Jamaica. Clin. Infect. Dis. 2002;34:1551–1557. doi: 10.1086/340537. [DOI] [PubMed] [Google Scholar]
- 35.Paiva A.M., Assone T., Haziot M.E.J., Smid J., Fonseca L.A.M., Luiz O.C., de Oliveira A.C.P., Gasseb J. Risk factors associated with HTLV-1 vertical transmission in Brazil: Longer breastfeeding, higher maternal proviral load and previous HTLV-1-infacted offspring. Sci. Rep. 2018;8:7742. doi: 10.1038/s41598-018-25939-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosadas C., Taylor G.P. Mother-to-child HTLV-1 transmission: Unmet research needs. Front. Microbiol. 2019;10:999. doi: 10.3389/fmicb.2019.00999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boostani R., Sadeghi R., Sabouri A., Ghabeli-Juibary A. Human T-lymphotropic virus type I and breastfeeding; systematic review and meta-analysis of the literature. Iran. J. Neurol. 2018;17:174–179. doi: 10.18502/ijnl.v17i4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kusuhara K., Sonoda S., Takahashi K., Tokugawa K., Fukushige J., Ueda K. Mother-to-child transmission of human T cell leukemia virus type I (HTLV-I): A fifteen-year follow-up study in Okinawa, Japan. Int. J. Cancer. 1987;40:755–757. doi: 10.1002/ijc.2910400607. [DOI] [PubMed] [Google Scholar]
- 39.Ando Y., Matsumoto Y., Nakano S., Saito K., Kakimoto K., Tanigawa T., Ekuni Y., Kawa M., Toyama T. Long-term follow up study of vertical HTLV-I infection in children breast-fed by seropositive mothers. J. Infect. 2003;46:177–179. doi: 10.1053/jinf.2002.1107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used in this study can be found in the full-text articles used in this systematic review.