1. Introduction
Alcohol use is prevalent among HIV-positive individuals or people living with HIV/AIDS (PLWHA). Alcohol abuse is associated with poor adherence to antiretroviral therapy (ART) [1], which in turn can lead to increased viral replication [2]. The other mechanisms by which alcohol abuse can exacerbate HIV replication are oxidative stress pathway [3] and disease progression [4,5]. In addition, alcohol is known to disrupt the integrity of the blood-brain barrier (BBB) [6], which can potentially increase infiltration of the virus into the central nervous system, where it infects perivascular macrophages and microglia.
2. Alcohol–ART interaction
Alcohol consumption not only exacerbates HIV pathogenesis but is also associated with decreased efficacy and increased toxicity of antiretrovirals (ARVs) [7] through drug–drug interactions mediated by cytochrome P450 (CYP) enzymes [8]. Although multi-sectoral approach such as biological, behavioral, psychosocial, and pharmacological treatments have been suggested for PLWHA to abstain from alcohol abuse [9], more than half the population do not adhere to the recommendation. Moreover, there are no current personalized guidelines for using ARVs in patients who consume alcohol. Therefore, due to the exacerbating effects of chronic alcohol consumption, there is a need to develop guidelines for ART treatment in patients with alcohol addiction. To this end, a greater understanding of the role of CYP enzymes and drug transporters in ARVs-alcohol interaction is crucial.
2.1. Role of CYP2E1, CYP3A4, and MDR1 in ART–alcohol interaction
CYP enzymes play a major role in xenobiotic metabolism, including alcohol and ARVs such as non-nucleotide reverse transcriptase inhibitors (NNRTIs) and protease inhibitors (PIs) [10]. Alcohol is primarily metabolized in the liver by alcohol dehydrogenase. However, alcohol is also metabolized by CYP2E1, and to a lesser extent by CYP3A4, which are induced by alcohol by several folds in alcohol drinkers [3,11]. Alcohol-inducible CYP2E1 plays a major role in ethanol metabolism leading to generation of reactive oxygen species (ROS), which can cause liver damage in chronic alcohol users [12]. Alcohol-mediated liver damage and other toxicities are further exacerbated in HIV-infected individuals taking ARVs [7]. Moreover, alcohol-induced oxidative damage can increase HIV replication [3], thus indirectly decreasing the efficacy of ART. Some NNRTIs and PIs are substrates, inducers, and inhibitors of CYP3A4 [10]. While the metabolism of NNRTIs and PIs by CYP3A4 can produce ROS, CYP3A4 inhibitors can decrease the elimination of ARVs, leading to increased drug half-lives and toxic drug accumulation, both of which lead to liver toxicity [10]. On the other hand, CYP3A4 inducers lead to suboptimal drug concentrations due to faster metabolism, resulting in reduced therapeutic effect of ARVs [10]. A case-control study consisting of 41 HIV-positive subjects revealed that chronic ethanol use has significantly affected the steady-state plasma concentrations of stavudine, lamivudine, and nevirapine [13]. Intracellular ARV drug concentrations are also dictated by the activity of efflux transporters such as ATP-binding cassette (ABC) proteins [14]. ARVs can act as substrates as well as inducers for membrane transporters, especially efflux transporters [14], which account for, at least in part, the reported high intracellular drug variability in HIV-positive subjects [15]. Kumar group has demonstrated that ethanol exposure increases the expression of ABC transporter protein, ABCC1, in U937 macrophages [16]. Therefore, the intracellular levels of ARVs may be decreased in alcoholic patients due to greater efflux, further decreasing their efficacy. However, these interactions may vary in the presence of genetic mutations which alter ABCC1 or ethanol metabolizing enzyme activities.
2.2. Effect of alcohol on CYP3A4-PI and CYP3A4-integrase strand transfer inhibitors (INSTI) interactions
Ethanol is known to interact with many medications, including PIs, through CYP3A4 induction leading to altered drug metabolism and toxicity in the liver. Kumar group has shown that ethanol increases CYP3A4 activity and protein expression in monocyte-derived macrophages (MDM), which are reservoirs of HIV [16]. Further, they have also demonstrated the effect of ethanol on CYP3A4–PI binding [17]. PIs upon binding to CYP3A4 can exhibit type I or type II spectral changes. Type I is non-covalent binding of ligand with the heme-Fe of CYP3A4 by replacement of a water molecule and characterized by a relatively strong binding affinity, whereas type II is characterized by covalent interaction between the heme-Fe of CYP3A4 and ligands. Ethanol exhibits differential effects on binding and inhibition of CYP3A4 with the PIs. Ethanol did not alter spectral binding affinity and inhibition constant (IC50) of type I PIs (atazanavir, lopinavir, saquinavir, and tipranavir). However, ethanol significantly decreased the IC50 of type II PIs, indinavir and ritonavir, and markedly increased the IC50 of spectrally unbound (amprenavir and darunavir) PIs. These findings suggest that alcohol can alter the metabolism and efficacy of type II and spectrally unbound PIs [17]. Moreover, being a strong inhibitor of CYP3A4, ritonavir (RTV)-boosted HIV therapy has been widely used to achieve optimum efficacy, while minimizing the dose of co-administered PIs. Therefore, ARVs given along with RTV should be administered cautiously in alcohol drinking patients to avoid ARV induced toxicity. Kumar group also conducted another study which showed that ethanol alone elevates HIV replication, but this effect was nullified with the addition of darunavir (DRV) or DRV + RTV [18]. Therefore, DRV could be administered to control HIV viral load in HIV-positive subjects with alcohol addiction. However, the reduction in concentration of DRV may be considered in alcohol-addicted patients as alcohol can increase IC50 of DRV [17]. Further, another commonly used ARV, elvitegravir (EVG), is metabolized by CYP3A4 and a strong CYP3A4 inhibitor such as cobicistat (COBI) is co-administered as a booster to increase EVG bioavailability. Recently, Kumar group showed that the ethanol exposure decreases the intracellular levels of EVG alone and also in combination with COBI in HIV-infected macrophages [19,20]. Subsequently, co-exposing cells to EVG or EVG + COBI along with ethanol showed increased viral replication by 31% and 19%, respectively, relative to control groups that did not receive ethanol [19]. Therefore, EVG level should be monitored in HIV patients who consume alcohol to attain sustained virological suppression.
3. Conclusion
Though the complete abstinence from alcohol is the cornerstone of therapy to control the alcohol exacerbated viral replication in PLWHA receiving ARVs, majority of the patients fail to achieve it. Successful treatment of alcohol dependence generally requires a multimodal approach often consisting of behavioral interventions, pharmacotherapy, and participation in self-help program. Available pharmacotherapies for alcohol dependence include disulfiram, naltrexone, and acamprosate. Naltrexone has been shown to mitigate alcohol-induced viral infection of T lymphocytes in vitro studies [21]. Acamprosate and disulfiram are both effective in treating alcohol dependence; however, data on their effect in HIV-infected patients is lacking. In addition, in vitro data suggest that alcohol does not affect spectral binding affinity and IC50 of type I PIs (atazanavir, lopinavir, saquinavir, and tipranavir). Therefore, they may be used to control HIV replication in this subpopulation. However, the in vitro findings should be validated in clinical trials before implementing in clinics. Further, the prevalence of alcohol abuse in PLWHA population has serious effects on their health outcomes, particularly because of the greater likelihood of poor adherence to HIV medication and poor virological response to ARV. Therefore, newer approaches aimed at improving adherence, such as use of electronic sensor in medicine bottles, formulation of multiple drugs in single pill, and extended drug delivery strategy, have been adopted. These approaches are showing promising results in improving adherence to ARVs.
4. Expert opinion
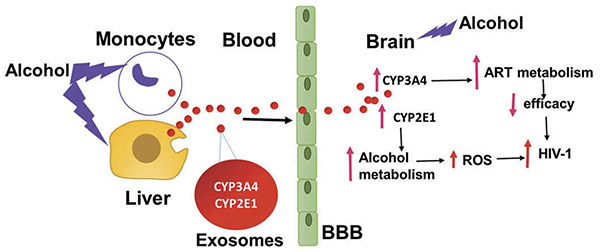
Liver dysfunction is the major concern with the use of alcohol and the toxicity is exacerbated with the use of ARVs such as NNRTIs, specifically nevirapine [22]. Complete abstinence from alcohol is the cornerstone of therapy for alcohol-induced liver diseases. Alcohol-ARVs interactions mediated via CYP pathways reduce the efficacy and increase the toxicity of ARVs. Moreover, alcohol can damage the BBB, which can potentially increase viral replication in the CNS where the efficacy of ARVs is low. Therefore, in addition to multimodal approach to treat alcohol addiction, targeting CYP pathways and delivery methods to improve ARVs levels in the CNS would provide better therapeutic options to control HIV replication in alcohol abusers. Recently, Kumar group observed a high abundance of CYP enzymes, especially CYP2E1 and 3A4, in plasma-derived exosomes [23]. Similar observation was also reported by Rowland A. et al., especially for CYP3A4, which further strengthened a potential role of exosomal CYPs in drug metabolism and drug–drug interactions [24]. Exosomes, small extracellular nanovesicles, have recently been recognized as extremely valuable targets for biological research due to their ability to package and transport diverse biological cargos such as proteins, mRNA, miRNA, and small molecules from donor cells to other cells, including the brain cells. Further, there is a possibility of alcohol-induced exosomal CYP enzymes crossing BBB and affecting metabolism of ARVs in the brain which may result in decreased efficacy of ARVs (Figure 1). Therefore, it is critical to study the role of exosomal CYP enzymes in alcohol-mediated modulation of pharmacokinetics of ARVs in these populations. Further, targeting exosomal CYP3A4 may reduce CYP3A4-ARVs interactions. Another approach is to load ARVs in exosomes, which can help to escape the metabolism of ARVs by circulatory CYP3A4 as well as can transport the ARVs to the CNS leading to their increased efficacy in the CNS. The findings from these approaches will provide a basis for future studies that would help to tailor ARV regimens in alcohol abusers.
Figure 1. Proposed model for role of exosomal CYPs in alcohol-induced neuro-AIDS.
Upon alcohol exposure, hepatic and monocytic cells secrete exosomes containing CYP enzymes which can circulate via plasma and cross the BBB. The transported exosomes release CYP enzymes in the brain which can exacerbate HIV pathogenesis via oxidative stress and by altering ART metabolism.
Acknowledgments
Funding
The authors are funded by National Institutes of Health grant AA022063.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer Disclosures
A referee has received research support from Gilead Sciences. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Hendershot CS, Stoner SA, Pantalone DW, et al. Alcohol use and antiretroviral adherence: review and meta-analysis. J Acquir Immune Defic Syndr. 2009;52:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalichman SC, Grebler T, Amaral CM, et al. Viral suppression and antiretroviral medication adherence among alcohol using HIV positive adults. Int J Behav Med. 2014;21:811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ande A, Sinha N, Rao PSS, et al. Enhanced oxidative stress by alcohol use in HIV + patients: possible involvement of cytochrome P450 2E1 and antioxidant enzymes. AIDS Res Ther. 2015;12:29.•• This ex vivo study demonstrates the potential role of CYP2E1 in alcohol-induced HIV replication in the clinical samples.
- 4.Molina PE, Simon L, Amedee AM, et al. Impact of alcohol on HIV disease pathogenesis, comorbidities and aging: integrating preclinical and clinical findings. Alcohol Alcohol. 2018;53:439–447.• This is a comprehensive review that covers the role of alcohol use with HIV disease progression, comorbidities, and aging.
- 5.Probst C, Parry CDH, Rehm J. HIV/AIDS mortality attributable to alcohol use in South Africa: a comparative risk assessment by socioeconomic status. BMJ Open. 2018;8:e017955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haorah J, Knipe B, Leibhart J, et al. Alcohol-induced oxidative stress in brain endothelial cells causes blood-brain barrier dysfunction. J Leukoc Biol. 2005;78:1223–1232.• This study demonstrates that alcohol increases the permeability of the BBB.
- 7.Braithwaite RS, Bryant KJ. Influence of alcohol consumption on adherence to and toxicity of antiretroviral therapy and survival. Alcohol Res Health. 2010;33:280–287. [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar S, Jin M, Ande A, et al. Alcohol consumption effect on antiretroviral therapy and HIV-1 pathogenesis: role of cytochrome P450 isozymes. Expert Opin Drug Metab Toxicol. 2012;8:1363–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durvasula R, Miller TR. Substance abuse treatment in persons with HIV/AIDS: challenges in managing triple diagnosis. Behav Med. 2014;40:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar S, Rao PSS, Earla R, et al. Drug-drug interactions between anti-retroviral therapies and drugs of abuse in HIV systems. Expert Opin Drug Metab Toxicol. 2015;11:343–355.• This review covers drug–drug interactions between ART and drugs of abuse, potentially through CYP pathway.
- 11.Jin M, Ande A, Kumar A, et al. Regulation of cytochrome P450 2e1 expression by ethanol: role of oxidative stress-mediated pkc/jnk/sp1 pathway. Cell Death Dis. 2013;4:e554.•• This is the first study that demonstrates the role of alcohol in the induction, and underlying mechanism, of CYP2E1 in non-hepatic cells monocytes and astrocytes.
- 12.Ceni E, Mello T, Galli A. Pathogenesis of alcoholic liver disease: role of oxidative metabolism. World J Gastroenterol. 2014;20:17756–17772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bbosa GS, Kyegombe DB, Anokbonggo WW, et al. Chronic ethanol use in alcoholic beverages by HIV-infected patients affects the therapeutic window of stavudine, lamivudine and nevirapine during the 9-month follow-up period: using chronic alcohol-use biomarkers. J Basic Clin Physiol Pharmacol. 2014;25:1–12. [DOI] [PubMed] [Google Scholar]
- 14.Kis O, Robillard K, Chan GNY, et al. The complexities of antiretroviral drug-drug interactions: role of ABC and SLC transporters. Trends Pharmacol Sci. 2010;31:22–35.• This study demonstrates the role of efflux and influx transportters on drug-drug interactions.
- 15.Fayet Mello A, Buclin T, Franc C, et al. Cell disposition of raltegravir and newer antiretrovirals in HIV-infected patients: high inter-individual variability in raltegravir cellular penetration. J Antimicrob Chemother. 2011;66:1573–1581. [DOI] [PubMed] [Google Scholar]
- 16.Jin M, Arya P, Patel K, et al. Effect of alcohol on drug efflux protein and drug metabolic enzymes in U937 macrophages. Alcohol Clin Exp Res. 2011;35:132–139.• This is the first report demonstrating the induction of CYPs and efflux transporter by ethanol in monocytic cells.
- 17.Kumar S, Kumar A. Differential effects of ethanol on spectral binding and inhibition of cytochrome P450 3A4 with eight protease inhibitors antiretroviral drugs. Alcohol Clin Exp Res. 2011;35:2121–2127.•• This study demonstrates differential interactions between ART drugs, PIs, and alcohol via CYP3A4, which is based upon the characteristics of PIs.
- 18.Midde NM, Gong Y, Cory TJ, et al. Influence of Ethanol on darunavir hepatic clearance and intracellular PK/PD in HIV-Infected monocytes, and CYP3A4-Darunavir interactions using inhibition and in silico binding studies. Pharm Res. 2017;34:1925–1933.•• This finding in this study demonstrates the effect of alcohol on reduced efficacy of darunavir via CYP3A4.
- 19.Midde NM, Sinha N, Lukka PB, et al. Alterations in cellular pharmacokinetics and pharmacodynamics of elvitegravir in response to ethanol exposure in HIV-1 infected monocytic (U1) cells. PLoS ONE. 2017;12:e0172628.• This finding in this study demonstrates the effect of alcohol on reduced efficacy of elvitegravir via CYP3A4.
- 20.Rao PSS, Kumar S. Chronic effects of ethanol and/or Darunavir/Ritonavir on U937 monocytic cells: regulation of cytochrome P450 and antioxidant enzymes, oxidative stress, and cytotoxicity. Alcohol Clin Exp Res. 2016;40:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Douglas SD, Peng J-S, et al. Naltrexone inhibits alcohol-mediated enhancement of HIV infection of T lymphocytes. J Leukoc Biol. 2006;79:1166–1172. [DOI] [PubMed] [Google Scholar]
- 22.Núñez M Hepatotoxicity of antiretrovirals: incidence, mechanisms and management. J Hepatol. 2006;44:S132–S139. [DOI] [PubMed] [Google Scholar]
- 23.Kumar S, Sinha N, Gerth KA, et al. Specific packaging and circulation of cytochromes P450, especially 2E1 isozyme, in human plasma exosomes and their implications in cellular communications. Biochem Biophys Res Commun. 2017;491:675–680.•• This study demonstrates, for the first time, the specific packaging of CYPs, especially CYP2E1, in plasma exosomes, which may have an important function in the distant cells.
- 24.Rowland A, Ruanglertboon W, van Dyk M, et al. Plasma extracellular nanovesicle (exosome) derived biomarkers for drug metabolism pathways: a novel approach to characterise variability in drug exposure. Br J Clin Pharmacol. 2018;85:216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]