Abstract
Respiratory diseases are leading causes of death and disability around the globe, with a diverse range of health problems. Treatment of respiratory diseases and infections has been verified to be thought-provoking because of the increasing incidence and mortality rate. Hydrogen sulfide (H2S) is one of the recognized gaseous transmitters involved in an extensive range of cellular functions, and physiological and pathological processes in a variety of diseases, including respiratory diseases. Recently, the therapeutic potential of H2S for respiratory diseases has been widely investigated. H2S plays a vital therapeutic role in obstructive respiratory disease, pulmonary fibrosis, emphysema, pancreatic inflammatory/respiratory lung injury, pulmonary inflammation, bronchial asthma and bronchiectasis. Although the therapeutic role of H2S has been extensively studied in various respiratory diseases, a concrete literature review will have an extraordinary impact on future therapeutics. This review provides a comprehensive overview of the effective role of H2S in respiratory diseases. Besides, we also summarized H2S production in the lung and its metabolism processes in respiratory diseases.
Keywords: hydrogen sulfide, respiratory diseases, metabolism processes, signaling pathways
1. Introduction
Respiratory diseases are generally the most common disorders, mainly characterized by lesions located in the trachea, bronchi, lungs and chest. Typical clinical symptoms include cough, asthma and chest pain. Severe cases involve difficulty in breathing or even respiratory failure. Overall, respiratory diseases are physiologically categorized as obstructive or restrictive [1]. Obstructive diseases usually inhibit the flow rate into and out of the lungs, while the obstructive circumstances cause a reduction in the functional lung volume. Common respiratory diseases are pneumo-thorax, pulmonary bulla, emphysema, lung cancer, pulmonary heart disease, respiratory failure, pulmonary embolism, lung abscess, pneumonia, neonatal pneumonia, pediatric pneumonia, bronchitis, asthma, tuberculosis, pneumoconiosis and interstitial lung disease. Moreover, the respiratory tract infections can further be discriminated by the location (i.e., upper or lower tract infections) affected by bacterial or viral infections [2,3]. Over the past three decades, respiratory diseases’ incidence has increased progressively in developed countries and attracted much attention. The World Health Organization (WHO) has defined chronic respiratory diseases as one of the chief diseases smiting the human world and has drawn much attention to their prevention, identification and treatment [4,5,6].
Hydrogen sulfide (H2S) is found in a gaseous state, is soluble in water, and has a distinctive odor like rotten eggs. Formerly, H2S was understood to be toxic due to its respiratory complex activities in mitochondria, resulting in cellular incapability to metabolize oxygen in an oxidative manner [7,8,9]. However, in recent years, there has been increasing evidence that H2S plays an essential role in various physiological and pathological processes, such as inflammation [10,11,12,13], neuromodulation [14], injury repair [15] and hypertension [16]. Furthermore, Szabo et al. threw light on cancer, disclosing that cystathionine beta-synthase (CBS), one of the critical enzymes involved in the formation of H2S, is highly expressed in colorectal cancer cells in comparison with nearby adjacent normal mucosal margin cells [17]. On the other hand, both shRNA silencing and pharmacological treatment-mediated CBS inhibition can induce the suppression of the multiplication of cancerous cells in the colon both in vitro and in vivo.
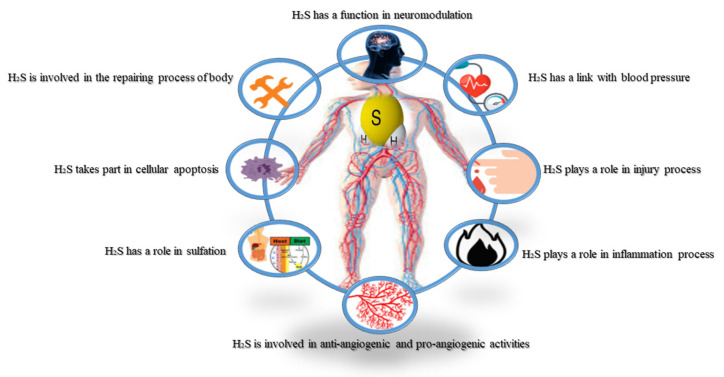
Furthermore, both gene silencing of CBS and the pharmacological inhibition of CBS cause distinct energy conversion at the cell level in cancerous colon cells [17]. The ratio of CBS to H2S plays a vital role in cancer progression, including in ovarian cancer [18] and breast cancer [19]. Otherwise, cystathionine γ-lyase (CSE) overexpression, another H2S-producing enzyme, has also been reported in melanoma [20]. Moreover, H2S plays a vital role in cellular activities such as proliferation, angiogenesis, the function of the mitochondria and vascular relaxation, and is thought to be an essential factor in cancer biology [21,22,23,24,25,26,27]. H2S is reported to induce both suppressive and inhibitory effects in human aorta SMC via the ERK/EGFR/MMP-2 and Akt/PTEN signaling pathways, and H2S activates MAPK and caspase-3 to initiate apoptosis [28]. Meanwhile, sulfhydration of NF-κB by H2S promotes anti-apoptotic activities [29]. It has been reported that phosphorylation of protein kinase Akt by H2S can also induce anti-angiogenic properties [30,31,32]. The antioxidant capacity of H2S has been investigated in specific animal experiments [33,34]. Nevertheless, the mechanisms by which H2S is produced inside tumor cells and enhances cancer cell growth, are still being unraveled. Figure 1 highlights the essential roles of H2S in the human body.
Figure 1.
A schematic diagram showing the roles of H2S in human physiology and pathology. H2S is formed throughout the body and moderates signaling processes in various tissues, including neuromodulation, blood pressure, injury, inflammation, anti-angiogenesis, pro-angiogenesis and sulfhydration apoptosis repair processes of the human body (H2S: hydrogen sulfide).
H2S is closely related to respiratory activities and can affect the outcome of various respiratory diseases. For example, several studies have indicated that serum levels of H2S in patients with chronic obstructive pulmonary disease (COPD) are low. This event is correlated with reduced chronic inflammation of the airway and vascular remodeling. Such activity shows the curative effects on pulmonary hypertension and asthma [35,36,37,38]. H2S in the respiratory tract induces anti-apoptosis and anti-inflammatory effects and regulates vascular permeability. According to recent reports, patients with acute exacerbation of COPD have significantly low serum H2S, while serum levels of H2S in smokers are much lower than in non-smokers [39].
Several pathways describe the damage of H2S in respiratory diseases. However, some mechanisms are still not fully clarified. H2S inhibits respiratory rhythm in neonates through the medulla [40]. H2S can reduce other substances and is oxidized via circulating oxidants. H2S, together with NO, CO and cyanide, are highly toxic, and micromolar concentrations can ultimately inhibit mitochondrial respiration [7]. H2S is found in combination with sulfate and sulfur species. The compounds have variable forms ranging from persulfide and polysulfide to elemental sulfur. The compounds are reactive [41]. A species of sulfane-sulfur acts as a store of H2S, which maintains toxicity and allows H2S to react with biological signals through sulfhydration [42,43]. Similarly, the sulfur compounds act through S-sulfhydration and are involved in most activities of H2S [44]. Altered biosynthesis of H2S is also linked with sulfate-sulfur levels based on pathophysiology, suggesting a close relationship.
The defective production of H2S initiates several systemic disorders, and such a situation reveals the advance of effective pharmacological mediators that increase H2S levels. H2S modulation in pharmacy is a recent dynamic field that is well-reported and examined for specific significance [45,46,47]. Currently, a considerable number of natural and artificial compounds have been documented as potent H2S donors [48], and many of them are in clinical trials for the treatment of cardiovascular disease (SG-1002 for heart failure) [48] and cancer (sulforaphane) [49]. In this review, the production and metabolism of H2S in the lung are highlighted. Besides, the mechanisms and roles associated with the effects of H2S in respiratory diseases are further explored.
2. Regulation of H2S and H2S-Mediated Sulfhydration in the Lung
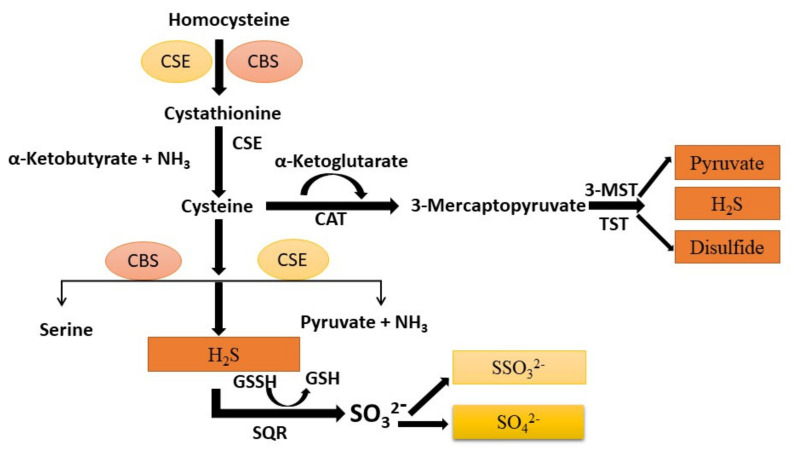
H2S is produced via CSE, CBS and 3-mercaptopyruate transferase (3-MPST) in the lung, but various species or cell types show different expression levels of the three enzymes. Their catalytic activities are reinforced by the reducing enzymes, mainly sulfide-quinone reductase (SQR) and thiosulfate sulfide or thiocyanate (TST) as shown in Figure 2 [50].
Figure 2.
Illustration of vascular synthesis of H2S formed by a catalytic process in several enzymes (CSE, CBS, and 3-MSPT) in the lung. Cysteine is generated from homocysteine through transculturation pathways intervened by CBS and CSE. H2S forms from homocysteine and cysteine via CBS and CSE. 3-MSPT forms 3-MST-cysteine persulfide (MST-SSH) using mercapto pyruvate, which is formed from cysteine via CAT. H2S is formed from MST-SSH via a non-enzymatic reaction. H2S is oxidized via sulfide oxidation to form thiosulfate and sulfate. H2S is produced from thiosulfate through a non-enzymatic reaction through reductants via the catalytic activity of thiosulfate sulfurtransferase or 3-MST. H2S: hydrogen sulfide; SQR: sulfide-quinone reductase; CBS: cystathionine beta-synthase; CSE: cystathionine γ-lyase; 3-MPST: 3-mercaptopyravute sulfurtransferase; TST: thiosulfate sulfurtransferase; CAT: cysteine aminotransferase; GSSH: glutathione.
The CSE is localized in the endothelium and smooth muscle, while 3-MPST is present in the mitochondria and cytoplasm [51]. Both human airway smooth muscle cells (SMCs) and human lung primary fibroblast MRC-5 cells express CSE and CBS. Immunohistochemical staining shows that CSE is present in the peripheral lung tissues of the airway and pulmonary vessels in rat lung, and mutually CSE and CBS are primarily expressed in pulmonary blood vessels, SMCs and endothelial cells, and airway SMCs in mouse lungs [52]. The catabolic process of H2S in the mitochondria is thiosulfate production, which can be further converted to sulfide and then sulfate by rhodanese enzyme action. Besides, methylation of H2S by thiol S-methyl transferase can produce dimethyl sulfide.
Most of the cellular responses mediated by H2S initiate after sulfhydration and post-translational modification of proteins [53]. Persulfides of H2S can modify proteins containing the thiol group. The mechanism through which H2S targets a particular thiol protein for S-sulfhydration is in its infancy. Some experimental data suggest that H2S attacks thiol-containing proteins, which oxidise as thiolate ions for S-sulfhydration. For example, cysteine residues with a low pKa exist as thiolate anions in typical situations and hence are more definitely confronted by numerous oxidants and are susceptible to S-sulfhydration [54]. The acid–base idea might offer a latent clarification of the mechanism of protein S-sulfhydration. The S-sulfhydration of tissue/cell-specific proteins may occur due to the altered enzymatic activity of H2S-producing proteins.
3. The Role of H2S in Respiratory Diseases
3.1. H2S and Chronic Obstructive Pulmonary Diseases
Pulmonary diseases such as emphysema, COPD and chronic bronchitis affect the respiratory tract’s airflow. The hindrance is inferred in developing common chronic diseases such as respiratory failure, and pulmonary and heart diseases [55]. One of the key preventable causes of COPD is smoking. However, the mechanism of COPD is not entirely understood. Generally, COPD results from airway inflammation interacting with reactive oxygen species (ROS) [39]. Evidence shows that serum H2S levels are significantly reduced in patients with COPD with acute exacerbations. Wang et al. show that H2S acts efficiently to improve respiration and reduce histopathological variations, such as lung edema and permeability.
Oxidative stress, inflammation and airway remodeling also decrease via H2S treatment [56]. H2S exerts both pro- and anti-inflammatory effects. H2S is anti-inflammatory and cyto-protective due to its ability to act as an antioxidant and a reducing agent, and its scavenging features [57]. NaHS, a donor of H2S, inhibits the in vitro production of intracellular oxidation and cellular damage induced by nitrates, hypochlorous acid and nitrous oxide (NO). It also inhibits the activity and expression of nicotinamide adenine dinucleotide phosphate (NADPH) and scavenges lipid peroxide [58]. Oleic acid induced lung injury in an animal model, while NaHS inoculation reduced lung injuries and plasma levels of interleukin (IL)-6 and IL-8, and the accumulation of inflammatory cells [59,60]. Moreover, in a murine model, the decrease in pro-inflammatory cytokine IL-1β and the rise of the anti-inflammatory cytokine IL-10 occurred after the administration of H2S in smoke and burn-induced lung injury [61].
Furthermore, the elevation in the expression of CSE, CBS and H2S levels in the pancreas, lung, liver, kidney and plasma of both mice and rats could cause acute inflammation [62]. The smoke of cigarettes is a primary etiological factor for the development of COPD in rat lungs. Treatment with NaHS can reduce lung inflammation and airway resistance caused by smoke [63]. A study shows that inhaled H2S develops lung function and prevents bronchial hyper-reactivity by moderating mast cells and fibroblast initiation [64]. Higher levels of serum H2S were positively correlated with severe COPD in stable COPD patients. Conversely, H2S levels in the serum are decreased in exacerbated COPD in a steady disease state [37].
In contrast, H2S levels in the sputum are higher in exacerbated COPD than in steady-state COPD, non-smokers and healthy subjects [65]. NaHS also protects against oxidative stress, airway inflammation, remodeling and an enhanced development rate of emphysema induced by tobacco smoke [39]. H2S formation offers a new mechanism for suppressing airway smooth muscle (ASM) cell propagation and cytokine release. H2S donors inhibit propagation and cytokine release in COPD ASM cells by inhibiting CBS and 3-MPST. However, COPD ASM cells’ capacity to react to H2S donors is not significant in smoker and non-smoker cells [66]. H2S treatment inhibited elevated levels of transforming growth factor-beta 1 (TGF-β1) and Smad in a cigarette smoke-induced COPD model via the inhibition of TGF-β1 and Smad pathways.
It has been revealed that the serum levels of H2S in smoker subjects are much lower than in non-smokers [39]. The endogenous H2S is associated with the activity and severity of COPD [37]. Further studies showed that H2S could protect macrophages from exposure to inflammation and oxidative stress, thereby enhancing macrophages’ corticosteroid sensitivity [67]. Besides, low levels of H2S in exhaled gases can be used to predict eosinophilia in patients [68]. Moreover, the sputum-to-serum ratio of H2S can be used to predict obstructive neutrophilic inflammation and COPD progression [68]. The imbalance of H2S/Hcy may contribute to COPD pathogenesis combined with cardiovascular diseases, providing a new target for treatment [69]. The intrinsic enzymatic mechanism of H2S expression in human airway SMCs has shown the potential for H2S being exploited to treat obstructive pulmonary disease (Figure 3) [70].
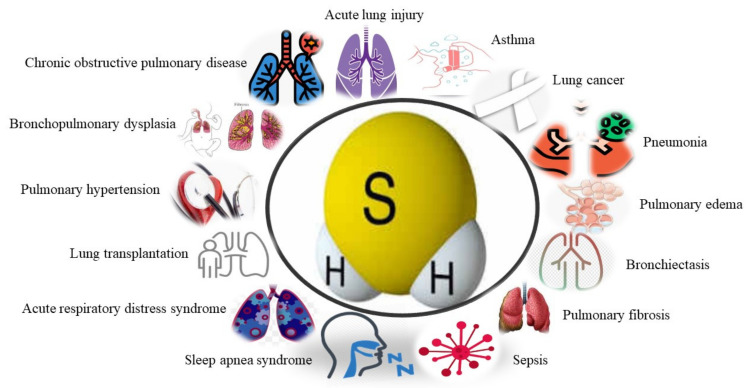
Figure 3.
The roles of H2S in human respiratory diseases, including COPD, ALI, asthma, lung cancer, pneumonia, pulmonary edema, bronchiectasis, pulmonary fibrosis, sepsis, SAS, ARDS, lung transplantation, pulmonary hypertension and bronchopulmonary dysplasia. COPD: chronic obstructive pulmonary disease; ALI: acute lung injury; ARDS: acute respiratory distress syndrome; SAS: sleep apnea syndrome; BPD: bronchopulmonary dysplasia.
3.2. H2S and Acute Lung Injury (ALI)
ALI is considered to be a set of medical symptoms, such as hikes in the permeability of the epithelial and pulmonary vascular system, acute inflammation and microvascular damage, leading to acute respiratory failure and respiratory distress syndrome [65]. Many clinical diseases can cause ALI, such as pancreatic inflammatory lung injury, ventilator lung injury and burn lung injury [71,72].
H2S reduces lung injury through numerous signaling pathways [73,74]. H2S also assisted in reducing oxidative stress and inflammation to control LPS-induced acute ALI [75]. Exogenous H2S prevented ALI by reducing mitochondrial lipid peroxidation and attenuating pro-inflammatory responses positively related to the H2S dose to protect the cell structure in LPS-induced rat models [76]. H2S inhalation prevents ALI by regulating p38 MAPK signal transduction and Nox-2 expression and synergistic inhibition of ROS formation [77]. Treating rats with H2S reduced the transcription of iNOS mRNA, iNOS and nitric oxide (NO); inhibited the activation of NF-κB p65 and attenuated oxidative stress, thereby preventing ALI [78]. H2S significantly reduces inflammation and pulmonary edema by regulating the TLR-4-Myd88-NF-κB pathway and AQP-1/AQP-5 expression [79]. Another study showed that thiosulfate inhibits NF-ҝB signaling in the pulmonary vascular endothelium to prevent ALI [80]. Simultaneously, the inhalation of H2S triggers genes for anti-apoptosis and anti-inflammation via the regulation of activating transcription factor-3 (ATF-3), demonstrating that ATF3 is noticeably involved in H2S-mediated protection [81]. Moreover, it has been revealed that dexamethasone can activate the PI3K pathway to improve the induction of H2S in developing acute ALI by increasing the expression of claudin-5 [82]. Similarly, NaHS treatment inhibits the inflammation and oxidation reactions via activation of Nrf-2 cell signaling in preventing lung injury after explosive limb trauma [83]. Furthermore, it has been shown that H2S also plays a role in preventing damage escalation in the alveoli and pulmonary hypertension (PHT) for lung injury induced by O2 [84]. While in another study, it was reported that intraperitoneal injection of 1 mg/kg NaHS improved the pulmonary levels of H2S and oxidative stress-related signs (ROS, myeloperoxidase (MPO) and malondialdehyde (MDA)) in a time-dependent way. Liu et al. pointed out that H2S attenuates oleic acid (OA)-induced lung injury by protective and upregulated endoplasmic reticulum proteins [85]. H2S also protects against ALI by reducing the expression of MMP-2 and MMP-9 [86]. Some studies reported that H2S induces a low metabolic status in anesthetized rats and prevents ventilator-induced lung injury by reducing lung inflammation unrelated to body temperature [87]. H2S can also reduce lung L/R injury pressure by reducing lung oxidation [15]. Alternatively, a study showed that an increment in the phosphorylation of myosin light chain (MLC) is protective against the toxicity of NaHS at the cellular level [33]. Meanwhile, an elevation in SMC properties, for instance, the expression of transgelin and motility, or a decrease in actomyosin improves cell survival after exposure to NaHS [87].
3.2.1. Pancreatic Inflammatory Lung Injury
Pancreatic inflammatory lung injury encompasses a set of inflammatory diseases, such as acute pancreatitis. Severe acute pancreatitis is dangerous with poor prognosis, high mortality and early multiple organ failure, especially ALI. After acute pancreatitis, the H2S synthase enzyme CSE in the pancreas induces morphological lung changes due to alveolar thickening and inflammatory cell infiltration [10]. Up to one third of all pancreatitis patients develop ALI or acute respiratory distress syndrome (ARDS), accounting for 60% of pancreatitis-associated deaths [88]. Bhatia et al. reported a good correlation between the level of H2S and the severity of pancreatitis, and indicated that the pro-inflammatory effects of H2S might be mediated by chemokines [89]. Tamizhselvi suggested that H2S may exhibit potent vasodilation activity through the vascular smooth muscle KATP channel, thereby affecting acute pancreatitis and the associated lung injury [90]. Endogenous H2S blocks sulfur mustard (SM)-induced oxidative damage through the Nrf-2 pathway [91]. Inhibition of CSE indicated anti-inflammatory outcomes in a murine model of pancreatitis-induced lung injury [10]. Bhatia et al. described that a high dose of NaSH (10 mg/kg I.P.) initiated lung inflammation and histological injury in mice, and this inflammation reverted to the baseline in 6 h post-injection, indicating that lethal consequences are due to high sulfide concentrations by the H2S donor (NaHS), which were rapidly cleared [92]. The administration of NaHS or H2S-releasing ACS15 [93] as a pre-treatment (10–15 mg/kg) decreased inflammation in pancreatitis-induced ALIs [89,93]. The study design influenced the role of NaHS. The high dose of 10 mg/kg of the H2S donor NaHS caused ephemeral lung inflammation in healthy subjects. At the same time, its administration as a pre-treatment induced anti-inflammatory effects in successive pancreatitis-induced ALI.
3.2.2. Inhalation-Induced Lung Injury
This refers to the degree of tracheal, bronchial and pulmonary parenchymal damage caused by various inhalation of harmful substances. Mild cases may only irritate cough and chest tightness; however, airway obstruction and pulmonary inflammation may also occur in severe cases. Even acute emphysema syndrome and multiple organ dysfunction syndromes (MODS) endanger the lives of patients. Burning is associated with a higher expression of CSE mRNA in the liver. Although H2S administration reduces tissue damage and inflammation, H2S donors exacerbate lung injuries caused by burns and smog inhalation in sheep [94,95]. On the other hand, treatment with Na₂S suppresses ALI caused by burns and smoke by attenuating iNOS expression, peroxynitrite formation, acute respiratory distress syndrome, nitro yield (lysine measurement), protein (oxidized protein carbonyl formation) and PARP-1 activity in vivo [94]. H2S biosynthesis inhibitors such as AOAA and the mitochondria-targeted H2S donor AP39 reduce intracellular and pulmonary plasma oxidative stress measured as MDA levels and cause organ infiltration into leukocytes (pulmonary MPO levels), pro-inflammatory and anti-inflammatory effects (circulating IL-6 and IL-10 levels), and liver and kidney dysfunction index (ALP and creatinine, respectively) [96]. Therapeutic administration of H2S biosynthesis the inhibitor PAG in mice with lung injuries caused by burns reduces systemic inflammatory non-MPO activity [91].
3.2.3. Ventilator-Induced Lung Injury (VILI)
Although lung-protective ventilation strategies (LPVS) are regularly practiced for patients with ARDS, ventilator-induced lung injury (VILI) has received widespread attention as a common complication. H2S relieves VILI by decreasing autophagy and endoplasmic reticulum stress in L2 cells and rats by decreasing PERK, PERK phosphorylation and nuclear expression ATF4 after treatment with NaHS [73]. The cyclin strain initiates nuclear NF-ҝβ, MAPK, JNK, p65, p38, and ERK; whereas the ER stress inhibitor 4-PBA or NaHS suppress them. The H2S donor NaHS and inhaled H2S prevent ALI caused by ventilators [39]. H2S decreases the migration of neutrophils and the release of cytokines, thus exerting anti-inflammatory effects [97]. H2S limits lung injury due to ventilators by hindering ROS production via the PI3K/Akt signaling pathway [74]. Francis, R.C. et al. recommended systemic endovascular treatment with Na2S, which prevents ventilator-induced lung injury and lung glutathione depletion by activating Nrf-2-dependent antioxidant gene transcription [98].
3.3. H2S and Asthma
Asthma affects about 334 million individuals and has a high global death ratio [99]. Therefore, asthma is a leading international health, economic and social concern. However, asthma’s pathogenesis includes allergies, chronic airway inflammation, responsiveness, airway neuromodulator disorders, genetic mechanisms, respiratory viral infections, neural signal transduction mechanisms and airway remodeling, and is not fully understood. The H2S metabolism influences the physiology of the lung and the development of asthma. Condensed endogenous H2S levels caused by the decline in H2S-producing enzymes may start an infective asthma infection aspect [52]. Alternatively, another study shows that a high dose (300 ppm) of H2S diffuses into the bloodstream through the lung membrane and causes hypoxemia, vasodilation and vasoconstriction [100].
Further evidence reveals that H2S stimulates the mitochondria to produce superoxide, which is converted to hydrogen peroxide (H₂O₂) to mediate hypoxic vasoconstriction [51]. A previous experimental study in asthmatic patients also found that exogenous H2S inhibits cell propagation and IL-8 release by attenuating the phosphorylation of ERK1/2 and p38 [101]. Clinical trials showed a strong association between serum H2S levels and forced expiratory volume (FEV 1.0), and a negative correlation with sputum cell count and sputum neutrophil percentage in acute asthma patients [30]. Wang, P. et al. reported that oxidative stress and mitochondrial dysfunction are related to asthma’s progress and development [52]. Similarly, antioxidants decrease mitochondrial dysfunction and oxidative stress in asthma [102]. A previous study revealed that endogenous H2S reduces airway inflammation and renovation in rat asthma models [39].
Besides, asthmatic mice characterized by inflammation and ovalbumin (OVA) decreased H2S production and CSE expression. Similarly, exogenous administration of NaHS reduced inflammation, and decreased airway infiltration by the neutrophils and eosinophils. Furthermore, NaHS reduced OVA, which initiates lung iNOS activation, restricting airway alterations. These facts indicate that H2S formed from CSE acts as an anti-remodeling and anti-inflammatory mediator in asthma’s pathogenicity [36]. In stable asthmatic patients or patients with acute exacerbation, H2S level is lower in their serum. On the other hand, serum [52] or an exhaled air H2S level [103] showed a positive relationship with forced expiratory volume and a negative association with neutrophil count [38]. The same results were recorded in small children having asthma [102].
3.4. H2S and Lung Cancer
Lung cancer is one of the most prevalent malignities globally and is a prominent source of cancer-associated mortalities. Recent studies indicated that the expression of different H2S-producing enzymes in cancer cells of different tissue types is high, suggesting the gas’s potential in developing the disease [83]. Szczesny et al. showed that severe mitochondrial DNA damage in lung cancer cells is linked to H2S and that normal lung epithelial cells do not have elevated cell A549/DDP cells (compared with A549 cells) [104].
3.5. H2S and Pneumonia
Pneumonia is an inflammation of the terminal respiratory tract, alveoli and interstitial lungs caused by microbes, physical and chemical factors, drug allergies and immune damage. Depending on the type of pathogen, it can be divided into fungal pneumonia, bacterial pneumonia, mycoplasma pneumonia and viral pneumonia. However, bacterial pneumonia is the most common kind of pneumonia and is a paramount public contagious infection. H2S has pro-inflammatory effects in various inflammatory models [105,106,107]. In the inflammatory model, plasma H2S levels, tissue H2S synthesis activity and CSE expression increased. Some of the literature has stated the anti-inflammatory effects of H2S treatments such as using s-diclofenac, ATB-429 and H2S donors (NaHS, Lawson’s reagent, N-acetyl cysteine) in inflammation to produce anti-inflammatory activity [58,95,107,108]. Recent studies have also shown a biphasic dose–response effect of H2S in inflammation [109].
H2S has an anti-inflammatory effect in a dose-dependent manner on pulmonary inflammation [110]. It has been shown that supplementation with H2S or inhibition of iNOS-induced elevation of the GSH/GSSG ratio is a possible mechanism for defending the airways from oxidative stress and inflammatory lung disease [111]. Prophylactic and therapeutic use of NaHS reduced total cell growth induced by ozone, containing macrophages and neutrophils. This type of treatment also reduces cytokine levels in broncho-alveolar lavage fluid, including TNF-α, factor (CXC motif) ligand 1, IL-1β and IL-6 levels; inhibits them bronchially; attenuates the ozone-induced increase in total MDA in broncho-alveolar lavage fluid and reduces the ratio of condensed glutathione/oxidized glutathione in the lung. Besides, NaHS can block and reverse the phosphorylation of p38 MAPK and heat shock protein [103]. This shows that H2S might have protective and therapeutic significance in treating airway diseases based on oxidative stress.
In 2013, Aslami et al. reported that NaHS might promote ATP synthesis and mitochondrial biogenesis by protecting oxidative phosphorylation to reduce organ damage in pulmonary sepsis caused by pneumococci [112]. Another study suggested that H2S produced by Streptococcus pneumoniae causes hemolysis via the enzymatic activity of HapE (a protein similar to cysteine desulfurase) [113]. In respiratory syncytial virus (RSV), H2S was found to have an overall inhibitory effect on paramyxoviruses (for example, human metapneumovirus (hMPV) and Nipah virus (NiV)) [114]. Pediatric cystic fibrosis can be chemically active, anoxic and highly condensed due to H2S formation [115], and H2S can upregulate cytokine and chemokine production, and aggravates NF-κB activation by participating in systemic inflammatory sepsis [116,117]. NaHS protects rat lungs from inflammatory responses through hemorrhagic shock, inhibiting oxidative stress, and Fas/FasL apoptotic signaling pathways [107]. In summary, different doses of NaHS and downregulation in lung inflammation were achieved through a reduction in pro-inflammatory chemokines and adhesion molecules.
3.6. H2S and Pulmonary Edema
Pulmonary edema is caused by the accumulation of tissue fluid and the loss of the intrapulmonary tract. A considerable quantity of tissue fluid cannot be absorbed through the pulmonary lymphatic vessels and the pulmonary venous system quickly if it is extravagated from the pulmonary capillaries, and collects in the alveoli, interstitial lungs and small bronchi on the lungs [118,119]. Ventilation causes serious obstacles. The clinical manifestations include breathing difficulties, cyanosis, extreme dyspnea, paroxysmal coughing and excessive sweating with a large amount of white/pink foamy sputum and double-lung balanced wet voice coverage. Experiments have shown that inhibition of transepithelial Na+ transportation gives a mechanism that improves edema development in H2S-exposed lungs [120,121,122]. NaHS decreases airway inflammation remodeling and tobacco smoke-induced oxidative stress, and enhances emphysema and developmental hypertension [39]. These protective outcomes are connected with improved phosphorylation of Akt and hindering of the downregulation of antioxidant molecules.
3.7. H2S and Bronchiectasis
Bronchiectasis relates to the destruction of the bronchial wall muscles and elastic tissues affected by chronic suppurate inflammation and fibrosis of the bronchus and its adjacent lung tissues, resulting in bronchial deformation and continued expansion. Typical symptoms are chronic cough and repeated hemoptysis. There are two different perspectives on the effects of H2S on bronchiectasis. Firstly, it leads to bronchodilation via regulation of the K-ATP channel and β-adrenergic receptors [123]. Secondly, the relaxation effect of NaHS is inactivated by Ca2+ influx and cholinergic receptor blockade [124]. Bronchiectasis is associated with increased Cl– and IκB phosphorylation. H2S regulates Cl– levels and decreases phosphorylated IκB expression, inhibiting the upregulation of pro-inflammatory cytokines in epithelial airway cells [125].
3.8. H2S and Pulmonary Fibrosis
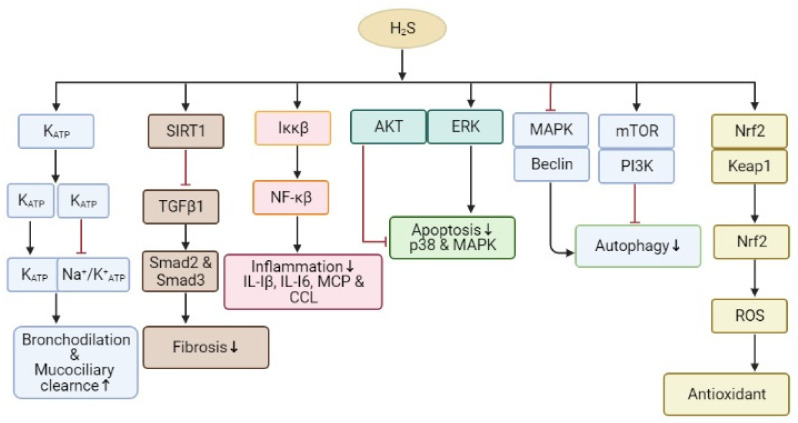
Pulmonary fibrosis is the most common form of interstitial lung illness. It involves a slow exchange of normal lung parenchyma and fibrotic tissues, leading to an irreparable reduction in oxygen diffusion ability. The causes of pulmonary fibrosis are diverse, and there are many triggers, e.g., chemicals, allergens, radiation and environmental particles [126]. The anti-fibrotic effect of H2S on pulmonary fibrosis is that H2S protects against oxidative stress and inflammation [127]. Studies indicate that the H2S donor induces the nuclear buildup of Nrf-2 in lung tissues, thereby upregulating the expression of the Nrf-2-regulated antioxidant genes HO-1and Trx-1 in smoking rats [91,126]. Moreover, H2S can decrease cigarette smoke-induced inflammation by preventing ERK1/2, JNK and p38 MAPK phosphorylation, and adversely regulating NF-ҝβ activation, thereby preventing pulmonary fibrosis in smoking rats (Figure 4) [128]. Wang et al. also have shown that the anti-fibrotic effect of H2S relates to the inhibition of the TGFβ/Smad pathway [129]. In contrast, a high concentration of H2S (50–500 ppm) may produce occlusive bronchiolitis and pulmonary edema, leading to chronic inflammation and pulmonary fibrosis [130].
Figure 4.
The signaling pathways underlying H2S regulation of inflammation, fibrosis, apoptosis, autophagy, antioxidant activity and bronchodilation. H2S has an anti-inflammatory outcome with diverse biological results, directly and indirectly decreasing activities such as Nrf2 activation. Abbreviations: ROS: reactive oxygen species, NF-ҝβ: nuclear factor-kappa B; Nrf2: nuclear factor erythroid-2 related factor 2; HO-1: heme oxygenase-1; PI3K: phosphoinositide 3-kinase; AMPK: AMP-activated protein kinase; ERK: extracellular signal-regulated kinase; TNF-α: tumor necrosis factor; TGF-β1: transforming growth factor-beta 1; Keap1: Kelch-like-ECH-associated protein; IL: interleukin; IKK: IҝB kinase.
3.9. H2S and Sepsis
The incidence of sepsis is high, with more than 19 million severe sepsis cases occurring worldwide each year [131]. The underlying pathogenesis of sepsis remains unclear. It involves complex systemic inflammatory network effects, genetic polymorphisms, immune dysfunction and coagulopathy. H2S activates the selective transient receptor potential vanilloid 1 (TRPV1) by enhancing the upregulation of COX-2 and PGEM, coordinating with the neurogenic inflammatory response. The overproduction of substance P initiates a neuro-inflammatory process, namely ERK-NF-Κβ. ERK-NF-κB is activated in a TRPV1-dependent manner and significantly increases sepsis severity [90,132]. H2S upregulates substance P by activating the substance P receptor to coordinate the inflammatory response, leading to lung inflammation and sepsis damage [133]. The failure of exogenous H2S to prevent neutrophil migration caused a noteworthy decrease in mortality in a mouse model of ALI [90].
3.10. H2S and Lung Transplantation
Among the lung transplantation diseases, COPD, idiopathic pulmonary fibrosis (IPF), cystic fibrosis, sap-1 antitrypsin deficiency and idiopathic pulmonary hypertension are the main predisposing factors. In clinical practice, lung transplantation is a surgical process, either as single lung transplantation, double lung transplantation, cardiopulmonary transplantation or live lung transplantation. Lung ischemia reperfusion (IR) injury is still the main reason for the early mortality of lung transplantation [134]. A preliminary study reported an experimental model for the application of H2S inhalation after long-term ischemia. In this study, lungs pretreated with inhaled H2S showed an improvement in graft function during reperfusion, indicating the therapeutic use of H2S in the lung transplantation experimental model [135].
Similarly, pretreatment in a rat model with intra-peritoneal NaHS administration significantly improved pulmonary function, and decreased lipid peroxidation and MPO activity after lung transplantation. Besides, NaHS inhibits interleukin 1β but increases interleukin 10 levels in graft lung tissues [136]. The rat model of diabetes mellitus suffering from ischemia reperfusion after lung transplantation decreased ischemi-reperfusion-related oxidative stress after treatment with a slow-releasing H2S, GYY4137 [134]. H2S attenuated lung IR injury in Type 2 diabetic disorder through the initiation of lung SIRT1 signaling, which upregulates the Nrf2/HO-1 and eNOS-mediated antioxidant signaling pathways, therefore decreasing cell apoptosis and inflammation, and finally having a protective lung function.
3.11. H2S and Pulmonary Hypertension
Pulmonary hypertension (PH) is a chronic infection described by central pulmonary vascular pressure and may be caused by various disease processes. Regardless of the cause, PH is a progressive disease. New therapeutic drugs are often decompensated in the advanced stage and usually have a poor prognosis [137]. A decrease in the endogenous H2S pathway in hypertension and pulmonary vascular structural remodeling caused a high pulmonary blood flow in mice [138]. H2S inhibits arterial elastin expression in its extracellular matrix [35]. In a hypoxic rat model with pulmonary artery smooth muscle cells, H2S effectively inhibited a hypoxia-induced increase in cell proliferation, migration and oxidative stress in PASMCs [139]. H2S can enhance total antioxidant capacity by attenuating the GSSG content levels in hypoxia-induced pulmonary hypertensive rats’ lung tissue and exert an antioxidation effect [140]. Endogenous H2S is downregulated in PH, and pulmonary vascular remodeling is influenced through high pulmonary blood flow. NaHS and endogenous H2S can also prevent elevated pulmonary hypertension, pulmonary vascular remodeling and high pulmonary blood flow due to chronic hypoxia [39].
3.12. H2S and Sleep Apnea Syndrome (SAS)
According to the American Sleeping Society, SAS refers to the complete collapse of the upper airway, with the disappearance of airflow but the presence of respiratory motion, characterized by the airflow disappearing for more than 10 s, with significant chest breathing or esophageal pressure fluctuations. Central sleep apnea syndrome (CSAS) is characterized by the complete disappearance of airflow and respiratory movements that disappear for more than 10 s; the airway is not entirely blocked when ventilation is insufficient and airflow is weakened. Arousal and hypoxemia (>3% SaO2) occur frequently. Mice deficient in HO-2 produce the gaseous molecule carbon monoxide (CO) and exhibit sleep apnea, categorized through high apnea and hypopnea indices [141]. The glomus cells in the primary sensory organ, the carotid body (CB), are responsible for monitoring arterial blood O2, CO2 and pH levels. In rodents, obstruction of H2S production through CSE and pharmacologic or genetic methods inhibits carotid body activity, and hypertension is induced through intermittent hypoxia. During hypoxia, ROS triggers carbon monoxide synthesis by HO-2 and inhibits the synthesis of H2S by inhibiting CSE [142]. During hypoxia, as compared with normoxia, HO-2 produces less CO, subsequently augmenting the production of H2S, which motivates CB activity, resulting in increased respiration rate, heart rate and blood pressure. It has been reported that a decrease in CO and an increase in CB in H2S generation led to sleep apnea in HO-2 knockout mice and impulsively hypertensive mice [141].
3.13. H2S and Acute Respiratory Distress Syndrome
ARDS is a clinical disorder categorized by obstinate hypoxemia. It has attracted much attention due to its high mortality rate. The causes of acute respiratory distress syndrome are numerous, and the pathogenesis of ARDS caused by different reasons is also different. Oxidative stress, including the formation of superoxide (O2•−), might play a vital role in the pathogenesis of ARDS. H2S gas intoxication develops ARDS, demanding a high rate of percussive ventilation [143]. In addition to direct vasoconstriction, O2•− also reacts with NO to form peroxynitrite and other reactive nitrogen, effectively reducing NO bioavailability. H2S inhibited O2•− formation in porcine aorta-derived endothelial cells, and the adenylate cyclase-PKA pathway upregulated NADPH oxidase. H2S-donating sildenafil may effectively treat ARDS through increasing cAMP and preventing Type 5 phosphodiesterase activity [144].
3.14. H2S and Bronchopulmonary Dysplasia (BPD)
BPD is a chronic lung infection that causes persistent respiratory distress. It is caused mainly by hyperoxia, mechanical ventilation and inflammation, and categorized through impaired alveolar growth and complex pulmonary hypertension (PHT) [84,145]. It exhibits substantial streaks and overexpansion characteristics in X-rays. H2S showed a protective effect in a BPD rodent model through HO-1 [145]. GYY4137 preserved and restored mitochondrial function in alveolar epithelial cells and normal alveolar development in mice pups exposed to hyperoxia for 2 weeks after birth [84]. The effect of NaHS on the migration of alveolar Type II (ATII) cells was reduced by glibenclamide, implicating ion channels, and was accompanied by Akt activation, suggesting two probable mechanisms of H2S action. Such work triggers more study of H2S as an applicant interventional approach to bind the prevented alveolarization linked with BPD [146].
4. H2S in the Physiopathology of Airways
H2S regulates some airways’ physiological processes, both in human and animal models, as summarized in Table 1. Disorders of the endogenous formation of H2S are connected to pathological procedures and the development of numerous ailments, including hypertension, hypoxic pulmonary hypertension and myocardial injury [35,147,148]. H2S mediates smooth muscle relaxation via high airway activity inhibition caused by smoke from cigarettes, ozone and ovum albumin. On the other hand, H2S intensifies the said effects if inhibited [149]. This relaxation was due to endogenous H2S production in porcine airways [150]. The precursors of H2S, such as L-cysteine, also produce relaxation in the airway, but an inhibitor of CBS, amino oxy-acetic acid, inhibits the relaxation activity. The relaxation of smooth muscle also involves the inhibition of H2S, which relaxes the smooth muscle inhibition of Ca2+ release via InsP3 receptors [151] and the K+ channel [150]. Tracheal smooth muscle cells of mice showed hyper-movement through the potassium channels by stimulating the large-conductance calcium-activated potassium channel (BKCa) after treatment with NaHS. Such action causes the inhibition of Ca2+ influx and hyper-polarization of cells [152]. In contrast, relaxation was caused by H2S via opening the KATP channels in smooth muscle cells of human airways [70]. The inhibition of phosphorylation of extracellular p38MAPK and ERK1/2 by H2S has an inverse effect on the multiplication of smooth muscle cells and interleukin-8 release induced in fetal calf serum [101]. H2S also regulates the physiological function of vessels, thus acting as a vaso-relaxant agent [153]. H2S also enhances NO signaling in vessels [45] and vasodilatation of the pulmonary artery in rat [154].
Table 1.
Pathophysiological actions of H2S in the lung.
| Action | H2S | References |
|---|---|---|
| Vasodilation | ↑ | [153,154] |
| Stable asthma | ↓ | [35,36,38,52,68,102,174] |
| Bronchodilation | ↑ | [103] |
| Angiogenic activity | ↑ | [22] |
| Pro-inflammatory action | ↑ | [61] |
| Anti-inflammatory action | ↑ | [61] |
| Airway hyper-reactivity | ↑ | [63,64] |
| Asthma exacerbation | ↓ | [35,36,38,52,68,102,174] |
| Stable COPD | ↑ | [65] |
| COPD exacerbation | ↓ | [37] |
↑ = Increased, ↓ = decreased.
5. H2S in Pulmonary Inflammation
Endogenous and exogenous H2S acts in the respiratory system via controlling mucolytic function. H2S can make the mucus less tacky, as it supports mucin cracking through connections with disulfide bonds [155]. H2S activates electrolyte absorption via the imitation of ATP-sensitive potassium channels (KATP) and prevents the Na+/K+-ATPase and calcium-sensitive potassium channels in human bronchiolar epithelia [156]. The function of exogenous H2S in lung ailments has been considered by using H2S donor representatives. The significance of slow or fast H2S-releasing elements in inflammatory reactions was generally evaluated by consuming molecules that are capable of producing H2S with deliberate and continuous discharge kinetics. Treatment with NaSH, “a fast releasing” H2S donor, encourages a significant provocative and inflammatory response in rats, as estimated through amplified MPO activity and the occurrence of leukocytes in the lungs [105].
Furthermore, the slow-releasing H2S elements such as GYY4137 produced anti-inflammatory effects in vivo and decreased pro-inflammatory cytokines (IL-I6, IL-Iβ, and TNF) in LPS-induced pulmonary inflammation in a mouse model. Similarly, treatment with GYY4137 produces noticeable antioxidant effects by reinstating the antioxidant enzymes catalase and SOD in lung tissues, strengthening the balance between reduced and oxidized GSH [157]. GYY4137 also reduced pro-inflammatory genes’ expression via moderating the initiation of NF-ҝβ and IFN regulatory factor-3 (IRF-3) [114]. Post-transcriptional NF-ҝβ is a new mark of H2S to reverse vascular inflammation. H2S blocked the initiation of the NF-ҝβ pathways in a model of nanoparticles. Pyrrole induced an inflammatory reaction in pulmonary artery endothelial cells via the sulfidation of IK-ҝβ of Cys179 residue, therefore preventing IK-ҝβ action. These types of process give clues about defending initiation against pulmonary vascular inflammation, pulmonary arterial hypertension and vascular modeling in vivo [158]. Moreover, treatment with GYY4137 prohibited lung injury and neutrophil migration, decreasing chemoattractant signaling molecules in vitro in the lung tissue of a mouse model of LPS-induced acute lung injury [157]. Remarkably, H2S moderates the entry of leukocytes from the bloodstream to swollen tissues [159], and this consequence depends on the initiation of annexin-1 pro-resolving pathways [160]. H2S considerably reduces pro-inflammatory cytokines such as IL-6 and IL-8 and augmented anti-inflammatory IL-10 in the plasma and lung. H2S directly repressed the pro-inflammatory reaction and ROS development in neutrophils, emphasizing the valuable prospective H2S donors as acute lung injury prophylactics (Figure 4). H2S promotes anti-inflammatory consequences via epigenetic changes.
H2S regulates the methylation and acetylation of histones, which governs the production of pro-inflammatory elements. Hence, H2S contributes to decreasing cytokine discharge and subsequent improvement of LPS in rats [155,157]. Treatment with diallyl disulfide (DADS) and arylthioamides as H2S donor induced a protective result in naphthalene-induced lung injury [161,162]. Therapy with DADS increases GSH levels in the lung tissue, preventing pro-inflammatory cytokine (IL-6, IL-8, and TNF) release relating to overcoming lung inflammatory cell deployment and precise neutrophil infiltration [163]. Sulforaphane, a naturally occurring isothiocyanate capable of generating H2S [164], reduced the release of pro-inflammatory mediators in a mouse model of LPS-induced acute lung injury. Sulforaphane mediates lung protection through transcription factor Nrf-2 by regulating mitochondrial function and energy use. Nrf-2 is accountable for inducing the expression of multiple antioxidant genes and averting oxidative injury. This kind of mechanism of action has also been defined for synthetic thiocyanate, whose H2S donor profile has been extensively discussed [147,165,166,167,168,169,170,171]. Anethiole dithiolethione has often been an H2S donor or a compound for developing H2S-releasing hybrid drugs with the non-steroidal anti-inflammatory agents H2S-diclofenac and H2S-aspirin. The previously mentioned new drugs possess anti-inflammatory outcomes compared with the “parent drugs” aspirin and diclofenac, showing their efficacy in decreasing lung MPO activity in a rat model of LPS-induced diclofenac-associated septic shock after H2S-diclofenac administration [172]. According to a recent study, PM considerably improved airway inflammation and emphysema in mice, calculated through the alveolar destruction index, total cell pro-inflammatory cytokinesis (IL-6, IL-8 and TNF), neutrophil counts and CXCL1 broncho-alveolar lavage fluid. H2S decreased particulate matter (PM)-induced mouse emphysema and airway inflammation by decreasing oxidative stress as assessed by 8-OHdG concentration in lung tissues. H2S plays a protective role in PM-induced rat emphysema and airway inflammation by preventing NLRP3 inflammasome development and apoptosis produced through fine particulate matter(pM2.5) contact with A549 cells but not in Nrf2-silenced cells [173].
6. Clinical Trials of H2S Donors
Ik-1001(Na2S) was the first compound chosen to administer as an H2S donor in a clinical trial in 2009. Directed IK-1001 (NCT00879645) was the first clinical trial conducted, which was soon terminated, as it was incapable of reducing the sulfide level. Not being capable to consistently measure sulfide is a serious issue for a compound’s approval. The main concerns raised by the scientific community were because of the highly volatile and quickly absorbed nature of exogenous sulfide [174]. Numerous sulfide compounds are present in biological systems, and sulfide participates in several chemical processes [175], which reveals that these endogenous compounds are highly dynamic.
In contrast, the exogenous administration of H2S might result in the equilibrium of this entire system in ways that we have not wholly known until now. After that, IK-1001(NCT00858936), during secondary trials, affected coronary artery bypass and stopped with this issue. A later clinical trial was established using ST-elevation myocardial infarction (NCT010074610), which was also stopped due to safety issues. Therefore, an aqueous solution of liquid H2S called IK-1001 can be typically administered with H2S-releasing salts or inhaled H2S. Neither administration of H2S through inhalation nor injection of H2S donors will possibly be utilized in the clinical trials due to airway mucosal injury. Still, there is the possibility of poisonous sulfide concentrations eventually being produced [174]. Inhalation of 330 ppm H2S via sub-lethal administration has been used as a model to study lung injury [86,176]. Efforts to evade the airway inflammation of gaseous H2S were applied to an extracorporeal membrane for lung ventilation in a pre-clinical study. However, there was limited development in the effect of cardiopulmonary bypass [100]. The trial utilized a combination of organic sulfide-releasing compounds and salts (named SG-1002(NCT01989208), which has been used in heart failure studies. This trial proved to be safe and well-tolerated in Phase 1 trials; however, it failed in the Phase II (NCT02278276) trial. An excellent prospect for H2S-based treatments is the reassessment of H2S donors or compounds that are now clinically permitted and have only recently been recognized to be capable of releasing H2S, such as sodium thiosulfate (STS) [177,178], which helped in cyanide detoxification and cisplatin overdosage (ammonium tetra-thiomolybdate (ATTM) [179,180], and is allowed for Wilson’s disorder (a copper metabolism ailment), and zofenopril [181], an inhibitor of angiotensin-altering enzymes permitted for hypertension. These compounds all have been verified widely and are recognized to have worthy safety profiles. For example, Dyson et al. revealed that ATTM led to a 50% decrease in infarct size in mouse models of myocardia and cerebral I/R in addition to the improved persistence of later hemorrhage [180].
The good wellbeing profile of STS [182], specifically, might be connected to the circumstance that thiosulfate itself is an endogenous intermediate of oxidative H2S metabolism [177] and is recommended as a molecule with valuable H2S results [183], specifically in hypoxic circumstances [177]. The clinical trial of IK-1001 in renal injury utilized thiosulfate as an unintended measure of H2S release by their compound, though this was finally found to be ineffective. STS is presently in a Phase II clinical trial to preserve cardiac function in SREMI. Concerning the lung, as revealed before, STS was helpful in murine models of intratracheal LPS and CLP [80]. While Sakaguchi et al.’s results supported these effects, they determined a practical consequence of STS in the lung, i.e., enhanced gas altercation and lung processes in an interpreter-related large animal model of hemorrhagic shock. Therefore, STS is a compound providing hope for the advancement of therapeutic H2S administration in ALI in clinical settings [80].
In animal experiments, NSAIDs conjugated to H2S (for example, celecoxib and naproxen) revealed a robust protective effect on gastrointestinal epithelium as matched with the parent’s lethal drug results [184]. For example, the H2S-releasing naproxen known as ATB-346, which releases H2S through a hydrolytic mechanism [184], was confirmed to have better anti-inflammatory results in animal models, decreasing leukocyte migration and decreasing TNF-α and TNF-αβ expression [184,185,186]. Another H2S donor is S-mesalamine (ATB-429), which is utilized for the treatment of inflammatory colitis. ATB-429 played a protective role in the gastrointestinal mucosa and had more remarkable anti-inflammatory outcomes than the parent drug [106]. Hence, ATB-429 might be a worthy applicant for decreasing inflammation [187]. Correspondingly, NBS-1120 had an excellent protective role in an animal model of inflammation compared with aspirin [188]. While GYY4137 was show to directly obstruct inflammation in a mouse model by inhibiting different inflammatory molecules [189], GYY4137 can reduce LPS-evoked septic shock [190]. These previously discussed studies emphasized the curative prospects of H2S donors for the treatment of inflammation and respiratory diseases. However, extra in vivo analyses and studies are necessary to endorse the effectiveness of these H2S donors, their safety, and their possible use in such diseases.
7. Perceptions, Limitations and Prospects
H2S executes a broad range of pathophysiological functions, including vasodilatation to lower the blood pressure, initiation of angiogenesis, signal regulation of neuronal action and regulation of glucose homeostasis, which have been widely demonstrated beside NO and CO. H2S was previously considered an environmental contaminant but is now widely recognized as an important biological and pharmacological medium, and is considered to be the third endogenous gas transmitter in mammals, There is increasing confirmation that H2S plays a crucial part in respiratory diseases, revealing that the metabolic machinery and mechanisms of H2S are an essential research topic in respiratory diseases. H2S is mainly metabolized by CSE, CBS and 3-MST in mammals. The metabolic pathway of H2S is different in different organs and tissues. There is increasing evidence that H2S plays a crucial role in respiratory diseases. Investigating the metabolic machinery and mechanisms of H2S in respiratory diseases is an important research topic that may help develop new drugs. The scientific relationships of H2S in mammals should be widely observed and studied experimentally to elucidate the expression and function of H2S-producing enzymes in different organs and tissues, and provide new ideas for the better development of new H2S donors and targeted clinical therapies.
Respiratory disorders are general and often-occurring ailments with a relatively high mortality rate. The primary lesions are in the trachea, bronchus, lungs and chest. In addition to the existing pathophysiological mechanisms, further research into and clarification of the new underlying mechanisms and new signaling pathways associated with respiratory diseases are needed. At present, some progress has been made by using animal models to study the molecular mechanism of H2S in respiratory injury. The molecular targets of H2S in the respiratory system also require further investigation. Because altered amounts of H2S-releasing compounds may produce various therapeutic outcomes, further appropriate dose ranges should be studied to achieve better therapeutic results. Moreover, new H2S-releasing donors should be designed and identified to increase the therapeutic effect by mediating H2S concentrations in human disease, and whether this effect would reduce long-term disease and mortality.
The present evidence proposes that H2S has a function in regulating and maintaining vital biological progressions in animals. Despite the noteworthy development of H2S donors, there is still an absence of compounds that can address all the requirements for the perfect H2S donor in clinical studies. There are major gaps in our understanding that obstruct the clinical usage of H2S donors. Many questions need to be answered, such as (i) what the H2S-releasing compounds are, (ii) the therapeutic concentrations of H2S and its compounds, (iii) the concentrations at which H2S and its donors become toxic, (iv) the level of toxicity, (v) the mechanisms of H2S release from the H2S donors and drugs, (vi) the administration of H2S in vivo at a constant rate, (vii) the mechanism action of H2S, (viii) the monitoring of plasma levels of H2S and its products, (ix) what the differences between H2S administration in vivo and in vitro are, (x) assessing the sensitivity and specificity of H2S, (xi) the selection of patients for assessing the effectiveness of H2S drugs, and (xii) finding appropriate doses of H2S or its donors for treatment over a reasonable period in respiratory disease, and in pre-clinical and clinical studies. In conclusion, a deeper understanding of the exact molecular mechanisms behind the role of H2S in the development, progression, prevention and treatment of respiratory diseases is important for using appropriate doses of H2S or its donors to improve its clinical efficacy.
Abbreviations
| H2S | hydrogen sulfide |
| CBS | cystathionine beta-synthase |
| CSE | cystathionine γ-lyase |
| 3-MPST | 3-mercaptopyruavte sulfurtransferase |
| COPD | chronic obstructive pulmonary disease |
| SQR | sulfide-quinone reductase |
| TST | thiosulfate sulfurtransferase |
| CAT | cysteine aminotransferase |
| GSSH | glutathione |
| SMC | smooth muscle cells |
| ROS | reactive oxygen species |
| ALI | acute lung injury |
| NO | nitric oxide |
| ATF-30 | transcription factor-3 |
| OA | oleic acid |
| ECM | extracellular matrix |
| MLC | myosin light chain |
| PHT | pulmonary hypertension |
| SM | sulfur mustard |
| NSAID | non-steroidal anti-inflammatory drugs |
| MODS | multiple organ dysfunction syndrome |
| MDA | malondialdehyde |
| MPO | myeloperoxidase |
| ARDS | acute respiratory distress syndrome |
| LPVS | lung-protective ventilation strategies |
| VILI | ventilator-induced lung injury |
| FEV | forced expiratory volume |
| LTx | lung transplantation |
| PH | pulmonary hypertension |
| RSV | respiratory syncytial virus |
| IPF | idiopathic pulmonary fibrosis |
| CSAS | central sleep apnea syndrome |
| SAS | sleep apnea syndrome |
| hMPV | human metapneumovirus |
| CB | carotid body |
| CO | carbon monoxide |
| BPD | bronchopulmonary dysplasia |
| ATII | alveolar Type II |
| NaHS | sodium hydrosulfide |
| HO-1 | heme oxygenase-1 |
| STAT-3 | signal transducer and activator of transporter-1 |
| Nrf-2 | nuclear factor erythroid-2 related factor |
| NF-қβ | nuclear factor-kappa B |
| PI3K | phosphoinositide 3-kinase |
| ERK | extracellular signal-regulated kinase |
| AMPK | AMP-activated protein kinase |
| TNF-α | tumor necrosis factor-α |
| TGF-β1 | transforming growth factor beta 1 |
| SOD | superoxide dismutase |
| IL | interleukin |
| IKK | IқB kinase |
| Keap1 | Kelch-like-ECH-associated protein |
| ADT-OH | 5-(hydroxyphenyl)-3H-1:2-dithiole-3-thione |
| ATB-429 | 4-(5-sulfanylidenedithiol-3-y) phenyl 5-amino-2-hydroxybenzoate |
| STS | sodium thiosulfate |
Author Contributions
D.-D.W. and X.-Y.J. conceived the concept of the review and supervised the project. S.K., Q.-Q.Z., M.S., P.M., E.E.N., N.H.K., S.R., Y.-Z.W., H.-W.Q., and D.W. reviewed the literatures and extracted the data. S.K. and Q.-Q.Z. drafted the manuscript. D.-D.W., A.A. and X.-Y.J. revised the manuscript and provided intellectual input in the review. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Nos 81802718 and U1504817), the Foundation of Science and Technology Department of Henan Province, China (Nos 192102310151 and 202102310480) and the Training Program for Young Backbone Teachers of Institution of Higher Learning in Henan Province, China (No. 2020GGJS038).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Scano G., Innocenti-Bruni G., Stendardi L. Do obstructive and restrictive lung diseases share common underlying mechanisms of breathlessness? Respir. Med. 2010;104:925–933. doi: 10.1016/j.rmed.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 2.Dasaraju P.V., Liu C. Infections of the Respiratory System. 4th ed. University of Texas Medical Branch at Galveston; Galveston, TX, USA: 1996. [PubMed] [Google Scholar]
- 3.Bartlett J.G. Management of Respiratory Tract Infections. Williams & Wilkins; Baltimore, MD, USA: 1997. [Google Scholar]
- 4.Perez-Padilla R.P.B., Marks G., Wong G., Bateman E., Jarvis D., Prabhakaran D., Anand S., Gaziano T.A., Mbanya J.-C., Wu Y., et al. Cardiovascular, Respiratory, and Related Disorders. 3rd ed. Volume 5. World Bank; Washington, DC, USA: 2017. Chronic Lower Respiratory Tract Diseases; pp. 263–285. [Google Scholar]
- 5.Zar H.J., Ferkol T.W. The global burden of respiratory disease-Impact on child health. Pediatr. Pulmonol. 2014;49:430–434. doi: 10.1002/ppul.23030. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization . WHO Strategy for Prevention and Control of Chronic Respiratory Diseases. World Health Organization; Geneva, Switzerland: 2002. [Google Scholar]
- 7.Abou-Hamdan A., Guedouari-Bounihi H., Lenoir V., Andriamihaja M., Blachier F., Bouillaud F. Oxidation of H2S in Mammalian Cells and Mitochondria. Methods Enzymol. 2015;554:201–228. doi: 10.1016/bs.mie.2014.11.042. [DOI] [PubMed] [Google Scholar]
- 8.Ramazzini B. De morbis artificum Bernardini Ramazzini diatribe. University of Chicago Press; Chicago, IL, USA: 1940. [Google Scholar]
- 9.Reiffenstein R.J., Hulbert W.C., Roth S.H. Toxicology of hydrogen sulfide. Annu. Rev. Pharmacol. Toxicol. 1992;32:109–134. doi: 10.1146/annurev.pa.32.040192.000545. [DOI] [PubMed] [Google Scholar]
- 10.Bhatia M., Wong F.L., Fu D., Lau H.Y., Moochhala S.M., Moore P.K. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J. 2005;19:1–17. doi: 10.1096/fj.04-3023fje. [DOI] [PubMed] [Google Scholar]
- 11.Wu D., Luo N., Wang L., Zhao Z., Bu H., Xu G., Yan Y., Che X., Jiao Z., Zhao T., et al. Hydrogen sulfide ameliorates chronic renal failure in rats by inhibiting apoptosis and inflammation through ROS/MAPK and NF-κB signaling pathways. Sci. Rep. 2017;7:455. doi: 10.1038/s41598-017-00557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L., Moore P.K. Could hydrogen sulfide be the next blockbuster treatment for inflammatory disease? Expert Rev. Clin. Pharmacol. 2013;6:593–595. doi: 10.1586/17512433.2013.842126. [DOI] [PubMed] [Google Scholar]
- 13.Fiorucci S. Hydrogen sulfide: From physiology to pharmacology. Inflamm. Allergy Drug Targets. 2011;10:77–84. doi: 10.2174/187152811794776277. [DOI] [PubMed] [Google Scholar]
- 14.Abe K., Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu D., Wang J., Li H., Xue M., Ji A., Li Y. Role of Hydrogen Sulfide in Ischemia-Reperfusion Injury. Oxidative Med. Cell. Longev. 2015;2015:1–16. doi: 10.1155/2015/186908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhong G.Z., Chen F.R., Cheng Y.Q., Tang C.S., Du J.B. The role of hydrogen sulfide generation in the pathogenesis of hypertension in rats induced by inhibition of nitric oxide synthase. J. Hypertens. 2003;21:1879–1885. doi: 10.1097/00004872-200310000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Szabo C., Coletta C., Chao C., Módis K., Szczesny B., Papapetropoulos A., Hellmich M.R. Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. USA. 2013;110:12474–12479. doi: 10.1073/pnas.1306241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhattacharyya S., Saha S., Giri K., Lanza I.R., Nair K.S., Jennings N.B., Rodriguez-Aguayo C., Lopez-Berestein G., Basal E., Weaver A.L., et al. Cystathionine Beta-Synthase (CBS) Contributes to Advanced Ovarian Cancer Progression and Drug Resistance. PLoS ONE. 2013;8:e79167. doi: 10.1371/journal.pone.0079167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sen S., Kawahara B., Gupta D., Tsai R., Khachatryan M., Roy-Chowdhuri S., Bose S., Yoon A., Faull K., Farias-Eisner R., et al. Role of cystathionine β-synthase in human breast Cancer. Free. Radic. Biol. Med. 2015;86:228–238. doi: 10.1016/j.freeradbiomed.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 20.Panza E., De Cicco P., Armogida C., Scognamiglio G., Gigantino V., Botti G., Germano D., Napolitano M., Papapetropoulos A., Bucci M. Role of the cystathionine γ lyase/hydrogen sulfide pathway in human melanoma progression. Pigment Cell Melanoma Res. 2015;28:61–72. doi: 10.1111/pcmr.12312. [DOI] [PubMed] [Google Scholar]
- 21.Baskar R., Bian J. Hydrogen sulfide gas has cell growth regulatory role. Eur. J. Pharmacol. 2011;656:5–9. doi: 10.1016/j.ejphar.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 22.Coletta C., Papapetropoulos A., Erdelyi K., Olah G., Modis K., Panopoulos P., Asimakopoulou A., Gero D., Sharina I., Martin E., et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc. Natl. Acad. Sci. USA. 2012;109:9161–9166. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szabo C. Gasotransmitters in cancer: From pathophysiology to experimental therapy. Nat. Rev. Drug Discov. 2016;15:185–203. doi: 10.1038/nrd.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Módis K., Coletta C., Asimakopoulou A., Szczesny B., Chao C., Papapetropoulos A., Hellmich M.R., Szabo C. Effect of S-adenosyl-L-methionine (SAM), an allosteric activator of cystathionine-β-synthase (CBS) on colorectal cancer cell proliferation and bioenergetics in vitro. Nitric Oxide. 2014;41:146–156. doi: 10.1016/j.niox.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szabó C., Papapetropoulos A. Hydrogen sulphide and angiogenesis: Mechanisms and applications. Br. J. Pharmacol. 2011;164:853–865. doi: 10.1111/j.1476-5381.2010.01191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szczesny B., Módis K., Yanagi K., Coletta C., Le Trionnaire S., Perry A., Wood M.E., Whiteman M., Szabo C. AP39, a novel mitochondria-targeted hydrogen sulfide donor, stimulates cellular bioenergetics, exerts cytoprotective effects and protects against the loss of mitochondrial DNA integrity in oxidatively stressed endothelial cells in vitro. Nitric Oxide. 2014;41:120–130. doi: 10.1016/j.niox.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang M.-J., Cai W.-J., Li N., Ding Y.-J., Chen Y., Zhu Y.-C. The Hydrogen Sulfide Donor NaHS Promotes Angiogenesis in a Rat Model of Hind Limb Ischemia. Antioxid. Redox Signal. 2010;12:1065–1077. doi: 10.1089/ars.2009.2945. [DOI] [PubMed] [Google Scholar]
- 28.Yang G., Sun X., Wang R. Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen-activated protein kinases and caspase-3. Faseb J. 2004;18:1782–1784. doi: 10.1096/fj.04-2279fje. [DOI] [PubMed] [Google Scholar]
- 29.Sen N., Paul B.D., Gadalla M.M., Mustafa A.K., Sen T., Xu R., Kim S., Snyder S.H. Hydrogen sulfide-linked sulfhydration of NF-κB mediates its antiapoptotic actions. Mol. Cell. 2012;45:13–24. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai W.-J., Wang M.-J., Moore P.K., Jin H.-M., Yao T., Zhu Y.-C. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc. Res. 2007;76:29–40. doi: 10.1016/j.cardiores.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 31.Isenberg J.S., Jia Y., Field L., A Ridnour L., Sparatore A., Del Soldato P., Sowers A.L., Yeh G.C., Moody T.W., A Wink D., et al. Modulation of angiogenesis by dithiolethione-modified NSAIDs and valproic acid. Br. J. Pharmacol. 2007;151:142–151. doi: 10.1038/sj.bjp.0707198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papapetropoulos A., Pyriochou A., Altaany Z., Yang G., Marazioti A., Zhou Z., Jeschke M.G., Branski L.K., Herndon D.N., Wang R., et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc. Natl. Acad. Sci. USA. 2009;106:21972–21977. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimura Y., Goto Y.-I., Kimura H. Hydrogen Sulfide Increases Glutathione Production and Suppresses Oxidative Stress in Mitochondria. Antioxid. Redox Signal. 2010;12:1–13. doi: 10.1089/ars.2008.2282. [DOI] [PubMed] [Google Scholar]
- 34.Wu D., Zheng N., Ziqiang S., Cheng H., Sun Z., Gao B., Zhang Y., Pang W., Huangfu C., Ji S., et al. Exogenous hydrogen sulfide mitigates the fatty liver in obese mice through improving lipid metabolism and antioxidant potential. Med. Gas. Res. 2015;5:1–8. doi: 10.1186/s13618-014-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J., Zhang H., Yu W., Chen L., Wang Z., Zhang T. Expression of pulmonary arterial elastin in rats with hypoxic pulmonary hypertension using H2S. J. Recept. Signal. Transduct. 2020;40:383–387. doi: 10.1080/10799893.2020.1738482. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y.-H., Wu R., Geng B., Qi Y.-F., Wang P.-P., Yao W.-Z., Tang C.-S. Endogenous hydrogen sulfide reduces airway inflammation and remodeling in a rat model of asthma. Cytokine. 2009;45:117–123. doi: 10.1016/j.cyto.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y.-H., Yao W.-Z., Geng B., Ding Y.-L., Lu M., Zhao M.-W., Tang C.-S. Endogenous Hydrogen Sulfide in Patients With COPD. Chest. 2005;128:3205–3211. doi: 10.1378/chest.128.5.3205. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J., Wang X., Chen Y., Yao W. Correlation between levels of exhaled hydrogen sulfide and airway inflammatory phenotype in patients with chronic persistent asthma. Respirology. 2014;19:1165–1169. doi: 10.1111/resp.12372. [DOI] [PubMed] [Google Scholar]
- 39.Han W., Dong Z., Dimitropoulou C., Su Y. Hydrogen Sulfide Ameliorates Tobacco Smoke-Induced Oxidative Stress and Emphysema in Mice. Antioxid. Redox Signal. 2011;15:2121–2134. doi: 10.1089/ars.2010.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen L., Zhang J., Ding Y., Li H., Nie L., Yan X., Zhou H., Zheng Y. KATP channels of parafacial respiratory group (pFRG) neurons are involved in H2S-mediated central inhibition of respiratory rhythm in medullary slices of neonatal rats. Brain Res. 2013;1527:141–148. doi: 10.1016/j.brainres.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 41.Iciek M., Bilska-Wilkosz A., Górny M. Sulfane sulfur—New findings on an old topic. Acta Biochim. Pol. 2019;66:533–544. doi: 10.18388/abp.2019_2909. [DOI] [PubMed] [Google Scholar]
- 42.Ishigami M., Hiraki K., Umemura K., Ogasawara Y., Ishii K., Kimura H. A Source of Hydrogen Sulfide and a Mechanism of Its Release in the Brain. Antioxid. Redox Signal. 2009;11:205–214. doi: 10.1089/ars.2008.2132. [DOI] [PubMed] [Google Scholar]
- 43.Toohey J.I. Sulfur signaling: Is the agent sulfide or sulfane? Anal. Biochem. 2011;413:1–7. doi: 10.1016/j.ab.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 44.Toohey J.I. The conversion of H2S to sulfane sulfur. Nat. Rev. Mol. Cell Biol. 2012;13:803. doi: 10.1038/nrm3391-c1. [DOI] [PubMed] [Google Scholar]
- 45.Szabo C. Hydrogen sulfide, an enhancer of vascular nitric oxide signaling: Mechanisms and implications. Am. J. Physiol. Physiol. 2017;312:C3–C15. doi: 10.1152/ajpcell.00282.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng Y., Yu B., De La Cruz L.K., Choudhury M.R., Anifowose A., Wang B. Toward Hydrogen Sulfide Based Therapeutics: Critical Drug Delivery and Developability Issues. Med. Res. Rev. 2018;38:57–100. doi: 10.1002/med.21433. [DOI] [PubMed] [Google Scholar]
- 47.Gojon G., Morales G.A. SG1002 and Catenated Divalent Organic Sulfur Compounds as Promising Hydrogen Sulfide Prodrugs. Antioxid. Redox Signal. 2020;33:1010–1045. doi: 10.1089/ars.2020.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olhemus D.J., Li Z., Pattillo C.B., Gojon G., Sr., Gojon G., Jr., Giordano T., Krum H. AA Novel Hydrogen Sulfide Prodrug, SG1002, Promotes Hydrogen Sulfide and Nitric Oxide Bioavailability in Heart Failure Patients. Cardiovasc Ther. 2015;33:216–226. doi: 10.1111/1755-5922.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang X., Liu Y., Ma L., Ji R., Qu Y., Xin Y., Lv G. Chemopreventive activity of sulforaphane. Drug Des. Dev. Ther. 2018;12:2905–2913. doi: 10.2147/DDDT.S100534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szabó C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 51.Madden J.A., Ahlf S.B., Dantuma M.W., Olson K.R., Roerig D.L. Precursors and inhibitors of hydrogen sulfide synthesis affect acute hypoxic pulmonary vasoconstriction in the intact lung. J. Appl. Physiol. 2012;112:411–418. doi: 10.1152/japplphysiol.01049.2011. [DOI] [PubMed] [Google Scholar]
- 52.Wang P., Zhang G., Wondimu T., Ross B., Wang R. Hydrogen sulfide and asthma. Exp. Physiol. 2011;96:847–852. doi: 10.1113/expphysiol.2011.057448. [DOI] [PubMed] [Google Scholar]
- 53.Mustafa A.K., Gadalla M.M., Sen N., Kim S., Mu W., Gazi S.K., Barrow R.K., Yang G., Wang R., Snyder S.H. H2S Signals Through Protein S-Sulfhydration. Sci. Signal. 2009;2:ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ju Y., Untereiner A., Wu L., Yang G. H2S-induced S-sulfhydration of pyruvate carboxylase contributes to gluconeogenesis in liver cells. Biochim. Biophys. Acta. 2015;1850:2293–2303. doi: 10.1016/j.bbagen.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 55.Pauwels R.A., Buist A.S., Calverley P.M., Jenkins C.R., Hurd S.S. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am. J. Respir. Crit. Care. Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 56.Wang L., Meng J., Wang C., Yang C., Wang Y., Li Y., Li Y. Hydrogen sulfide alleviates cigarette smoke-induced COPD through inhibition of the TGF-β1/smad pathway. Exp. Biol. Med. 2020;245:190–200. doi: 10.1177/1535370220904342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Viegas J., Esteves A.F., Cardoso E.M., Arosa F.A., Vitale M., Taborda-Barata L. Biological Effects of Thermal Water-Associated Hydrogen Sulfide on Human Airways and Associated Immune Cells: Implications for Respiratory Diseases. Front. Public Heal. 2019;7:128. doi: 10.3389/fpubh.2019.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whiteman M., Winyard P.G. Hydrogen sulfide and inflammation: The good, the bad, the ugly and the promising. Expert Rev. Clin. Pharmacol. 2011;4:13–32. doi: 10.1586/ecp.10.134. [DOI] [PubMed] [Google Scholar]
- 59.Li T., Zhao B., Wang C., Wang H., Liu Z., Li W., Jin H., Tang C., Du J. Regulatory Effects of Hydrogen Sulfide on IL-6, IL-8 and IL-10 Levels in the Plasma and Pulmonary Tissue of Rats with Acute Lung Injury. Exp. Biol. Med. 2008;233:1081–1087. doi: 10.3181/0712-RM-354. [DOI] [PubMed] [Google Scholar]
- 60.Grommes J., Soehnlein O. Contribution of Neutrophils to Acute Lung Injury. Mol. Med. 2011;17:293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Esechie A., Kiss L., Olah G., Horvath E., Hawkins H., Szabo C., Traber D. Protective effect of hydrogen sulfide in a murine model of combined burn and smoke inhalation-induced acute lung injury. Clin. Sci. 2008;115:91–97. doi: 10.1042/CS20080021. [DOI] [PubMed] [Google Scholar]
- 62.Rose P., Moore P.K., Zhu Y.Z. H2S biosynthesis and catabolism: New insights from molecular studies. Cell. Mol. Life Sci. 2017;74:1391–1412. doi: 10.1007/s00018-016-2406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen Y.-H., Wang P.-P., Wang X.-M., He Y.-J., Yao W.-Z., Qi Y.-F., Tang C.-S. Involvement of endogenous hydrogen sulfide in cigarette smoke-induced changes in airway responsiveness and inflammation of rat lung. Cytokine. 2011;53:334–341. doi: 10.1016/j.cyto.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 64.Roviezzo F., Bertolino A., Sorrentino R., Terlizzi M., Matteis M., Calderone V., Mattera V., Martelli A., Spaziano G., Pinto A., et al. Hydrogen sulfide inhalation ameliorates allergen induced airway hypereactivity by modulating mast cell activation. Pharmacol. Res. 2015;100:85–92. doi: 10.1016/j.phrs.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 65.Saito J., Mackay A.J., Rossios C., Gibeon D., Macedo P., Sinharay R., Bhavsar P.K., A Wedzicha J., Chung K.F. Sputum-to-serum hydrogen sulfide ratio in COPD. Thorax. 2014;69:903–909. doi: 10.1136/thoraxjnl-2013-204868. [DOI] [PubMed] [Google Scholar]
- 66.Perry M.M., Tildy B., Papi A., Casolari P., Caramori G., Rempel K.L., Halayko A.J., Adcock I., Chung K.F. The anti-proliferative and anti-inflammatory response of COPD airway smooth muscle cells to hydrogen sulfide. Respir. Res. 2018;19:1–10. doi: 10.1186/s12931-018-0788-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Kirkham P.A., Whiteman M., Winyard P.G., Caramori G., Gordon F., Ford P.A., Barnes P.J., Adcock I.M., Chung K.F. Impact of theophylline/corticosteroid combination therapy on sputum hydrogen sulfide levels in patients with COPD. Eur. Respir. J. 2014;43:1504–1506. doi: 10.1183/09031936.00131513. [DOI] [PubMed] [Google Scholar]
- 68.Zhang J., Wang X., Chen Y., Yao W., Rosner E., Mastropietro C.W. Exhaled Hydrogen Sulfide Predicts Airway Inflammation Phenotype in COPD. Respir. Care. 2014;60:251–258. doi: 10.4187/respcare.03519. [DOI] [PubMed] [Google Scholar]
- 69.Chen Y., Liu S., Yao W. Imbalance of Endogenous Hydrogen Sulfide and Homocysteine Pathway in Chronic Obstructive Pulmonary Disease Combined with Cardiovascular Disease; Proceedings of the American Thoracic Society 2012 International Conference; San Francisco, CA, USA. 18–23 May 2012; New York, NY, USA: American Thoracic Society; 2012. p. 3985. [Google Scholar]
- 70.Fitzgerald R., DeSantiago B., Lee D.Y., Yang G., Kim J.Y., Foster D.B., Chan-Li Y., Horton M.R., Panettieri R.A., Wang R., et al. H2S relaxes isolated human airway smooth muscle cells via the sarcolemmal KATP channel. Biochem. Biophys. Res. Commun. 2014;446:393–398. doi: 10.1016/j.bbrc.2014.02.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wheeler A.P., Bernard G.R. Acute lung injury and the acute respiratory distress syndrome: A clinical review. Lancet. 2007;369:1553–1564. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- 72.Mackay A., Al-Haddad M. Acute lung injury and acute respiratory distress syndrome. Contin. Educ. Anaesth. Crit. Care Pain. 2009;9:152–156. doi: 10.1093/bjaceaccp/mkp028. [DOI] [Google Scholar]
- 73.Ge X., Sun J., Fei A., Gao C., Pan S., Wu Z. Hydrogen sulfide treatment alleviated ventilator-induced lung injury through regulation of autophagy and endoplasmic reticulum stress. Int. J. Biol. Sci. 2019;15:2872–2884. doi: 10.7150/ijbs.38315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spassov S.G., Donus R., Ihle P.M., Engelstaedter H., Hoetzel A., Faller S. Hydrogen sulfide prevents formation of reactive oxygen species through PI3K/Akt signaling and limits ventilator-induced lung injury. Oxid. Med. Cell. Longev. 2017;2017:3715037. doi: 10.1155/2017/3715037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang H.-X., Liu S.-J., Tang X.-L., Duan G.-L., Ni X., Zhu X.-Y., Liu Y.-J., Wang C.-N. H2S Attenuates LPS-Induced Acute Lung Injury by Reducing Oxidative/Nitrative Stress and Inflammation. Cell. Physiol. Biochem. 2016;40:1603–1612. doi: 10.1159/000453210. [DOI] [PubMed] [Google Scholar]
- 76.Faller S., Zimmermann K.K., Strosing K.M., Engelstaedter H., Buerkle H., Schmidt R., Spassov S.G., Hoetzel A. Inhaled hydrogen sulfide protects against lipopolysaccharide-induced acute lung injury in mice. Med. Gas. Res. 2012;2:26. doi: 10.1186/2045-9912-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zimmermann K.K., Spassov S.G., Strosing K.M., Ihle P.M., Engelstaedter H., Hoetzel A., Faller S. Hydrogen Sulfide Exerts Anti-oxidative and Anti-inflammatory Effects in Acute Lung Injury. Inflammation. 2017;41:249–259. doi: 10.1007/s10753-017-0684-4. [DOI] [PubMed] [Google Scholar]
- 78.Han Z.-H., Jiang Y., Duan Y.-Y., Wang X.-Y., Huang Y., Fang T.-Z. Protective effects of hydrogen sulfide inhalation on oxidative stress in rats with cotton smoke inhalation-induced lung injury. Exp. Ther. Med. 2015;10:164–168. doi: 10.3892/etm.2015.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qi Q.Y.C., Chen W., Li X.L., Wang Y.W., Xie X.H. H₂S protecting against lung injury following limb ischemia-reperfusion by alleviating inflammation and water transport abnormality in rats. Biomed. Environ. Sci. 2014;27:410–418. doi: 10.3967/bes2014.070. [DOI] [PubMed] [Google Scholar]
- 80.Sakaguchi M., Marutani E., Shin H.-s., Chen W., Hanaoka K., Xian M., Ichinose F. Sodium thiosulfate attenuates acute lung injury in mice. Anesthesiology. 2014;121:1248–1257. doi: 10.1097/ALN.0000000000000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spassov S., Pfeifer D., Strosing K., Ryter S., Hummel M., Faller S., Hoetzel A. Genetic Targets of Hydrogen Sulfide in Ventilator-Induced Lung Injury—A Microarray Study. PLoS ONE. 2014;9:e102401. doi: 10.1371/journal.pone.0102401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Geng P., Ma T., Xing J., Jiang L., Sun H., Zhu B., Zhang H., Xiao H., Wang J., Zhang J. Dexamethasone ameliorates H2S-induced acute lung injury by increasing claudin-5 expression via the PI3K pathway. Hum. Exp. Toxicol. 2017;37:626–635. doi: 10.1177/0960327117721961. [DOI] [PubMed] [Google Scholar]
- 83.Ning J., Mo L., Zhao H., Lu K., Lai X., Luo X., Zhao H., Ma D. Sodium Hydrosulphide Alleviates Remote Lung Injury Following Limb Traumatic Injury in Rats. PLoS ONE. 2013;8:e59100. doi: 10.1371/journal.pone.0059100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vadivel A., Alphonse R.S., Ionescu L., Machado D.S., O’Reilly M., Eaton F., Haromy A., Michelakis E.D., Thébaud B. Exogenous Hydrogen Sulfide (H2S) Protects Alveolar Growth in Experimental O2-Induced Neonatal Lung Injury. PLoS ONE. 2014;9:e90965. doi: 10.1371/journal.pone.0090965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu Z.-W., Wang H.-Y., Guan L., Zhao B. Regulatory effects of hydrogen sulfide on alveolar epithelial cell endoplasmic reticulum stress in rats with acute lung injury. World J. Emerg. Med. 2015;6:67–73. doi: 10.5847/wjem.j.1920-8642.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang J., Zhang H., Su C., Chen J., Zhu B., Zhang H., Xiao H., Zhang J. Dexamethasone Ameliorates H2S-Induced Acute Lung Injury by Alleviating Matrix Metalloproteinase-2 and -9 Expression. PLoS ONE. 2014;9:e94701. doi: 10.1371/journal.pone.0094701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aslami H., Heinen A., Roelofs J.J.T.H., Zuurbier C.J., Schultz M.J., Juffermans N.P. Suspended animation inducer hydrogen sulfide is protective in an in vivo model of ventilator-induced lung injury. Intensiv. Care Med. 2010;36:1946–1952. doi: 10.1007/s00134-010-2022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shields C.J., Winter D.C., Redmond H.P. Lung injury in acute pancreatitis: Mechanisms, prevention, and therapy. Curr. Opin. Crit. Care. 2002;8:158–163. doi: 10.1097/00075198-200204000-00012. [DOI] [PubMed] [Google Scholar]
- 89.Bhatia M., Sidhapuriwala J.N., Sparatore A., Moore P.K. Treatment with H2S-releasing diclofenac protects mice against acute pancreatitis-associated lung injury. Shock. 2008;29:84–88. doi: 10.1097/shk.0b013e31806ec26. [DOI] [PubMed] [Google Scholar]
- 90.Tamizhselvi R., Moore P.K., Bhatia M. Inhibition of Hydrogen Sulfide Synthesis Attenuates Chemokine Production and Protects Mice against Acute Pancreatitis and Associated Lung Injury. Pancreas. 2008;36:e24–e31. doi: 10.1097/MPA.0b013e31816857bb. [DOI] [PubMed] [Google Scholar]
- 91.Meng W., Pei Z., Feng Y., Zhao J., Chen Y., Shi W., Xu Q., Lin F., Sun M., Xiao K. Neglected role of hydrogen sulfide in sulfur mustard poisoning: Keap1 S-sulfhydration and subsequent Nrf2 pathway activation. Sci. Rep. 2017;7:1–17. doi: 10.1038/s41598-017-09648-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bhatia M., Zhi L., Zhang H., Ng S.-W., Moore P.K. Role of substance P in hydrogen sulfide-induced pulmonary inflammation in mice. Am. J. Physiol. Cell. Mol. Physiol. 2006;291:L896–L904. doi: 10.1152/ajplung.00053.2006. [DOI] [PubMed] [Google Scholar]
- 93.Sidhapuriwala J.N., Ng S.W., Bhatia M. Effects of hydrogen sulfide on inflammation in caerulein-induced acute pancreatitis. J. Inflamm. 2009;6:35. doi: 10.1186/1476-9255-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Esechie A., Enkhbaatar P., Traber D.L., Jonkam C., Lange M., Hamahata A., Djukom C., Whorton E.B., Hawkins H.K., Traber L.D. Beneficial effect of a hydrogen sulphide donor (sodium sulphide) in an ovine model of burn-and smoke-induced acute lung injury. Br. J. Pharmacol. 2009;158:1442–1453. doi: 10.1111/j.1476-5381.2009.00411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Song Z.J., Ng M.Y., Lee Z.-W., Dai W., Hagen T., Moore P.K., Huang D., Deng L.-W., Tan C.-H. Hydrogen sulfide donors in research and drug development. Med. Chem. Comm. 2014;5:557–570. doi: 10.1039/C3MD00362K. [DOI] [Google Scholar]
- 96.Ahmad A., Szabo C. Both the H 2 S biosynthesis inhibitor aminooxyacetic acid and the mitochondrially targeted H 2 S donor AP39 exert protective effects in a mouse model of burn injury. Pharmacol. Res. 2016;113:348–355. doi: 10.1016/j.phrs.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 97.Faller S., Ryter S.W., Choi A.M.K., Loop T., Schmidt R., Hoetzel A. Inhaled Hydrogen Sulfide Protects against Ventilator-induced Lung Injury. Anesthesiology. 2010;113:104–115. doi: 10.1097/ALN.0b013e3181de7107. [DOI] [PubMed] [Google Scholar]
- 98.Francis R.C., Vaporidi K., Bloch K.D., Ichinose F., Zapol W.M. Protective and detrimental effects of sodium sulfide and hydrogen sulfide in murine ventilator-induced lung injury. Anesthesiology. 2011;115:1012–1021. doi: 10.1097/ALN.0b013e31823306cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Asher I., Pearce N. Global burden of asthma among children. Int. J. Tuberc. Lung Dis. 2014;18:1269–1278. doi: 10.5588/ijtld.14.0170. [DOI] [PubMed] [Google Scholar]
- 100.Derwall M., Francis R.C., Kida K., Bougaki M., Crimi E., Adrie C., Zapol W.M., Ichinose F. Administration of hydrogen sulfide via extracorporeal membrane lung ventilation in sheep with partial cardiopulmonary bypass perfusion: A proof of concept study on metabolic and vasomotor effects. Crit. Care. 2011;15:R51. doi: 10.1186/cc10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Perry M.M., Hui C., Whiteman M., Wood M.E., Adcock I., Kirkham P., Chung K.F. Hydrogen Sulfide Inhibits Proliferation and Release of IL-8 from Human Airway Smooth Muscle Cells. Am. J. Respir. Cell Mol. Biol. 2011;45:746–752. doi: 10.1165/rcmb.2010-0304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tian M., Wang Y., Lu Y.-Q., Yan M., Jiang Y.-H., Zhao D.-Y. Correlation between serum H2S and pulmonary function in children with bronchial asthma. Mol. Med. Rep. 2012;6:335–338. doi: 10.3892/mmr.2012.904. [DOI] [PubMed] [Google Scholar]
- 103.Zhang P., Li F., Wiegman C.H., Zhang M., Hong Y., Gong J., Chang Y., Zhang J., Adcock I., Chung K.F., et al. Inhibitory Effect of Hydrogen Sulfide on Ozone-Induced Airway Inflammation, Oxidative Stress, and Bronchial Hyperresponsiveness. Am. J. Respir. Cell Mol. Biol. 2015;52:129–137. doi: 10.1165/rcmb.2013-0415OC. [DOI] [PubMed] [Google Scholar]
- 104.Ma Y., Yan Z., Deng X., Guo J., Hu J., Yu Y., Jiao F. Anticancer effect of exogenous hydrogen sulfide in cisplatin-resistant A549/DDP cells. Oncol. Rep. 2018;39:2969–2977. doi: 10.3892/or.2018.6362. [DOI] [PubMed] [Google Scholar]
- 105.Bhatia M. Role of Hydrogen Sulfide in the Pathology of Inflammation. Scientifica. 2012;2012:1–12. doi: 10.6064/2012/159680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fiorucci S., Orlandi S., Mencarelli A., Caliendo G., Santagada V., Distrutti E., Santucci L., Cirino G., Wallace J.L. Enhanced activity of a hydrogen sulphide-releasing derivative of mesalamine (ATB-429) in a mouse model of colitis. Br. J. Pharmacol. 2007;150:996–1002. doi: 10.1038/sj.bjp.0707193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xu D.-Q., Gao C., Niu W., Li Y., Wang Y.-X., Gao C.-J., Ding Q., Yao L.-N., Chai W., Li Z.-C. Sodium hydrosulfide alleviates lung inflammation and cell apoptosis following resuscitated hemorrhagic shock in rats. Acta Pharmacol. Sin. 2013;34:1515–1525. doi: 10.1038/aps.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bhatia M. H2S and Inflammation: An Overview. Handb. Exp. Pharmacol. 2015;230:165–180. doi: 10.1007/978-3-319-18144-8_8. [DOI] [PubMed] [Google Scholar]
- 109.Guo F., Yu T., Hong J., Fang J. Emerging Roles of Hydrogen Sulfide in Inflammatory and Neoplastic Colonic Diseases. Front. Physiol. 2016;7:156. doi: 10.3389/fphys.2016.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saglam K., Alhan E., Turkyilmaz S., Vanizor B.K., Ercin C. The anti-inflammatory effect of hydrogen sulphide on acute necrotizing pancreatitis in rats. Turk. J. Surg. 2017;33:158–163. doi: 10.5152/UCD.2017.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Campos D., Ravagnani F.G., Gurgueira S.A., Vercesi A.E., Teixeira S.A., Costa S.K., Muscará M.N., Ferreira H.H. Increased glutathione levels contribute to the beneficial effects of hydrogen sulfide and inducible nitric oxide inhibition in allergic lung inflammation. Int. Immunopharmacol. 2016;39:57–62. doi: 10.1016/j.intimp.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 112.Aslami H., Pulskens W.P., Kuipers M.T., Bos A.P., Van Kuilenburg A.B.P., Wanders R.J.A., Roelofsen J., Roelofs J.J.T.H., Kerindongo R.P., Beurskens C.J.P., et al. Hydrogen Sulfide Donor NaHS Reduces Organ Injury in a Rat Model of Pneumococcal Pneumosepsis, Associated with Improved Bio-Energetic Status. PLoS ONE. 2013;8:e63497. doi: 10.1371/journal.pone.0063497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Großhennig S., Ischebeck T., Gibhardt J., Busse J., Feussner I., Stülke J. Hydrogen sulfide is a novel potential virulence factor of M ycoplasma pneumoniae: Characterization of the unusual cysteine desulfurase/desulfhydrase HapE. Mol. Microbiol. 2016;100:42–54. doi: 10.1111/mmi.13300. [DOI] [PubMed] [Google Scholar]
- 114.Oliver E., Ma Y., Escaffre O., Ivanciuc T., Komaravelli N., Kelley J.P., Coletta C., Szabo C., Rockx B., Garofalo R.P., et al. Role of Hydrogen Sulfide in Paramyxovirus Infections. J. Virol. 2015;89:5557–5568. doi: 10.1128/jvi.00264-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cowley E.S., Kopf S.H., Lariviere A., Ziebis W., Newman D.K. Pediatric Cystic Fibrosis Sputum Can Be Chemically Dynamic, Anoxic, and Extremely Reduced Due to Hydrogen Sulfide Formation. mBio. 2015;6:e00767-15. doi: 10.1128/mBio.00767-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gaddam R.R., Fraser R., Badiei A., Chambers S., Cogger V.C., Le Couteur D.G., Ishii I., Bhatia M. Cystathionine-Gamma-Lyase Gene Deletion Protects Mice against Inflammation and Liver Sieve Injury following Polymicrobial Sepsis. PLoS ONE. 2016;11:e0160521. doi: 10.1371/journal.pone.0160521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang H., Zhi L., Moochhala S., Moore P.K., Bhatia M. Hydrogen sulfide acts as an inflammatory mediator in cecal ligation and puncture-induced sepsis in mice by upregulating the production of cytokines and chemokines via NF-κB. Am. J. Physiol. Lung Cell Mol. Physiol. 2007;292:L960–L971. doi: 10.1152/ajplung.00388.2006. [DOI] [PubMed] [Google Scholar]
- 118.El-Chemaly S., Levine S.J., Moss J. Lymphatics in Lung Disease. Ann. N. Y. Acad. Sci. 2008;1131:195–202. doi: 10.1196/annals.1413.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Reed H.O., Wang L., Sonett J., Chen M., Yang J., Li L., Aradi P., Jakus Z., D’Armiento J.M., Hancock W.W., et al. Lymphatic impairment leads to pulmonary tertiary lymphoid organ formation and alveolar damage. J. Clin. Investig. 2019;129:2514–2526. doi: 10.1172/JCI125044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Erb A., Althaus M. Actions of Hydrogen Sulfide on Sodium Transport Processes across Native Distal Lung Epithelia (Xenopus laevis) PLoS ONE. 2014;9:e100971. doi: 10.1371/journal.pone.0100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Althaus M., Clauss W.G., Fronius M. Amiloride-Sensitive Sodium Channels and Pulmonary Edema. Pulm. Med. 2010;2011:1–8. doi: 10.1155/2011/830320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Egli M., Duplain H., Lepori M., Cook S., Nicod P., Hummler E., Sartori C., Scherrer U. Defective respiratory amiloride-sensitive sodium transport predisposes to pulmonary oedema and delays its resolution in mice. J. Physiol. 2004;560:857–865. doi: 10.1113/jphysiol.2004.066704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kubo S., Doe I., Kurokawa Y., Kawabata A. Hydrogen sulfide causes relaxation in mouse bronchial smooth muscle. J. Pharmacol. Sci. 2007;104:392–396. doi: 10.1254/jphs.SC0070199. [DOI] [PubMed] [Google Scholar]
- 124.Gharib-Naseri M.K., Saberi S., Mard S.A., Latifi S.M. Bronchodilatory Effect of Hydrogen Sulfide in Rat. Iran. J. Basic Med. Sci. 2012;15:907–915. [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang Y.-L., Chen P.-X., Guan W.-J., Guo H.-M., Qiu Z.-E., Xu J.-W., Luo Y.-L., Lan C.-F., Xu J.-B., Hao Y., et al. Increased intracellular Cl− concentration promotes ongoing inflammation in airway epithelium. Mucosal Immunol. 2018;11:1149–1157. doi: 10.1038/s41385-018-0013-8. [DOI] [PubMed] [Google Scholar]
- 126.Zhou X., An G., Chen J. Inhibitory effects of hydrogen sulphide on pulmonary fibrosis in smoking rats via attenuation of oxidative stress and inflammation. J. Cell. Mol. Med. 2014;18:1098–1103. doi: 10.1111/jcmm.12254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Song K., Li Q., Yin X.-Y., Lu Y., Liu C.-F., Hu L.-F. Hydrogen Sulfide: A Therapeutic Candidate for Fibrotic Disease? Oxidative Med. Cell. Longev. 2015;2015:1–10. doi: 10.1155/2015/458720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou X., Cao Y., Ao G., Hu L., Liu H., Wu J., Wang X., Jin M., Zheng S., Zhen X. CaMKKβ-dependent activation of AMP-activated protein kinase is critical to suppressive effects of hydrogen sulfide on neuroinflammation. Antioxid. Redox Signal. 2014;21:1741–1758. doi: 10.1089/ars.2013.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang Z., Yin X., Gao L., Feng S., Song K., Li L., Lu Y., Shen H. The protective effect of hydrogen sulfide on systemic sclerosis associated skin and lung fibrosis in mice model. SpringerPlus. 2016;5:1084. doi: 10.1186/s40064-016-2774-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang S., Pan C., Zhou F., Yuan Z., Wang H., Cui W., Zhang G. Hydrogen Sulfide as a Potential Therapeutic Target in Fibrosis. Oxidative Med. Cell. Longev. 2015;2015:1–12. doi: 10.1155/2015/593407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fleischmann M.C., Scherag A., Adhikari N.K.J., Hartog C.S., Tsaganos T., Schlattmann P., Angus D.C., Reinhart K. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016;193:259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 132.Ang S.-F., Moochhala S.M., MacAry P.A., Bhatia M. Hydrogen sulfide and neurogenic inflammation in polymicrobial sepsis: Involvement of substance P and ERK-NF-κB signaling. PLoS ONE. 2011;6:e24535. doi: 10.1371/journal.pone.0024535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang H., Hegde A., Ng S.W., Adhikari S., Moochhala S.M., Bhatia M. Hydrogen sulfide up-regulates substance P in polymicrobial sepsis-associated lung injury. J. Immunol. 2007;179:4153–4160. doi: 10.4049/jimmunol.179.6.4153. [DOI] [PubMed] [Google Scholar]
- 134.Jiang T., Yang W., Zhang H., Song Z., Liu T., Lv X. Hydrogen Sulfide Ameliorates Lung Ischemia-Reperfusion Injury Through SIRT1 Signaling Pathway in Type 2 Diabetic Rats. Front. Physiol. 2020;11:596. doi: 10.3389/fphys.2020.00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.George T.J., Arnaoutakis G.J., Beaty C.A., Jandu S.K., Santhanam L., Berkowitz D.E., Shah A.S. Inhaled hydrogen sulfide improves graft function in an experimental model of lung transplantation. J. Surg. Res. 2012;178:593–600. doi: 10.1016/j.jss.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu J.-X., Wei J., You X., Chen X., Zhu H., Zhu X., Liu Y., Xu M. Inhibition of hydrogen sulfide generation contributes to lung injury after experimental orthotopic lung transplantation. J. Surg. Res. 2013;182:e25–e33. doi: 10.1016/j.jss.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 137.Wanamaker B., Cascino T., McLaughlin V., Oral H., Latchamsetty R., Siontis K.C. Atrial Arrhythmias in Pulmonary Hypertension: Pathogenesis, Prognosis and Management. Arrhythmia Electrophysiol. Rev. 2018;7:43–48. doi: 10.15420/aer.2018.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xiaohui L., Junbao D., Lin S., Jian L., Xiuying T., Jianguang Q., Bing W., Hongfang J., Chaoshu T. Down-Regulation of Endogenous Hydrogen Sulfide Pathway in Pulmonary Hypertension and Pulmonary Vascular Structural Remodeling Induced by High Pulmonary Blood Flow in Rats. Circ. J. 2005;69:1418–1424. doi: 10.1253/circj.69.1418. [DOI] [PubMed] [Google Scholar]
- 139.Wu J., Pan W., Wang C., Dong H., Xing L., Hou J., Fang S., Li H., Yang F., Yu B. H2S attenuates endoplasmic reticulum stress in hypoxia-induced pulmonary artery hypertension. Biosci. Rep. 2019;39:BSR20190304. doi: 10.1042/BSR20190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wei H.-L., Zhang C.-Y., Jin H.-F., Tang C.-S., Du J.-B. Hydrogen sulfide regulates lung tissue-oxidized glutathione and total antioxidant capacity in hypoxic pulmonary hypertensive rats. Acta Pharmacol. Sin. 2008;29:670–676. doi: 10.1111/j.1745-7254.2008.00796.x. [DOI] [PubMed] [Google Scholar]
- 141.Peng Y.-J., Zhang X., Gridina A., Chupikova I., McCormick D.L., Thomas R.J., Scammell T.E., Kim G., Vasavda C., Nanduri J., et al. Complementary roles of gasotransmitters CO and H2S in sleep apnea. Proc. Natl. Acad. Sci. USA. 2017;114:1413–1418. doi: 10.1073/pnas.1620717114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yuan G., Peng Y.-J., Khan S.A., Nanduri J., Singh A., Vasavda C., Semenza G.L., Kumar G.K., Snyder S.H., Prabhakar N.R. H2S production by reactive oxygen species in the carotid body triggers hypertension in a rodent model of sleep apnea. Sci. Signal. 2016;9:ra80. doi: 10.1126/scisignal.aaf3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Claudet I., Marcoux M.-O., Karsenty C., Rittié J.-L., Honorat R., Lelong-Tissier M. Intoxication accidentelle grave à l’hydrogène sulfuré: Un cas pédiatrique de survie. Ann. Françaises D’anesthésie Et De Réanimation. 2012;31:255–258. doi: 10.1016/j.annfar.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 144.Muzaffar S., Jeremy J.Y., Sparatore A., Del Soldato P., Angelini G.D., Shukla N. H2 S-donating sildenafil (ACS6) inhibits superoxide formation and gp91phox expression in arterial endothelial cells: Role of protein kinases A and G. Br. J. Pharmacol. 2008;155:984–994. doi: 10.1038/bjp.2008.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lin H., Wang X. The effects of gasotransmitters on bronchopulmonary dysplasia. Eur. J. Pharmacol. 2020;873:172983. doi: 10.1016/j.ejphar.2020.172983. [DOI] [PubMed] [Google Scholar]
- 146.Madurga A., Mižíková I., Ruiz-Camp J., Vadász I., Herold S., Mayer K., Fehrenbach H., Seeger W., Morty R.E. Systemic hydrogen sulfide administration partially restores normal alveolarization in an experimental animal model of bronchopulmonary dysplasia. Am. J. Physiol. Cell. Mol. Physiol. 2014;306:L684–L697. doi: 10.1152/ajplung.00361.2013. [DOI] [PubMed] [Google Scholar]
- 147.Calderone V., Martelli A., Testai L., Citi V., Breschi M.C. Using hydrogen sulfide to design and develop drugs. Expert Opin. Drug Discov. 2015;11:163–175. doi: 10.1517/17460441.2016.1122590. [DOI] [PubMed] [Google Scholar]
- 148.Citi V., Piragine E., Testai L., Breschi M.C., Calderone V., Martelli A. The Role of Hydrogen Sulfide and H2S-donors in Myocardial Protection against Ischemia/Reperfusion Injury. Curr. Med. Chem. 2018;25:4380–4401. doi: 10.2174/0929867325666180212120504. [DOI] [PubMed] [Google Scholar]
- 149.Zhang G., Wang P., Yang G., Cao Q., Wang R. The Inhibitory Role of Hydrogen Sulfide in Airway Hyperresponsiveness and Inflammation in a Mouse Model of Asthma. Am. J. Pathol. 2013;182:1188–1195. doi: 10.1016/j.ajpath.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 150.Rashid S., Heer J., Garle M., Alexander S., Roberts R. Hydrogen sulphide-induced relaxation of porcine peripheral bronchioles. Br. J. Pharmacol. 2013;168:1902–1910. doi: 10.1111/bph.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Castro-Piedras I., Perez-Zoghbi J.F. Hydrogen sulphide inhibits Ca2+release through InsP3receptors and relaxes airway smooth muscle. J. Physiol. 2013;591:5999–6015. doi: 10.1113/jphysiol.2013.257790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Huang J., Luo Y.-L., Hao Y., Zhang Y.-L., Chen P.-X., Xu J.-W., Chen M., Luo Y.-F., Zhong N.-S., Xu J., et al. Cellular mechanism underlying hydrogen sulfide induced mouse tracheal smooth muscle relaxation: Role of BKCa. Eur. J. Pharmacol. 2014;741:55–63. doi: 10.1016/j.ejphar.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 153.Hosoki R., Matsuki N., Kimura H. The Possible Role of Hydrogen Sulfide as an Endogenous Smooth Muscle Relaxant in Synergy with Nitric Oxide. Biochem. Biophys. Res. Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 154.Wang Y.-F., Mainali P., Tang C.-S., Shi L., Zhang C.-Y., Yan H., Liu X.-Q., Du J.-B. Effects of nitric oxide and hydrogen sulfide on the relaxation of pulmonary arteries in rats. Chin. Med. J. 2008;121:420–423. doi: 10.1097/00029330-200803010-00010. [DOI] [PubMed] [Google Scholar]
- 155.Costantino M., Lampa E., Nappi G. Effectiveness of sulphur spa therapy with politzer in the treatment of rhinogenic deafness. Acta Otorhinolaryngol. Ital. 2006;26:7–13. [PMC free article] [PubMed] [Google Scholar]
- 156.Pouokam E., Althaus M. Epithelial Electrolyte Transport Physiology and the Gasotransmitter Hydrogen Sulfide. Oxidative Med. Cell. Longev. 2016;2016:1–13. doi: 10.1155/2016/4723416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Faller S., Hausler F., Goeft A., Von Itter M.-N.A., Gyllenram V., Hoetzel A., Spassov S.G. Hydrogen sulfide limits neutrophil transmigration, inflammation, and oxidative burst in lipopolysaccharide-induced acute lung injury. Sci. Rep. 2018;8:1–10. doi: 10.1038/s41598-018-33101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang D., Wang X., Chen S., Chen S., Yu W., Liu X., Yang G., Tao Y., Tang X., Bu D., et al. Endogenous hydrogen sulfide sulfhydrates IKKβ at cysteine 179 to control pulmonary artery endothelial cell inflammation. Clin. Sci. 2019;133:2045–2059. doi: 10.1042/CS20190514. [DOI] [PubMed] [Google Scholar]
- 159.Zanardo R.C.O., Brancaleone V., Distrutti E., Fiorucci S., Cirino G., Wallace J.L. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. Faseb J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 160.Brancaleone V., Mitidieri E., Flower R.J., Cirino G., Perretti M. Annexin A1 Mediates Hydrogen Sulfide Properties in the Control of Inflammation. J. Pharmacol. Exp. Ther. 2014;351:96–104. doi: 10.1124/jpet.114.217034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Benavides G.A., Squadrito G.L., Mills R.W., Patel H.D., Isbell T.S., Patel R.P., Darley-Usmar V.M., Doeller J.E., Kraus D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA. 2007;104:17977–17982. doi: 10.1073/pnas.0705710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Martelli A., Testai L., Citi V., Marino A., Pugliesi I., Barresi E., Nesi G., Rapposelli S., Taliani S., Da Settimo F., et al. Arylthioamides as H2S Donors: L-Cysteine-Activated Releasing Properties and Vascular effects in vitro and in vivo. ACS Med. Chem. Lett. 2013;4:904–908. doi: 10.1021/ml400239a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu Y., Li A., Feng X., Sun X., Zhu X., Zhao Z. Pharmacological Investigation of the Anti-Inflammation and Anti-Oxidation Activities of Diallyl Disulfide in a Rat Emphysema Model Induced by Cigarette Smoke Extract. Nutrients. 2018;10:79. doi: 10.3390/nu10010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lucarini E., Micheli L., Trallori E., Citi V., Martelli A., Testai L., De Nicola G.R., Iori R., Calderone V., Ghelardini C., et al. Effect of glucoraphanin and sulforaphane against chemotherapy-induced neuropathic pain: Kv7 potassium channels modulation by H2 S release in vivo. Phytother. Res. 2018;32:2226–2234. doi: 10.1002/ptr.6159. [DOI] [PubMed] [Google Scholar]
- 165.Citi V., Corvino A., Fiorino F., Frecentese F., Magli E., Perissutti E., Santagada V., Brogi S., Flori L., Gorica E., et al. Structure-activity relationships study of isothiocyanates for H2S releasing properties: 3-Pyridyl-isothiocyanate as a new promising cardioprotective agent. J. Adv. Res. 2021;27:41–53. doi: 10.1016/j.jare.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Citi V., Martelli A., Gorica E., Brogi S., Testai L., Calderone V. Role of hydrogen sulfide in endothelial dysfunction: Pathophysiology and therapeutic approaches. J. Adv. Res. 2021;27:99–113. doi: 10.1016/j.jare.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Citi V., Martelli A., Testai L., Marino A., Breschi M.C., Calderone V. Hydrogen Sulfide Releasing Capacity of Natural Isothiocyanates: Is It a Reliable Explanation for the Multiple Biological Effects of Brassicaceae? Planta Med. 2014;80:610–613. doi: 10.1055/s-0034-1368591. [DOI] [PubMed] [Google Scholar]
- 168.Citi V., Piragine E., Pagnotta E., Ugolini L., Mannelli L.D.C., Testai L., Ghelardini C., Lazzeri L., Calderone V., Martelli A. Anticancer properties of erucin, an H2S-releasing isothiocyanate, on human pancreatic adenocarcinoma cells (AsPC-1) Phytother. Res. 2019;33:845–855. doi: 10.1002/ptr.6278. [DOI] [PubMed] [Google Scholar]
- 169.Martelli A., Citi V., Testai L., Brogi S., Calderone V. Organic Isothiocyanates as Hydrogen Sulfide Donors. Antioxid. Redox Signal. 2020;32:110–144. doi: 10.1089/ars.2019.7888. [DOI] [PubMed] [Google Scholar]
- 170.Martelli A., Piragine E., Citi V., Testai L., Pagnotta E., Ugolini L., Lazzeri L., Mannelli L.D.C., Manzo O.L., Bucci M., et al. Erucin exhibits vasorelaxing effects and antihypertensive activity by H2S-releasing properties. Br. J. Pharmacol. 2020;177:824–835. doi: 10.1111/bph.14645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sestito S., Pruccoli L., Runfola M., Citi V., Martelli A., Saccomanni G., Calderone V., Tarozzi A., Rapposelli S. Design and synthesis of H2S-donor hybrids: A new treatment for Alzheimer’s disease? Eur. J. Med. Chem. 2019;184:111745. doi: 10.1016/j.ejmech.2019.111745. [DOI] [PubMed] [Google Scholar]
- 172.Takayama K., Hirose A., Suda I., Miyazaki A., Oguchi M., Onotogi M., Fotopoulos G. Comparison of the Anti-Inflammatory and Analgesic Effects in Rats of Diclofenac-Sodium, Felbinac and Indomethacin Patches. Int. J. Biomed. Sci. 2011;7:222–229. [PMC free article] [PubMed] [Google Scholar]
- 173.Jia G., Yu S., Sun W., Yang J., Wang Y., Qi Y., Chen Y. Hydrogen Sulfide Attenuates Particulate Matter-Induced Emphysema and Airway Inflammation Through Nrf2-Dependent Manner. Front. Pharmacol. 2020;11:29. doi: 10.3389/fphar.2020.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.McCook O., Radermacher P., Volani C., Asfar P., Ignatius A., Kemmler J., Möller P., Szabó C., Whiteman M., Wood M.E., et al. H2S during circulatory shock: Some unresolved questions. Nitric Oxide. 2014;41:48–61. doi: 10.1016/j.niox.2014.03.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nagy P., Pálinkás Z., Nagy A., Budai B., Tóth I., Vasas A. Chemical aspects of hydrogen sulfide measurements in physiological samples. Biochim. Biophys. Acta. 2014;1840:876–891. doi: 10.1016/j.bbagen.2013.05.037. [DOI] [PubMed] [Google Scholar]
- 176.Wu N., Du X., Wang D., Hao F. Myocardial and lung injuries induced by hydrogen sulfide and the effectiveness of oxygen therapy in rats. Clin. Toxicol. 2011;49:161–166. doi: 10.3109/15563650.2011.565419. [DOI] [PubMed] [Google Scholar]
- 177.Olson K.R., DeLeon E.R., Gao Y., Hurley K., Sadauskas V., Batz C., Stoy G.F. Thiosulfate: A readily accessible source of hydrogen sulfide in oxygen sensing. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305:R592–R603. doi: 10.1152/ajpregu.00421.2012. [DOI] [PubMed] [Google Scholar]
- 178.Szabo C., Papapetropoulos A. International Union of Basic and Clinical Pharmacology. CII: Pharmacological Modulation of H2S Levels: H2S Donors and H2S Biosynthesis Inhibitors. Pharmacol. Rev. 2017;69:497–564. doi: 10.1124/pr.117.014050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xu S., Yang C.-T., Meng F.-H., Pacheco A., Chen L., Xian M. Ammonium tetrathiomolybdate as a water-soluble and slow-release hydrogen sulfide donor. Bioorganic Med. Chem. Lett. 2016;26:1585–1588. doi: 10.1016/j.bmcl.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dyson A., Dal-Pizzol F., Sabbatini G., Lach A.B., Galfo F., Cardoso J.D.S., Mendonça B.P., Hargreaves I., Pinto B.B., Bromage D.I., et al. Ammonium tetrathiomolybdate following ischemia/reperfusion injury: Chemistry, pharmacology, and impact of a new class of sulfide donor in preclinical injury models. PLoS Med. 2017;14:e1002310. doi: 10.1371/journal.pmed.1002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Donnarumma E., Ali M.J., Rushing A.M., Scarborough A.L., Bradley J.M., Organ C.L., Islam K.N., Polhemus D.J., Evangelista S., Cirino G., et al. Zofenopril Protects Against Myocardial Ischemia–Reperfusion Injury by Increasing Nitric Oxide and Hydrogen Sulfide Bioavailability. J. Am. Hear. Assoc. 2016;5:003531. doi: 10.1161/JAHA.116.003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Neuwelt E.A., Gilmer-Knight K., Lacy C., Nicholson H.S., Kraemer D.F., Doolittle N.D., Hornig G.W., Muldoon L.L. Toxicity profile of delayed high dose sodium thiosulfate in children treated with carboplatin in conjunction with blood-brain-barrier disruption. Pediatr. Blood Cancer. 2006;47:174–182. doi: 10.1002/pbc.20529. [DOI] [PubMed] [Google Scholar]
- 183.Marutani E., Yamada M., Ida T., Tokuda K., Ikeda K., Kai S., Shirozu K., Hayashida K., Kosugi S., Hanaoka K., et al. Thiosulfate Mediates Cytoprotective Effects of Hydrogen Sulfide Against Neuronal Ischemia. J. Am. Heart Assoc. 2015;4:e002125. doi: 10.1161/JAHA.115.002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wallace J.L., Caliendo G., Santagada V., Cirino G. Markedly reduced toxicity of a hydrogen sulphide-releasing derivative of naproxen (ATB-346) Br. J. Pharmacol. 2010;159:1236–1246. doi: 10.1111/j.1476-5381.2009.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ekundi-Valentim E., Mesquita F.P., Santos K.T., Paula M.A.V.D., Florenzano J., I Zanoni C., Rodrigues L., De Nucci G., A Teixeira S., Ferreira H.H., et al. A comparative study on the anti-inflammatory effects of single oral doses of naproxen and its hydrogen sulfide (H2S)-releasing derivative ATB-346 in rats with carrageenan-induced synovitis. Med. Gas. Res. 2013;3:24. doi: 10.1186/2045-9912-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wallace J.L., Nagy P., Feener T.D., Allain T., Ditrói T., Vaughan D.J., Muscara M.N., De Nucci G., Buret A.G. A proof-of-concept, Phase 2 clinical trial of the gastrointestinal safety of a hydrogen sulfide-releasing anti-inflammatory drug. Br. J. Pharmacol. 2020;177:769–777. doi: 10.1111/bph.14641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kashfi K., Chattopadhyay M., Kodela R. NOSH-sulindac (AVT-18A) is a novel nitric oxide- and hydrogen sulfide-releasing hybrid that is gastrointestinal safe and has potent anti-inflammatory, analgesic, antipyretic, anti-platelet, and anti-cancer properties. Redox Biol. 2015;6:287–296. doi: 10.1016/j.redox.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kodela R., Chattopadhyay M., Velázquez-Martínez C.A., Kashfi K. NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid has enhanced chemo-preventive properties compared to aspirin, is gastrointestinal safe with all the classic therapeutic indications. Biochem. Pharmacol. 2015;98:564–572. doi: 10.1016/j.bcp.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Whiteman M., Li L., Rose P., Tan C.-H., Parkinson D.B., Moore P.K. The Effect of Hydrogen Sulfide Donors on Lipopolysaccharide-Induced Formation of Inflammatory Mediators in Macrophages. Antioxid. Redox Signal. 2010;12:1147–1154. doi: 10.1089/ars.2009.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Li L., Salto-Tellez M., Tan C.-H., Whiteman M., Moore P.K. GYY4137, a novel hydrogen sulfide-releasing molecule, protects against endotoxic shock in the rat. Free Radic. Biol. Med. 2009;47:103–113. doi: 10.1016/j.freeradbiomed.2009.04.014. [DOI] [PubMed] [Google Scholar]