Abstract
Exosomes are cell-derived vesicles containing heterogeneous active biomolecules such as proteins, lipids, mRNAs, receptors, immune regulatory molecules, and nucleic acids. They are typically range in size 30–150 nm in diameter. An exosome’s surfaces can be bioengineered with antibodies, fluorescent dye, peptides, and tailored for small molecule and large active biologics. Exosomes have enormous potential as a drug delivery vehicle due to enhanced biocompatibility, excellent payload capability, and reduced immunogenicity compared to alternative polymeric-based carriers. Due to active targeting and specificity, exosomes are capable of delivering their cargo to exosome-recipient cells. Additionally, exosomes can potentially act as early-stage disease diagnostic tools as the exosome carries various protein biomarkers associated with a specific disease. In this review, we summarized recent progress on exosome composition, biological characterization, and isolation techniques. Finally, we have outlined the exosome’s clinical applications and preclinical advancement to provide an outlook on the importance of exosomes for use in targeted drug delivery, biomarker study, and vaccine development.
Keywords: Exosome, clinical translation, drug delivery, biomarker, diagnosis, vaccine
Graphical Abstract
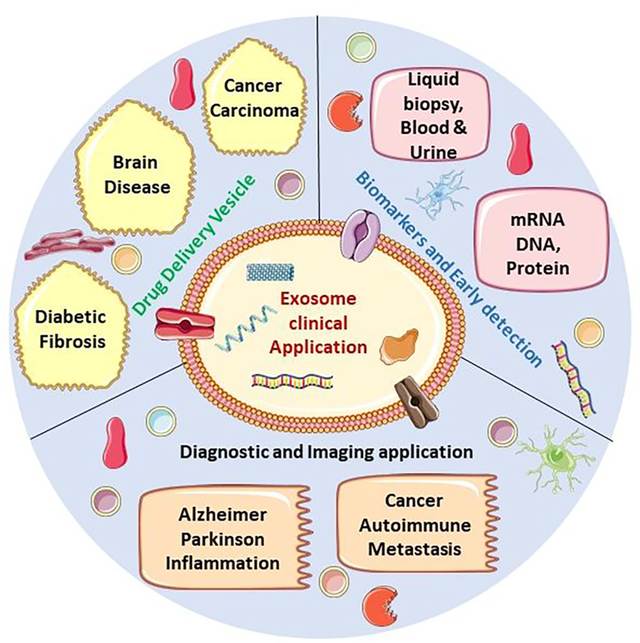
The exosome is a bio-inspired and biomimetic material consisting of proteins, lipids, and other various cellular derivatives and has potential as a therapeutic and diagnostic tool. Due to its vast biocompatibility and origin from biological cells, the exosome has many advantages over synthetic and semi-synthetic polymeric biomaterials used in biomedical applications.
1. Introduction:
With recent development and progress, biomarkers are an emerging tool for drug discovery and development. Given that the exosome embodies various proteins and lipids that are cell derived, these specific proteins, receptors, signaling molecules, and lipids can be identified and potentially used for diagnostic measures of abnormalities on the cellular level when compared with a healthy control1. Therefore, exosome-mediated detection technologies have emerging potential in the early-stage disease diagnosis field. Early detection via biomarker identification is considered a robust tool for efficient treatment of various chronic diseases such as cancer, auto-immune, infectious, and inflammatory diseases2–4. Besides, biomarkers are being widely used as diagnostic tools, personalized medicine platforms, and substitute endpoints for clinical research5.
Over the last decade, there have been many exciting developments in drug delivery. Synthetic biopolymers stand out among these innovations due to their ability to act as a drug delivery platform with improved abilities in drug targeting and controlled release6. Also, a range of exosome mediated drug formulations is being developed and currently undergoing preclinical and clinical trials. Unfortunately, drug-loaded synthetic polymers will opsonize with other biomolecules (protein) in the bloodstream which can results in three distinct issues: toxicity, immunogenicity, and mononuclear phagocyte system (MPS) rapid clearance7,8. In hopes of addressing these issues, the exosome has been singled out as a potential candidate as a bioinspired, bioengineered, and biomimetic drug delivery solution9,10.
Exosomes usually range from 30 to 150 nm. The intraluminal vesicle (ILV) is a circular lipid bilayer vesicle released from cells that differs from other extracellular vesicles such as microvesicles and apoptotic bodies, in composition and biogenesis11,12. First described as small vesicles by which maturating sheep reticulocytes discard obsolete cellular components13,14, further studies have shown that exosomes and other secreted extracellular vesicles are the prominent and universal form of cell to cell communication15. When exosomes are released, they are immediately internalized by surrounding cells or enter systemic circulation for intercellular communication16. Exosome secretion is a constitutive mechanism involved in both pathological and physiological conditions, regulating exosome surface markers and contents1,17. Exosomes can transport biologically active molecules, including proteins, fragmented DNA, antigens, and nucleic acids that regulate gene expression and cellular function in target cells18–22. As such, exosomes mediates autocrine, paracrine, and endocrine effects, classifying them as potential therapeutics18. For example, mesenchymal stem cells (MSCs) and other progenitor cells used in cell therapy mediated cytoprotective, angiogenic, and regenerative effects that can be recapitulated by the exosomes they release23. Indeed, exosomes have been found and investigated in numerous bodily fluids, including bile acid, blood, breast milk, urine, cerebrospinal fluid, and saliva, suggesting that exosomes play a prominent role in physiological regulation response and disease progression1,11,24–26. Recently, exosomes’ pathophysiological role in diseases, especially cancers, neurodegenerative, inflammatory, and infectious diseases, has emerged26–29. Exosomes function as diagnostic biomarkers, imaging tools, therapeutic targets, tissue repairing agent, drug delivery platforms and can be used in vaccine development. This would eventually lead to preclinical and clinically trials as avenues of new investigation as a result of their unique biological and pathophysiological characteristics30–35. However, thus far, there is no review currently available about the progress of exosome research and potential applications in a clinical setting. In this review, we have laid out a comprehensive study on the status of exosome clinical trials and their preclinical application to various diseases. More information on exosome classification, biological composition, relevant markers can be found at http://www.isev.org (International Society for Extracellular Vesicles), http://microvesicles.org (Vesiclepedia, a compendium for EVs with continuous community annotation) 36, http://www.exocarta.org (ExoCarta, a web-based compendium of exosomal cargo) 37, and http://exrna.org (Extracellular RNA communication program). Also, we state how exosome surface engineering can act as a translational medicine agents due to advancement in bio-engineering techniques like cationic pullulan, cationic linkers (DBCO-amine/dye), aptamer-based DNA tether, and click chemistry38–41.
2. Exosome Composition, Biogenesis, and Mechanism of Action
About 98% of all potential therapeutic medicines related to central nervous system (CNS) diseases have failed to reach the market due to an inability to cross the BBB42. While drug formulations have managed to overcome the barrier43,44, they have their own drawbacks, including significant toxicity and rapid clearance by the mononuclear phagocyte system (MPS) 7. Similar immediate clearance phenomena are observed in animal models for targeted drug delivery, cell therapy, and tumor therapy45,46. On the contrary, exosomes (30–150 nm) and cell origin vesicles offer intrinsic characteristics of an ideal drug delivery method for intracellular platform47,48. Exosomes as delivery vesicles provide: i) good tolerance in the body because of their wide distribution in bodily fluids (like milk, urine, blood, saliva, etc )4,50–53, ii) proper internalization in distant cells54, iii) reliable delivery of cargo like proteins55, mRNA56, lipids57, drugs6, nucleic acids, and iv) an extended circulation half-life via i.v. injections 58. Thus, naturally occurring exosomal intrinsic properties enable targeted delivery and diminish the rapid clearance of drugs59–61. In Figure 1, we illustrate what a typical exosome contains. From our understanding, the composition varies in its protein, lipid, and nucleic acid content depending on cell origin, cell homeostasis, and its current pathological condition. On their surface, exosomes carry immune regulatory molecules, membrane trafficking molecules, and tetraspanin. These molecules either help the exosome to bind or pass-through the recipient membrane for delivering its cargo. Exosomes carry multiple forms of these molecules inside them, including nucleic acids, signaling molecules, chaperons, and enzymes to bring the message to the neighboring cells. These chemical messengers can both modulate cell physiology and carry information about any foreign invaders. Exosomes originating from immune cells can activate or inactivate T-cells, depending on immune cell physiological condition. This is why we found multiple studies on exosome proteomics and lipidomics that explore exosome composition for either biomarker study or targeted drug delivery. Exosomes also play a crucial role in cell-cell communication using protein chaperones, cDNA, nucleic acid, and mRNA content to connect with neighboring and distant cells62. Exosomes deliver their protein, lipid, and cytoplasmic content to recipient cells through membrane fusion and modify physiological and pathological functions of targeted cells63. The exosome’s cargo is determined by its cell origin, cell physiological condition, and intercellular release site1. Exosome biogenesis begins with early endosomal maturation to microvesicles (MVB) and late endosomes to exosomes, during which endosomal membrane transforms into intraluminal vesicles (ILVs) in the lumen of the organelles through multiple pathways64. The most studied endosomal pathways are associated with endosomal complexes ESCRT-0, ESCRT-I, ESCRT-II, ESCRT-III, and AAA ATPase Vps4 associated complex for transport65–68. In ESCRT RNAi screening, a total of 23 ESCRT and ESCRT-associated proteins have been identified in HeLa cells69. In another study after shRNA transfection, secreted exosome trapped with anti-CD63 beads and screen result identified 7 ESCRT protein with a role in exosome secretion70. One research study shows that the depletion of both ESCRT-0 protein Hrs and ESCRT-1 protein STAM1 resulted in reduced exosome secretion69.
Figure 1.
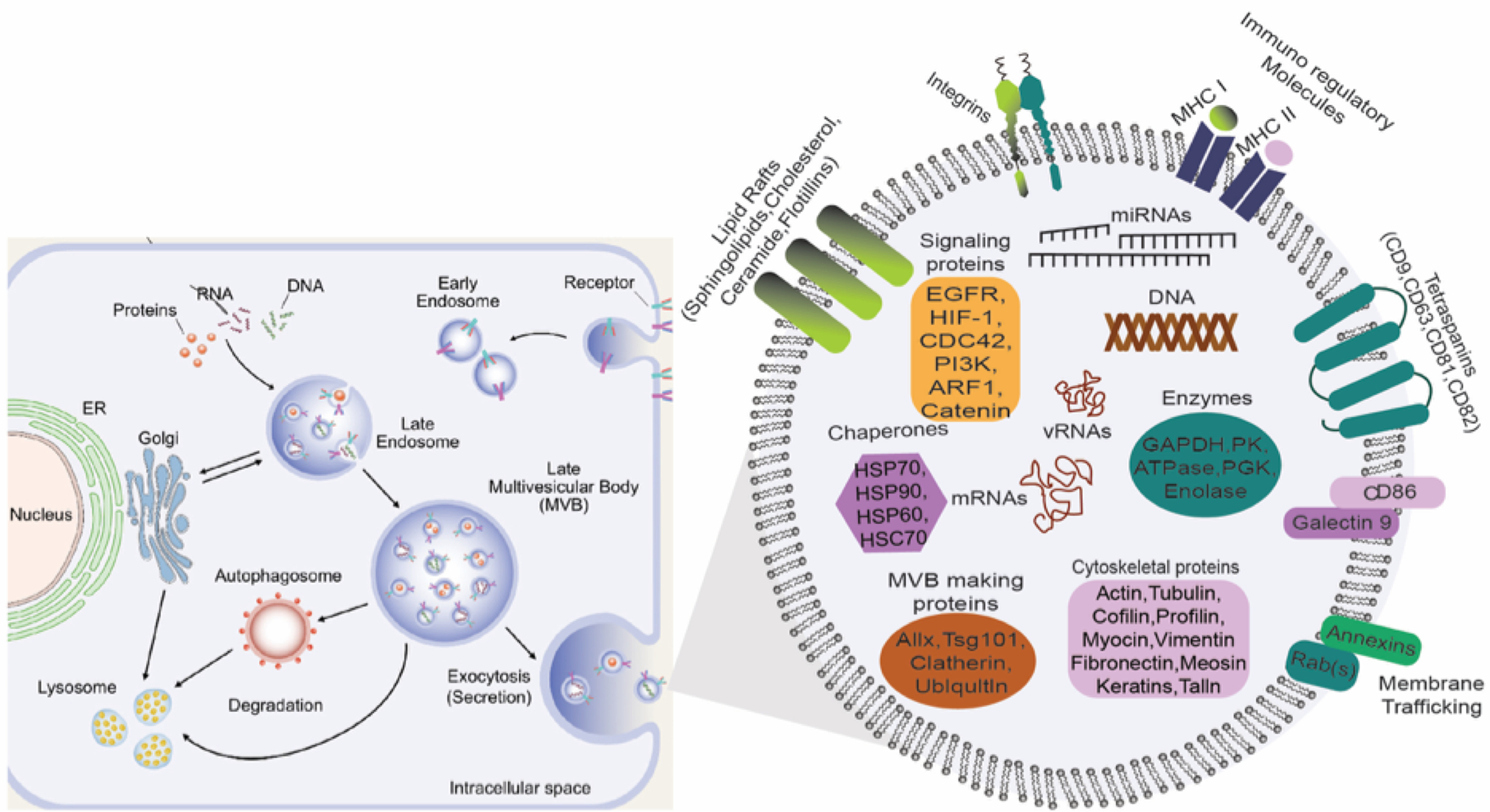
Exosome biogenesis begins with the formation of intraluminal vesicles (ILs) in late endosomes following cargo sorting. Both ESCRT dependent and ESCRT independent lipid-driven pathways are involved in creating multivesicular bodies. Exocytic MVCs fuse with the plasma membrane in Rab GTPases regulated miRNAs; exosome content depends on cell type and cells’ physiological and pathological conditions. Here we illustrate the components of exosomes identified in multiple proteomic studies and different cell content. Adapted from Gurunath et al. (2019) 49, Copyright @ 2019 MDPI and modified to accompany our review on exosome biogenesis and composition.
On the contrary, knockdown of ESCRT-III and associated proteins-like VSP4B, VTA1, and ALIX increased exosome secretion69. In the same study, after further investigation, the authors found that Hrs, TSG101, and STAM1 depletion decreased exosome secretion, whereas VPS4B knockdown increased production. Those proteins were purified by ultracentrifugation and analyzed via western blot (WB) and qRT-PCR69–71. The endosomal membrane transiently recruited ESCRT proteins from the cytoplasm, where their function is to sort the transmembrane protein and from MVB. ESCRT-0 binds with a ubiquitin-protein programmed for degradation, executing a sorting of MVB in the first set of steps62. Knockdown of ESCRT-0 protein Hrs from dendritic cells results in fewer exosomes secreted, which can be measured by the exosomal level of ubiquitinated proteins: TSG101, and VPS4B72. ESCRT -I and II promote the budding process and start the enzymatic de-ubiquitous cargo protein before forming (ILVs) microvesicles in the intracellular compartment73. The ESCRT-3 complex drives the final stage of membrane invagination and separation74.
An integral membrane protein of the lysosome has been suggested to play a role in exosome formation. A higher amount of exosome secretion was observed after transfection of COS cells with SIMPLE lipopolysaccharide-induced TNF factor (LITAF) and mutation of SIMPLE interfered with proper MVB formation75. Also, syndecans, the membrane proteins carrying heparan sulfate chains, are mediated by their binding to syntenin. Syntenin is a multivalent soluble protein that binds ALIX to build a link between syndecans and ESCRT machinery76. Another study determines that the syndecan–syntenin–ALIX mechanism in MCF-7 cells was responsible for l-50% of the secreted exosomes77. In addition to proteins, lipids also play an essential role in vesicular transport78,79, and both act intrinsically for vesicle transportation like membrane deformation, fission, and fusion80. The exosome membrane is enriched in sphingomyelin, tetraspanin, integrin, cholesterol, immune regulatory molecules, and ceramide, whereas inside, it contains chaperons, mRNA, cDNA, and proteins49,81,82. Exosomes released from a cell are taken up through catherin-independent endocytosis or micropinocytosis by neighboring cells19,83–85. Once internalized by recipient cells, exosomes release their cargo, resulting in the altered regulation of the recipient cell’s various biological functions86,87. The biogenesis of exosomes is often described as either an ESCRT-dependent or ESCRT-independent mechanism88, but these pathways might ineterplay89. Current research also suggests that these pathways may work synergistically in the different subpopulations of exosomes depending on the origin of the various biogenesis machinery90. Phospholipids and sphingolipids are also involved in the formation of exosomes91–93.
For example, following epidermal growth factor (EGF) stimulation, EGF receptor (EGFR) was not sorted into the ILVs of ESCRT-depleted cells, suggesting diversity in exosome formation pathways90. The late endosomal lipid marker, bismonoacylglycerophosphate (BMP), also known as lyso-bisphosphatidic acid (LBPA), was found to co-localize with EGF containing exosomes. However, other studies have suggested that LBPA-carrying MVBs are distinct from EGF, providing MVBs are developed after EGFR stimulation (EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation)94,95. Multiple studies have been done on both ESCRT dependent and independent pathways of exosome biogenesis69,96–98. Finally, the comparatively smaller size and unified shape allow exosomes to successfully escape clearance by the MPS, prolonging their circulation time and implying their cell-cell communication superiority. Remember, the biogenesis pathways work synergistically, meaning that the subpopulation of exosomes depends on a different mechanism. The cell homeostasis and physiological conditions are also essential factors to consider, which control exosome release and secretion pathways99,100. For example, silencing of ALIX protein modulates exosome cargo selection rather than affecting their secretion. Decreasing ALIX expression in a shRNA-expressing cells increases the content of MCH class II content on the exosome surface69. Another study by Hoshino et al. showed the exosome populations were reduced by Hrs knockdown in head and neck squamous cell carcinoma cells, using NTA analysis101. Epithelial cells can secrete exosomes apically and basolaterally to eliminate unfavorable lipid and proteins from entering into the lumen102. Another study suggests that inflammation induced by IL-1β can be counteracted by primary bone marrow macrophages-derived exosomes carrying MHC II membrane protein103. The study also confirms that MHC II expression is lower in healthy tissue than in inflamed regions. Exosomes will play a vital role in the future of precision and personalized-based medicine against cancer, infectious, rare, and immune diseases.
3. Exosome Isolation and Characterization
Recently, exosomes gained much attention for their intrinsic properties such as cell-cell communication, immune response, and antigen presentation across various disease models104. Like cells, exosomes are composed of a lipid bilayer that can facilitate loading both hydrophobic and hydrophilic drugs57. Exosomes are widely distributed in human blood, serum, urine, and bodily fluid. They typically have low immunogenicity and a longer half-life than many other available drug delivery vehicles105. Furthermore, the exosomes have advantages over similar polymeric vehicles due to their inherited surface markers and receptors with its target cells, thus increasing targeted drug delivery to specific tissue/cells106. Important points to remember, due to the variation in the size of different cell-secreted vesicles, the exosome’s (30–150 nm) related purification and isolation processes are critical. The size of the particle plays a crucial role in targeted drug delivery. It is essential to use around 100–200 nm particles for the exosome delivery method104–106. Robust methods of purifying exosomes from cell culture media rely on minimizing co-purifying protein aggregates and other membranous particles. Thankfully, different laboratory-based isolation protocols are available, like differential ultracentrifugation107, size exclusion chromatography108,109, immunoaffinity-based capture110,111, exosome precipitation112, polymer precipitation113, microfluidic-based isolation114, and commercially available kits that scientists use to yield exosomes.
When a heterogeneous mixture (suspension) is centrifuged, more abundant and denser particulate constituents in the suspension will precipitate first (Figure 2). Centrifugation is employed to isolate and purify exosomes and enzyme hydrodynamic properties of polymeric particles like proteins and nucleic acids115–117. Depending on the centrifuge force, exosomes can be separated according to their size and viscosity. Ultracentrifugation (UC) is a centrifugation process optimized for high centrifugal forces up to 1,000,000 × g. There are two branches of ultracentrifugation: analytical and preparative118. Analytical ultracentrifugation is an isolation process depending on particulate material physicochemical properties and molecular interactions of polymeric materials. Preparative ultracentrifugation plays a crucial part since it is used to separate small particles such as viruses, bacteria, subcellular organelles, and exosomes49,111,118. Ultracentrifugation-based isolation is considered the benchmark and most studied isolation method in published research 119. In brief, the culture supernatants were cleared of cell debris, large proteins, dead cells, and large vesicles by sequential centrifugation at 300 g for 10 min (to remove cells), then 1000 g for 20 min (to remove apoptotic bodies), and finally, 10,000 g for 30 min (to remove microvesicles), followed by filtration using either 220 or 450 nm syringe filters. Then, the cleared sample are spun at 100,000 g for 1–2 h to pellet the exosomes120. To avoid contamination by the FBS-derived exosomes, FBS was spun at 100,000g for two hours to remove exosomes before the cell culture experiment48,121. Differential filtration is also applied to separate exosomes from cell culture medium or serum. Firstly, dead-end (normal) filtration uses a 100 nm membrane filter, depleting floating cells and large cell debris. Secondly, the filtrate undergoes tangential flow filtration via 500 kDa molecular weight cut-off (MWCO) hollow fibers122. Then concentrated samples are further filtered using biofiltration. Size exclusion chromatography (SEC) separation technique is also applied to exosome isolation. In SEC, stationary phase gels like sucrose or Sepharose are utilized to sort differential molecular size.
Figure 2.
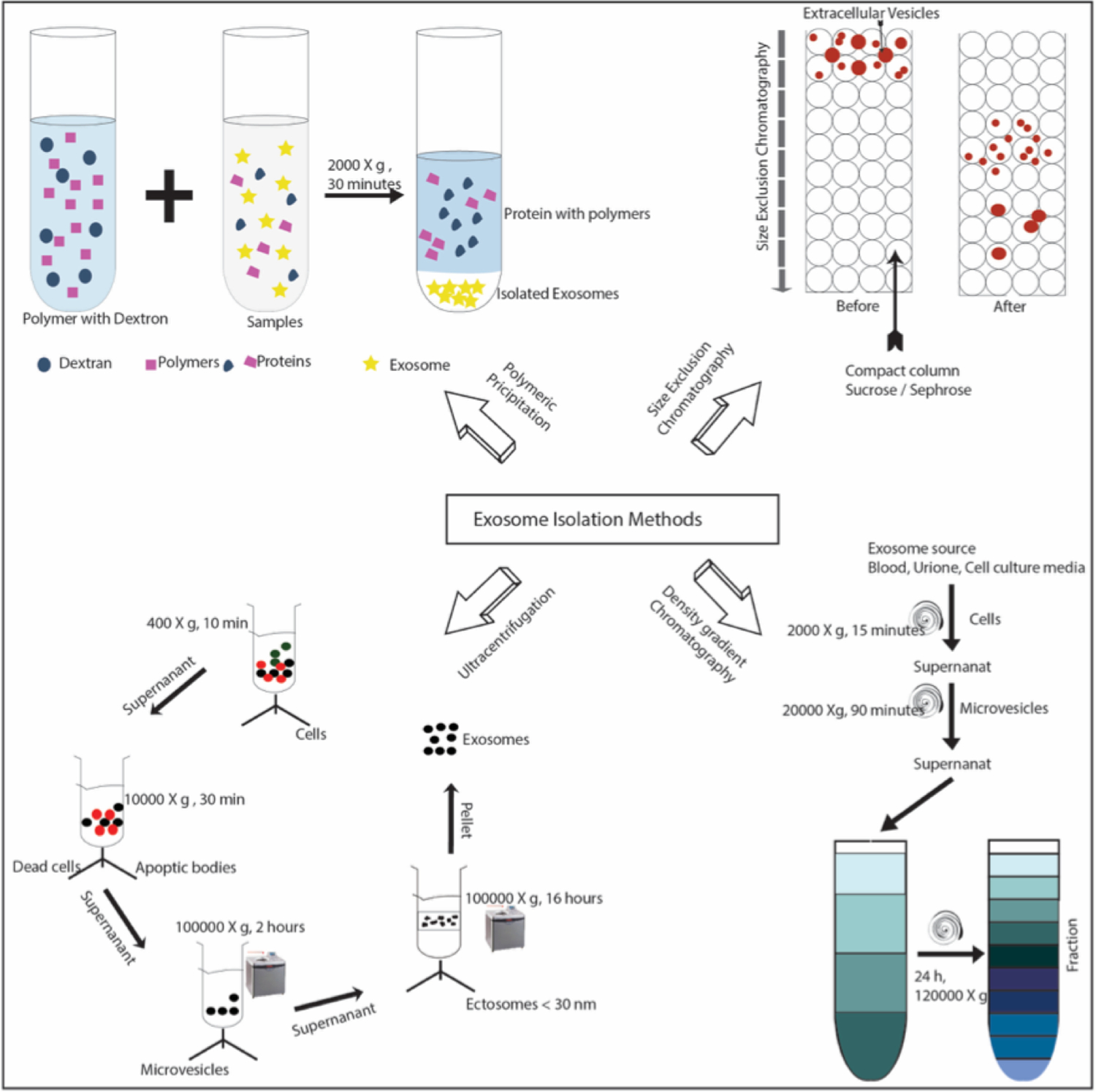
Schematic summary of standard laboratory methods for exosome purification. Four different isolation techniques are demonstrated here: Polymeric precipitation116, (top left), column for size exclusion chromatography123, (top right), density gradient chromatography110, (bottom right) and differential ultracentrifugation110, (bottom left). Temperature maintained at 4°C for most of the protocol110.
samples with small radii will get trapped in the pore opening, letting larger particles go down fast. When this technique is performed using organic solvents, it is called gel permeation chromatography (GPC)125. The main application of GPC is found in polymer analysis125. When size-exclusion chromatography is performed utilizing an aqueous solvents column, the method is called gel filtration123. The disadvantages of these methods are (i) the susceptibility of the chromatography column to contamination, (ii) the need to collect and analyze a larger fraction of exosome to obtain a larger exosome sub-population, and (iii) the length of time for post exosome isolation115. Immunomagnetic isolation uses antibody-labeled magnetic beads and captures exosome with stained antibody using the magnetic field111. To isolate and purify polymers from other unwanted materials, polymeric precipitation is a technique used to form a mesh-like net structure that embeds exosomes between 60 and 180 nm. Polymeric precipitation isolation methods have advantages in detecting biomarkers of identified exosomes113. Several immune-isolation assays based on either magnetic beads or microfluidic devices have been able to use antibody-based affinity capture for rapid exosome isolation126,127. These methods depend on the availability of specific exosomal surface proteins or antibodies for discrimination between the exosomes of interest and other vesicles’ sizes in the fluids126,128,129. In Figure 3, we try to rationalize from a recent study, where authors have compared the ultracentrifugation method with the commercially available isolation kit ExoQuick. The study confirms how the commercial kit more precisely isolates exosomes. Immunoblot of purified exosomes isolated by ExoQuick shows a wider band than that of the exosomes isolated by UC. Finally, we will mention the commercial kits available for exosome isolation. Some of the prevalent kits typically used like ExoQuick™, Ultra exosome precipitation solution (EXQ)130 by System Biosciences, total Exosome Isolation for serum or plasma (TEI)131, exoRNeasy Serum/Plasma Midi Kit (EXR)132, and RIBO™ Exosome Isolation Reagent (REI)133 yield relatively pure isolation.
Figure 3:
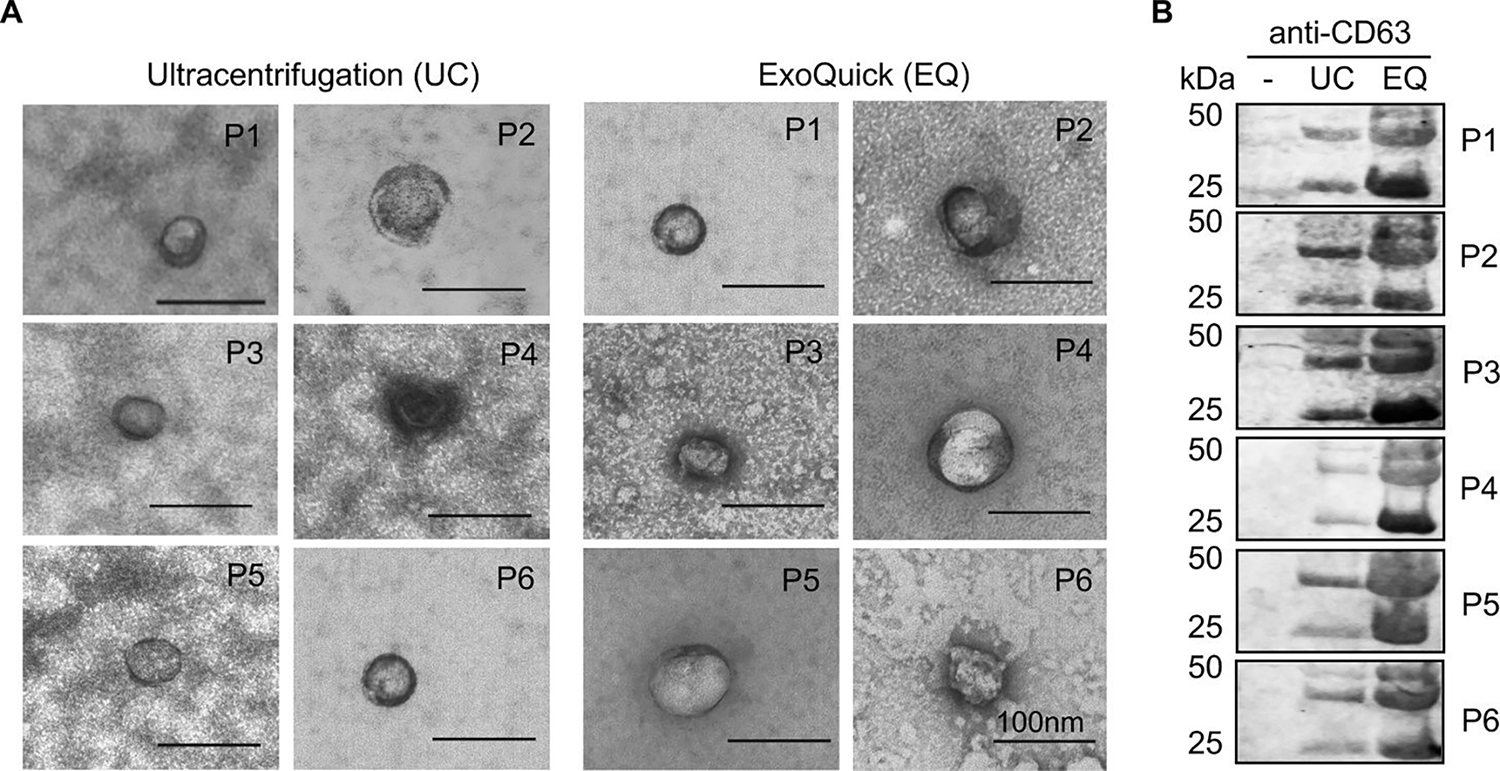
Validation of exosome enrichment from human cell-free sera. (A) TEM micrographs of exosomes in ultracentrifugation (UC) and ExoQuick (EQ) preparations. Data for 6 independent patient samples are shown (P1–6). Exosomes confirmed by size (30–100nm) and appearance. Scale bar in each image represents 100 nm. (B) Immunoblot of CD63 in unprocessed cell-free serum alone (−), UC, and EQ exosome preparations. Adapted from Prendergast et al. (2018)124, Copyright @ 2018, PLOS.
Exosome characterization is very challenging due to the heterogeneity of the exosome population, different isolation techniques, the mixed-size-distribution, and the difficulty in cargo profiling. For exosome characterization, general instrumental methods used for particle size, hydrodynamic diameter, and surface zeta potentials are nanoparticle tracking analysis (NTA)134 and dynamic light scattering (DLS)135. For morphology characterization, available techniques are scanning electron microscopy (SEM)134 and transmission electron microscopy (TEM)135,136. Western blot analysis136,137 and mass spectrometry136,138 have been widely used for biological characterization and proteomics. Electron microscopy technique is the gold standard for characterization of exosome morphology. However, morphology observed by TEM contradicts that of the morphology observed by SEM. TEM images show that exosomes are cup-shaped whereas SEM images show that they are roughly round shaped. One drawback of the TEM/SEM technique is that the system requires a thin sample; therefore, sample preparation is tedious, affecting exosome properties. Nanoparticle tracking analysis (NTA) technique is another way of determining sizes of exosomes. NTA utilizes Brownian movement of the exosomes to determine the size and particle concentration139. DLS is also based on a similar principle where the hydrodynamic radii of exosome solution determine the fluctuations in reflected laser transmission caused by the Brownian motion of the particles. Different molecular profiling approaches were applied for proteomic analysis of exosomes. In particular, two-dimensional gel electrophoresis (2DGE) and liquid chromatography coupled tandem mass spectroscopy (LC-MS) are predominantly used140–143. But compared to proteomic analysis, lipid and metabolite analysis of exosomes is underutilized. The main limitation of proteomics and lipidomics is the risk of contamination of other extracellular vesicles, mainly caused by the isolation techniques. Exosome isolation purification can be determined by western blotting (WB) or RT-qPCR. Both techniques develop bands from protein or RNA purified from exosomes. Fluorescent imaging is another characterization assay that uses lipophilic dye like PKH67, Dil, DiD, or DiR embedded in the lipid bilayer of the exosomes. For drug delivery application, characterization assays like NTA, WB, TEM, and RT-qPCR are enough to demonstrate various physical and composition properties. For biomarker analysis, WB or PCR are used to identify specific protein/metabolite expression in pathogenic exosomes.
This section provided an overview of exosome isolation techniques, and characterization methods that are opening a new window towards developing safer and more advanced strategies and devices for more cost-effective, time-saving, and efficient isolations of exosomes from biological fluids.
4. Exosome Drug Loading Techniques
One of the most promising forms of targeted drug delivery revolves around implementing insoluble drug loading in lipid-based systems for enhanced accumulation in the diseased tissues. Exosomes gained much interest in the scientific community of drug delivery because they can carry various molecules, including carbohydrates, proteins, lipids, and nucleic acids24. Besides, the exosomes themselves can vary in size from 30 to 150 nm in diameter, depending on the type. This variability in the potential transport vehicles creates opportunities for the loading and targeting of a diverse array of biomolecules to provide therapy to targeted organs in the body144 (Figure 4). A reliable means to load small hydrophobic molecules has been found using sonication, which works by causing shear forces in the exosome that allow drug molecules to accumulate in the lipid layer of its membrane145. Effective methodologies have utilized a direct probe and a set, consisting of 30 seconds of sonication and 30 seconds of rest, repeated six times 145. This method was used to load macrophages with paclitaxel (PTX), a potent chemotherapeutic agent and an eminently hydrophobic compound. The study showed the most significant relative particle size (287.7±0.7nm) that displayed the highest encapsulation efficiency (EE) (28.29±1.38%). This method’s efficiency was significantly higher than other loading methods of the same drug, including electroporation or incubation, with neither reaching above 6% EE 145. Similarly, the catalase for Parkinson’s study used an almost identical method, producing only moderate sizes (179.0 ± 10.6 nm unloaded, 183.7 ± 13.8 nm exoCAT loaded), but also received the highest relative loading capacity (<200 μg catalase/mg exosome). The nature of this loading method allows for drug fusion to the membrane, which may inhibit total controlled release due to an initial burst phase. Incubation has been attempted in the previous study, which involved shaking for one hour at 37°C. This resulted in a significantly smaller particle (132.2±2.3 nm) with a spare loading capacity (1.44±0.38%)48. In the same article, the authors had showed that the catalase in the Parkinson’s study was added to 250 μL of exosomes for a final concentration of 0.1 mg/ml complete protein. Before the addition of catalase, the macrophages were diluted in PBS (0.15 mg/ml total protein). The sample was then incubated at room temperature for 18 hours. Sizing was 108±14.3 nm, and loading was measured by enzymatic activity, which was rated very low (>20 μg catalase/mg exosome). A side note is that post-loading sizes of incubated exosomes were relatively similar; however, this may be due to this method’s deficient efficacy level. SEM images show this method creates abnormal non-spherical shapes, which may have unintended effects in a therapeutic context. The freeze-thaw loading cycle was attempted in the catalase for Parkinson’s study, which involved adding the exosomes and catalase identically to the incubation loading, allowed them to incubate for 30 min, and then to freeze at −80°C rapidly, and then to thaw at room temperature (RT). This cycle was repeated three times and was somewhat successful, with an average size of 147.0±10.0 nm unloaded, 158.0 ± 11.0 nm loaded, and ~100 μg catalase/mgexosome. Continuous freeze-thaw cycles have been shown to cause fluctuations in fluorescence due to lipid-dilution ratio changes. Extrusion is performed by placing the catalase mixture in an Avanti lipids extruder with 200 nm pore diameter and then purifying it using gel-filtration chromatography with Sepharose 6 BCL. Sizing was consistent and small (134.0±7.5 nm unloaded, 154.8±11.0 nm loaded), and loading (190–200 μg catalase/mg exosome) was the 2nd most effective drug loading method after sonication48. Morphology data shows spherical and consistent shape. Electroporation was attempted in the PTX study with abysmal results. The exosomes and PTX were added to a chilled 4 mm electroporation cuvette and subsequently electroporated using an Eppendorf evaporator at 1000kV for 5ms, and then incubated at 37°C for 30 minutes to allow for the recovery of the exosomal membrane 147. This method resulted in an average size of 145.3±1.0 nm, but encapsulation was low (5.3±0.48%). In brief, exosomes could be loaded with drugs either in vitro in purified exosomes or in vivo during biogenesis (Figure 4).
Figure 4:
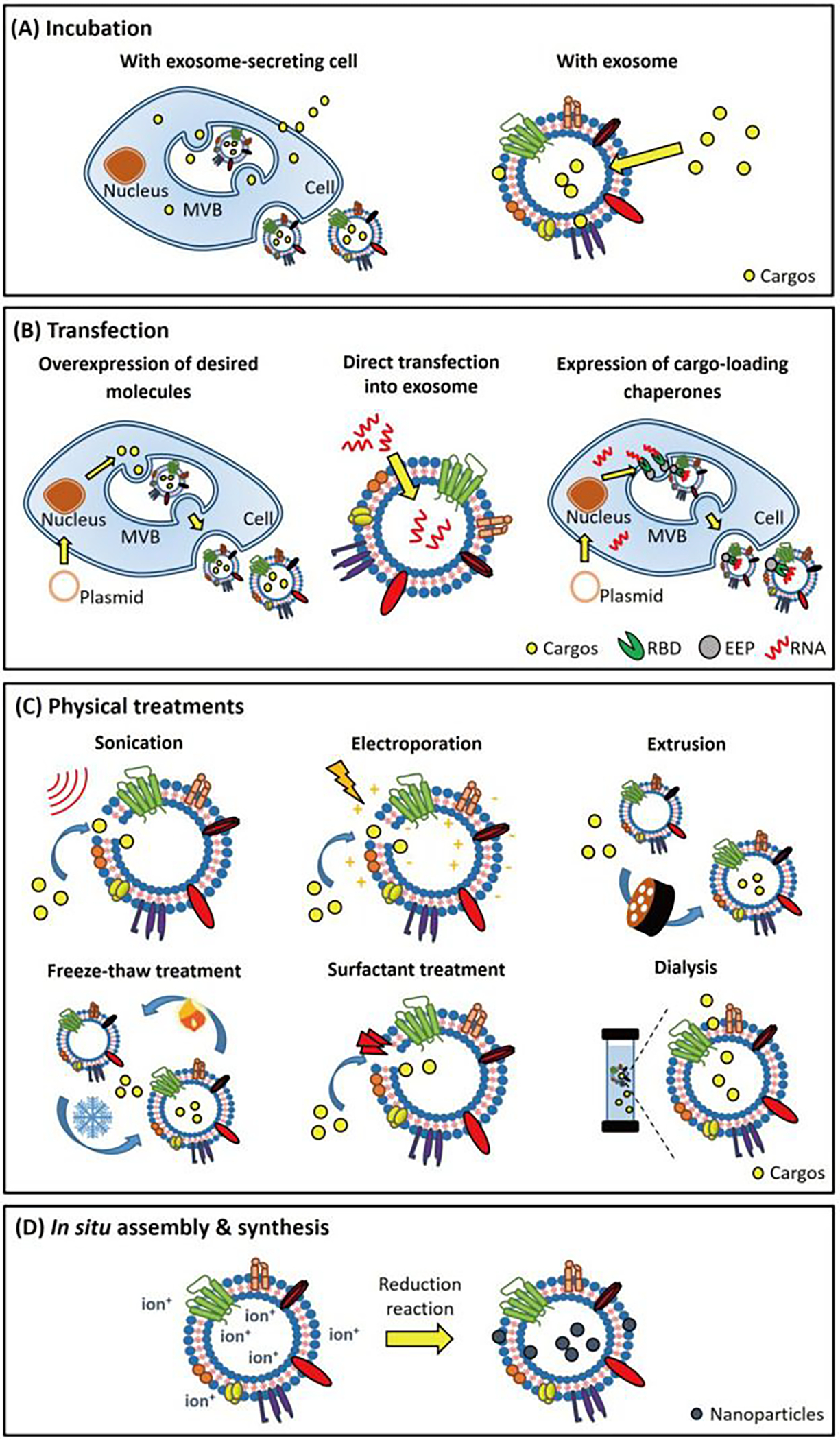
Exosome drug loading techniques, (A) Exosome-secreting cells or exosomes incubated with desired cargos. Cargos diffuse across the cell and exosomal membrane and are subsequently packaged within the exosomes. (B) Desired nucleic acids can be loaded into exosomes via a transfection-based strategy. Transfected with vectors, the donor cell generates RNAs/proteins and packages these products into exosomes using endogenous expression and sorting machinery of donor cell, respectively. Exosomes can be directly transfected with small RNAs for cargo loading purposes. (C) Cargos can be loaded into exosomes directly through physical treatments. Electroporation, sonication, and surfactant treatment generate pores on the exosomal membranes that facilitate cargo loading. Freeze-thaw treatment, extrusion, and dialysis enhance cargo loading into exosomes during membrane recombination processes. Adapted from Shengyang Fu et al.(2020)146, Copyright @ ELSEVIER.
Transfection is a technique for loading proteins, peptides, and nucleic acids into exosomes. Using specific transfecting agents like plasmids or tethers, the cell can be transduced to ectopically express desired proteins, lipids, or nucleic acids which will later undergo exocytosis from the cell via exosomes. For example, Bellavia et al. transduced human embryonic kidney 293 (HEK293) cells using BCR-ABL siRNA and later collected exosomes from cell medium148. Yang et al. has also showed that different transfecting cells with mRNA can produce a 50-fold higher exosome amount compared to the naïve cell culture technique. These exosomes carrying PTEN mRNA restore tumor-suppression function in the brain, increase animal survival, and enhance tumor-growth inhibition149. Except for nucleic acids, we can introduce specific proteins or lipids via transfection techniques. For example, HEK293 cells were transfected with CD9-human antigen R (HuR) to facilitate the loading of miR-155 into exosomes150. Another study showed that HEK293 cells transfected with vascular stomatitis virus glycoprotein(VSVG) enabled exosomes to penetrate the plasma membrane of recipient cells151. Further, exosome cargo can also modulate by expressing cargo-sorting proteins onto exosome surfaces via cell transfection. This cargo loading technique is promising, yet its cargo loading efficiency is low due to cargo selectivity and chemical impurity due to transfection.
Electroporation is another technique for loading DNA, mRNA, siRNA, and RNAi into exosomes. In this technique, the electric field is applied to increase permeability for small molecule drugs and large molecule biologics through the membrane of exosomes. For drug loading, exosome and payload (drug/protein) need resuspension in electroporation buffer. The electroporation buffer can be trehalose pulse medium (TPM; 50 mM trehalose (Sigma-Aldrich, Cat. No. T0167) in PBS) or (1.15 mM potassium phosphate, pH = 7.2, 25 mM potassium chloride, 21% Optiprep) or cytomix electroporation buffer (120 mM KCl, 0.15 mM CaCl2, 10 mM KPO4, 25 mM HEPES, 2 mM EGTA and 5 mM MgCl2, adjusted to pH 7.6 with KOH)152,153. Then electroporation is carried out using a GenePulser Xcell electroporator (E.g., from Bio-Rad). All samples are filtered using omega membrane Nanosep centrifugal devices (100–3000 MWCO, depending on the size of payload) to remove the excess payload of drug, DNA, or mRNA. Measuring the volume of samples being loaded into the electroporation buffer is also important. Depending on the loading protein, the voltages and capacitances of the electroporator will differ154. Some studies report that electroporation leads to exosome aggregation, resulting in a lower loading efficiency. That is why it is recommended to filter the electroporated sample with a 450/220 μL filter.
Surfactant treatment is another technique for exosome drug loading. Surfactants like saponin or triton are used to increase the membrane permeability of the exosome through simple incubation methods155,57. Incubation with surfactant can be used to facilitate the loading of antioxidant, catalase into exosomes, and provide neuroprotective efficiency post intranasal administration in Parkinson’s disease (PD) animal model48. Although, the surfactant enhances higher loading efficiency within the exosome, there exist some limitations in the technique. Surfactants may inactivate/degrade the potential function of therapeutic or loading cargo and excessive surfactant may cause in vivo hemolysis. Additional purification methods may need to be implemented after incubating with surfactant155.
Hypotonic dialysis is another drug loading method widely used for exosome drug loading. The basic principle is that an exosome and drug mixture is placed in a dialysis tube and continuously stirred to allow for drug loading. This method can load 11 folds higher drug content than room temperature incubation loading method 156. This loading system is also suitable for reducing intra-exosomal pH by rehydrating and dehydrating the exosome in acidic citrate and ethanol buffer. This pH gradient of exosome helps to load miRNA and siRNA157. Some studies report that the dialysis loading method may induce protein degradation due to the pH change of the exosomes158. Therefore, this method is considered as a highly effective drug loading method, yet proper validation is needed to identify the experimental conditions and exosomal cargo selection.
Today, numerous drug loading techniques have been developed in light of the exosome’s intrinsic properties for drug loading and delivery (see Figure 4 and discussion earlier in this section for details). In incubation methods, drug loading efficiency depends on the proportion of drug and exosome protein concentration. The loading efficiency of incubation methods is poor, and certain factors influence efficiency. First, in gradient-based cargo diffusion, the concentration of cargo is curved due to the saturated concentration of the drug, indicating the enhanced drug loading profile. Second, the membrane integrity of the exosome restricts most of the hydrophilic drug to the influx. To increase the loading efficiency, we need physical triggering methods like sonication, extrusion, electroporation, surfactant treatment, dialysis, etc. Multiple studies conducted in parallel demonstrate that drug loading efficiency increased with these physical treatment methods compared to the general incubation methods48,159. Despite of higher drug loading efficiency, the physical methods have many disadvantages for drug delivery application of exosomes. First, surfactant treatment may introduce impurities in the exosome, which may cause toxicity during therapy. Second, electroporation may destabilize the exosome membrane integrity or cause severe aggregation. Third, dialysis treatment may cause the inactivation or degradation of the protein loaded. Fourth, the ultracentrifugation method provides us a mixture of extracellular vesicles e.g., range from 30–150, which is satisfactory for biomarker analysis, but not for drug delivery where we need a precise range of particles. Fifth, the freeze-thaw method, due to multiple freezing and thawing cycles, can cause degradation of the exosome membrane and a leaky structure. Transfection is another way to increase the loading efficiency of protein, lipids, and nucleic acid via transducing cells or exosomes with nucleic acids or proteins expressed by a plasmid. However, this technique is costly and time-consuming, making it unstable for small-scale research purposes. Overall, exosome loading techniques can improve desirable cargo but introduce impurities that affect exosomal properties. Therefore, we need to use particular loading techniques depending on the exosome application and consider the implications of the introduced impurities for drug loading. The purification of exosomes is laborious due to their intrinsic biological properties, making them more difficult to use for drug loading. Engineered exosomes provide an alternative means to overcome drug loading issues. If we can develop or utilize current techniques like plasmid, tether, bio-ortholog click chemistry, we can generate the desired exosomes from cell culture. In this way, we can avoid drug loading steps and have stabilized exosome treatment that can be used to combat cancer, immune, and rare diseases. We can also consider how to remove natural exosomal cargo during exocytosis, allowing us to load more therapeutic payload during the drug loading step. However, the optimization of exosome loading strategies is limited by our insufficient understanding of exosome biology, structure, biogenesis, and lagging exosome-related research and development tools60. We need a standardized drug loading protocol for getting uniform and stable results in drug delivery applications both in preclinical and clinical studies.
5. Pre-Clinical Research Developments
5.1. Role of Exosomes in the Immune System
Exosomes play an important role in immune regulation, eliciting both positive and negative “unwanted” immune responses, including tolerance and evasion160–162. Individually, exosomes can act as immunoregulating agents by modulating immune activation, antigen presentation, suppression, and surveillance163–166. The exact mechanisms for many of these actions are not entirely understood. Several studies have begun to understand how these vesicles play necessary and frequently pivotal roles in initiating various immune responses167–169. The inflammatory response is often signaled by exosomes, meaning that these vesicles play a crucial role in several pathological states, including cancer, diabetes, obesity, and neurodegenerative disease28,170–174. For example, microRNAs regulate cells gene expression after transcribing and exosomes deliver microRNAs to recipient cells. A study by Alexander et al. shows dendritic cell-derived exosomes delivering miR-155 and miR-146α to recipient dendritic cells to promote endotoxin-induced inflammation in mice171. In neurodegenerative Alzheimer’s disease, exosomes carry pathological misfolded proteins to neighboring neurons, thus promoting a cascade of exosomes carrying pathological misfolded proteins to other neighboring neurons, initiating disease onset and propagation175. The study of exosome cargo release may lead to the identification of biomarkers for many of these diseases. Since exosome cargo is a continuously excreted substance via fluids, saliva or urine collection may be valuable pathological screening tools for biomarker identification176. We will discuss exosome biomarker applications in more detail in section 6.4 of this review. Exosomes also play an essential part in cardiovascular disease recovery by promoting tissue repair and regeneration177,178. Exosomes originating from immune cells play a significant role in prompt immune response and inflammation, unlike stem cells and cardiomyocytes179. Although these mechanisms are not well studied and only a small number of exosomes directly related to immune response regulation have been discovered, what is known is that exosomes demonstrate cardioprotective effects against post-infarction and atherosclerosis. One example of exosome-based manipulations may found in a study published in Allergy180, where B cell-derived exosomes with pMHC-II found on FDCs could stimulate CD4+ T cells, which aided their development. Scientists believe that pMHC-II found on the FDCs likely allowed the exosomes to engage the T lymphocytes, modulating immune memory to expand their collection of antigens. Activation of the immune system may also be triggered by exosome activity181. Dendritic cell (DC) exosomes classified as “mature” are significantly more effective than their younger counterparts when inducing specific antigen T-cell activation181. This phenomenon is most likely due to distinct differences in protein composition that accumulate as the cell matures181. These changes can help in tumor suppression; however, these same effects have occasionally been hijacked by tumor cells, allowing for uncontrolled growth without a proper response. Recently, a study found that tumor cells can bypass the typical immune response by upregulating the surface expression of programmed death-ligand 1 (PD-L1), allowing the tumor to mask itself by eliciting the immune checkpoint response 120. Effective quantification of PD-L1 could be used as a possible tool for helping in tumor treatment decisions based on the amount observed at specific sites182. This line of investigation closely follows the migration and composition of these vesicles. Peptide transfer acting as a form of cell-to-cell communication via exosome migration can have profound biological effects183. For example, prion proteins from the exosome walls may be transferred to uninfected cells by fusing with their uninfected counterparts184. In pregnant women, placenta-derived exosomes circulate T cell activating markers including Fas ligands and HLA-DR. These exosomes also show greater suppression of JAK3 and CD3-zeta (T-cell co-receptor) than pre-pregnant circulating placenta exosomes185. Dendritic and lymphoid cell-derived exosomes regulate immune activation. Tumor-derived exosomes (TEX) have also been considered as a vaccine platform due to their effects on T lymphocytes, suppression CD3-zeta and JAK3 expression. Thus TEX expressing tumor antigens can suppress T cell signaling and induce apoptosis for potential use as a tumor vaccine186. In another study, the authors compare the molecular profile of TEX with healthy controls circulating exosomes. They found TEX downregulates both CD3-zeta and JAK3 expression of activated T cells and Fas/FasL-dependent apoptosis. TEX were incubated with activated T-cells, CD56(+) CD16(+) NK (natural killer) cells or conventional CD4(+) CD25(neg) T-cells res. Also, the authors showed how TEX promote CD4(+) CD25(neg) T-cell proliferation but suppress it when they transform into CD4(+) CD25(hi)FOXP3+ (FOXP3 is forkhead box P3) Treg cells (regulatory T-cells). Therefore TEX have immunosuppressive properties that depend on the T cell activation state187. Tumor cells escape immune checkpoint by upregulating PD-L1, which interacts with program death-1 (PD-1) T cell receptor188,189. Anti-PD-1 or Anti-PD-L1 antibodies have shown promising results in treating tumors190. Along the same lines, metastatic melanomas releasing exosomes containing PD-L1, can suppress CD8 T cells, preventing proliferating tumor growth via IFN-ϒ stimulation183. This study unveiled a mechanism for how tumor cells suppress the immune system initially and how exosome PD-L1 is a potential target for anti-PD-1 therapy. In autoimmune diseases study, T cell regulation is a key mediator of diseases treatment and some of the mechanisms are suppressed by Treg cells, apoptosis of overactivated T cells by cytokines destitution, immune checkpoints like PD-1, and CTLA-4 expression191,192. Multiple previously published reviews and research articles conclud that exosomes released from immune cells play both preventive and developmental roles in autoimmune diseases193,194. Mesenchymal stromal cell (MSC) exosomal immune properties are well studied. Zhang et al. study showed that MSC derived exosomes induced production of CD4+CD25+Foxp3+ Treg or CD4+CD25+ T cells via allogeneic APC-enriched CD11C+ cells through T cells activation195. This activation is both exosome and APC dependent.
Exosomes’ intrinsic properties of cell-to-cell communication allow for the transfer of potentially toxic proteins without the need for direct contact. However, this type of communication may also be used in a manner beneficial to the immune system by allowing for a more robust and adaptable transfer of antigenic markers between cells, which would bypass the need for a more abrasive communication route. Overall, understanding of the role that exosomes play in immune system response is still in its infancy. A great deal of research must be done to gain insight into the complex interactions that elicit the varied responses discovered. This field’s foundation will need to focus on mechanistic and response-oriented inquiry to understand how these vesicles can be fully utilized.
5.2. Role of Exosomes in Blood-Brain Barrier (BBB) Penetration
The BBB is a protective mechanism that helps maintain a stable chemical environment in the brain196,197. No other body organ or tissue is as protective and dependent on maintaining the internal environment as the brain196. For blood and proteins to reach the brain through brain capillaries, these products must cross three barriers, (i) the endothelium of the capillary wall, (ii) external capillaries of the wall covered by relatively thick basal lamina, and (iii) the bulbous “feet” of the astrocytes clinging to the capillaries (Figure 5). Nutrients such as glucose, electrolytes, and essential amino acids can penetrate the BBB via passive diffusion through the endothelium cell membrane198–200. On the contrary, small nonessential amino acids and potassium ions are prevented from entering the brain. They are actively pumped out from the brain through endothelium capillary action198. Transport across the BBB is catalyzed by transport processes such as carrier-mediated/receptor-mediated transport, and active efflux transport205–207. Efflux transport protects the brain from endogenous substances such as neurotransmitters and hormones and is also vital for drug transportation to a diseased brain region208. At places of high glutamate presence in the diseased brain, the brain’s glutamate levels are regulated by the BBB through the use of excitatory amino acid transporters (EAATs 1–4)205. Due to the limited ability of most drug delivery methods, an alternate approach is required. Thus exosomes may work as a cloak, which can have elevated drug loading amounts and better-targeted delivery48. Recent advances in exosome research regarding their intercellular communication and their organotrophic behavior, opened a new door in targeted drug delivery research209,210. For cell-cell communication, the surface of the exosome is enriched with cell-adhesion targeting molecules (tetraspanin and integrin), antigen-presenting molecules (MHC I and II), membrane trafficking molecules, and receptor proteins211 For example, tetraspanin proteins CD9, CD63, and CD81 isolated from brain endothelial HCMEC/D3 cells, play a crucial role in communication between primary astrocytes and cortical neurons201. Exosomes derived from neuronal glioblastoma (GBM) and neuroectodermal cells cannot cross the BBB, whereas exosomes derived from endothelium cells that have a tetraspanin marker as CD63 can212. Also, endothelium cell exosomes can pass through the BBB using cell-specific proteins via receptor-mediated endocytosis212. Hypoxic GBM U87 cells releases exosomes through VEGF-A induced BBB permeability for tumor invasion, endangering brain health integrity. Authors found GBM exosomes alter/reduce the expression of claudin-5 and promote BMVECs204. In zebrafish, exosome loaded doxorubicin, and paclitaxel, show promising ability to cross the BBB, whereas neither of the drugs showed brain uptake by themselves201. In figure 6213, the authors show that the CSF can carry exosomes and constituents, observed by TEM imaging. Interestingly, NTA analysis confirms exosome population increase in CSF due to systemic LPS injection compared to control CSF. This experiment validates our conclusion that exosome number increases due to disease state. miRNA analysis also confirms that exosomes can carry payloads like miRNA and mRNA proteins. These data validates the exosome’s capability of drug delivery of active biologics to the brain in a disease condition.
Figure 5.
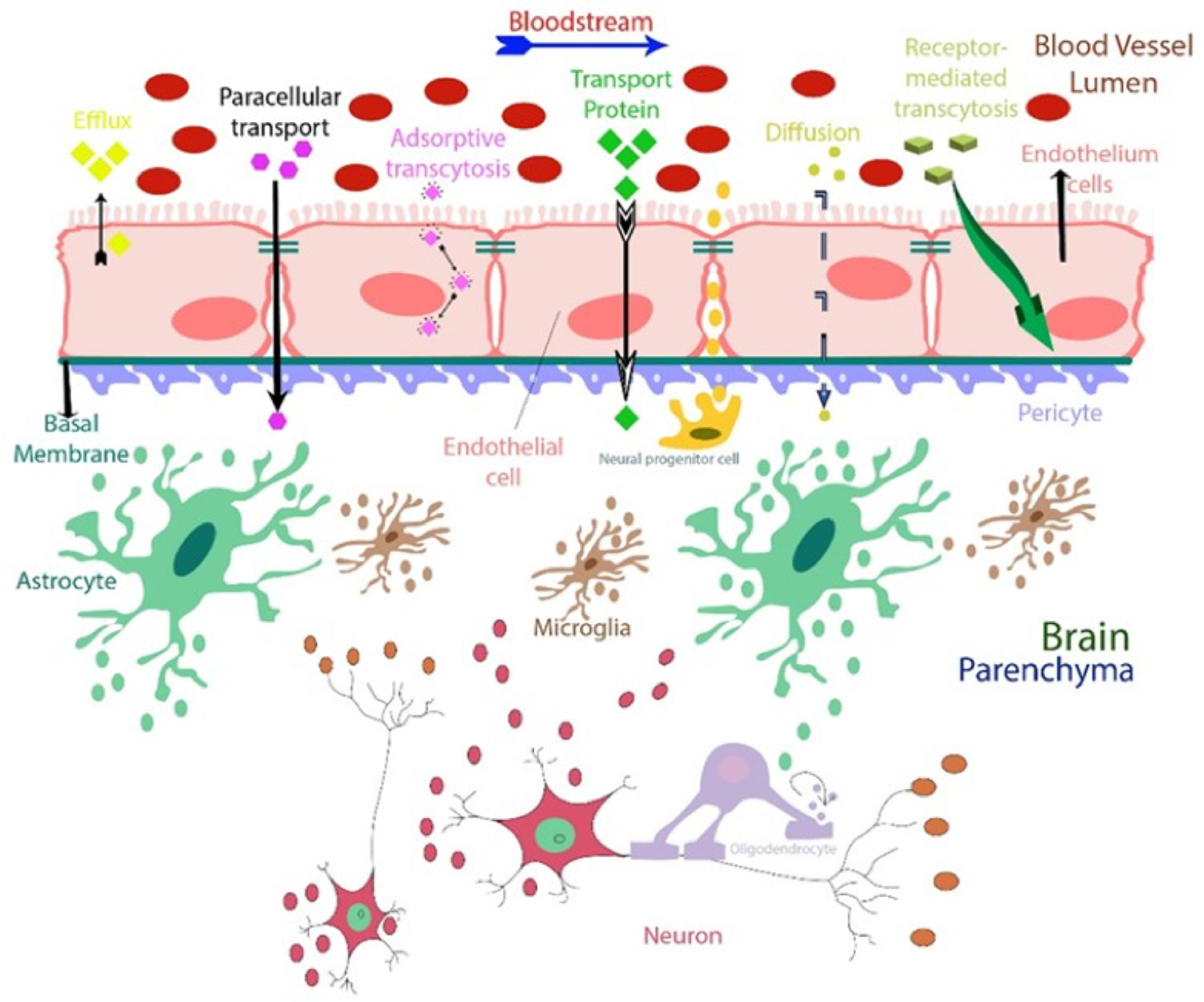
Recent studies confirm that exosomes can pass through the blood-brain barrier (BBB)201–204 in both directions. This means that specific exosomes detected in the cerebrospinal fluid (CSF), or in the blood from the brain can release into the bloodstream and vice versa. Each cell type releases a specific type of exosome(s) that are released and communicates with neighboring cells, acting as the messenger. This characteristic makes exosomes attractive as new sources of biomarkers and therapeutic targets suitable for use in clinical practice, such as liquid biopsy that could replace current invasive diagnostic methods. Exosomes also have a potential role in drug delivery for brain disease models, and their membrane markers can be used to identify their cellular origin.
Figure 6:
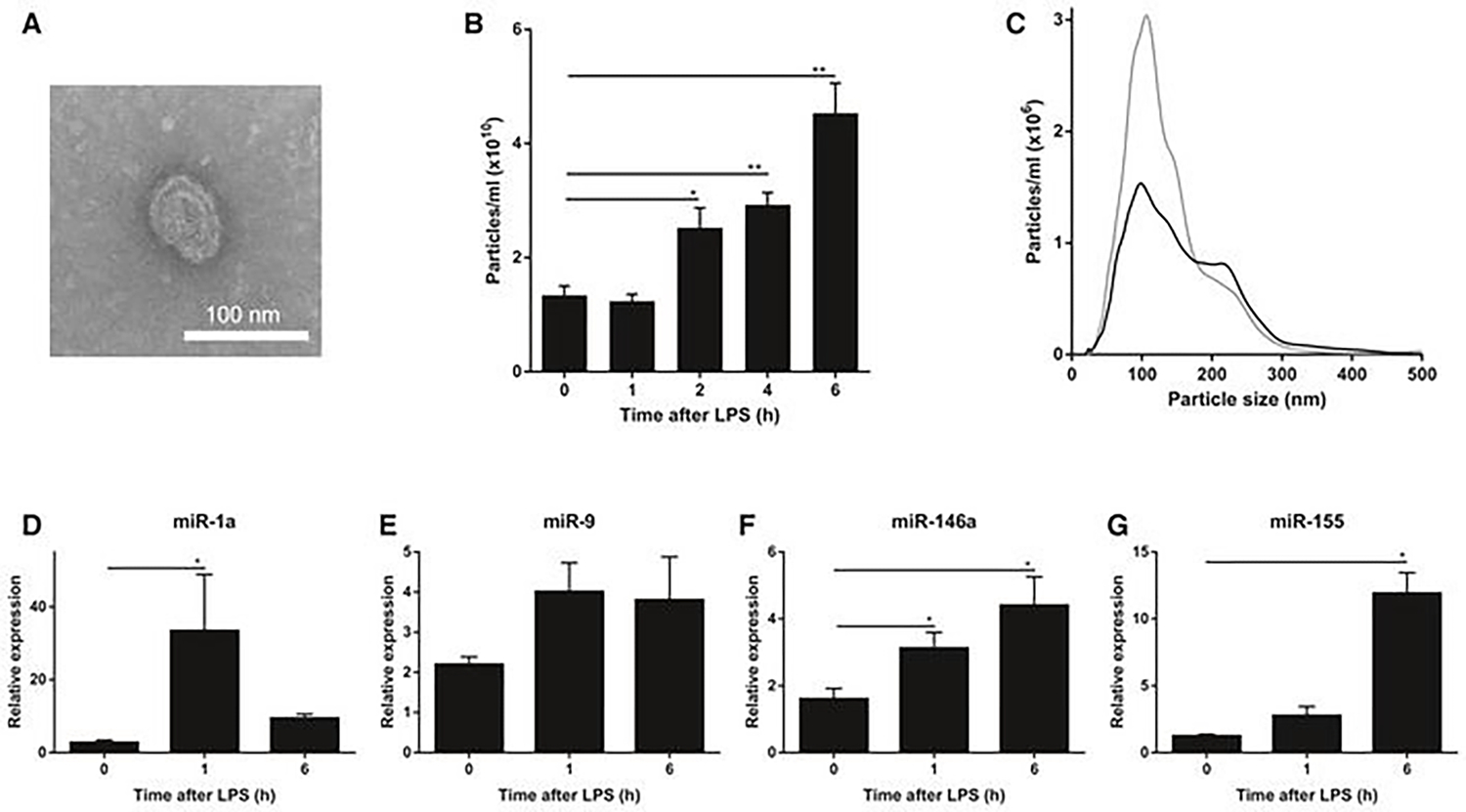
LPS injection induces changes in extracellular vehicles (exosomes) and miRNAs in the cerebrospinal fluid (CSF) A. Representative transmission electron microscope (TEM) image showing the presence of EVs in the CSF in two independent experiments. B. NanoSight quantification of the number of particles in the CSF at 0, 1, 2, 4, and 6 h after i.p. LPS injection (n = 3–5). C. Size distribution of the EVs in vivo in the CSF before (black; n = 5) and 6 h after (gray; n = 3) LPS treatment determined by NanoSight analysis. D–G. Quantitative real-time polymerase chain reaction analysis of miR-1a (D), miR-9 (E), miR-146a (F), and miR-155 (G) (n = 4). RNA was isolated from pooled CSF (50 μl) from different mice (n = 3). Data information: Data in (B, D-G) are displayed as mean ± SEM and analyzed by Student’s t-test. Significance levels are indicated on the graphs: *0.01 ≤ P < 0.05; **0.001 ≤ P < 0.01. Adapted from Balusu et al. (2016) 213, Copyright @ EMBO Press.
In HIV patients, the role of amyloid-beta (Aβ) deposition is one of the characteristics, and the BBB plays a critical role in Aβ homeostasis within the brain. It was reported that HIV-1 infection increases exosome release from brain endothelial cells and higher Aβ cargo in the brain compared to a healthy control214. This study concludes that exosomes carried cargo across the BBB and successfully delivered it to the brain. Aβ plaques accumulation is also a pathological characterization of Alzheimer’s disease215. The review by Badwar et al. summarized how blood exosomes could be a potential source of the biomarkers for Alzheimer’s disease. The authors compiled about 26 previously published studies on blood exosome biomarkers and other sources such as neuron, astrocyte, and brain vasculature exosomes biomarker screening. This study provides a correlation of blood exosomes with exosomes derived from other brain fluid sources216. Parkinson’s disease (PD) is another deadly brain disease and a common movement disorder. Dopamine administration is one of the main treatment used for PD. Qu et al. have reported that dopamine loaded in blood exosomes showed and improved therapeutic result in the PD mouse model and reduce systemic toxicity compared to free dopamine administration. Blood exosome (40–200 nm) shows a promising targeted drug delivery approach for PD treatment217. In Figure 7, the study shows that the authors investigated the correlation of miRNA expression due to peripheral inflammation in the brain region. The authors also found systemic TNF injection increases the total amount of exosomes released and found a significant increase in the expression of miR146a and miR155 due to LPS injection in vivo213.
Figure 7:
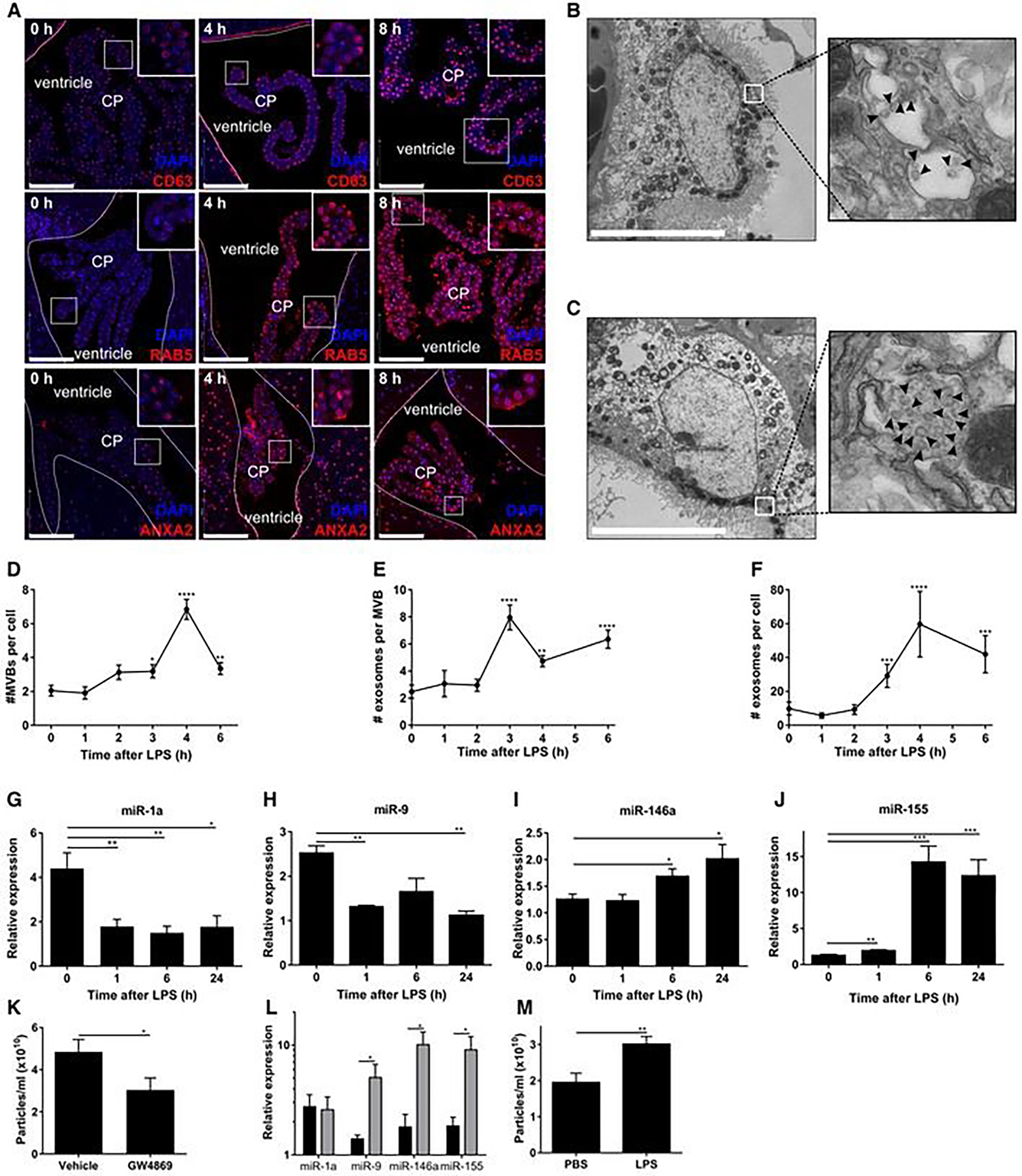
Systemic inflammation activates the exosomal machinery in the choroid plexus. A. Representative confocal images of CD63, RAB5, and ANXA2 (red) in the choroid plexus (CP) at 0, 4, and 8 h after LPS treatment. Hoechst (blue) was used to stain the nucleus. The dotted line indicates the ependymal cells that line the ventricle, and the square boxes indicate the zoomed insert images displayed at the right corner of each image. Scale bars, 100 μm. B, C. Representative TEM images showed the presence of MVBs in the CPE cells before (B) and 6 h after (C) LPS administration in vivo. Black arrowheads point to exosomes present in MVBs. Scale bars, 9 μm. D–F. Quantification of number of MVBs per cell section (D), number of exosomes per MVB (E), and number of exosomes per cell section (F), based on TEM analysis of several adjacent cells (0 h, n = 20; 3 h, n = 21; 4 h, n = 13; 6 h, n = 23). G–J. Quantitative real-time polymerase chain reaction (qPCR) analysis of miR-1a (G), miR-9 (H), miR-146a (I), and miR-155 (J). Data is presented as relative expression normalized with housekeeping miRs by TaqMan qPCR assay (0 h, n = 4; 1 h, n = 5; 6 h, n = 5; 24 h, n = 3). K. NanoSight analysis of CSF isolated from LPS-injected mice followed by icv injection of vehicle or GW4869, a neutral sphingomyelinase inhibitor that inhibits exosome secretion (n = 8). L. qPCR analysis of the expression of miR-1a, miR-9, miR-146a, and miR-155 in the choroid plexus of mice injected with LPS and then icv injected with vehicle (black) or GW4869 (gray) (n = 4). M. NanoSight analysis of the supernatant of choroid plexus explants from PBS- or LPS-injected mice (n = 6). Data information: Data in (D–M) are displayed as mean ± SEM and analyzed by Student’s t-test. Significance levels are indicated on the graphs: *0.01 ≤ P < 0.05; **0.001 ≤ P < 0.01; ***0.0001 ≤ P < 0.001; ****P < 0.0001. Adapted from Balusu et al. (2016)213, Copyright @ EMBO Press.
From the above discussion, we found that exosomes can cross the BBB and carry payloads back and forth from the inner and outer lumens. Thus exosomes provide another avenue for therapeutic drug delivery to fight against brain diseases and brain-related cancers that are untreatable with current therapeutic agents212,217.
5.3. Role of Exosomes as a Drug Delivery Vehicle:
Currently, the most preferred drug delivery systems are based on biodegradable liposomes or biological exosomes. Due to novel developments through exosomal research, several exosome-based drug formulations are currently in clinical trials, and recently some have been approved for clinical use218. Exosome bilayer-based drug delivery benefits the payload alternation of its biodistribution and higher encapsulation capacity218. Biological exosomes are also commonly used as drug delivery vehicles because of their overall bioavailability, improved drug encapsulation coupled with a controlled release, longer circulation time, and lessened toxicity219–221. Biodegradable nanoparticles like exosomes have successfully encapsulated bioactive molecules such as curcumin222, paclitaxel223, neurotoxin-I224, and dexamethasone225, all of which improve biodistribution and controlled release. Additionally, biodegradable nanoparticles are also utilized as drug delivery vesicles for multiple disease models of cancers226,227, diabetes228, and brain diseases such as Alzheimer’s229, Prions230, and Parkinson48. Most of these medications have translated into clinical trials, and some have already been introduced to the American market231.
On the other hand, liposomes, PLGA, PLA, or poly(lactic-co-glycolic acid) are the most common and well-studied nanoparticles (NPs) for targeted drug delivery applications232–235. Many liposomal and PLGA NPs mediated formulations have been successfully translated to the clinic and have obatined FDA approval: Doxil (Liposomal Doxorubicin)236, DaunoXome (Liposomal daunorubicin)237, Onivyde (liposomal nanoformulations of irinotecan)238, Cimzia (a PEGylated blocker of tumor necrosis factor-alpha (TNF-α))239, Neulasta (PEGylated form of filgrastim)240, Vivitrol (PLGA L/G 75:25 with active ingredient naltrexone)241,242, and Signifor LAR (PLGA with active ingredient pasireotide pamoate, treatment for acromegaly)243,244. One of the main challenges in translating polymer-based formulation is the behavioral difference between in vivo models compared to in vitro. To overcome the existing challenges in biocompatibility, diffusion, cell internalization, and tissue transportation, further studies are needed to thoroughly investigate utilizing different animal models245. These biodegradable polymeric vehicles accumulate in the reticuloendothelial system (RES), including the liver, spleen, kidney, lymph nodes, and bone marrow. Polymeric NPs are cleared by resident APCs, like macrophages, via direct interaction and increase immunosuppression and risk of infection232,246,247. Plasma proteins also play a pivotal role in clearing polymer-based drug formulations from the RES via opsonization248,249. Liposome and PLGA NPs also interact with immune cells in the blood and resulting in antibody production against NPs different functional components due to repeated injection45,250–252. This phenomenon is called the “accelerate blood clearance (ABC)” phenomenon. Dams et al. first observed the ABC phenomenon when animal models were administered with empty PEGylated liposomes, it influenced biodistribution and pharmacokinetic behavior of the 2nd dose of PEGylated liposomes after seven days253. Some polymeric NPs also induce innate immune response due to subsequent activation of the complementary system known as complement activation–related pseudoallergy (CARPA)254. CARPA has been observed from clinically approved liposome formulations (e.g., DaunoXome® and Doxil®)255. Another challenge, specifically for tumor-targeting polymeric NPs, arises from the complexity and heterogeneity of the tumor’s microenvironment, resulting in the accumulation of NPs in neighboring healthy cells256,257. Lastly, polymeric and biodegradable nanoparticle delivery systems’ development and marketability, even with their ability to evade the host immune system with extended circulation, stability, and low toxicity, have remained elusive258.
To overcome the limitations of most biodegradable/polymeric nanoparticles259, exosome-mediated drug delivery210 provides superior features including long circulation half-life260, enhanced cell-specific targeted delivery145, increased biocompatibility104,261, reduced/low toxicity262,263, ability to stimulate an immune response against pathogens161, anti-tumor modulation161, and antigen presentation264, etc. Exosomes have been utilized as a drug delivery vesicle in multiple studies using low-molecular-weight drugs, active biologics (lipids, nucleic acids, siRNA, proteins), and larger antibodies41,201,265–269. For example, the exosome-mediated delivery systems using curcumin have already shown great potential over conventional drug delivery systems270. Curcumin, an antioxidant that has chemotherapeutic properties, is a natural polyphenol found in the rhizomes of turmeric271–273. Alvarez-Erviti et al. reported expressing a neuron targeting a protein on the exosome surface with post-loading using siRNA, followed by injection into the mouse bloodstream. The authors have achieved specific gene knockdown in the brain and proof of the exosomal capability of crossing the BBB without inducing any immune response268. Another ongoing challenge part in delivery science is targeting the subcellular compartment of specific cells. For instance, targeting nuclease and delivering the CRISPR-Cas9 system is very attractive to the scientific world and has higher precision for gene editing. Scientists can deliver large plasmids, including the CRISPR–Cas9 expression vectors loaded in exosome, to mesenchymal stem cells269. This study validates that exosomes can deliver cargo to recipient cells and gives insight into in vivo gene editing potentials against multiple diseases269. Delivery of antibodies and active biologics are also a promising platform in the drug delivery field. Wan et al. has reported on modification of aptamer-based DNA on exosomal surfaces by DNA hybridization chain reaction, enhancing exosome functionality and showing potential for broader biomedical applications like targeted drug delivery, cell-free therapy, and gene knockdown41. Acquired drug resistance is a challenging mechanism against cancer chemotherapeutics and it has been reported that exosomes play a critical role in this drug-resistance transfer among cancer cells. Ming-lv et al. has showed that drug-sensitive MCF-7 cells (MCF-7/s) become drug-resistant after treatment with exosome isolates from the docetaxel-resistant variant MCF-7 cell line (MCF-7/DOC)274. The authors also found that P-glycoprotein (P-GP) expression is higher in exosomes from MCF-7/s cells after treatment with MCF-7/doc exosomes, indicating P-GP has a role in drug-resistance transfer among the cells. In 1996, Raposo et al. first observed the role exosomes play in adaptive immune system stimulation via antigen presentation275. Exosomes also carried and presented MHC-I/-II to modulate the antigen-specific CD8+ and CD4+ via direct and cross-presentation276. Bianco et al. showed that immature dendritic cell-derived exosomes inhibit inflammation in a murine footpad model via inflammatory cytokines IL-10 and IL-4277. Another study by Chen et al. showed mesenchymal stem cell-derived (MSC) exosomes increased the concentration of anti-inflammatory factor TGF-β and suppressed the secretion of pro-inflammatory factor IL-1β and TNF-α278. Besides, MSC exosomes also induced the transition of Th1 to Th2 cells and reduced the potential to differentiate into interleukin 17-producing effector T cells (Th17). Thus, MSC exosomes have the intrinsic properties of modulating the tumor microenvironment’s immune response and providing immune protection via exosomes. T and B-cell-derived exosomes also play a vital role in immune modulation. For example, mouse B lymphoma cell-derived exosomes carry heat shock protein 70, modulating the anti-tumor immune response in T-cells279. In another study, dendritic cell-derived exosomes primed with acid-eluted tumor peptides eradicated tumors in mice280. Exosomes from T-cells also improves immune response with the help of communication with endothelium cells by destroying tumor stroma and preventing tumor metastasis281. Immune modulation is achieved by bioactive lipids and proteins of the exosome and exosome mRNA. Archer et al. reported that human macrophage exosomes functionally inhibits cancer cells proliferation by delivering miRNAs to hepato-carcinoma cells (HCCs)282. Another study based on MSC-derived exosomes showed that the paclitaxel-loaded exosome inhibit in vitro tumor growth283. The study by Pascucci L et al. showed the effect of murine MSC SR4987 line exosomes loaded with paclitaxel (PTX) and delivered to the human pancreatic cell line CFPAC-1, which possessed intense anti-proliferation activity against CFPAC-1283. PTX loaded MSC exosomes showed higher cell target specificity as well. Rani et al. also reported that MSC-derived exosomes play a crucial role in its paracrine function266. Another study by Kalimuthu et al. showed paclitaxel (PTX) loaded with MSC-derived exosomes could accelerate anticancer treatment against breast cancer (MDA-MB-231) cells observed both in vitro and in vivo265. Exosomes have been extensively studied for brain drug delivery to improve brain disease and inflammation treatment. A study by Yang et al. has shown that exosomes derived from mouse brain endothelial cell line (bEND3) loaded with doxorubicin significantly reduced growth and proliferation of U-87 MG cancer cell compared to embryos treated with buffer control or drug only. Chemotherapy is the standard and most effective method for cancer treatment, and the above discussion validates that exosome-chemotherapeutic drug delivery reduces side effects through a targeted drug delivery strategy which reduces the overall drug dose needed for the treatment 284. Zhuang X et al. demonstrated that exosomes encapsulated with curcumin (Exo-cur) and STAT3 inhibitor JSI124 (Exo- JSI124) via LPS induced brain inflammation via microglia cells in a mouse model. The authors have reported the delivery method of Exo-cur and Exo- JSI124 induced apoptosis of microglial cells285. Additionally, exosome-mediated drug delivery systems have been utilized for curcumin delivery, which forms a complex with curcumin that enhances both loading efficiency and the safe transportation for patients in clinical trials286. Other great applications of exosomes involve their immune-protective and regenerative effects. MSCs are derived from multiple sources like bone marrow, adipose tissue, cord blood, and other sources and are getting much attention as potential candidates for regenerative medicine287–289. Cardiosphere-derived cells (CDCs) derived exosomes produce a range of cardio-protective measures like anti-oxidant, anti-fibrotic, anti-apoptotic, and anti-inflammatory effects 290,291. Another highlight of exosomes research is delivery of siRNA292,293. For example, Shtam et al. demonstrated that HeLa cell-derived exosomes delivering siRNA for RAD51 and RAD52 activate apoptosis of recipient cancer cells294. Wahlgren et al. also showed that peripheral blood exosomes mediated siRNA delivery efficiently silences the target MAPK gene in lymphocytes and monocytes295. Another interesting finding is that analysis of protein and mRNA confirm exosomal mediated siRNA delivery, targeting successful knockdown of BACE1, a therapeutic target of Alzheimer’s disease296. Also, a recent study by Hanet et al. reported that catalase loaded into exosomes can cross the BBB, improving the disease outcomes in a Parkinson’s mouse model48. Recent studies have also found that the targeted delivery of streptavidin-FasL (SA-FasL) via exosomes could substantially enhance the therapeutic effects of the SA-FasL protein while minimizing its potential off-target effects often caused by its solubility when doses are delivered by injection297. Regarding exosome-mediated vaccine development and delivery, Li et al. reported that the exosomes can transfer TNF-ϒ and induce antiviral activity298. Exosomes derivate from DCs also show promising potential for targeted immune responses against tumor cells and increased therapeutic effect compared with cell and non-cell based therapeutic strategies299. Specifically, mature and activated DC-derived exosomes carry MHC-I and MHC-II molecules and co-stimulatory molecules like CD40, CD80, CD86 and deliver cargo to active cytotoxic T- and natural killer (NK) cells in vitro and in vivo via potent antigen-specific T- and B-cell responses300,301. Genetically engineered autologous or allogeneic T cells expressing chimeric antigen receptors (CARs) or T-cell receptors (TCRs) as cellular immunotherapy may also be considered as a promising for cancer treatment method302. Z. Lu et al. recently reported that exosomes from hepatocellular carcinoma (HCC) antigen-modified DCs could be used as cell-free vaccines for HCC and opens the window for HCC immunotherapy303. Another study by Geis-Asteggiante et al. demonstrated myeloid-derived suppressor cells (MDSC) derived exosomes using protein mRNA and miRNA, can induce immune suppression function304. Anticoli et al. used an engineered exosome with the E7 protein of human papilloma virus (HPV). The E7 protein elicited a strong and effective antigen-specific cytotoxic T lymphocyte (CTL) immunity305. A DNA vector expressing HPV-E7 and fused at the C-terminus of an exosome-anchoring protein name Nefmut was injected to mice306. In this study, the authors provide evidence that injection of Nefmut/E7 DNA induces similar antigen-specific cytotoxic T lymphocytes like mice implanted with TC-1 tumor cells. Integrin αvβ6 can convert the latent transforming growth factor (TGF)-β to promote the development of Treg cells307. The authors demonstrated that the delivery of cardiovascular exosomes carrying integrin αvβ6 promote the generation of the donor antigen-specific immune tolerance. On the same line, another study showed DC-mediated exosomes promote heart allograft survival308. The authors have finally demonstrated that donor-derived peripheral exosomes carrying MMP1a promoted the allograft heart survival via inducing donor antigen-specific Treg to attenuate the T helper (Th)2 pattern inflammation309.
Exosomes offer enormous promise as a contemporary yet promising area for small and large biological molecules’ therapeutic drug delivery. As a drug delivery vehicle, exosomes provides an added advantage over polymeric vehicles due to lack of accumulation of exosomes in the RES, especially the liver, which helps them avoid first-pass metabolic effects before reaching target sites297. It is also essential to note that exosome-mediated drug delivery offers a comparatively longer circulation half-life, induces a robust immune response against pathogens, and facilitates subcellular-specific targeted delivery of therapeutics (e.g. to mitochondria and nucleus)15. However, there is a need for additional investigations into how exosomes react to the body’s immune responses before these therapies are accepted as permanent therapeutic methods27. This section demonstrates the exosome’s robust immune response, drug delivery capacity to any specific target, carrying of extensive biologics and antibodies, and discusses how scientists can utilize the exosome platform for designing an adjuvant vaccine and therapeutic delivery. Surface modification and engineered exosomes added a plethora of applications for drug delivery, disease diagnosis, and facilitate immunotherapy. Nevertheless, significant effort is required to develop exosome as a personalized therapeutic modality based on patient disease history.
5.4. Exosome as Disease Biomarker:
The National Institutes of Health Biomarkers Definitions Working Group in 1998 defined a biomarker as a quantifiable measure of a normal biological process, pathological process, of pharmacological response to a therapeutic administration310. Currently, both invasive and noninvasive methods are employed for biomarker identification. For example, serum analysis of blood samples from cancer patients is well established for monitoring the location and stage of cancer. Exosomes reignite the field of biomarker study. Naturally, the question arises, why do exosomes have advantages in biomarker screening applications? First, MHC-expressing exosomes have the ability of antigen presentation via both direct and indirect pathways127,311. Second, exosomes contain cell-specific surface markers, that carry protein and RNA cargo, and are highly stable in storage condition312,313. The exosome was initially considered an unnecessary protein excreted from cells. However, recent studies confirm the importance of exosomes in cell-cell communication by transporting microRNA, mRNA, and proteins. The membrane bilayer and luminal content of exosomes are protected from extracellular proteases. Multiple exosome sources contribute to the biomarker study; they are urine, saliva, cerebrospinal fluid, blood, body fluid, amniotic fluid, ascites, and cells used to identify and validate biomarker screening. Exosomes contain a variety of lipids, nucleic acids, mRNA, proteins of cytosolic, cell signaling, and membrane trafficking, reflecting its cell type and condition. In the a PubMed search conducted on January 20th, 2021, 4767 papers are generated related to exosomes and biomarker studies. As a biomarker, exosomes are getting more attention from various groups of scientists as more evidence is emerging that exosomes contain protein and nucleic acids associated with cancer, liver, kidney, neurodegenerative, infectious, and metabolic diseases. Exosomes are easy to analyze and can be stored at −80°C for one week to 1–2 years ( depending on the exosome source) for future use314. More information on exosome biomarkers can be found on http://www.exocarta.org (ExoCarta, a web-based compendium of exosomal cargo), and http://exrna.org (Extracellular RNA communication program). Biomarker screening studies utilize multiple tools to analyze specific markers relevant to the disease model. Protein, mRNA, and microRNA content of exosomes are used as a diagnostic tool for biomarker analysis. Most general approaches are flow cytometry (FACS), immunohistochemistry, biochemical analysis (microarray studies, RT-qPCR, western blotting), surface resonance Raman spectroscopy (SERS), and principal component analysis (PCA). These assays are based on the type of exosome source and disease-specific biomarker.
Proteins found in exosomes from both healthy and disease states are diverse and resemble various disease conditions related to cancer, liver, renal, kidney, and brain diseases. Several proteins have been identified as a diagnostic marker for exosomes. Scaffolding membrane proteins Tetraspanin are enriched on the exosome surface. The study shows plasma CD63+ expression elevated in patients with melanoma compared with a healthy control315. Recent research also stated that a higher level of CD63+ in different cancer types consolidate as a potential biomarker for cancer316. CD81, another biomarker, was found to be higher in chronic hepatitis C patients and associated with fibrosis and inflammation317–319. In a lung cancer diagnostic biomarker study, authors found higher expression of CD151, CD171, and tetraspanin 8 in serum exosome blood collected from 581 cancer patients (431 with lung cancer and 150 controls)320. This study is suggests exosomal protein is a promising biomarker for non-small-cell lung carcinoma (NSCLC). Glypican-1 (GPC1)-positive exosomes serve as potential biomarkers in early-stage pancreatic cancer. Exosomes isolated from systemic circulation of 250 pancreatic patients showed a higher correlation of GPC1 in cancer patients than the healthy control321. In another biomarker proteomic study, urine exosomes collected from a mouse liver damage model were utilized. The authors demonstrated that CD26, CD81, S1C3A1, and CD10 could be used as a potential biomarker for hepatic damage322. On the same line, a urine exosome biomarker study revealed that some specific markers are most frequently associated with ALIX ( ALG2-interacting protein X), CD24, CD9, flotillin-1, HSP70, TSG101 (tumor susceptibility gene 101), LAMP1 (lysosome-associated membrane protein 1), gp330 precursor, uromodulin, pro-epidermal growth factor precursor, MME Neprilysin, and Beta-galactosidase precursor323–329. In a gastric cancer biomarker study, the authors found that metastatic AZ-P7a cells release let-7 miRNA, which activates CD-97 associated pathways to promote oncogenesis330–332. Many studies prove that glioblastoma (GBM) is malignant and exosome mRNA content provides us more insight on GBM and how biomarker identification will lead to an effective treatment333. Studies show MiR-21 plays a key role in GBM pathways. Also, exosomal marker non-coding RNA (RNU6–1) and microRNA (miR-320, miR-574–3p) are significantly associated with GBM diagnosis. More evidence is showing an exosome role in carcinogenesis pathways like ERK, PI3K/AKT, STAT3, and PTEN333–336. Breast cancer is a highly prevalent disease, and early diagnosis gives a better outcome of treatment. Serum exosome microRNA or non-coding RNA analysis shows promising results in breast cancer biomarker identification337. In another study, the authors found exosomal miRNA-21 with 105 expressions higher tissue of metastasis patients than non-metastasis and healthy donors, which implies that liquid biopsy based on circulating exosomes can be a complementary diagnostic biomarker tool for a breast cancer study338. Another plasma exosome analysis study revealed that exosomal microRNA MiR-21, MiR-1246, are more significantly elevated in human breast cancer patients and can serve as a plasma biomarker for breast cancer.
Exosomes harbor different proteins, lipids, nucleic acids that are present in most body fluids. It has been proven that exosomes play a role as a critical signal transduction promoter to recipient cells via transporting proteins, lipids, mRNA, microRNA, etc. Research on exosomal biology and functions makes it ideal as a biomarker-screening tool. Compared with traditional biomarker specimens like serum or urine, exosome offer higher sensitivity and specificity to their excellent stability. The use of biomarker screening utilizing exosomes will expand since they are found in mammalian cells and a diverse range of pathological microorganisms 339–341. In conclusion, exosomes for use as biomarkers are in a very early stage of discovery, and their potential clinical value waits to be fully explored.
5.5. Exosome Applications in Medical Imaging and Tracking:
Exosomes were thought to be waste materials from cells until they were revealed to transfer various biomolecules to various cavities342. To engage in intercellular communication by overcoming the natural biological barrier (e.g., blood-brain-barrier), exosomes have become an emerging effective diagnostic and therapeutic nanocarrier13,343. However, there is a limited understanding of how and where exogenously administered exosomes are distributed in vivo344. The current method to assess exosome-mediated delivery success is to evaluate therapeutic symptoms or repeated post-mortem histopathological examination. Therefore, real-time, non-invasive exosome imaging is a prerequisite to making exosomal therapy clinically relevant. In recent years, scientists have developed an efficient labeling and tracking method of exosomes using various imaging modalities (Figure 8). The imaging tools can provide information on exosomes such as bio-distribution, migration capability, physiological functions, in-vivo behavior, and enhance the opportunities to find an optimal administration route for exosome-mediated drug delivery345. Optical imaging is a widely used imaging and diagnostic technique that is cost-effective, highly sensitive, and includes images at the molecular level. There are mainly two types of optical imaging: fluorescent imaging and bioluminescence imaging. Fluorescence imaging is based on a fluorescence probe that is excited upon the laser irradiation, by which it produces fluorescent signals observed by the optical imaging system. Fluorescence imaging is non-invasive and is used in real-time with the use of non-ionizing light sources346. There are two ways to label exosomes with fluorescent materials (e.g., proteins, dyes, or nanoparticles). (1) Indirect labeling is to have parent cells express a fluorescence protein, including green fluorescent protein or red fluorescent protein, to excrete the exosomes with their biological mechanism of visualization capability passed down to their daughter cells. (2) Direct labeling is another type of labeling in which fluorescence dyes (or nanoparticles) are used to label to the secreted exosomes after isolation via surface modification or physical interaction to observe their bio-distribution and tissue uptake347,348. Anchordoquy et al. demonstrated that lipophilic carbocyanine DiOC18(7) (DiR) dyes can be used to successfully labeled exosomes derived from breast cancer cells and the resulting the bio-distribution in tumor-bearing xenografts could be observed. In comparison with DiR dye-labeled liposomes, they found most exosomes accumulated in the liver and spleen after 1 hour of intravenous injection. They also observed higher fluorescence sensitivity of exosomes than liposomes within tumor regions349. Bioluminescence imaging utilizes the natural light emission process from some living organisms that bioluminesce (e.g., firefly, bacteria). Bioluminescence images are generated by detecting photons emitted internally from enzyme-catalyzed reactions with an optical imaging system350. Takakura groups from Kyoto University constructed exosomes with intense luciferase activity via indirect labeling. In their study, B16-f10 murine melanoma cells were transfected with the plasmid expressing a fusion protein of gLuc-lactadherin. The secreted exosomes from the cells were collected by the ultracentrifugation isolation method. After a 4-hour systemic injection, they found the exosomes accumulated first to the liver and then to the lungs with fast clearance from systemic circulations 351. Due to the penetration depth limitation of light in optical imaging, other whole-body imaging modalities are needed. Computed tomography (CT) is a crucial imaging technique for disease diagnostics with a high temporal and spatial resolution based on measuring X-ray absorptions throughout the body. X-ray CT imaging has been widely used in the clinic to visualize bone structures and has now been adapted to quantify cell tracking with gold nanoparticle labeling352. A recent publication showed that stem cell derived-exosomes labeled with gold nanoparticles could track the migration and homing patterns in brain disorders353. Betzer et al. studied the efficient labeling of glucose-coated gold nanoparticles to exosomes and demonstrated the enhanced exosomal accumulation at the lesion site using CT imaging on a mouse model of focal brain ischemia after 24 h of intranasal administration354. Gold nanostructure can be potentially used for photoacoustic imaging (PAI) of tracking of exosomes, using the strong light absorption to generate sound waves. Based on a ‘light in/sound out’ approach, PAI can efficiently combine optical imaging’s spectral contrast, and the anatomical penetration depth of ultrasound imaging (up to multiple centimeters)355.
Figure 8.
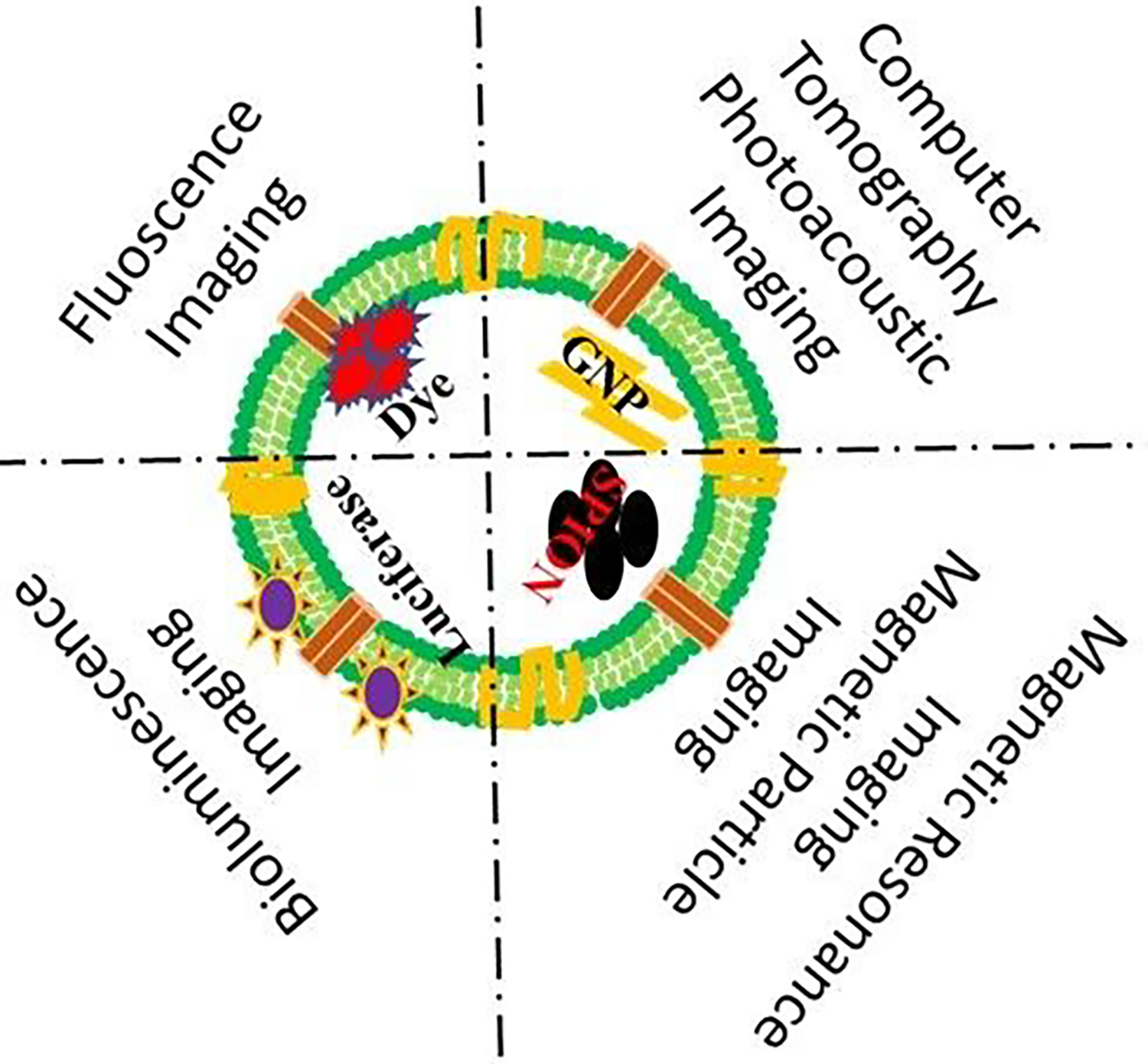
Exosome visualization using various imaging modalities. Fluorescence dye-labeled or luciferase-expressing exosomes visualize the biodistribution or tissue uptake under optical imaging systems (fluorescence imaging or bioluminescence imaging). Gold nanoparticles (GNPs) labeling exosomes observe the whole-body tracking in deep tissues under computer tomography (CT) or photoacoustic imaging (PAI). Superparamagnetic iron oxide nanoparticles (SPIONs) labeled exosomes show the active cell migration or homing to target regions in vivo under magnetic resonance imaging (MRI) or magnetic particle imaging (MPI).
Another whole-body imaging technique is magnetic resonance imaging (MRI), which offers advantages with radiation-free, high spatial resolution. To be detected by MRI, exosomes are required to be labeled with superparamagnetic iron oxide nanoparticles (SPIONs). In one study, Hu et al. utilized the electroporation technique to allow melanoma exosomes to be loaded with SPIONs and detected by MRI. They revealed the SPION-exosome exhibited a much more efficient homing and retention in sentinel lymph nodes than the free SPION 48 h after initial footpad injection356. Furthermore, in a newer magnetic imaging modality, magnetic particle imaging (MPI), SPIONs can create a linearly quantifiable signal that is difficult to achieve in MRI without any tissue background signal. In a recent study, Jung et al. labeled SPIONs in MDA-MB-231 cancer cells’ culture in hypoxic conditions and showed hypoxic cancer cells take up these exosomes more avidly. They showed these exosomes are quantitatively visualized with MPI signals from SPIONs to monitor the whole-body distribution and in vivo targeting of exosome-mediated drug (Olaparib) delivery to tumors357. Exosomes plays a pivotal role in analyzing cancer biology and brain diseases. It is crucial to diminish false-positive exosomal signals from exosome labeling as an imaging tool.
Besides, exosome-based multimodal imaging can be considered more effective and specific for noninvasive disease diagnostics. Cao et al reported that nucleus targeted exosome engineered vanadium quantum dots nanocomposites showed effective multimodal (PAI and MRI) image - uided efficient cancer therapeutic potency. Besides, nanocomposites showed good biocompatibility and long circulation time. As a result, it can target a high number of cancer cells and have demonstrated efficacy in escaping the endosome, and advancement into the nucleus. Due to their NIR absorbance and good photostability, nanocomposites-treated mice exhibited a 2.11-fold higher PAI signal in the tumor site than the control group. In contrast, due to the 3d1 electronic configuration of quadrivalent vanadium (V) and the quantum mechanical confinement, nanocomposites displayed 3.73-fold higher in vivo T1 MRI contrast than the control groups. Their overall results indicated that nanocomposites could be attractive agents for multimodal imaging (Figure 9)341. New exosomal imaging techniques will be developed for preclinical and clinical settings with t continuous development in the field of exosome research.
Figure 9:
In vitro and in vivo Photoacoustic imaging and Magnetic Resonance Imaging of exosome loaded nanocomposites (V2C-TAT@Ex-RGD) (A) In vitro PAI images and the quantitative curve of PA intensity of the V2C-TAT at different concentrations. (B) In vivo PA images of mice 12 h after intravenous injection of PBS, V2C-TAT (10 mg/kg), and V2C-TAT@Ex-RGD (V2C-TAT, 10 mg/kg). (C) Quantification of the PA signals from the tumor sites from different groups treated with (1) PBS, (2) V2C-TAT (10 mg/kg), and (3) nanocomposites (V2C-TAT, 10 mg/kg). (D, E) In vivo PA images and the responding signal intensities of mice at different times after intravenous injection of nanocomposites (V2C-TAT, 10 mg/kg). (F) T1-weighted MR images of mice 24 h after intravenous injection of PBS, V2C-TAT (V2C-TAT, 10 mg/kg), and nanocomposites (V2C-TAT, 10 mg/kg). (G) Quantification of the MR signals from the tumor sites from different groups. Adapted from Cai et al (2019) 358, Copyright @2019, American Chemical Society.
5.6. Role of Exosomes in Vaccine Development and Delivery:
Almost all living organisms, including viruses and bacteria, shed exosomes in the extracellular matrix. As mentioned before, exosomes carry larger proteins, lipids, and nucleic acids in their cargo. Lipids, proteins, membrane trafficking, nucleic acid-like signal transducers, anti-apoptosis molecules, and T-cell stimulations found on the exosome surface also have some immune-modulatory effects. Exosomes also contains a high level of triacylglycerol (TAG), cardiolipin, and cholesterol (CE), and where TAG and CE are found in the lipid droplet core and cardiolipin in mitochondria65. Both cell-specific and ubiquitous proteins are selectively expressed in exosomes from their native cells. They also include cytosolic proteins like tubulin, flotillin, and membrane transport proteins like annexin, actin, and RAB proteins 1. Exosomes also carry heat shock proteins like HSP20, HSP27, HSP70, and HSP90, involved in loading and binding antigen peptide onto MHC molecules, antigen presentation, maturation dendritic cells, and translocation of NF-κβ into the nucleus through CD91359,360. Another abundant protein family present on the exosome surface is tetraspanin like CD9, CD63, CD81, and CD82, interacting with multiple proteins like integrin and MHC class I & II361. Exosomes that are released from viral cells also carry viral miRNA, proteins, and even entire virion. For example, the major oncoprotein of EVB (a gamma herpesvirus), latent membrane protein 1 (LMP1), was identified in exosomes isolated from EVB infected cells362. Immune modulating cells like macrophages and monocytes abundant with exosomes can modulate antigen presentation and affect myeloid cell differentiation and proliferation. During antigen presentation, B-cell-derived exosomes first interact with antigens and modulate T-cell activation and cytokine secretion363,364. The study also shows that immune-derived exosomes take cytokines like TGF-β, IL-1α, TNF, and IL-1 β. On the contrary, infected cell-derived exosomes can carry viral molecules and microbials365.
Exosomes also play a critical role in the chronic inflammatory process. For example, DC-derived exosomes behave as antigen-presenting molecules and can perpetuate Th2 cells response to DC to regulate of immunity and inflammation366. Mast and B-cell-derived exosomes also drive Th2 responses and promoted the Th2 environment367.
Exosomes also play a role in processes like angiogenesis, stromal cell activation, and tumor growth metastasis. The release of exosomes is increased when a cell is under stress, particularly at stress conditions due to disease-related changes368. In the current therapeutic paradigms, cancer cells become non-responsive to chemotherapeutics and radiation after repeated exposures and are exosomes are one of the major players in cancer progression. For example, pancreatic cell-derived exosomes carrying tetraspanin-8 promote vessel branching. Tetraspanin-8 also modulates uptake and binding of cancer exosomes by endothelial cells369. In the tumor microenvironment, cancer-associated fibroblasts (CAF) differentiate into myofibroblasts to facilitate tumor progression. The study shows CAF exosomes promote or activate multiple signaling pathways. For example, Luga et al. have shown that CD81 positive exosomes from stromal cells activate several signaling pathways in breast cancer tumor. This activated signaling promotes cancer cell motility, metastasis, and tumor growth370. Mature and activated DC-derived exosomes carry MHC-I and MHC-II molecules and co-stimulatory molecules like CD40, CD80, CD86, among others which activate natural killer (NK) cells and cytotoxic T-cells in vitro and in vivo via potent antigen-specific T- and B-cell responses301,371. Therefore, exosomes play both an immune activation and a cancer progress role depending on cell type origin. However, exosomes also suppress immune cell activation. Studies show that cancer exosomes also elevate the differentiation of mature dendritic cells without its antigen presentation to the myeloid cell, which produces TGF-beta for T cells suppression372. Thus, exosomes use signaling between tumor cells and surrounding cells to promote the tumor microenvironment. In an infectious disease, the pathogen faces a hostile situation and invades cell signaling. Along the same line, the study shows pathogen use of exosomes for differentiation, growth control, transmission, and virulence coordination to infection. For example, exosomes from malaria are actively taken up by endothelium and monocytes, altering vascular properties, promoting virulence via malaria exosomes and stimulating DNA-sensing pathways via microRNA373. Parasite or virus-infected cells or parasites themselves release exosomes to activate immune cells via antigens to present to APC. In contrast, exosomes from microbial molecules carrying leishmania GP63, or HIV Nef can activate apoptosis of immune effector cells like helper T cells and effector B cells or inhibit T-cells374.
Vaccines delivers the natural or inactive form of proper antigens adjuvant; partial viral particles typically elicit a potent immune response. In cancer immunotherapy, a tumor-associated antigen (TAA) can be a potential delivery particle as a vaccine. For vaccine applications, some TAA targeting proteins such as HER2, p53, CEA, RAS, MUC1, etc., are immunosuppressive and poorly antigenic375. For example, Hartman et al. has proposed to generate recombinant adenoviral vectors expressing the extracellular domain (ECD) of carcinoembryonic antigen (CEA) or HER2 linked to the C1C2 domain of lactadherin in addition to native unlinked ECD versions of CEA and HER2. The authors found adenoviral expression of a C1C2 modified CEA/ECD, and HER2/ECD resulted in higher expression of the protein in the exosome fraction in transgenic murine model than the control. This study signifies low immunogenicity of soluble TAAs in cancer patients and opens the cancer vaccine platform via improving anti-tumor immune response376. Recently, exosomes are getting much more attention due to their intrinsic properties as drug carriers and immunomodulators. Anticoli et al. has used engineered exosomes with the E7 protein of the Human Papilloma Virus (HPV). The study demonstrates that E7 protein elicited solid and effective antigen-specific cytotoxic T lymphocyte (CTL) immunity. Genetically engineered autologous or allogeneic T cells expressing chimeric antigen receptors (CARs) or T-cell receptors (TCRs) as cellular immunotherapy agents are promising as a new treatment method for multiple ranges of cancers302. Despite T-cell efficiency, T-cell therapies show unique toxicities like cytokine release syndrome (CRS) and CAR-T-related encephalopathy syndrome (CRES). Previous studies also showed human T-cell-derived exosomes in cytotoxic T lymphocyte (CTL)-target cell interactions377,378. CTL-derived exosomes containing CTL surface membrane molecules (CD3, CD8, and the TCRs) result in tumor cell death as a consequence of interactions between the TCR and proper antigen/MHC combination. The authors’ data validate that CAR-containing exosomes derived from CAR-T cells can be used as cancer-targeting agents and can improve the therapeutic efficacy of a potential cancer vaccine platform262. Another study by Yawen Li et al. demonstrates that an exosome derived from Toxoplasma Gondii that modulates the immune response. The exosomes were isolated from T. Gondi and incubated with macrophage RAW264.7 cells. After T. Gondi exosomal treatment, production of IL-12, TNF-α, and IFN-γ in macrophage cells increased and the level of IL-10 decreased, as determined using an enzyme-linked immunosorbent assay (ELISA). The authors have concluded that T. Gondi exosome modulated macrophage activation in vitro triggers humoral and cellular immune responses and results in partial protection against acute parasite infection in mice. The results suggest that exosomes may serve as a potential candidate against toxoplasmosis379.
S. Roier et al. showed how outer membrane vesicles form in gram-negative bacteria emerging in the research field and significantly impact future applications such as outer membrane vesicle (OMV)-based vaccines. OMVs derived from heterologous H. influenza strains were thoroughly characterized for size distribution and quantity of vesicle production among strains Rd KW20, Hib strain Eagan, and NTHi 2019-R strain. The presence of vaccine candidate 13 ATP-binding cassettes (ABC) transporter proteins and eight lipoproteins in H. influenzae OMVs supports their potential to act as a vaccine against H. influenzae infections. In contrast, the presence of essential virulence factors like serine protease HtrA and vaccine candidates OMP 26 and protein D indicates that OMVs in H. influenza pathogenesis have potential vaccine application via exosome380. This above discussion demonstrates that the exosomes can play an important role in immune modulation, serve as potential vaccine development platforms, and be used as a delivery vehicle. Exosomes can increase the efficiency of vaccines to generate antibodies against multiple diseases and are a versatile platform for developing more vaccines.
In the pre-clinical study discussion, we found exosomes play a critical role in both physiological and pathological conditions, as they can carry pathogenic messages that indicate disease condition. Therefore, exosomes can be utilized as a biomarker and a disease marker to target for therapeutic efficiency. Pre-clinical studies of exosomes for use as a biomarker, in early detection and drug delivery methods, and in vaccine development platform makes them suitable candidates in clinical settings. We believe exosomes can link bridges between our knowledge gaps in different disease conditions. The pre-clinical data are evident that exosomes have the potential for application in cancer immunotherapy, infectious disease, and brain disease treatment.
6. Exosomes in Clinical Trials
The wide range of biological content found and released from exosomes in physiological conditions has various applications in biomedical and drug delivery contexts, such as finding new biomarkers, creating new imaging tools, and developing therapeutic carriers for cancers and brain disease. Up until January 2021, we have found 205 clinical trials related to exosomal research. Out of 205 trials, around 87 trials involve cancer-related studies, 18 include brain pathologies, and 100 include diabetic, cardiovascular, lung, or kidney diseases, including a novel coronavirus study.
6.1. Exosomes for Use in Clinical Trials for Cancer Patients:
In cancer-related trials, there are about 87 clinical trials in the pipeline. The trials include using exosomes for various processes, such as studies on angiogenesis, tumor growth metastasis, and stromal cell activation, and facilitating cancer progression368. When a tumor starts growing, it quickly becomes hypoxic, which triggers the regulation factors of both pro-angiogenic and anti-angiogenic cytokines like vascular endothelial, fibroblast, pericytes, and endothelial growth factors (EGF)381,382. Exosomes are one of the key players in cancer progression, drug resistance383, and prognosis384,385. For example, pancreatic cell-derived exosomes carrying tetraspanin-8 promote vessel branching. Tetraspanin-8 also modulates the binding and uptake of cancer exosomes by endothelial cells30. Al Nedawi et al. found lung cancer exosomes delivered mutated EGF receptor to pulmonary endothelial cells, activating EGF receptor, and signaling through AKT and MAP kinase pathways. This activation is misleading to VEGF secretion and endothelial cell response to tumor progression386. Therefore, cancer cell-derived exosomes can provide effective treatment of anti-angiogenic therapy387. Lucero et al. demonstrated glioblastoma (GBM) cell-derived exosomes deliver angiogenic mRNA and translate it to protein into recipient cells388. Authors identified possible GBM exosomal mRNA as a liquid biopsy biomarker, which shows a trace of post-transcriptional gene silencing. Another study found colorectal exosomes deliver angiogenic mRNA to endothelial cells and enhance proliferation of tubular formation389. With angiogenic mRNA transfer via interaction with α4 and β1 integrins, angiogenesis is stimulated via eNOS and the PI3K/AKT signaling pathway. Table 1 shows clinical trials based on the use of exosomes as a diagnostic tool, biomarkers, and therapeutic intervention for cancer research. Early detection is a powerful tool to fight against cancer progression. In early detection statistics, there was more than a 70% mortality decrease due to early detection and identification of novel markers of cancer. Exosomes have been used as a biomarker for cancer for decades. That is why we found more than 50 clinical studies of multiple phases looking for biomarkers like protein expression, mRNA, tumor-circulating exosomes, and tumor-derived exosomes—the first study was based on exosomes role as a therapeutic tool for those with advanced unresectable or metastatic melanoma. Exosomes from senescent melanoma cells will be utilized to study the process of drug resistance and relapse, as a therapeutic tool for melanoma for personalized medicine for patients. Another study in Table 1 (No. 4), in early lung cancer research, uses a blood sample exosome of lung cancer patients for identifying biomarkers (NCT03542253)390. No. 8 in the table will use urine exosomes, utilizing clear cell renal cell carcinoma to identify diagnostic biopsy tools for early detection (NCT04053855). Another study on Proteinosis Gallbladder Carcinoma uses the blood exosome as a biomarker for a correlation study (NCT03581435)391. In breast neoplasm, tumor-derived exosomes are used as diagnostic and prognostic markers against receiving neoadjuvant chemotherapy (NCT01344109)392. In gastric cancer, gastric cancer-derived exosomes are used as diagnostic tools for early detection (NCT01779583)393. In bone metastasis, scientists also utilize circulating tumor exosomes to identify deregulated miRNAs as a biomarker for use in subsequent bioinformatic tool development (NCT03895216)394. Another study on carcinoma ovarian cancer was based on the analysis of miRNA and lncRNA expression in exosomes395. These biomarkers will be employed as biomarkers for early detection as well (NCT0373831).
Table 1:
Exosome clinical application in cancer research
| No | Disease or Conditions | Interventions and Exosome source | Therapeutic and disease model | Clinical Trial Identification | Status & Clinical Phase |
|---|---|---|---|---|---|
| 1 | Metastasis (stage IV), melanoma (stage iiic) | Biological test blood | Exosome for developing theranostic tools | NCT02310451 | Unknown & Not applicable |
| 2 | Pancreatic cancer | Endoscopic ultrasound-guided portal venous blood sampling exosome | Feasibility and safety of sampling portal venous blood, detecting CTCs, and analyzing mRNA markers | NCT03821909 | Recruiting & Not applicable |
| 3 | Colon cancer | Curcumin, Curcumin conjugated with plant exosome. | Exosome loaded curcumin on the immune modulation, cellular metabolism, and phospholipid profile of normal vs. Malignant | NCT01294072 | Active, not recruiting, Phase 1 |
| 4 | Early lung cancer | CT chest scans and blood, exosome detection | We are using peripheral blood exosomes in identifying early biomarkers for lung cancer. | NCT03542253 | Not yet recruiting & Not applicable |
| 5 | Sarcoma | Blood samples | Evaluate cancer pathogenesis, progression, and treatment efficiency of the serum-derived exosomes. | NCT03800121 | Recruiting & not applicable |
| 6 | Prostate cancer | Urine exosomes | Validate a non-DRE exosome gene expression test of prostate cancer in a prostate needle biopsy | NCT02702856 | Completed & not applicable |
| 7 | Pancreatic cancer, Benign pancreatic disease | Blood sample from patients and healthy controls | Exosome purification for such as proteomics and RNA sequencing. | NCT02393703 | Active, not recruiting & not applicable |
| 8 | Clear cell renal cell carcinoma | Urine samples | Detecting tumor exosomes in urine, a new biopsy tool for early diagnosis | NCT04053855 | Not yet recruiting & |
| 9 | Lung metastases osteosarcom a | Blood sample | Identifying the levels and mutations of circulating exosomal RNA with or without lung metastasis. | NCT03108677 | Recruiting & Not applicable |
| 10 | Proteinosis gallbladder carcinoma | Exosomes from blood specimens | Correlations between exosome biomarkers and gallbladder carcinoma. | NCT03581435 | Recruiting & Not applicable |
| 11 | Metastatic & ductal (stage IV) Pancreatic adenocarcin oma, Pancreatic cancer AJCC v8 | Mesenchymal stromal cells-derived exosomes with KRAS G12D siRNA | Mesenchymal stromal cells-derived exosomes with krasg12d siRNA (exosomes) in treating participants with pancreatic cancer with krasg12d mutation | NCT03608631 | Not yet recruiting & Phase 1 |
| 12 | Breast neoplasms | Tumor-derived exosomes | Evaluating the use of tumor-derived exosomes as a marker for response to therapy in women receiving neoadjuvant chemotherapy | NCT01344109 | Withdrawn & Not applicable |
| 13 | Cholangiocar cinoma benign biliary stricture | Cholangiocarcino ma derived exosomes | Characterize the ncRNAs of cholangiocarcinoma obtained exosomes as a diagnostic tool and evaluate the prognostic and predictive value of cholangiocarcinoma exosome levels in plasma, before and after surgical resection. | NCT03102268 | Recruiting & Not applicable |
| 14 | Pancreatic ductal adenocarcin oma (PDAC) | The portal vein blood sample | Test 3 CTC isolation methods and analyses by flow cytometry for onco-exosomes in pancreatic cell lines’ culture media. | NCT03032913 | Completed & Not applicable |
| 15 | Sleep apnea syndromes, obstructive cancer | Blood samples | To evaluates exosomal PD-1/PD-L1 expression in patients | NCT03811600 | Not yet recruiting & Not applicable |
| 16 | Gastric cancer | Gastric cancer-derived exosomes | Characterize gastric cancer-derived exosomes’ molecular profile as diagnostic tools and evaluate gastric cancer exosomes levels in plasma prognostic and predictive value. | NCT01779583 | Unknown & Not applicable |
| 17 | Bone metastases | Circulating tumor exosome | Identify deregulated miRNAs, subsequent bioinformatics analysis to identify their potential role in tumor progression | NCT03895216 | Recruiting & Not applicable |
| 18 | Oropharyngeal cancer | Exosomes from blood and saliva | Detect specific HPV proteins in the blood or saliva exosomes to help improve the detection of OPSCC. | NCT02147418 | Recruiting & Not applicable |
| 19 | Larynx, lip, oral cavity, pharynx | Metformin Hydrochloride, Placebo, Cancer exosomes. | Identify the role of metformin hydrochloride and exosomes in cancer cells’ metabolic activity and surrounding supportive tissues. | NCT03109873 | Active, not recruiting & Early Phase 1 |
| 20 | Carcinoma ovarian cancer, benign gynecologic diseases | Ovarian cancer exosomes, | Analyze the expression of micro-RNA and long non-coding RNA of exosomes by next-generation sequencing | NCT03738319 | Recruiting & Not applicable |
| 21 | Oral mucositis, head, and neck cancer, | Grape extract exosomes, Drug: Lortab, Fentanyl patch, mouthwash | The ability of plant (grape) exosomes to prevent oral mucositis of head and neck cancer. | NCT01668849 | Active, Not recruiting & Phase 1 |
| 22 | Non-small cell lung cancer (NSCLC) | Plasma exosome, liquid biopsy | Plasma exosome, new radiotherapy combining with immunotherapy. | NCT02890849 | Unknown & Not applicable |
| 23 | Non-small cell lung cancer (NSCLC) | Plasma exosomes radiotherapy | plasma exosome level before and after radiotherapy, PD-L1 mRNA levels in Pexo, and radiotherapy practice combined with immunotherapy. | NCT02869685 | Unknown & Not applicable |
| 24 | Non-small cell lung cancer | Dendritic cell-derived exosome | Phase I trials showed no induction of T cells could be monitored in patients. | NCT01159288 | Completed & Phase 2 |
| 25 | Rectal cancer | Blood sample from participants, Neoadjuvant chemoradiation therapy | Characterize exosomal biomarker levels in patients and compare exosomal expression rates before, during, and after chemoradiation therapy. | NCT03874559 | Recruiting & Not applicable |
| 26 | Breast cancer leptomening eal metastasis | CSF and blood | Use of proteomic profile issued from cerebrospinal fluid microvesicles for diagnosis of leptomeningeal metastases. | NCT03974204 | Not yet recruiting & Not applicable |
| 27 | Metastatic castrate resistant prostate cancer, | Blood samples, Abiraterone and Enzalutamide | Detection of arv7 splice variant transcripts from exosomes in the circulation of MCRPC patients pre-and post-treatment with selective Androgen pathway inhibitors. | NCT03236688 | Active, not recruiting & Not applicable |
| 28 | Bladder cancer | Urine samples | Urine samples analysis compared to the results of cystoscopy. | NCT04155359 | Not yet recruiting |
| 29 | Lung cancer (diagnosis) | Exosomes from plasma, human bronchial epithelium & cancer cells | Serum exosomes noncoding RNA as a biomarker’s sensitivity and specificity for the determination of lung cancer | NCT03830619 | Recruiting & Not applicable |
| 30 | Carcinoma, non-small-cell lung | Blood samples | Feasibility identifying EML4-ALK fusion transcripts and T790M EGFR mutation from exosomes in NSCLS patients’ circulation. | NCT03236675 | Active, not recruiting & Not applicable |
| 31 | Cancer | Blood and urine | HSP70-exosome can be used for the early diagnosis of patients with a solid malignant tumor. | NCT02662621 | Recruiting & Not applicable |
| 32 | Thyroid cancer | Urine sample, Urine exosomal thyroglobulin and galectin-3 | Identifying urinary exosomal proteins, including thyroglobulin and galectin 3 | NCT03488134 | Active, not recruiting & Not applicable |
| 33 | New tumor diagnostics from human plasma samples | Plasma samples | Protein profiling on the isolated exosomes and isolate nucleic acids from exosomes for analysis. | NCT04081194 | Recruiting & Not applicable |
| 34 | Prostate cancer | Genetic analysis for the detection of prostasomes | Purification of prostasomes from prostate cancer patients and their ability to determine the grade of the prostate tumors. | NCT03694483 | Recruiting & Not applicable |
| 35 | Prostate cancer | Exodx Prostate Intelliscore, Urine samples | Investigating a new and validated urine test that predicts the likelihood of high-grade prostate cancer on an initial prostate biopsy | NCT03031418 | Recruiting & Not applicable |
| 36 | Colon cancer, Liver tumors | Blood draws, colectomy or hepatectomy, fibroscan test | Novel ways of diagnosing colon cancer and predicting its propensity to spread to other organs such as the liver. | NCT03432806 | Recruiting & Not applicable |
| 37 | Prostate cancer | Exodx prostate (intelliscore), urine samples | Validated urine test to predicts the likelihood of high-grade prostate cancer on an initial prostate biopsy. | NCT03235687 | Active, not recruiting & Not applicable |
| 38 | Pancreatic carcinoma &intraductal papillary mucinous neoplasm | Optical Coherence tomography, blood samples | How well ultra-high-resolution optical coherence tomography works to detect micrometer-sized early-stage pancreatic cancer in participants with pancreatic cancer. | NCT03711890 | Not yet recruiting & Not applicable |
| 39 | Prostatic neoplasms | Drug: 18F- dcfpyl PET/CT, | Identify the sensitivity and specificity of 18F-dcfpyl PET/CT, basis, and characterize ctdna and exosome. | NCT03824275 | Recruiting & Phase 2, Phase 3 |
| 40 | Prostate Cancer | Urine and serum exosomes | Investigate the relationship between urinary exosome and the aggressiveness of prostate cancer. | NCT03911999 | Recruiting & Not recruiting |
| 41 | Triple-negative breast cancer | Merck 3475 Pembrolizumab, intraoperative radiation therapy (IORT), serum exosomes | Assess response to pembrolizumab in both primary tumor, normal breast stroma, circulating lymphocytes, and serum exosomes | NCT02977468 | Recruiting & Phase 1 |
| 42 | Renal fibrosis, Kidney transplant failure | Kidney transplantation urine exosomes | Urinary exosomes and the degree of graft fibrosis to determine biomarkers | NCT03870542 | Recruiting & Not applicable |
| 43 | No small lung cancer | Blood and serum sample, high-dose radiotherapy, cisplatin-doublet therapy, and radiotherapy | Markers (molecular and immunological) of ICD or anti-tumor immunity (exosomal or molecular) can be detected in the serum | NCT02921854 | Completed & not applicable |
| 44 | Thyroid cancer | Urine exosome protein biomarker | Evaluate new therapeutic mechanism and medications for poorly differentiated or anaplastic thyroid cancer. | NCT02862470 | Active, not recruiting & Not applicable |
| 45 | Oncology | Interstitial tissue fluid of pancreatic cancer site | A short OMICS analysis of PDAC (all stages confounded) uses a "modified EXPEL" procedure. | NCT03791073 | Recruiting & Not applicable |
| 46 | Lymphoma, T-Cell | MK-3475, Copanlisib, Blood samples, peripheral blood lymphocytes PD-1 expression, peripheral blood T-cell and NK-cell | PD-1 and PD-L1 expression on tumor tissue; tumor-infiltrating lymphocytes and gene expression as prognostic and predictive biomarkers. | NCT02535247 | Recruiting & Phase 1, Phase 2 |
| 47 | Ovarian cancer ovarian neoplasms | Blood samples | To see if monocytes taken from the blood of people with ovarian cancer can kill tumor cells. | NCT02063464 | Completed & Not applicable |
| 48 | Lung cancer | Blood samples | Diagnosis stages I-IV lung cancer drug efficacy, surgical effect evaluation, recurrence monitoring, prognosis judgment, medication guidance, and molecular classification differentiation via analyzing blood ctDNA. | NCT03317080 | Recruiting & Not applicable |
| 49 | Carcinoma, Hepatocellular, kidney and Colorectal neoplasms,melanoma, | Blood samples collected before, after, and during radiotherapy, Blood sample exosome | This study will follow-up immune cell populations secreted factors and released nanovesicles in the blood back, during, and after high dose radiation therapy. | NCT02439008 | Terminated & Not applicable |
| 50 | Thyroid cancer | Metformin, hydrochloride, radioactive Iodine, placebo, saliva, and serum samples | Metformin hydrochloride works against radioactive iodine treatment of differentiated thyroid cancer. | NCT03109847 | Recruiting & Phase 2 |
| 51 | Prostate cancer | Whole-body MRI, blood exosomes | Compare diagnostic concordance of whole body multi‐parametric Magnetic Resonance Imaging (MRI) with current conventional multi-modality reference standard imaging | NCT02935816 | Unknown & Not applicable |
| 52 | Pancreatic neoplasms | Diagnostic Test: MRI/MRCP, serum, and blood | MRI/MRCP to screen for early stage pancreatic cancer or precursor lesions by analyzing blood samples from pancreatic cancer | NCT03250078 | Recruiting & Not applicable |
| 53 | Prostate cancer | Urine sample | Validate the performance characteristics of the mir Scientific Sentinel™ CS test and mir Scientific Sentinel™ PCA test. | NCT04100811 | Not yet recruiting & Not applicable |
| 54 | Pancreatic cancer, Pancreatic diseases, Pancreatitis | Blood draw, cyst fluid, tumor tissue collection, functional DNA repair assays | Blood samples were analyzed for various biomarkers. First biomarkers like proteins and proteases, exosomes, stromal elements, circular RNAs, and circulating tumor DNA | NCT03334708 | Recruiting & Not applicable |
| 55 | Lip, oral cavity squamous pharynx, larynx, squamous arcinoma | Nivolumab, IDO1 Inhibitor BMS986205, therapeutic conventional surgery | Change in exosome composition and abundance in the peripheral blood | NCT03854032 | Recruiting & Phase 2 |
| 56 | Metastatic colorectal cancer | Toripalimab, stereotactic body radiotherapy | Use of stereotactic body radiation therapy in combination with ICI in colorectal cancer patients with oligometastatic | NCT03927898 | Recruiting & Phase 2 |
| 57 | Soft tissue sarcoma | MDM2, AMG-232, radiation therapy | Side effects of MDM2 inhibitor AMG-232 and radiation therapy in treating patients with soft tissue sarcoma. | NCT03217266 | Recruiting & Phase 1 |
| 58 | Non-small cell lung cancer | Olmutinib | Evaluate the efficacy of Olmutinib (Olita®) in patients with T790M-positive non-small cell lung cancer confirmed using DNA extracted from the exosomes in bronchoalveolar lavage fluid. | NCT03228277 | Completed & Phase 2 |
| 59 | Recurrent inflammatory breast carcinoma, HER2/neu negative, stage IV breast cancer, stage IV | Ipilimumab, laboratory biomarker analysis, Nivolumab | ctDNA and immune signature assessed by exosome analysis in blood samples. | NCT02892734 | Terminated Has result & Phase 2 |
| 60 | Renal cell cancer | Urine and serum samples | Metastatic renal cell cancer (RCC) treatment uses five kinase inhibitors sunitinib, everolimus, temsirolimus, sorafenib, and pazopanib, which are now approved for clinical application. | NCT02071719 | Terminated & Not applicable |
| 61 | Advanced solid tumor, Advanced/m etastatic colorectal cancer | Drug: AL3810 | Study of personalized medicine evaluation system establishment for liver cancer, gastric cancer, and nasopharynx cancer. | NCT03260179 | Phase 1 |
| 62 | Prostate cancer | Drug: steroids switch, Blood samples exosomes | The change of prednisone to dexamethasone in CRPC patients that progress biochemically to AA + prednisone can improve. | NCT02928432 | Completed & Phase 2 |
| 63 | Recurrent lung non-small cell carcinoma, stage II, IIA, IIB, IIIA & IIIB Cancer AJCC v7 | Image-guided radiation therapy, intensity-modulated radiation therapy, laboratory biomarker analysis | Radiation dose is delivered on the body, pass into the tumor, and through the body. | NCT01629498 | Recruiting & Phase 1, Phase 2 |
| 64 | Prostate cancer | Samples with DNA, human tissue, body fluids, and fresh blood tissue | This study is to collect healthy and cancerous tissues. | NCT00578240 | Active, not recruiting & Not applicable |
| 65 | Metastatic triple-negative breast carcinoma, stage IV breast cancer AJCC v6 and v7 | Enobosarm, laboratory biomarker analysis, Pembrolizumab | Giving pembrolizumab and enobosarm may work better than pembrolizumab alone in treating androgen receptor-positive triple-negative breast cancer. | NCT02971761 | Active, not recruiting & Phase 2 |
| 66 | Tumors refractory solid tumors, cancer, neoplasms, recurrent solid tumors | IT-141 | Deliver more drugs via exosomes to the tumor with reduced toxicity on healthy tissues. | NCT03096340 | Recruiting & Phase 1 |
| 67 | Pancreatic cancer, pancreatic resectable & pancreatic ductal adenocarcin oma. | Ascorbic acid, paclitaxel protein-bound, cisplatin, gemcitabine | Combination of paclitaxel protein-bound (also known as nab-paclitaxel), gemcitabine, and cisplatin, effective in individuals with untreated metastatic pancreatic cancer. | NCT03410030 | Recruiting & Phase 1, Phase 2 |
| 68 | Prostate cancer, obesity | Robotic radical prostatectomy | How fat cells communicate with prostate cancer cells to look at how exosomes communication. | NCT04167722 | Recruiting & Not applicable |
| 69 | Recessive dystrophic epidermolysi s bullosa | Drug: Rigosertib Sodium Other: Quality-of-life assessment | How rigosertib sodium works in treating patients with recessive dystrophic epidermolysis bullosa (RDEB) with locally advanced squamous cell carcinoma. | NCT04177498 | Not yet recruiting & Early Phase 1 |
| 700 | Rectal neoplasm, malignant Carcinoma, adenocarcin oma | Radiation, capecitabine-Irinotecan combination, plasma exosome | Research on Biomarkers for Predicting the efficacy and toxicities of Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer Based on Tissue and Plasma Exosomal RNA | NCT04227886 | Recruiting & Not applicable |
| 71 | HER2-positive breast cancer | Acquisition of blood samples and tumor tissue samples (biopsies) | Blood tests for anti-HER2 treatments, instead of invasive tissue biopsies | NCT04288141 | Recruiting & Not applicable |
| 72 | Lung cancer | Blood exosome | Molecular and cellular biomarkers (exosomes antigens, circulating tumor cells -, the panel of mutations in circulating free DNA), and radiomic signature are complementary to assist early detection of lung cancer LDCT. | NCT04315753 | Recruiting & Not applicable |
| 73 | Prostate cancer obesity | Exosome from biological samples | The investigators will be collecting prostate and fat tissue from participants undergoing radical prostatectomy to culture and study in the laboratory. | NCT04167722 | Recruiting & Not applicable |
| 74 | Metastasis breast cancer genomic analysis | Tissue and Blood samples | Metastatic breast cancer with genetic tests including WES, RNAseq, ctDNA, and exosomes | NCT04258735 | Recruiting & not applicable |
| 75 | Prostate cancer | Liquid biopsies sample | Correlation of the exodx Prostate test results with the outcome of prostate biopsies. | NCT04357717 | Recruiting & Not applicable |
| 76 | Lung cancer | Exosome antigen analysis | Molecular and cellular biomarkers (exosomes, protein signatures, circulating tumor cells - CTCs, microRNA) | NCT04323579 | Recruiting & Not applicable |
| 77 | Cancer | Distress exosome from patients | Benefits of these psychological interventions on changes in exosomes | NCT04298398 | Not yet recruiting & Not applicable |
| 78 | Prostate cancer | Urine and semen biomarkers | Perform urinary and seminal genome, exosomes, methylome, and transcriptome analysis to identify novel molecular signatures associated with prostate cancer imaging endotypes | NCT04340245 | Not yet recruiting & Not applicable |
| 79 | Colorectal cancer | Blood sample exosomes | Exosome protein marker for the diagnostic tool | NCT04394572 | Not yet recruited & Not applicable |
| 80 | Lung cancer | Blood and serum sample | The exosome is a liquid biopsies diagnosis tool for lung cancer | NCT04529915 | Active, not recruiting |
| 81 | Untreated advanced NSCLC patients | Plasma exosomes | Performance of exosomes loaded with EML4-ALK fusion in NSCLC diagnosis. | NCT04499794 | Recruiting, |
| 82 | Squamous cell carcinoma of the head and neck | Serum, cell fluid exosomes | Use of the hemopurifier to clear immunosuppressive exosomes in combination with pembrolizumab (Keytruda) | NCT04453046 | Recruiting, not applicable |
| 83 | NSCLC patients | Plasma exosomes, pabolizumab, nafulizumab. | Plasma exosomes PD-L1 and mRNA as a biomarker after therapeutic efficacy against NSCLC. | NCT04427475 | Recruiting, not applicable |
| 84 | Lung cancer | Blood exosomes | Blood exosomes analysis to determine hypoxia as a potential biomarkers for early detection. | NCT04629079 | Recruiting, not applicable |
| 85 | Breast, digestive, gynecologic cancer circulating, tumor DNA, exosomes | Blood samples | Blood exosome as an early biomarkers for digestive and gynecological/ breast cancer. | NCT04530890 | Not yet recruiting and not applicable |
| 86 | Prostate cancer | Blood sample | Blood exosome biomarker for early detection. | NCT04556916 | Not yet recruiting and not applicable |
| 87 | Pancreas adenocarcin oma | Venous sampling | Tumor cells secret exosome and long RNA, small RNA, miRNA, tRNA, piRNA analysis as a biomarker. | NCT03711890 | Recruiting and not applicable |
Other examples include a lung cancer study (NCT03830619), prostate cancer biomarker correlation study (NCT03911999), renal fibrosis study based on urine exosome biomarkers (NCT03870542), non-small lung cancer biomarker study for early detection (NCT02921854), and a colorectal cancer biomarker study using blood exosome samples (NCT04394572). These clinical studies have been in multiple phases, and success in these studies will accelerate current cancer treatment many folds. Another cancer research topic uses exosomes in a clinical study to deliver and measure certain drugs effectiveness in cancer treatment. For example, a review of MK-3475 (Pembrolizumab) on the triple-negative breast tumor microenvironment analyzing both primary tumor, normal breast stroma, circulating lymphocytes, and serum exosomes are in phase 1 (NCT02535247). Another study on NK and T-cell Non-Hodgkin lymphoma, using MK-3475 alone or in combination with copanlisib, analyzes PD-1 expression of peripheral blood lymphoma and T-cell exosomes in phases 1 & 2 (NCT02535247). Another study on thyroid cancer (NCT03109847) targets the side effects of radioactive iodine treatment of differentiated thyroid cancer, aiming to mitigate them using Metformin hydrochloride validated by serum and saliva exosomes in phase 2. Another exciting research study is based on patients with stage II-IV squamous cell cancer of the head and neck using Nivolumab and BMS986205, designed to analyze the abundance of exosomes and composition in the peripheral blood for identifying exosomal biomarkers. This study is in phase 2 with clinical identification number NCT03854032. The last review we will discuss here is colorectal cancer patients with oligometastasis. The study used Ripalimab plus stereotactic body radiotherapy for clinical therapeutic intervention. It is also a phase 2 study and identification number NCT03927898396. Other drug analysis studies based on exosome applications include a study on MDM2 inhibitor AMG-232 treating soft tissue sarcoma (NCT03217266) and another on evaluating the efficacy of Olmutinib using DNA extracted from exosomes of bronchoalveolar lavage fluid on T790M-positive non-small cell lung cancer (NCT03228277). These drug efficiency studies using exosomes give the researcher a suitable and versatile option for treatment. Most of these drug-testing studies are either in phase 1 or 2 and show promising data. Additional exciting applications of exosomes are in vaccines and cancer imaging. In the previous section, we discussed exosomes’ immune modulation capabilities and anti-tumor properties. We found some studies of exosomes for use in vaccine applications. One study used tumor antigen-loaded dendritic cell-derived exosomes as vaccination candidates for non-small cell lung cancer immunotherapy. This is a phase 2 study with successful phase 1 data on lung cancer patients and identification number NCT01159288. In the case of imaging and early detection, exosomes also play a promising role. For example, a study combined CT and exosome diagnosis in early lung cancer and found exosomal micro-A was highly expressed in early stage lung cancer tissues and was significantly higher than paracancerous tissues (NCT03542253). The subsequent study dealt with metastatic, castrate-resistant prostate cancer using the detection of ARv7 in the plasma through blood sample analysis (NCT03236688). Another study used ultra-high-resolution optical coherence tomography in detecting micrometer-sized early-stage pancreatic cancer using urine and serum exosomes (NCT03911999). We found 87 clinical trials on cancer biomarker studies, immunotherapies, or combination therapies. Unfortunately, we do not have access to finished clinical trial data that are not published publicly. The number and outcome of publicly available clinical trials clinical trials are promising. Exosome-based cancer immunotherapies show very promising outcomes that can translate as clinically applicable products in the near future.
6.2. Clinical Trials Addressing Brain and Inflammation Diseases:
In a brain disease and inflammation study, we found a total of 19 clinical study designs (Table 2). Penetrating the BBB and delivering drugs or active biologics is an interesting are for more more investigation because of the unique challenges. From our prior discussion, exosomes are suitable candidates for detection and drug delivery for brain diseases. For example, MSC-derived exosomes enriched with miR-124 are used in treating cerebrovascular disorders (NCT03384433). A previous study on MSC-derived exosomes shows promising data on wound healing, cell-free therapy against lung fibrosis, and skeleton muscle regeneration397,398. The next promising study is a biomarker study on Parkinson’s disease. The study identifies LRRK2 and other novel exosomal protein expressions utilizing exosome biomarker screening (NCT01860118). Another study on Parkinson’s disease estimates the prevalence of ARMD in a sample of Parkinson’s patients and identifies the correlation between L-DOPA treatment and ARMD (NCT01860118). In an Alzheimer’s disease study, curcumin’s benefits due to the inhibition of several potential disease pathways in Alzheimer’s disease and exosomes are employed in analyzing potential therapeutic applications (NCT01811381). In this trial, the investigator looks for specific blood biomarker changes due to curcumin and yoga’s combined effect. Another Alzheimer’s disease therapeutic application is allogenic adipose MSC-exosome safety and efficacy in Alzheimer’s patients with mild to moderate dementia for improving cognitive function (NCT04388982). Investigators also look for abnormal kidney and liver function due to exosomal treatment. The next study in Table 2 focuses on using exosomes to enhance the delivery of anti-inflammatory agents and growth factors to targets by using focused transcranial ultrasound for neuralga before intravenous infusion of exosomes (NCT04202783). In this trial, the investigator utilizes a brief pain inventory (BPI) scale to measure the pain due to transcranial ultrasound treatment399,400. For cancer immunotherapy of malignant glioma neoplasms, a comparative phase 1 study with conventional treatment and a boost with immunotherapy used brain cancer-derived exosomes (NCT01550523). Scientists isolate the patient’s tumor cells and treat them with an anti-sense molecule (IGF-1R/AS ODN) to remove a targeted tumor receptor on the tumor cells’ surface. After re-implanting the treated cells in the same patients, tumor cells activated apoptosis and released exosome-carrying tumor antigens. Due to antigen release, T cells activated to eliminate the tumor. By training our immune system to recognize tumors in the future, the patient will be protected from another tumor invasion via immune surveillance401,402. Pre-clinical data reveal that exosomes can cross the BBB and deliver payload within the brain. Thus, exosomes have the immense possibility of overcoming the therapeutic drawbacks of brain-related diseases.
Table 2:
Clinical application of exosomes in Brain related diseases research
| No | Disease and Conditions | Interventions and Exosome source | Therapeutic used in the study | Trial Identification Number | Status & Clinical Phase |
|---|---|---|---|---|---|
| 1 | Cerebrovascular disorders | Mesenchymal stem cell exosomes | MSC derived exosomes enriched by miR-124 | NCT03384433 | Not yet recruiting & Phase1, Phase 2 |
| 2 | Lymphoma, B-cell, aggressive non-hodgkin (B-NHL) | Blood samples | Exosome carries therapeutic targets (CD20, PDL-1) and could act as “decoy-receptors” for immunotherapy and identify aggressive B-NHL. | NCT03985696 | Recruiting & Not applicable |
| 3 | Parkinson’s disease (PD), LRRK2 kinase inhibitor sunitinib | PD patients and controls proteomes | Exosome proteomes derived from PD patients versus controls, if LRRK2 expression and phosphorylation are significantly lowered in exosomes of treated with the potent LRRK2 kinase inhibitor sunitinib. | NCT01860118 | Completed & Not applicable |
| 4 | Macular degeneration, senile | Optical coherence tomography, Color retinography, Fundus autofluorescence imaging | The L-Dopa regulates the cell’s exosomal and endosomal pathways, decreases the RPE’s exosome release significantly. | NCT02863640 | Terminated & Not applicable |
| 5 | Parkinson disease, age-related macular degeneration | Optical coherence tomography, Fundus autofluorescence imaging | Estimate the prevalence of ARMD in Parkinson’s Patients and explore a possible causal link between L-DOPA treatment and ARMD. | NCT03415984 | Completed & Not applicable |
| 6 | Malignant glioma of brain | IGF-1R/AS ODN, bio-diffusion chamber | Compared to traditional treatment alternatives for tumor recurrence, including a boost of further radiation and more chemotherapy. | NCT01550523 | Completed & Phase 1 |
| 7 | Malignant glioma neoplasms | Drug: IGF-1R/AS ODN | Within 24 hours of craniotomy, implanted for 48 hours, surgery with tissue harvest and implantation of 20 diffusion chambers in the rectus sheath with IGF-1R/AS ODN, | NCT02507583 | Active, not recruiting & Phase 1 |
| 8 | Healthy subjects, systemic autoimmune diseases | Exosomes from plasma and urine sample | To constitute a Healthy Volunteers cohort to compare with systemic autoimmune diseases cohort into molecular clusters instead of clinical entities by determining molecular profiles using several “Omics” techniques. | NCT02890147 | Completed & Not applicable |
| 9 | NCT02890134 | Unknown & Not applicable | |||
| 10 | NCT02890121 | Completed & Not applicable | |||
| 11 | Mild cognitive impairment, neurocognitive disorder, vascular dementia, alzheimer, dementia, age-related cognitive decline | Neurocognitive battery, EEG with event-related potential (ERP), Amyloid PET CT, blood, MRI, blood samples | study of older HK Chinese adults with cognitive impairment, with subjective cognitive decline and mild cognitive impairment. | NCT03275363 | Recruiting & Not applicable |
| 12 | Mild cognitive impairment | Curcumin, behavioral: aerobic yoga, non-aerobic yoga, Placebo | Study the clinical benefits of curcumin, inhibit several potential disease pathways in Alzheimer’s diseases, and determine how physical exercise programs impact individuals with early memory problems. | NCT01811381 | Recruiting & Not applicable |
| 13 | Relapsing multiple sclerosis | Blood sample | Auto-reactive T lymphocytes an early hallmark of MS, a link inflammation and neurodegeneration in a complex and inter-regulated circuit and the presence of a link between metabolism and immune responses. | NCT04121065 | Not yet recruiting & Not applicable |
| 14 | Neuralgia | Exosome analysis, focused transcranial ultrasound | Exosomes loaded anti-inflammatory and growth factor targeted delivery | NCT04202783 | Recruiting & Not applicable |
| 15 | Refractory depression, anxiety disorders, neurodegenerati ve diseases | Exosomes | Safety and efficacy of exosome deployment with concurrent transcranial ultrasound. | NCT04202770 | Recruiting & Not applicable |
| 16 | Multiple organ dysfunction syndrome | MSC derived exosomes | Safety and efficacy of exosomal of MSC | NCT04356300 | Not yet recruited & Phase 1, Phase 2 |
| 17 | Long-term memory decline, mild cognitive impairment | Neural exosomes | Identify changes in neuronally derived exosome levels induced by training | NCT04253587 | Not yet Recruiting & Not applicable |
| 18 | Alzheimer’s disease | Allogenic adipose mesenchymal stem cells exosome | Low/mild & high MSCs-Exos administrated for nasal drip Dosage | NCT04388982 | Not yet recruited & Phase 1& 2 |
6.3. Clinical Trials of Immune, Heart, Lung, Diabetes, Kidney, and Blood Diseases:
In table 3, we compiled multiple disease applications of exosomes either as biomarkers, diagnostics, therapeutics, or vaccine applications. We found a total of 100 clinical studies on type-2 diabetes, cardiovascular research, kidney, lung, heart diseases, ulcers, hypertension, etc. Here we will describe some essential studies to give a concise description of the clinical research. For example, for ulcer patients, investigators utilize plasma-derived exosomes on cutaneous wound healing (NCT02565264). In this trial’s pre-clinical study, scientists found serum-derived exosomes accelerate cutaneous wound healing in the BALB/c mice model. Scientists conclude from that study that exosome supplements to cutaneous ulcer diseases like peripheral arterial disease, decubitus, or burns have a significant therapeutic effect, and serum exosomes that will be collected from the patient’s own body will have more acceptance as a therapeutic403,404. The following study is on umbilical cord-blood MSC derived-exosomes on β-cells masses in type I diabetes mellitus (NCT02138331), now in phase 2 &3. Authors conclude that cell-free umbilical cord-blood derived MSC exosomes may improve inflammatory state and enhance β-cell mass of the pancreases along with glycemic control405. In the pre-clinical study of the trial, the authors observed that transplantation of MSC correlates an increase in T regulatory cells and both local and systemic reduction of autoaggressive T cell populations, i.e., the shift of cytokine profile from pro-inflammatory to anti-inflammatory type. Furthermore, MSC transplantation increases local pancreatic cell number and increases circulating epidermal growth factor (EGF). EGF lowers blood glucose and increases insulin secretion406. In both a type1 and type 2 diabetes mellitus study, circulating exosomes from β-cells were analyzed for biomarkers and therapeutic targets407–410. There is a study on preeclampsia, where the exosomes of peripheral blood will be compared to umbilical cord mesenchymal stem cells to identify miRNAs 136, 494, and 495 gene expression (NCT03562715, Table 3, No. 14). This specific trial is an example of biomarker screening utilizing the exosome profile. Recent data suggests that exosomes released from the placenta carry specific cargo responsible for causing preeclampsia. Isolating exosomes from the placenta and maternal blood and analyzing their biochemical and molecular mechanisms can provide important insight into the novel therapeutic intervention of preeclampsia associated with cardiovascular disease in normal and complicated pregnancies411–414. In another study based on exosomes, chronic kidney failure treatment is conducted using hemodiafiltration (OL-HDF) and by analyzing mRNA expression of serum exosomes (NCT03202212). MicroRNA content analysis of exosomes using RT-qPCR will give insight for any inflammatory markers due to treatment against chronic kidney disease. Obesity is another significant health care burden. Exosomes have also been studied for the development of a potential obesity treatment415. In the current study, to determine how meal timing affects these endpoints differentially during the daytime and nighttime, urine exosomes will be analyzed in 12-hour bins (NCT03459703, Table 3, No. 30). In this study, investigators will evaluate how mealtime influence obesity by conducting multiple behavioral studies like mood state, retention, depression, loneliness, appetite, adherence, urine content analysis (oxalate, sodium, potassium, creatinine, nitric oxide, albumin, nephrin, KIM-1) and urine exosomeal mRNA and microRNA content416,417. In a COPD study, exosome expression alteration was analyzed on epigenetic, mRNA, miRNA content using RT-qPCR and exosome profiling (NCT03049202 & NCT04183530, Table 3, Nos. 34 and 63). In both trials, the authors utilize exosomes from saliva, serum, urine, blood, and stool sources for biomarker analysis.
Table 3:
Exosome clinical study application on different diseases model other than brain and cancer
| No | Disease and Conditions | Interventions and Exosome source | Therapeutic used in the study | Trial Identification Number | Status & Clinical Phase |
|---|---|---|---|---|---|
| 1 | Ulcer | Plasma-derived exosomes | The objective is to evaluate the effect of autologous exosome rich plasma on cutaneous wound healing | NCT02565264 | Enrolling by invitation & Early Phase 1 |
| 2 | Myocardial infarction | Exosomes in peripheral blood of patients | Expression of miRNA with healthy volunteers, explore its relationship with the development of myocardial infarction | NCT04127591 | Not yet recruiting, & Not applicable |
| 3 | Sepsis | Drug: Antibiotics, Blood exosomes | To compare peripheral blood dendritic cell-derived exosome changes in patients with sepsis with healthy controls. | NCT02957279 | Unknown & Not applicable |
| 4 | Polycystic ovary syndrome | Ginger exosomes, Aloe exosomes placebo | Ginger or aloe plants exosomes will treat and improve the condition of polycystic ovary syndrome | NCT03493984 | Recruiting & Not applicable |
| 5 | Preeclampsia, cardiovascular disease | Exosomes from maternal blood and placental tissue in patients diagnosed with preeclampsia | The functional role of exosomal cargo in normal and pathological pregnancies and point towards novel therapeutic intervention strategies. | NCT04154332 | Not yet recruiting & Not applicable |
| 6 | Diabetes mellitus type 1 | Mesenchymal stem cells exosomes | Intravenous infusion of cell-free umbilical cord-blood derived MSC exosomes may reduce the inflammatory state and improve the β-cell mass. | NCT02138331 | Unknown & Phase 2, Phase 3 |
| 7 | Atrial fibrillation | Epicardial fat biopsy | Investigates the role of epicardial fat-derived exosomes in patients who suffer from atrial fibrillation. | NCT03478410 | Recruiting & Not applicable |
| 8 | Kidney transplantation | Urinary exosomes | Prevalence of NCC activation three months after transplantation inpatient treated by CNI. | NCT03503461 | Completed & Not applicable |
| 9 | Healthy | High salt diet followed by a low salt diet and vice versa | Changes in the epithelial sodium channel (ENaC) of the kidney are reflected in the urinary exosomes, | NCT02823613 | Active, not recruiting & Not applicable |
| 10 | Hemodynamic instability autophagy | Blood and urine specimens | Exosomes purified in blood and urine and proteomics studies to analyze autophagy and apoptosis-related biomarkers of exosomes by bioinformatics. | NCT03267160 | Active, not recruiting & Not applicable |
| 11 | Macular holes | Exosomes derived from mesenchymal stem cells from the human umbilical cord | Exosome isolation sequential ultracentrifugation confirmed via spectral-domain optical coherence tomography (OCT) and the minimum linear diameter (MLD). | NCT03437759 | Recruiting & Early Phase 1 |
| 12 | Blood coagulation, platelet function | Exosomes from red blood cells | Analyze the effect red blood cell exosomes units have on blood coagulation and platelet function. | NCT02594345 | Completed & Not applicable |
| 13 | Healthy | Erythropoietin, placebo | The diagnostic value of differentially regulated exosome proteins could be further validated against the existing IEF EPO WADA accredited tests. | NCT03700515 | Recruiting & Not applicable |
| 14 | Preeclampsia | Umbilical cord mesenchymal stem cell exosomes | To identify miRNAs 136, 494, and 495 genes expression in the exosomes between peripheral blood, andumbilical cord mesenchymal stem cells. | NCT03562715 | Completed & Not applicable |
| 15 | Sepsis with multiple organ dysfunction (MOD) | Exosomes from macrophage co-culture with human cells, blood, and urine. | Proteomics studies in exosomes from cell culture and clinical specimens. analyze ubiquitination, autophagic, and apoptosis-related biomarkers of exosomes by bioinformatics. | NCT03222986 | Recruiting & Not applicable |
| 16 | Prehypertension | Urine exosomes | To characterize changes in urine electrolytes and exosome protein. | NCT04142138 | Not recruiting yet & Not applicable |
| 17 | Exercise physiology | Age, Genes, Training, Tickborne Disease, and Endurance | Investigate potential relationships between age, training intensity, training volume, genes, exosomes, and history of tick-borne disease and physiological variables and endurance performance. | NCT03569566 | Enrolling by invitation & Not applicable |
| 18 | Overweight children with type 2 diabetes risk | Exosomes from blood samples. | microRNA profiling in circulating exosomes and in blood peripheral mononuclear cells in pre-adolescents with high risk to develop T2D. | NCT03027726 | Completed & Not applicable |
| 19 | Diabetic retinopathy (DR) | Hematological examination, ophthalmic examination. Serum exosomes | Significant associations between DR progression and different exosomal miRNA using various statistical methods. | NCT03264976 | Not recruiting yet & Not applicable |
| 20 | Spinal disease | Urine & blood exosomes | Exosomes have the potential of being simple biomarkers that can diagnose postoperative delirium and predict cognitive decline. | NCT04120272 | Not yet recruiting & Not applicable |
| 21 | CKDu, arterial stiffness | Arterial stiffness assessment, serum, and urine biomarker | To characterize their disease profile using analysis serum and renal urine biomarkers, exosomes, proteomics, and DNA adducts. | NCT02226055 | Completed & Not applicable |
| 22 | Normal cellular metabolism | Somatostatin glucagon, exosome derived from the arterial-venous supply of tissues. | Study the exosomes derived from the arterial-venous supply of tissues related to the TCA cycle activity. | NCT02748369 | Active, not recruiting & Phase 1 |
| 23 | Hypertension | Urine samples | The study is to determine the concentrations and variabilities of urinary exosomal sodium channels and plasma angiotensins. | NCT03034265 | Completed & Not applicable |
| 24 | Chronic kidney failure, dialysis related complication | Mixed online hemodiafiltration, High flux bicarbonate dialysis, plasma exosomes | Quantitative micro-RNA changes in plasmatic exosome/microvesicles assessed by quantitative real-time PCR, Quantitative changes in C-Reactive Protein, Neutrophil Gelatinase associated Lipocalin, Interleukin-6, Ferritin. | NCT03202212 | Completed & Phase 1, Phase 2 |
| 25 | Uveitis, vasculitis, ocular inflammatory disease | Optic fluid, exosomes present in vitreous and AC fluid in the eye | Kinds of cytokines, lymphokines, biomarkers, proteome, and exosomes present in vitreous and AC fluid in the eye with uveitis or other retinal diseases. | NCT00331331 | Completed & Not applicable |
| 26 | Port-wine stain | Biopsy sample from Port Wine Stain Birthmark, Blood exosomes | Blood samples to characterize exosomes and metabolites from Port Wine Stain. | NCT02051101 | Active, Not recruiting & Not applicable |
| 27 | Diabetes mellitus, type 1 diabetes diabetes. | Insulin deprivation in type 1 diabetic patients, exosomes blood | Transient insulin deprivation in adolescents and T1DM adults alter the circulating blood and metabolome exosome contents | NCT03392441 | Active, not recruiting & Not applicable |
| 28 | Type1 diabetes mellitus, type2 diabetes, | Human blood samples, beta-cell exosomes | In this study, beta-cell derived exosomes will be detected and characterized in human blood samples. | NCT03106246 | Unknown |
| 29 | Sepsis | Blood & serum exosome | miRNA expression levels in exosomes, serum, and blood cells. | NCT03280576 | Complete & Not applicable |
| 30 | Obesity | Early time-restricted feeding, structured weight loss program, urine exosome | Urine exosomes will be analyzed in 12-hour bins to determine how meal timing affects these endpoints differentially during the daytime and nighttime. | NCT03459703 | Recruiting & Not applicable |
| 31 | Barretťs esophagus, gastroesophageal reflux, esophageal adenocarcinoma | Blood exosomes | Measure for a biomarker called microRNA (miRNA) using exosomes. | NCT02464930 | Unknown & Not applicable |
| 32 | Childhood chronic kidney disease | Urine exosomes | Molecular value (ADMA & urine exosome miRNA), | NCT03227055 | Recruiting & Not applicable |
| 33 | Chronic Ulcer | Conditioned media, opical Antibiotic Combinations, Exosome from cell medium | Study to see the therapeutic potentials of Conditioned Medium Stem Cell as an additional growth factor in chronic skin ulcer healing and to compare the success of chronic ulcer healing | NCT04134676 | Not yet recruiting & Phase 1 |
| 34 | COPD, emphysema chronic bronchitis airway obstruction smoking, tobacco, gender | Exosomes from lung cells. | Alterations at the epigenetic, mRNA, microRNA, proteome, metabolome, and microbiome level will perform from multiple lung compartments | NCT03049202 | Recruiting & Not applicable |
| 35 | Hypertension | sodium phosphate sevelamer, sodium bicarbonate, sodium chloride Plasma and urine sample | Changes in NaPi-IIa assessed from urinary exosomes. | NCT02822131 | Completed & Not applicable |
| 36 | Thyroid disease, heart failure | Urine sample | Investigators will enroll clinical and subclinical thyroid disease with quarterly follow-up then detect urine exosomal proteins NT-proBNP. | NCT03984006 | Recruiting & Not applicable |
| 37 | Drug-resistant epilepsy | Blood sample | Expression profile of miRNAs in the plasma as well as in the exosomes. | NCT03419000 | Recruiting & Not applicable |
| 38 | Panic disorder | Serum sample | Changes in exosomal microRNAs (miRNAs) from serum samples taken before an Disorder d after CBT (PD) patients from Panic | NCT04029740 | Recruiting & Not applicable |
| 39 | Diabetes | Dual energy X-ray, adipose tissue biopsy, blood sample | New biomarkers of adult-onset autoimmune diabetes. | NCT03971955 | Recruiting & Not applicable |
| 40 | Aging, cognitive, ketones, blood sugar | Jardiance 25 mg, Plasma exosomes | The study is an increased expression of receptors and mediators of ketone metabolism in plasma exosomes. | NCT03852901 | Recruiting & Phase 1 |
| 41 | Diabetes mellitus, type 2 diabetes, cardiovascular diseases | Dapagliflozin 10 mg, Saxagliptin 5 mg, Urine exosomes | In addition to Dapagliflozin (additive effect), Saxagliptin may improve EPC number and function even more than Dapa alone, compared to placebo. | NCT03660683 | Recruiting & Phase 4 |
| 42 | Insulin resistance | Rosiglitazone versus placebo, response to amiloride infusion & furosemide infusion, Urine exosomes | The difference in the ENac abundance in exosomes in the urine measured after eight weeks of treatment with either rosiglitazone or placebo. | NCT00285805 | Completed & Not applicable |
| 43 | Childhood obesity, adolescent obesity | Behavioral: Exercise, Serum exosomes | Looking for circulating exosome-derived miRNA in plasma. | NCT03762629 | Recruiting & Not applicable |
| 44 | Fibrosis, kidney transplant failure, kidney allograft, and rejection | Observational (a urinary biomarker for kidney allograft fibrosis), urine exosomes | Urinary exosomes are isolated and analyzed transglutaminase type 2 | NCT03487861 | Recruiting & Not applicable |
| 45 | Obstructive sleep apnea, morbid obesity, epigenetic disorder | CPAP, bariatric surgery, blood sample, exosome mRNA | Differences in miRNA profile among patients with morbid obesity with or without OSA. | NCT03995836 | Completed & Not applicable |
| 46 | HIV infection, tuberculosis Infection | Detection of molecular biomarkers, plasma exosomes | Description of miRNA expression profile in a cohort of patients with an HIV infection and Tuberculosis and correlate it with their clinical evolution. | NCT03941210 | Recruiting & Not applicable |
| 47 | Obstructive sleep apnea of adult, hypoxia, sleep disorder, stroke, endothelial dysfunction, oxidative stress | Drug lowering cerebral blood flow (CBF) and normoxia sleep, urine exosomes, placebo, and intermittent hypoxia sleep. blood sample, | Vascular biomarkers exosome analysis or urinary prostaglandins before and after sleep under normoxia and intermittent hypoxia exposure with cerebral blood flow changes. | NCT03255408 | Not yet recruiting & Phase 1 & 2 |
| 48 | Muscular dystrophy, neuromuscular diseases, X-Linked genetic diseases, inborn | Cardiosphere-derived cell exosomes. | Evaluating the safety and efficacy of a cell therapy called CAP-1002. | NCT03406780 | Active not recruiting & Phase 2 |
| 49 | Heart Failure with Preserved Ejection Fraction | Drug: 0.9% Sodium Chloride, Furosemide 40 mg | Sodium transporters in Urinary exosomes will be characterized and compared between HFpEF patients and controls. | NCT03837470 | Recruiting & Early Phase 1 |
| 50 | Obesity, insulin resistance | Mediterranean diet, ketogenic diet, Blood and Adipose tissue exosomes | Signaling between organs and cells will be examined by isolating exosomes from blood and adipose tissue | NCT04131166 | Recruiting & Not applicable |
| 51 | Thoracic surgery, video-assisted | two-lumen catheter, chest tube, plasma sample exosomes | Diagnostic value and molecular characteristics of plasma exosome-derived miRNAs for these patients. | NCT03230019 | Recruiting & Not applicable |
| 52 | Healthy older adults ages 65–89 | Blood, CSF, and serum exosomes | Different biomarkers may relate to immune health and the aging process, the risk for cognitive decline, and Alzheimer’s disease. | NCT03944603 | Recruiting & Not applicable |
| 53 | Obesity, insulin resistance | Blood and adipose tissue exosomes | Signaling between organs and cells will be examined by isolating exosomes from blood and adipose tissue. | NCT02706262 | Recruiting & Not applicable |
| 54 | Body weight changes | Moderately high protein diet, low-fat diet, serum exosome mRNA | MicroRNAs levels in exosomes will be measured by NGS Illumina Myseq at baseline and the end of the bodyweight-loss period | NCT02737267 | Unknown & Not applicable |
| 55 | Rhinitis, allergic, perennial | Exosomes from blood, saliva, serum, and plasma | Exosome Isolation Reagent (for plasma or serum) will compare the change in exosomes before and after treatment. | NCT02653339 | Unknown & Not applicable |
| 56 | Exosome | Endometrial fluid collection, serum, blood exosomes | To describe the morphology, size distributions, and specific markers of the different vesicle’s populations present endometrial fluid (i.e., DNA, RNA, proteins, lipids) | NCT02797834 | Unknown & Not applicable |
| 57 | Non-alcoholic fatty liver disease | Tofogliflozin, glimepiride | Changes from baseline in microRNAs and exosome contents. | NCT02649465 | Recruiting & Phase 4 |
| 58 | Chordoma | Drug: Afatinib Blood samples | Pharmacokinetic study and translational studies on EGFR pathway activation and signaling on blood and tumor samples. | NCT03083678 | Recruiting & Phase 2 |
| 59 | Minimal Residual disease, recurrent acute&myelodysplasia recurrent childhood acute myeloid leukemia | Daratumumab, Donor Lymphocyte Infusion, Laboratory Biomarker Analysis | The donor lymphocytes and monoclonal antibodies, such as daratumumab, may kill the remaining cancer cells. | NCT03537599 | Recruiting & Phase 1&2 |
| 60 | Dystrophic epidermolysis bullosa | AGLE-103 is an allogeneic derived exosomes product derived from healthy donor mesenchymal stem cells (MSCs), placebo | To assess the safety and effectiveness of AGLE-103 vs. placebo on lesions in subjects with EB. | NCT04173650 | Not yet recruiting & Phase 1, Phase 2 |
| 61 | Type-1 diabetes | Circulating β-cell exosomes | To diagnose the disease and its progression in type 1 diabetes. | NCT04164966 | Not yet recruiting & Not applicable |
| 62 | Fasting | Time-restricted feeding (TRF) with dietary counseling, blood exosomes | Safety and compliance, as well as the efficacy of one specific IF intervention called time-restricted feeding. | NCT04184076 | Not yet recruiting & Phase 2 |
| 63 | COPD | Blood, stool, urine, saliva, serum exosomes | Exosome characterization of COPD patients and healthy controls. | NCT04183530 | Recruiting & Not applicable |
| 64 | Dry eye | Mesenchymal stem cells derived | Umbilical Mesenchymal Stem Cells (UMSC) derived exosomes for chronic Graft Versus Host Diseases (cGVHD) treatment | NCT04213248 | Not yet recruiting & Phase 1, Phase 2 |
| 65 | Sleep apnea, inflammation, atherosclerosis | Myeloid PTP1B expression analysis, blood exosomes | To investigate myeloid PTP1B involvement in the vascular pro-inflammatory process described in OSA. | NCT04235023 | Not yet recruiting, not applicable |
| 66 | Coronavirus | MSCs-derived exosomes | Efficacy and safety of aerosol inhalation of the exosomes derived from allogeneic adipose mesenchymal stem cells (MSCs-Exo) | NCT04276987 | Not yet recruited & Phase 1 |
| 67 | Corona infection, virus pneumonia | T-cell-derived exosomes | Safety and efficacy of this new targeted delivery by metered-dose inhaler | NCT04389385 | Active but not recruiting & Phase 1 |
| 68 | Pulmonary Nodule | Blood and alveolar lavage of lung nodules patients | Study the sensitivity, specificity, and diagnostic accuracy of ctDNA and exosome combined detection in the identification of benign and malignant pulmonary nodules | NCT04182893 | Recruiting & Not applicable |
| 69 | Periodontitis | Adipose-derived stem cells exosomes | The adipose stem cell exosomes are isolated autogenously from the patient to be injected locally into the periodontal pockets to evaluate their regenerative effect. | NCT04270006 | Recruiting & Early Phase 1 |
| 70 | Healthy control | Low level of MSCs-Exo, High level of MSCs-Exo | Safety and tolerance of aerosol inhalation of the exosomes derived from allogeneic adipose mesenchymal stem cells (MSCs-Exo) | NCT04313647 | Recruiting & Phase 1 |
| 71 | Healthy elderly | IkT-148009 & placebo, CNS-deriver exosomes | Investigates the safety tolerability movement of drug IkT-148009 in healthy elderly volunteers. | NCT04350177 | Not yet recruiting & Phase 1 |
| 72 | Heart failure | Exercise training, plasma exosome | Plasma exosomes will be isolated using microbead-based sorting techniques and characterized | NCT04334603 | Recruiting & Not applicable |
| 73 | Empagliflozin, hypoglycemic agents, sodium-glucose transporter 2 Inhibitors | Jardiance 25 mg, plasma exosomes | Determine expression of receptor and mediators of ketone metabolism in plasma exosomes. | NCT03852901 | Recruiting & Phase 1 |
| 74 | Multiple system atrophy | Blood, and plasma exosomes. | The study is to complete the target validation of insulin resistance for future treatment trials. | NCT04250493 | Not yet recruiting & Not applicable |
| 75 | Multiple organ failure | MSC exosomes | To evaluate the safety and efficacy of exosomes from MSCs to determine clinical dosage for patients with severe MODS | NCT04356300 | Not yet recruiting & Not applicable |
| 76 | Oocyte maturation | Follicular fluid exosomes | Investigate the miRNA in (follicular fluid) FF exosomes in young and aged women and their relationship to egg maturation | NCT04382872 | Not yet recruiting & Not applicable |
| 77 | Corona virus Infection, COVID-19, SARS | Organicell Flow, placebo | Safety and efficacy of intravenous infusion of Organicell Flow | NCT04384445 | Not yet recruited & Phase 1, 2 |
| 78 | Coronavirus | MSCs-derived exosomes | The efficiency of aerosol inhalation of the exosomes derived from allogeneic adipose mesenchymal stem cells (MSCs-Exo) | NCT04276987 | Complete, Phase 1 |
| 79 | Drug resistance exosomes | MPCs-derived | Evaluate the efficacy and safety of haMPC-exosome treatment with pulmonary infection caused by gram-negative bacilli resistant to carbapenems. | NCT04544215 | Recruiting, Phase 1 & 2 |
| 80 | Endothelial dysfunction, obese, OSA patients | Circulating exosome, mRNA analysis. | Evaluation of miRNA contained in exosomes | NCT04459182 | Not yet recruiting, not applicable |
| 81 | Kidney transplantation | Urine exosome analysis | Urine exosome analysis after kidney transplantation | NCT03503461 | Complete, not applicable |
| 82 | Covid19, SARS-CoV-2 pneumonia, COVID-19 | Mesenchymal stem cells exosomes | Inhalation of exosomes may reduce inflammation and damage to the lung tissue and stimulate the regenerative processes. | NCT04602442 | Enrolled by invitation, Phase 2 |
| 83 | Covid19, SARS-CoV-2 Pneumonia, COVID-19 | Mesenchymal stem cells exosomes | Inhalation of exosomes may reduce inflammation and damage to the lung tissue and stimulate the regenerative processes. | NCT04491240 | Complete, Phase 1& 2 |
| 84 | Acute respiratory distress syndrome | Human mesenchymal stem cell exosome | To evaluate allogeneic human mesenchymal stem cell exosomes (hMSC-Exos) in the treatment of acute respiratory distress syndrome | NCT04602104 | Not yet recruiting, Phase 1 &2 |
| 85 | Healthy control | Mesenchymal stem cells (MSCs) exosomes | Evaluate aerosol inhalation’s safety and tolerance of the exosomes derived from allogeneic adipose mesenchymal stem cells (MSCs-Exo) in healthy volunteers. | NCT04313647 | Recruiting, Phase 1 |
| 86 | Macular holes | Mesenchymal stem cells (MSCs) exosomes | Efficacy and safety of MSC-derived exosomes (MSC-Exos) for promoting healing of large and refractory macular holes (MHs) | NCT03437759 | Recruiting, early phase 1 |
| 87 | Blood coagulation, platelet function | Red derived exosomes blood- | To analyze the effect exosomes derived from red blood, cell units have blood coagulation and platelet function. | NCT02594345 | Complete, Not applicable |
| 88 | Metabolism, acute resistance exercise | Muscle exosomes | Exercise-induced skeletal muscle exosomes promote adipocyte lipolysis | NCT04500769 | Recruiting, not applicable |
| 89 | Allergic asthma, severe eosinophilic asthma | rEOS- and iEOS-derived exosomes | Qualitative and quantitative selected ncRNA levels in lung resident EOS- and inflammatory EOS-derived exosomes | NCT04542902 | Recruiting, Not applicable |
| 90 | Jaundice, Neonatal | Breast milk exosomes. | Analysis of breast milk exosomes miRNA for hyperbilirubinemia Neonatal | NCT04527536 | Notrecruiting yet, not applicable |
| 91 | Diabetes mellitus type 2, gestational diabetes, Overweight and obese, Pregnancy in diabetic, Insulin resistance, Insulin sensitivity, Pregnancy, High Risk | Serum plasma exosomes and | The level, content, and bioactivity of exosomes in serum and plasma Versus Insulin sensitivity | NCT04617405 | Not yet recruiting, not applicable |
| 92 | Lupus nephritis | Urine and serum exosomes | Analyze urine and serum exosome biomarkers | NCT04534647 | Recruiting, Not applicable |
| 93 | Acute myeloid leukemia | Bone marrow and peripheral blood exosomes | Analysis of exosomes and microvesicles derived from PB and bone marrow samples of AML patients | NCT04460963 | Not yet recruiting, not applicable |
| 94 | Polycystic kidney disease, autosomal dominant | Urine exosomes | Changes in polycystin-1 (PC-1) and polycystin-2 (PC-2) protein levels in urinary exosomes from baseline to Day 44 | NCT04536688 | Recruiting, Phase 1 |
| 95 | COVID-19 | Cardiosphere-derived cells (CDCs) exosomes | CDC exosome ability to be immunomodulatory, anti-fibrotic, and regenerative. | NCT04623671 | Recruiting, Phase 2 |
| 96 | Covid19 ARDS pneumonia, viral | Bone marrow-derived exosomes | Safety and efficacy of intravenous administration of bone marrow-derived exosomes | NCT04493242 | Not yet recruiting, Phase 2 |
| 97 | Non-alcoholic fatty liver disease, Metabolic syndrome, metabolically abnormal & metabolically normal obesity, obesity | Liver exosomes | To determine the specific cellular and organ system, metabolic and immunologic alterations via analyzing liver exosomes. | NCT01104220 | Recruiting, not applicable |
| 98 | SARS (Severe Acute Respiratory Syndrome) | Blood exosomes | Blood filtration using Hemopurifier and blood exosome analysis. | NCT04595903 | Not yet recruiting, not applicable |
| 99 | Systemic autoimmune diseases | Plasma and urine exosomes | Exosomes for biomarker screening, gene expression analysis | NCT02890134 | Unknown, not applicable |
| 100 | Systemic autoimmune diseases | Urine and plasma exosome | Exosomes for biomarker screening, gene expression analysis | NCT02890121 | Complete, Not applicable |
In pre-clinical studies of COPD, the authors identified either circulating exosomes content or microRNA expression (e.g., MiR-21) in analysing specific pathways related to COPD418,419. These identified markers can be used as diagnostic or therapeutic targets for novel therapeutics against COPD. In HIV and tuberculosis also, clinical trials currently underway are using the exosomal platforms. The study design analyzed changes in serum and tissue exosomal miRNA expression in HIV and tuberculosis patients for early detection for biomarkers (NCT03941210). In the current situation, COVID-19 has been spread worldwide, and still, outbreaks continue due to a lack of knowledge of its pathogen and vaccine absence. Scientists are working relentlessly to find a cure against SARS-CoV-2. We found 4 COVID-19 related clinical trials already based on the exosome platform, which confirms the exosome’s versatile application and capability.
The first one seeks to evaluate the safety and potential efficacy of Organicell flow Zofin via exosome analysis (NCT04384445). Zofin is a cellular product derived from human amniotic fluid. It contains over 300 growth factors, chemokines, cytokines, and exosomes derived from epithelial and amniotic cells. Surface marker analysis reveals the presence of exosome associated proteins CD9, CD133, CD63, and CD81, and completed sequencing revealed 102 commonly expressed miRNA. Major pro-inflammatory cytokines targeted by miRNA found in Zofin include TNF, IL-6, IL-8. Other targeted cytokines are VEGFA, IGF-1, FGF2, IL36a, CCL8, CXCL12, and IL37. Many published articles suggest that suppressing the abovementioned pro-inflammatory cytokines cascade will reduce the severity of elevated immune response420–424. The next trial is in severe patients with novel coronavirus pneumonia (NCP) to evaluate the safety and efficiency of aerosol inhalation of the exosomes derived from allogeneic adipose mesenchymal stem cells (MSCs-Exo) (NCT04276987). Human adipose MSCs derived exosomes (hASCs-Exo) can stimulate T cells in vitro and inhibit IFN-γ release and T cell proliferation. Thus hASCs-Exo can be considered as therapeutic against inflammation-related diseases425,426,427. The last trial is to test the safety and efficacy of T-cell-derived exosomes following targeted delivery by metered-dose inhaler on coronavirus patients. Investigators collect donor origin COVID-19 specific T-cells and expand them via viral peptide fragments with cytokines present. This will activate the T-cells, and stimulation will cause the release of IFN-ϒ in exosomes428–431. All these coronavirus studies are based on vaccine development clinical trials, and hopefully, we will see commercialized vaccines based on the exosome platform. We already have the coronavirus vaccines from Moderna, and Pfizer-BioNTech, and these vaccines were made available to the general public in January 2021. Third vaccine from Janssen also got emergency use authorization on February 27th, 2021. But there have been reporting of a very rare and serious blood clot in people who receive the Janssen vaccine. The Oxford COVID-19 vaccine shows a robust immune response in adults of 60–70 years old. In phase 3 studies of shows the vaccine to be 76% effective at preventing someone from COVID-19 infection. Table 3 also focuses on obesity, type 1 & 2 diabetes, obesity, cardiac disease, organ failure, hypertension, atrophy, muscle dystrophy, and an exosome study on insulin resistance application. Hopefully, this section can give a proper rationale for exosome application in disease diagnosis, biomarker screening, and therapeutic application phases.
We can see that exosomes have intrinsic advantages over traditional delivery methods, biomarker analysis, diagnosis, and medical imaging application from the three tables above. Even while some polymeric nanoparticles have been commercialized, exosomes still hold a better future in drug delivery and vaccine development. Recent studies also expect exosome-based personalized medicine for patients with cancer, neurodegenerative, and inflammatory diseases. Exosomes will answer many unknowns of the multiple conditions for which proper treatment or diagnosis is not available yet.
7. Limitation of Exosome Research in the Clinical Setting:
With substantial development of this field in the last two decades, exosomes bring us more possibilities in multiple deadly disease treatments and diagnosis. Although therapies are under development, we still don’t know its exact mechanism of biogenesis. Isolation techniques for exosomes are tedious and hard to translate to the clinical setting. We found multiple commercial exosome isolation kits available; still, we need considerable progress in this technology. Once we identify a universal isolation method, we can correlate clinical outcomes all over the world. The validation of promising findings by scientists is impossible until we have a unified isolation and characterization method in place. In our clinical review section, we found that, as of now, 205 clinical trials have been conducted based on exosome application. However, because of complexities and variations in methodologies, the reproducibility of the exosome is widely varied, which presents difficulties for interpretation of results. Therefore, we need standard operating procedures (SOPs) for exosomes isolation, storage, characterization, and analysis. With more in-depth knowledge of biogenesis and function, exosomes will open up significant opportunities in therapeutic application, and already recent studies have investigated exosomes as a biomarker and natural gene/drug delivery system. In conclusion, we need urgently an efficient and reliable isolation method to advance this research field.
8. Summary and Future Direction
Exosomes are widely disseminating and heterogeneous entities. However, exosome complexity is not thoroughly understood, especially the mechanisms responsible for sorting cargo into exosomes and releasing cargo into cells after exosome internalization. While many recent studies have focused on protein sorting in exosomes, executive functions might be associated with RNA delivery. Therefore, determining the mechanism that underlies RNA sorting in the exosomes holds excellent potential for developing various therapeutic applications. Due to their several advantages over traditional nanoparticles, exosomes are more than viable candidates for targeted drug delivery innovation. In clinical trials so far, most applications of exosomes are in their use as biomarkers. Exosomes have slowly garnered more attention in the drug delivery field due to their natural origins and the protein/lipids/receptors present on their surface. Besides, some research groups have taken this even further by working on exosome surface modification through genetic alterations, DNA tethers, etc. However, the clinical application of current exosome research is the scope of this review. These inquiries into the exosomes provide a field ripe for further innovation and exploration. In the future, more investigations will need to be conducted into both the physiological and pathological conditions and the mechanisms that interface with the release of exosomes and impairment of exosome-mediated cell-cell communication, which may prove to be the basis of a new class of personalized therapeutics.
Acknowledgment:
Dr. Nurunnabi would like to acknowledge research grant support by Lizanell and Colbert Colwell Foundation. Research reported in this publication was supported by the National Institute On Minority Health And Health Disparities of the National Institutes of Health under Award Number U54MD007592. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviation:
- Aβ
Amyloid Beta
- ABC
accelerate blood clearance
- BBB
Blood-brain barrier
- BMVEC
brain microvascular endothelial cells
- CD8
cluster of differentiation 8
- CDC
Cardiosphere-derived cells
- CRISPR
clustered regularly interspaced short palindromic repeats
- CSF
cerebrospinal fluid
- CT
Computed tomography
- CTLA-4
cytotoxic T-lymphocyte-associated protein 4
- EAAT
excitatory amino acid transporters
- EGF
epidermal growth factor
- ESCRT
endosomal pathways are associated with endosomal complex
- GPC
gel permeation chromatography
- IL-10
Interleukin 10
- ILV
Intraluminal vesicle
- MDSC
Myeloid-derived suppressor cells
- MHC-1
major histocompatibility complex class 1
- MPS
mononuclear phagocyte system
- MVBs
microvesicles
- mRNA
messenger ribonucleic acid
- miRNA
micro ribonucleic acid
- MWCO
molecular weight cut-off
- MSC
Mesenchymal stromal cell
- Nk cell
natural killer cell
- PD-L1
programmed death-ligand 1
- RES
reticuloendothelial system
- TEX
Tumor-derived exosomes
Footnotes
Conflict of Interest: Authors declare no conflict of interest.
References:
- (1).Zhang Y; Liu Y; Liu H; Tang WH Exosomes: Biogenesis, Biologic Function and Clinical Potential. Cell Biosci. 2019, 9 (1), 19. 10.1186/s13578-019-0282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Lin J; Li J; Huang B; Liu J; Chen X; Chen X-M; Xu Y-M; Huang L-F; Wang X-Z Exosomes: Novel Biomarkers for Clinical Diagnosis. Sci. World J. 2015, 2015, 657086. 10.1155/2015/657086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Lu M; Yuan S; Li S; Li L; Liu M; Wan S The Exosome-Derived Biomarker in Atherosclerosis and Its Clinical Application. J. Cardiovasc. Transl. Res. 2019, 12 (1), 68–74. 10.1007/s12265-018-9796-y. [DOI] [PubMed] [Google Scholar]
- (4).Lakhal S; Wood MJA Exosome Nanotechnology: An Emerging Paradigm Shift in Drug Delivery. BioEssays 2011, 33 (10), 737–741. 10.1002/bies.201100076. [DOI] [PubMed] [Google Scholar]
- (5).He C; Zheng S; Luo Y; Wang B Exosome Theranostics: Biology and Translational Medicine. Theranostics 2018, 8 (1), 237–255. 10.7150/thno.21945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Batrakova EV; Kim MS Using Exosomes, Naturally-Equipped Nanocarriers, for Drug Delivery. J. Control. Release 2015, 219, 396–405. 10.1016/j.jconrel.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Peng Q; Zhang S; Yang Q; Zhang T; Wei X-Q; Jiang L; Zhang C-L; Chen Q-M; Zhang Z-R; Lin Y-F Preformed Albumin Corona, a Protective Coating for Nanoparticles Based Drug Delivery System. Biomaterials 2013, 34 (33), 8521–8530. 10.1016/j.biomaterials.2013.07.102. [DOI] [PubMed] [Google Scholar]
- (8).Liechty WB; Kryscio DR; Slaughter BV; Peppas NA Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. 10.1146/annurev-chembioeng-073009-100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Jang SC; Kim OY; Yoon CM; Choi D-S; Roh T-Y; Park J; Nilsson J; Lötvall J; Kim Y-K; Gho YS Bioinspired Exosome-Mimetic Nanovesicles for Targeted Delivery of Chemotherapeutics to Malignant Tumors. ACS Nano 2013, 7 (9), 7698–7710. 10.1021/nn402232g. [DOI] [PubMed] [Google Scholar]
- (10).Jang SC; Gho YS Could Bioengineered Exosome-Mimetic Nanovesicles Be an Efficient Strategy for the Delivery of Chemotherapeutics? Nanomedicine 2014, 9 (2), 177–180. 10.2217/nnm.13.206. [DOI] [PubMed] [Google Scholar]
- (11).Doyle LM; Wang MZ Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8 (7), 727. 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Wang J; Chen D; Ho EA Challenges in the Development and Establishment of Exosome-Based Drug Delivery Systems. J. Control. Release 2020. 10.1016/j.jconrel.2020.10.020. [DOI] [PubMed] [Google Scholar]
- (13).Pan B-T; Johnstone RM Fate of the Transferrin Receptor during Maturation of Sheep Reticulocytes in Vitro: Selective Externalization of the Receptor. Cell 1983, 33 (3), 967–978. 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- (14).Trams EG; Lauter CJ; Norman Salem J; Heine U Exfoliation of Membrane Ecto-Enzymes in the Form of Micro-Vesicles. Biochim. Biophys. Acta - Biomembr. 1981, 645 (1), 63–70. 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- (15).Conlan RS; Pisano S; Oliveira MI; Ferrari M; Mendes Pinto I Exosomes as Reconfigurable Therapeutic Systems. Trends Mol. Med. 2017, 23 (7), 636–650. 10.1016/j.molmed.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Margolis L; Sadovsky Y The Biology of Extracellular Vesicles: The Known Unknowns. PLOS Biol. 2019, 17 (7), e3000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Duan P; Tan J; Miao Y; Zhang Q Potential Role of Exosomes in the Pathophysiology, Diagnosis, and Treatment of Hypoxic Diseases. Am. J. Transl. Res. 2019, 11 (3), 1184–1201. [PMC free article] [PubMed] [Google Scholar]
- (18).Maia J; Caja S; Strano Moraes MC; Couto N; Costa-Silva B Exosome-Based Cell-Cell Communication in the Tumor Microenvironment. Frontiers in Cell and Developmental Biology. 2018. 10.3389/fcell.2018.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Mittelbrunn M; Gutiérrez-Vázquez C; Villarroya-Beltri C; González S; Sánchez-Cabo F; González MÁ; Bernad A; Sánchez-Madrid F Unidirectional Transfer of MicroRNA-Loaded Exosomes from T Cells to Antigen-Presenting Cells. Nat. Commun. 2011, 2 (1), 282. 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Delcayre A; Shu H; Le Pecq J-B Dendritic Cell-Derived Exosomes in Cancer Immunotherapy: Exploiting Nature’s Antigen Delivery Pathway. Expert Rev. Anticancer Ther. 2005, 5 (3), 537–547. 10.1586/14737140.5.3.537. [DOI] [PubMed] [Google Scholar]
- (21).Morishita M; Takahashi Y; Matsumoto A; Nishikawa M; Takakura Y Exosome-Based Tumor Antigens–Adjuvant Co-Delivery Utilizing Genetically Engineered Tumor Cell-Derived Exosomes with Immunostimulatory CpG DNA. Biomaterials 2016, 111, 55–65. 10.1016/j.biomaterials.2016.09.031. [DOI] [PubMed] [Google Scholar]
- (22).Yim N; Ryu S-W; Choi K; Lee KR; Lee S; Choi H; Kim J; Shaker MR; Sun W; Park J-H; Exosome Engineering for Efficient Intracellular Delivery of Soluble Proteins Using Optically Reversible Protein–Protein Interaction Module. Nat. Commun. 2016, 7 (1), 12277. 10.1038/ncomms12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Ferreira JR; Teixeira GQ; Santos SG; Barbosa MA; Almeida-Porada G; Gonçalves RM Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-Conditioning. Front. Immunol. 2018, 9, 2837. 10.3389/fimmu.2018.02837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Rajagopal C; Harikumar KB The Origin and Functions of Exosomes in Cancer. Front. Oncol. 2018, 8, 66. 10.3389/fonc.2018.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Li M; Zeringer E; Barta T; Schageman J; Cheng A; Vlassov AV Analysis of the RNA Content of the Exosomes Derived from Blood Serum and Urine and Its Potential as Biomarkers. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369 (1652), 20130502. 10.1098/rstb.2013.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Schorey JS; Bhatnagar S Exosome Function: From Tumor Immunology to Pathogen Biology. Traffic 2008, 9 (6), 871–881. 10.1111/j.1600-0854.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Ha D; Yang N; Nadithe V Exosomes as Therapeutic Drug Carriers and Delivery Vehicles across Biological Membranes: Current Perspectives and Future Challenges. Acta Pharm. Sin. B 2016, 6 (4), 287–296. 10.1016/j.apsb.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Bellingham S; Guo B; Coleman B; Hill A Exosomes: Vehicles for the Transfer of Toxic Proteins Associated with Neurodegenerative Diseases?. Frontiers in Physiology. 2012, p 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Fleming A; Sampey G; Chung MC; Bailey C; van Hoek ML; Kashanchi F; Hakami RM The Carrying Pigeons of the Cell: Exosomes and Their Role in Infectious Diseases Caused by Human Pathogens. Pathog. Dis. 2014, 71 (2), 109–120. 10.1111/2049-632X.12135. [DOI] [PubMed] [Google Scholar]
- (30).Becker A; Thakur BK; Weiss JM; Kim HS; Peinado H; Lyden D Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30 (6), 836–848. 10.1016/j.ccell.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Li Y; Zheng Q; Bao C; Li S; Guo W; Zhao J; Chen D; Gu J; He X; Huang S Circular RNA Is Enriched and Stable in Exosomes: A Promising Biomarker for Cancer Diagnosis. Cell Res. 2015, 25 (8), 981–984. 10.1038/cr.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Thakur BK; Zhang H; Becker A; Matei I; Huang Y; Costa-Silva B; Zheng Y; Hoshino A; Brazier H; Xiang J; Double-Stranded DNA in Exosomes: A Novel Biomarker in Cancer Detection. Cell Res. 2014, 24 (6), 766–769. 10.1038/cr.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Masyuk AI; Masyuk TV; Larusso NF Exosomes in the Pathogenesis, Diagnostics and Therapeutics of Liver Diseases. J. Hepatol. 2013, 59 (3), 621–625. 10.1016/j.jhep.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).van Balkom BWM; Pisitkun T; Verhaar MC; Knepper MA Exosomes and the Kidney: Prospects for Diagnosis and Therapy of Renal Diseases. Kidney Int. 2011, 80 (11), 1138–1145. 10.1038/ki.2011.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Barile L; Vassalli G Exosomes: Therapy Delivery Tools and Biomarkers of Diseases. Pharmacol. Ther. 2017, 174, 63–78. 10.1016/j.pharmthera.2017.02.020. [DOI] [PubMed] [Google Scholar]
- (36).Kalra H; Simpson RJ; Ji H; Aikawa E; Altevogt P; Askenase P; Bond VC; Borràs FE; Breakefield X; Budnik V; Vesiclepedia: A Compendium for Extracellular Vesicles with Continuous Community Annotation. PLoS Biol. 2012. 10.1371/journal.pbio.1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Keerthikumar S; Chisanga D; Ariyaratne D; Al Saffar H; Anand S; Zhao K; Samuel M; Pathan M; Jois M; Chilamkurti N; ExoCarta: A Web-Based Compendium of Exosomal Cargo. J. Mol. Biol. 2016, 428 (4), 688–692. 10.1016/j.jmb.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Tamura R; Uemoto S; Tabata Y Augmented Liver Targeting of Exosomes by Surface Modification with Cationized Pullulan. Acta Biomater. 2017, 57, 274–284. 10.1016/j.actbio.2017.05.013. [DOI] [PubMed] [Google Scholar]
- (39).Khongkow M; Yata T; Boonrungsiman S; Ruktanonchai UR; Graham D; Namdee K Surface Modification of Gold Nanoparticles with Neuron-Targeted Exosome for Enhanced Blood–Brain Barrier Penetration. Sci. Rep. 2019, 9 (1), 8278. 10.1038/s41598-019-44569-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Smyth T; Petrova K; Payton NM; Persaud I; Redzic JS; Graner MW; Smith-Jones P; Anchordoquy TJ Surface Functionalization of Exosomes Using Click Chemistry. Bioconjug. Chem. 2014, 25 (10), 1777–1784. 10.1021/bc500291r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Wan S; Zhang L; Wang S; Liu Y; Wu C; Cui C; Sun H; Shi M; Jiang Y; Li L; Molecular Recognition-Based DNA Nanoassemblies on the Surfaces of Nanosized Exosomes. J. Am. Chem. Soc. 2017, 139 (15), 5289–5292. 10.1021/jacs.7b00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Zhou Y; Tian T; Zhu Y; Jaffar Ali D; Hu F; Qi Y; Sun B; Xiao Z Exosomes Transfer Among Different Species Cells and Mediating MiRNAs Delivery. J. Cell. Biochem. 2017, 118 (12), 4267–4274. 10.1002/jcb.26077. [DOI] [PubMed] [Google Scholar]
- (43).Schorey JS; Harding CV Extracellular Vesicles and Infectious Diseases: New Complexity to an Old Story. J. Clin. Invest. 2016, 126 (4), 1181–1189. 10.1172/JCI81132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Pardridge WM Drug Transport across the Blood–Brain Barrier. J. Cereb. Blood Flow Metab. 2012, 32 (11), 1959–1972. 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).La-Beck NM; Liu X; Wood LM Harnessing Liposome Interactions With the Immune System for the Next Breakthrough in Cancer Drug Delivery. Front. Pharmacol. 2019, 10, 220. 10.3389/fphar.2019.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Navarro SM; Darensbourg C; Cross L; Stout R; Coulon D; Astete CE; Morgan T; Sabliov CM Biodistribution of PLGA and PLGA/Chitosan Nanoparticles after Repeat-Dose Oral Delivery in F344 Rats for 7 Days. Ther. Deliv. 2014, 5 (11), 1191–1201. 10.4155/tde.14.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Tian Y; Li S; Song J; Ji T; Zhu M; Anderson GJ; Wei J; Nie G A Doxorubicin Delivery Platform Using Engineered Natural Membrane Vesicle Exosomes for Targeted Tumor Therapy. Biomaterials 2014, 35 (7), 2383–2390. 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- (48).Haney MJ; Klyachko NL; Zhao Y; Gupta R; Plotnikova EG; He Z; Patel T; Piroyan A; Sokolsky M; Kabanov AV; Exosomes as Drug Delivery Vehicles for Parkinson’s Disease Therapy. J. Control. Release 2015, 207, 18–30. 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Gurunathan S; Kang M-H; Jeyaraj M; Qasim M; Kim J-H Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells 2019, 8 (4), 307. 10.3390/cells8040307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Schiffelers R; Kooijmans S; Vader; van Dommelen; Van Solinge. Exosome Mimetics: A Novel Class of Drug Delivery Systems. Int. J. Nanomedicine 2012, 1525. 10.2147/IJN.S29661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Théry C; Amigorena S; Raposo G; Clayton A Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. Curr. Protoc. Cell Biol. 2006, 30 (1), 3.22.1–3.22.29. 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- (52).Rekker K; Saare M; Roost AM; Kubo A-L; Zarovni N; Chiesi A; Salumets A; Peters M Comparison of Serum Exosome Isolation Methods for MicroRNA Profiling. Clin. Biochem. 2014, 47 (1–2), 135–138. 10.1016/j.clinbiochem.2013.10.020. [DOI] [PubMed] [Google Scholar]
- (53).Abdel-Haq H Blood Exosomes as a Tool for Monitoring Treatment Efficacy and Progression of Neurodegenerative Diseases. Neural Regen. Res. 2019, 14 (1), 72–74. 10.4103/1673-5374.243709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Feng D; Zhao W-L; Ye Y-Y; Bai X-C; Liu R-Q; Chang L-F; Zhou Q; Sui S-F Cellular Internalization of Exosomes Occurs Through Phagocytosis. Traffic 2010, 11 (5), 675–687. 10.1111/j.1600-0854.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- (55).Yuan D; Zhao Y; Banks WA; Bullock KM; Haney M; Batrakova E; Kabanov AV Macrophage Exosomes as Natural Nanocarriers for Protein Delivery to Inflamed Brain. Biomaterials 2017, 142, 1–12. 10.1016/j.biomaterials.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Pegtel DM; Cosmopoulos K; Thorley-Lawson DA; van Eijndhoven MAJ; Hopmans ES; Lindenberg JL; de Gruijl TD; Wurdinger T; Middeldorp JM Functional Delivery of Viral MiRNAs via Exosomes. Proc. Natl. Acad. Sci. 2010, 107 (14), 6328–6333. 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Luan X; Sansanaphongpricha K; Myers I; Chen H; Yuan H; Sun D Engineering Exosomes as Refined Biological Nanoplatforms for Drug Delivery. Acta Pharmacol. Sin. 2017, 38 (6), 754–763. 10.1038/aps.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Lai CP; Mardini O; Ericsson M; Prabhakar S; Maguire CA; Chen JW; Tannous BA; Breakefield XO Dynamic Biodistribution of Extracellular Vesicles in Vivo Using a Multimodal Imaging Reporter. ACS Nano 2014, 8 (1), 483–494. 10.1021/nn404945r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Ishida T; Kashima S; Kiwada H The Contribution of Phagocytic Activity of Liver Macrophages to the Accelerated Blood Clearance (ABC) Phenomenon of PEGylated Liposomes in Rats. J. Control. Release 2008, 126 (2), 162–165. 10.1016/j.jconrel.2007.11.009. [DOI] [PubMed] [Google Scholar]
- (60).Lai RC; Yeo RWY; Tan KH; Lim SK Exosomes for Drug Delivery — a Novel Application for the Mesenchymal Stem Cell. Biotechnol. Adv. 2013, 31 (5), 543–551. 10.1016/j.biotechadv.2012.08.008. [DOI] [PubMed] [Google Scholar]
- (61).Johnsen KB; Gudbergsson JM; Skov MN; Pilgaard L; Moos T; Duroux M A Comprehensive Overview of Exosomes as Drug Delivery Vehicles — Endogenous Nanocarriers for Targeted Cancer Therapy. Biochim. Biophys. Acta - Rev. Cancer 2014, 1846 (1), 75–87. 10.1016/j.bbcan.2014.04.005. [DOI] [PubMed] [Google Scholar]
- (62).Schmidt O; Teis D The ESCRT Machinery. Curr. Biol. 2012, 22 (4), R116–R120. 10.1016/j.cub.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Wang H; Sui H; Zheng Y; Jiang Y; Shi Y; Liang J; Zhao L Curcumin-Primed Exosomes Potently Ameliorate Cognitive Function in AD Mice by Inhibiting Hyperphosphorylation of the Tau Protein through the AKT/GSK-3β Pathway. Nanoscale 2019, 11 (15), 7481–7496. 10.1039/C9NR01255A. [DOI] [PubMed] [Google Scholar]
- (64).Huotari J; Helenius A Endosome Maturation. EMBO J. 2011, 30 (17), 3481–3500. 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Henne WM; Stenmark H; Emr SD Molecular Mechanisms of the Membrane Sculpting ESCRT Pathway. Cold Spring Harb. Perspect. Biol. 2013, 5 (9), a016766–a016766. 10.1101/cshperspect.a016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Trajkovic K; Hsu C; Chiantia S; Rajendran L; Wenzel D; Wieland F; Schwille P; Brugger B; Simons M Ceramide Triggers Budding of Exosome Vesicles into Multivesicular Endosomes. Science (80-. ). 2008, 319 (5867), 1244–1247. 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- (67).Hurley JH ESCRTs Are Everywhere. EMBO J. 2015, 34 (19), 2398–2407. 10.15252/embj.201592484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Kowal J; Tkach M; Théry C Biogenesis and Secretion of Exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125. 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- (69).Colombo M; Moita C; van Niel G; Kowal J; Vigneron J; Benaroch P; Manel N; Moita LF; Théry C; Raposo G Analysis of ESCRT Functions in Exosome Biogenesis, Composition and Secretion Highlights the Heterogeneity of Extracellular Vesicles. J. Cell Sci. 2013, 126 (24), 5553–5565. 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- (70).Hessvik NP; Llorente A Current Knowledge on Exosome Biogenesis and Release. Cell. Mol. Life Sci. 2018, 75 (2), 193–208. 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).McAndrews KM; Kalluri R Mechanisms Associated with Biogenesis of Exosomes in Cancer. Mol. Cancer 2019, 18 (1), 52. 10.1186/s12943-019-0963-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Tamai K; Tanaka N; Nakano T; Kakazu E; Kondo Y; Inoue J; Shiina M; Fukushima K; Hoshino T; Sano K; Exosome Secretion of Dendritic Cells Is Regulated by Hrs, an ESCRT-0 Protein. Biochem. Biophys. Res. Commun. 2010, 399 (3), 384–390. 10.1016/j.bbrc.2010.07.083. [DOI] [PubMed] [Google Scholar]
- (73).Piper RC; Katzmann DJ Biogenesis and Function of Multivesicular Bodies. Annu. Rev. Cell Dev. Biol. 2007, 23 (1), 519–547. 10.1146/annurev.cellbio.23.090506.123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Wollert T; Wunder C; Lippincott-Schwartz J; Hurley JH Membrane Scission by the ESCRT-III Complex. Nature 2009, 458 (7235), 172–177. 10.1038/nature07836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Zhu H; Guariglia S; Yu RYL; Li W; Brancho D; Peinado H; Lyden D; Salzer J; Bennett C; Chow C-W Mutation of SIMPLE in Charcot–Marie–Tooth 1C Alters Production of Exosomes. Mol. Biol. Cell 2013, 24 (11), 1619–1637. 10.1091/mbc.e12-07-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Baietti MF; Zhang Z; Mortier E; Melchior A; Degeest G; Geeraerts A; Ivarsson Y; Depoortere F; Coomans C; Vermeiren E; Syndecan–Syntenin–ALIX Regulates the Biogenesis of Exosomes. Nat. Cell Biol. 2012, 14 (7), 677–685. 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- (77).Friand V; David G; Zimmermann P Syntenin and Syndecan in the Biogenesis of Exosomes. Biol. Cell 2015, 107 (10), 331–341. 10.1111/boc.201500010. [DOI] [PubMed] [Google Scholar]
- (78).Brügger B; Bankaitis VA Lipids and Vesicular Transport. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 2012, 1821 (8), 1039. 10.1016/j.bbalip.2012.05.005. [DOI] [PubMed] [Google Scholar]
- (79).Haucke V; Di Paolo G Lipids and Lipid Modifications in the Regulation of Membrane Traffic. Curr. Opin. Cell Biol. 2007, 19 (4), 426–435. 10.1016/j.ceb.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).McMahon HT; Boucrot E Membrane Curvature at a Glance. J. Cell Sci. 2015, 128 (6), 1065–1070. 10.1242/jcs.114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Skotland T; Sandvig K; Llorente A Lipids in Exosomes: Current Knowledge and the Way Forward. Prog. Lipid Res. 2017, 66, 30–41. 10.1016/j.plipres.2017.03.001. [DOI] [PubMed] [Google Scholar]
- (82).Skotland T; Hessvik NP; Sandvig K; Llorente A Exosomal Lipid Composition and the Role of Ether Lipids and Phosphoinositides in Exosome Biology. J. Lipid Res. 2019, 60 (1), 9–18. 10.1194/jlr.R084343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Kosaka N; Iguchi H; Yoshioka Y; Takeshita F; Matsuki Y; Ochiya T Secretory Mechanisms and Intercellular Transfer of MicroRNAs in Living Cells. J. Biol. Chem. 2010, 285 (23), 17442–17452. 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Costa Verdera H; Gitz-Francois JJ; Schiffelers RM; Vader P Cellular Uptake of Extracellular Vesicles Is Mediated by Clathrin-Independent Endocytosis and Macropinocytosis. J. Control. Release 2017, 266, 100–108. 10.1016/j.jconrel.2017.09.019. [DOI] [PubMed] [Google Scholar]
- (85).Nakase I; Noguchi K; Aoki A; Takatani-Nakase T; Fujii I; Futaki S Arginine-Rich Cell-Penetrating Peptide-Modified Extracellular Vesicles for Active Macropinocytosis Induction and Efficient Intracellular Delivery. Sci. Rep. 2017, 7 (1), 1991. 10.1038/s41598-017-02014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Webber J; Steadman R; Mason MD; Tabi Z; Clayton A Cancer Exosomes Trigger Fibroblast to Myofibroblast Differentiation. Cancer Res. 2010, 70 (23), 9621–9630. 10.1158/0008-5472.CAN-10-1722. [DOI] [PubMed] [Google Scholar]
- (87).Phuyal S; Hessvik NP; Skotland T; Sandvig K; Llorente A Regulation of Exosome Release by Glycosphingolipids and Flotillins. FEBS J. 2014, 281 (9), 2214–2227. 10.1111/febs.12775. [DOI] [PubMed] [Google Scholar]
- (88).Raposo G; Stoorvogel W Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013, 200 (4), 373–383. 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Kowal J; Arras G; Colombo M; Jouve M; Morath JP; Primdal-Bengtson B; Dingli F; Loew D; Tkach M; Théry C Proteomic Comparison Defines Novel Markers to Characterize Heterogeneous Populations of Extracellular Vesicle Subtypes. Proc. Natl. Acad. Sci. 2016, 113 (8), E968–E977. 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Stuffers S; Sem Wegner C; Stenmark H; Brech A Multivesicular Endosome Biogenesis in the Absence of ESCRTs. Traffic 2009, 10 (7), 925–937. 10.1111/j.1600-0854.2009.00920.x. [DOI] [PubMed] [Google Scholar]
- (91).Skotland T; Sagini K; Sandvig K; Llorente A An Emerging Focus on Lipids in Extracellular Vesicles. Adv. Drug Deliv. Rev. 2020. 10.1016/j.addr.2020.03.002. [DOI] [PubMed] [Google Scholar]
- (92).Verderio C; Gabrielli M; Giussani P Role of Sphingolipids in the Biogenesis and Biological Activity of Extracellular Vesicles. J. Lipid Res. 2018, 59 (8), 1325–1340. 10.1194/jlr.R083915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Burrello J; Biemmi V; Dei Cas M; Amongero M; Bolis S; Lazzarini E; Bollini S; Vassalli G; Paroni R; Barile L Sphingolipid Composition of Circulating Extracellular Vesicles after Myocardial Ischemia. Sci. Rep. 2020, 10 (1), 16182. 10.1038/s41598-020-73411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Kobayashi T; Beuchat M-H; Chevallier J; Makino A; Mayran N; Escola J-M; Lebrand C; Cosson P; Kobayashi T; Gruenberg J Separation and Characterization of Late Endosomal Membrane Domains. J. Biol. Chem. 2002, 277 (35), 32157–32164. 10.1074/jbc.M202838200. [DOI] [PubMed] [Google Scholar]
- (95).White IJ; Bailey LM; Aghakhani MR; Moss SE; Futter CE EGF Stimulates Annexin 1-Dependent Inward Vesiculation in a Multivesicular Endosome Subpopulation. EMBO J. 2006, 25 (1), 1–12. 10.1038/sj.emboj.7600759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Buschow SI; van Balkom BWM; Aalberts M; Heck AJR; Wauben M; Stoorvogel W MHC Class II-Associated Proteins in B-Cell Exosomes and Potential Functional Implications for Exosome Biogenesis. Immunol. Cell Biol. 2010, 88 (8), 851–856. 10.1038/icb.2010.64. [DOI] [PubMed] [Google Scholar]
- (97).Li S; Lin Z; Jiang X; Yu X Exosomal Cargo-Loading and Synthetic Exosome-Mimics as Potential Therapeutic Tools. Acta Pharmacol. Sin. 2018, 39 (4), 542–551. 10.1038/aps.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Ghossoub R; Lembo F; Rubio A; Gaillard CB; Bouchet J; Vitale N; Slavík J; Machala M; Zimmermann P Syntenin-ALIX Exosome Biogenesis and Budding into Multivesicular Bodies Are Controlled by ARF6 and PLD2. Nat. Commun. 2014. 10.1038/ncomms4477. [DOI] [PubMed] [Google Scholar]
- (99).Kanemoto S; Nitani R; Murakami T; Kaneko M; Asada R; Matsuhisa K; Saito A; Imaizumi K Multivesicular Body Formation Enhancement and Exosome Release during Endoplasmic Reticulum Stress. Biochem. Biophys. Res. Commun. 2016, 480 (2), 166–172. 10.1016/j.bbrc.2016.10.019. [DOI] [PubMed] [Google Scholar]
- (100).Baixauli F; López-Otín C; Mittelbrunn M Exosomes and Autophagy: Coordinated Mechanisms for the Maintenance of Cellular Fitness. Frontiers in Immunology. 2014, p 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Hoshino D; Kirkbride KC; Costello K; Clark ES; Sinha S; Grega-Larson N; Tyska MJ; Weaver AM Exosome Secretion Is Enhanced by Invadopodia and Drives Invasive Behavior. Cell Rep. 2013. 10.1016/j.celrep.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).van Niel G; Porto-Carreiro I; Simoes S; Raposo G Exosomes: A Common Pathway for a Specialized Function. J. Biochem. 2006, 140 (1), 13–21. 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- (103).Qu Y; Franchi L; Nunez G; Dubyak GR Nonclassical IL-1β Secretion Stimulated by P2X7 Receptors Is Dependent on Inflammasome Activation and Correlated with Exosome Release in Murine Macrophages. J. Immunol. 2007, 179 (3), 1913 LP – 1925. 10.4049/jimmunol.179.3.1913. [DOI] [PubMed] [Google Scholar]
- (104).Barros FM; Carneiro F; Machado JC; Melo SA Exosomes and Immune Response in Cancer: Friends or Foes? Front. Immunol. 2018, 9, 730. 10.3389/fimmu.2018.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Carmeliet P; Jain RK Molecular Mechanisms and Clinical Applications of Angiogenesis. Nature 2011, 473 (7347), 298–307. 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Tan A; Rajadas J; Seifalian AM Exosomes as Nano-Theranostic Delivery Platforms for Gene Therapy. Adv. Drug Deliv. Rev. 2013, 65 (3), 357–367. 10.1016/j.addr.2012.06.014. [DOI] [PubMed] [Google Scholar]
- (107).Bobrie A; Colombo M; Krumeich S; Raposo G; Théry C Diverse Subpopulations of Vesicles Secreted by Different Intracellular Mechanisms Are Present in Exosome Preparations Obtained by Differential Ultracentrifugation. J. Extracell. Vesicles 2012, 1 (1), 18397. 10.3402/jev.v1i0.18397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Novak JP; Nickerson C; Franzen S; Feldheim DL Purification of Molecularly Bridged Metal Nanoparticle Arrays by Centrifugation and Size Exclusion Chromatography. Anal. Chem. 2001, 73 (23), 5758–5761. 10.1021/ac010812t. [DOI] [PubMed] [Google Scholar]
- (109).Mamer OA Medical Applications of Mass Spectrometry. In Encyclopedia of Spectroscopy and Spectrometry; Elsevier, 2017; pp 772–780. 10.1016/B978-0-12-803224-4.00199-0. [DOI] [Google Scholar]
- (110).Greening DW; Xu R; Ji H; Tauro BJ; Simpson RJ A Protocol for Exosome Isolation and Characterization: Evaluation of Ultracentrifugation, Density-Gradient Separation, and Immunoaffinity Capture Methods. In Methods in molecular biology (Clifton, N.J.); United States, 2015; Vol. 1295, pp 179–209. 10.1007/978-1-4939-2550-6_15. [DOI] [PubMed] [Google Scholar]
- (111).Li P; Kaslan M; Lee SH; Yao J; Gao Z Progress in Exosome Isolation Techniques. Theranostics 2017, 7 (3), 789–804. 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Lobb RJ; Becker M; Wen Wen S; Wong CSF; Wiegmans AP; Leimgruber A; Möller A Optimized Exosome Isolation Protocol for Cell Culture Supernatant and Human Plasma. J. Extracell. Vesicles 2015, 4 (1), 27031. 10.3402/jev.v4.27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Deregibus MC; Figliolini F; D’antico S; Manzini PM; Pasquino C; De Lena M; Tetta C; Brizzi MF; Camussi G Charge-Based Precipitation of Extracellular Vesicles. Int. J. Mol. Med. 2016, 38 (5), 1359–1366. 10.3892/ijmm.2016.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Wang Z; Wu H; Fine D; Schmulen J; Hu Y; Godin B; Zhang JXJ; Liu X Ciliated Micropillars for the Microfluidic-Based Isolation of Nanoscale Lipid Vesicles. Lab Chip 2013, 13 (15), 2879. 10.1039/c3lc41343h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Konoshenko MY; Lekchnov EA; Vlassov AV; Laktionov PP Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. Biomed Res. Int. 2018, 2018, 1–27. 10.1155/2018/8545347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Sharma A; Sharma US; Stuffers S; Sem Wegner C; Stenmark H; Brech A; Date AA; Joshi MD; Patravale VB; Lee KS; Isolation of Exosomes by Differential Centrifugation: Theoretical Analysis of a Commonly Used Protocol. J. Control. Release 2015, 5 (31), 1697–1704. 10.1093/cvr/cvu167. [DOI] [Google Scholar]
- (117).Helwa I; Cai J; Drewry MD; Zimmerman A; Dinkins MB; Khaled ML; Seremwe M; Dismuke WM; Bieberich E; Stamer WD; A Comparative Study of Serum Exosome Isolation Using Differential Ultracentrifugation and Three Commercial Reagents. PLoS One 2017, 12 (1), e0170628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).CHAPTER 7 - Preparative and Analytical Ultracentrifugation**Chapter 7 Was Contributed by Rodes Trautman and Keith M. Cowan. In Methods in Immunology and Immunochemistry; WILLIAMS CA, CHASE MWBT-P and C. M., Eds.; Academic Press, 1968; Vol. 2, pp 81–118. 10.1016/B978-1-4831-9796-8.50008-0. [DOI] [Google Scholar]
- (119).Livshts MA; Khomyakova E; Evtushenko EG; Lazarev VN; Kulemin NA; Semina SE; Generozov EV; Govorun VM Isolation of Exosomes by Differential Centrifugation: Theoretical Analysis of a Commonly Used Protocol. Sci. Rep. 2015. 10.1038/srep17319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Denzer K; Kleijmeer MJ; Heijnen HF; Stoorvogel W; Geuze HJ Exosome: From Internal Vesicle of the Multivesicular Body to Intercellular Signaling Device. J. Cell Sci. 2000, 113 (19), 3365 LP – 3374. [DOI] [PubMed] [Google Scholar]
- (121).Tian T; Zhang H-X; He C-P; Fan S; Zhu Y-L; Qi C; Huang N-P; Xiao Z-D; Lu Z-H; Tannous BA; Surface Functionalized Exosomes as Targeted Drug Delivery Vehicles for Cerebral Ischemia Therapy. Biomaterials 2018, 150, 137–149. 10.1016/j.biomaterials.2017.10.012. [DOI] [PubMed] [Google Scholar]
- (122).Feng Y; Huang W; Wani M; Yu X; Ashraf M Ischemic Preconditioning Potentiates the Protective Effect of Stem Cells through Secretion of Exosomes by Targeting Mecp2 via MiR-22. PLoS One 2014, 9 (2), e88685. 10.1371/journal.pone.0088685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Batas B; Chaudhuri JB Protein Refolding at High Concentration Using Size-Exclusion Chromatography. Biotechnol. Bioeng. 1996, 50 (1), 16–23. . [DOI] [PubMed] [Google Scholar]
- (124).Prendergast EN; de Souza Fonseca MA; Dezem FS; Lester J; Karlan BY; Noushmehr H; Lin X; Lawrenson K Optimizing Exosomal RNA Isolation for RNA-Seq Analyses of Archival Sera Specimens. PLoS One 2018, 13 (5), e0196913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (125).Kennedy JA; Taylor AW Analysis of Proanthocyanidins by High-Performance Gel Permeation Chromatography. J. Chromatogr. A 2003, 995 (1–2), 99–107. 10.1016/S0021-9673(03)00420-5. [DOI] [PubMed] [Google Scholar]
- (126).Chen C; Skog J; Hsu C-H; Lessard RT; Balaj L; Wurdinger T; Carter BS; Breakefield XO; Toner M; Irimia D Microfluidic Isolation and Transcriptome Analysis of Serum Microvesicles. Lab Chip 2010, 10 (4), 505–511. 10.1039/B916199F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Clayton A; Court J; Navabi H; Adams M; Mason MD; Hobot JA; Newman GR; Jasani B Analysis of Antigen Presenting Cell Derived Exosomes, Based on Immuno-Magnetic Isolation and Flow Cytometry. J. Immunol. Methods 2001, 247 (1–2), 163–174. 10.1016/S0022-1759(00)00321-5. [DOI] [PubMed] [Google Scholar]
- (128).Tauro BJ; Greening DW; Mathias RA; Ji H; Mathivanan S; Scott AM; Simpson RJ Comparison of Ultracentrifugation, Density Gradient Separation, and Immunoaffinity Capture Methods for Isolating Human Colon Cancer Cell Line LIM1863-Derived Exosomes. Methods 2012, 56 (2), 293–304. 10.1016/j.ymeth.2012.01.002. [DOI] [PubMed] [Google Scholar]
- (129).Taylor DD; Zacharias W; Gercel-Taylor C Exosome Isolation for Proteomic Analyses and RNA Profiling. In Methods in Molecular Biology; 2011; pp 235–246. 10.1007/978-1-61779-068-3_15. [DOI] [PubMed] [Google Scholar]
- (130).Serrano-Pertierra E; Oliveira-Rodríguez M; Rivas M; Oliva P; Villafani J; Navarro A; Blanco-López M; Cernuda-Morollón E Characterization of Plasma-Derived Extracellular Vesicles Isolated by Different Methods: A Comparison Study. Bioengineering 2019, 6 (1), 8. 10.3390/bioengineering6010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Tang Y-T; Huang Y-Y; Zheng L; Qin S-H; Xu X-P; An T-X; Xu Y; Wu Y-S; Hu X-M; Ping B-H; Comparison of Isolation Methods of Exosomes and Exosomal RNA from Cell Culture Medium and Serum. Int. J. Mol. Med. 2017, 40 (3), 834–844. 10.3892/ijmm.2017.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Enderle D; Spiel A; Coticchia CM; Berghoff E; Mueller R; Schlumpberger M; Sprenger-Haussels M; Shaffer JM; Lader E; Skog J; et al. Characterization of RNA from Exosomes and Other Extracellular Vesicles Isolated by a Novel Spin Column-Based Method. PLoS One 2015, 10 (8), e0136133. 10.1371/journal.pone.0136133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Ding M; Wang C; Lu X; Zhang C; Zhou Z; Chen X; Zhang C-Y; Zen K; Zhang C Comparison of Commercial Exosome Isolation Kits for Circulating Exosomal MicroRNA Profiling. Anal. Bioanal. Chem. 2018, 410 (16), 3805–3814. 10.1007/s00216-018-1052-4. [DOI] [PubMed] [Google Scholar]
- (134).Sokolova V; Ludwig A-K; Hornung S; Rotan O; Horn PA; Epple M; Giebel B Characterisation of Exosomes Derived from Human Cells by Nanoparticle Tracking Analysis and Scanning Electron Microscopy. Colloids Surfaces B Biointerfaces 2011, 87 (1), 146–150. 10.1016/j.colsurfb.2011.05.013. [DOI] [PubMed] [Google Scholar]
- (135).Petersen KE; Manangon E; Hood JL; Wickline SA; Fernandez DP; Johnson WP; Gale BK A Review of Exosome Separation Techniques and Characterization of B16-F10 Mouse Melanoma Exosomes with AF4-UV-MALS-DLS-TEM. Anal. Bioanal. Chem. 2014, 406 (30), 7855–7866. 10.1007/s00216-014-8040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (136).Conde-Vancells J; Rodriguez-Suarez E; Embade N; Gil D; Matthiesen R; Valle M; Elortza F; Lu SC; Mato JM; Falcon-Perez JM Characterization and Comprehensive Proteome Profiling of Exosomes Secreted by Hepatocytes. J. Proteome Res. 2008, 7 (12), 5157–5166. 10.1021/pr8004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Fernández-Llama P; Khositseth S; Gonzales PA; Star RA; Pisitkun T; Knepper MA Tamm-Horsfall Protein and Urinary Exosome Isolation. Kidney Int. 2010, 77 (8), 736–742. 10.1038/ki.2009.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (138).Bandu R; Oh JW; Kim KP Mass Spectrometry-Based Proteome Profiling of Extracellular Vesicles and Their Roles in Cancer Biology. Exp. Mol. Med. 2019, 51 (3), 30. 10.1038/s12276-019-0218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Dragovic RA; Gardiner C; Brooks AS; Tannetta DS; Ferguson DJP; Hole P; Carr B; Redman CWG; Harris AL; Dobson PJ; Sizing and Phenotyping of Cellular Vesicles Using Nanoparticle Tracking Analysis. Nanomedicine 2011, 7 (6), 780–788. 10.1016/j.nano.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Encarnación S; Hernández M; Martínez-Batallar G; Contreras S; Vargas M. del C.; Mora J Comparative Proteomics Using 2-D Gel Electrophoresis and Mass Spectrometry as Tools to Dissect Stimulons and Regulons in Bacteria with Sequenced or Partially Sequenced Genomes. Biol. Proced. Online 2005, 7, 117–135. 10.1251/bpo110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (141).Kreimer S; Belov AM; Ghiran I; Murthy SK; Frank DA; Ivanov AR Mass-Spectrometry-Based Molecular Characterization of Extracellular Vesicles: Lipidomics and Proteomics. J. Proteome Res. 2015, 14 (6), 2367–2384. 10.1021/pr501279t. [DOI] [PubMed] [Google Scholar]
- (142).Chen Y; Xie Y; Xu L; Zhan S; Xiao Y; Gao Y; Wu B; Ge W Protein Content and Functional Characteristics of Serum-Purified Exosomes from Patients with Colorectal Cancer Revealed by Quantitative Proteomics. Int. J. cancer 2017, 140 (4), 900–913. 10.1002/ijc.30496. [DOI] [PubMed] [Google Scholar]
- (143).Yang C; Guo W-B; Zhang W-S; Bian J; Yang J-K; Zhou Q-Z; Chen M-K; Peng W; Qi T; Wang C-Y; Comprehensive Proteomics Analysis of Exosomes Derived from Human Seminal Plasma. Andrology 2017, 5 (5), 1007–1015. 10.1111/andr.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (144).Li X; Corbett AL; Taatizadeh E; Tasnim N; Little JP; Garnis C; Daugaard M; Guns E; Hoorfar M; Li ITS Challenges and Opportunities in Exosome Research—Perspectives from Biology, Engineering, and Cancer Therapy. APL Bioeng. 2019, 3 (1), 011503. 10.1063/1.5087122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).Bunggulawa EJ; Wang W; Yin T; Wang N; Durkan C; Wang Y; Wang G Recent Advancements in the Use of Exosomes as Drug Delivery Systems. J. Nanobiotechnology 2018. 10.1186/s12951-018-0403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).Fu S; Wang Y; Xia X; Zheng JC Exosome Engineering: Current Progress in Cargo Loading and Targeted Delivery. NanoImpact 2020, 20, 100261. 10.1016/j.impact.2020.100261. [DOI] [Google Scholar]
- (147).Kim MS; Haney MJ; Zhao Y; Mahajan V; Deygen I; Klyachko NL; Inskoe E; Piroyan A; Sokolsky M; Okolie O; Development of Exosome-Encapsulated Paclitaxel to Overcome MDR in Cancer Cells. Nanomedicine Nanotechnology, Biol. Med. 2016, 12 (3), 655–664. 10.1016/j.nano.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148).Bellavia D; Raimondo S; Calabrese G; Forte S; Cristaldi M; Patinella A; Memeo L; Manno M; Raccosta S; Diana P; Interleukin 3- Receptor Targeted Exosomes Inhibit in Vitro and in Vivo Chronic Myelogenous Leukemia Cell Growth. Theranostics 2017, 7 (5), 1333–1345. 10.7150/thno.17092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).Yang Z; Shi J; Xie J; Wang Y; Sun J; Liu T; Zhao Y; Zhao X; Wang X; Ma Y; Large-Scale Generation of Functional MRNA-Encapsulating Exosomes via Cellular Nanoporation. Nat. Biomed. Eng. 2020, 4 (1), 69–83. 10.1038/s41551-019-0485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (150).Li Z; Zhou X; Wei M; Gao X; Zhao L; Shi R; Sun W; Duan Y; Yang G; Yuan L In Vitro and in Vivo RNA Inhibition by CD9-HuR Functionalized Exosomes Encapsulated with MiRNA or CRISPR/DCas9. Nano Lett. 2019, 19 (1), 19–28. 10.1021/acs.nanolett.8b02689. [DOI] [PubMed] [Google Scholar]
- (151).Yang Y; Hong Y; Nam G-H; Chung JH; Koh E; Kim I-S Virus-Mimetic Fusogenic Exosomes for Direct Delivery of Integral Membrane Proteins to Target Cell Membranes. Adv. Mater. 2017, 29 (13). 10.1002/adma.201605604. [DOI] [PubMed] [Google Scholar]
- (152).Lamichhane TN; Raiker RS; Jay SM Exogenous DNA Loading into Extracellular Vesicles via Electroporation Is Size-Dependent and Enables Limited Gene Delivery. Mol. Pharm. 2015, 12 (10), 3650–3657. 10.1021/acs.molpharmaceut.5b00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (153).Johnsen KB; Gudbergsson JM; Skov MN; Christiansen G; Gurevich L; Moos T; Duroux M Evaluation of Electroporation-Induced Adverse Effects on Adipose-Derived Stem Cell Exosomes. Cytotechnology 2016, 68 (5), 2125–2138. 10.1007/s10616-016-9952-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (154).Wahlgren J; Statello L; Skogberg G; Telemo E; Valadi H Delivery of Small Interfering RNAs to Cells via Exosomes BT - SiRNA Delivery Methods: Methods and Protocols; Shum K, Rossi J, Eds.; Springer New York: New York, NY, 2016; pp 105–125. 10.1007/978-1-4939-3112-5_10. [DOI] [PubMed] [Google Scholar]
- (155).Fuhrmann G; Serio A; Mazo M; Nair R; Stevens MM Saponins as Cytotoxic Agents: A Review. J. Control. Release 2015, 205, 35–44. 10.1016/j.jconrel.2014.11.029. [DOI] [PubMed] [Google Scholar]
- (156).Fuhrmann G; Serio A; Mazo M; Nair R; Stevens MM Active Loading into Extracellular Vesicles Significantly Improves the Cellular Uptake and Photodynamic Effect of Porphyrins. J. Control. Release 2015, 205, 35–44. 10.1016/j.jconrel.2014.11.029. [DOI] [PubMed] [Google Scholar]
- (157).Jeyaram A; Lamichhane TN; Wang S; Zou L; Dahal E; Kronstadt SM; Levy D; Parajuli B; Knudsen DR; Chao W; Enhanced Loading of Functional MiRNA Cargo via PH Gradient Modification of Extracellular Vesicles. Mol. Ther. 2020, 28 (3), 975–985. 10.1016/j.ymthe.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (158).Xu M; Yang Q; Sun X; Wang Y Recent Advancements in the Loading and Modification of Therapeutic Exosomes. Front. Bioeng. Biotechnol. 2020, 8, 586130. 10.3389/fbioe.2020.586130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (159).Wang J; Chen D; Ho EA Challenges in the Development and Establishment of Exosome-Based Drug Delivery Systems. J. Control. Release 2021, 329, 894–906. 10.1016/j.jconrel.2020.10.020. [DOI] [PubMed] [Google Scholar]
- (160).Li X-B; Zhang Z-R; Schluesener HJ; Xu S-Q Role of Exosomes in Immune Regulation. J. Cell. Mol. Med. 2006, 10 (2), 364–375. 10.1111/j.1582-4934.2006.tb00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (161).Greening DW; Gopal SK; Xu R; Simpson RJ; Chen W Exosomes and Their Roles in Immune Regulation and Cancer. Semin. Cell Dev. Biol. 2015, 40, 72–81. 10.1016/j.semcdb.2015.02.009. [DOI] [PubMed] [Google Scholar]
- (162).Lema DA; Burlingham WJ Role of Exosomes in Tumour and Transplant Immune Regulation. Scand. J. Immunol. 2019, 90 (5), e12807. 10.1111/sji.12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (163).Utsugi-Kobukai S; Fujimaki H; Hotta C; Nakazawa M; Minami M MHC Class I-Mediated Exogenous Antigen Presentation by Exosomes Secreted from Immature and Mature Bone Marrow Derived Dendritic Cells. Immunol. Lett. 2003, 89 (2), 125–131. 10.1016/S0165-2478(03)00128-7. [DOI] [PubMed] [Google Scholar]
- (164).Smith VL; Cheng Y; Bryant BR; Schorey JS Exosomes Function in Antigen Presentation during an in Vivo Mycobacterium Tuberculosis Infection. Sci. Rep. 2017, 7 (1), 43578. 10.1038/srep43578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (165).Zhang H-G; Kim H; Liu C; Yu S; Wang J; Grizzle WE; Kimberly RP; Barnes S Curcumin Reverses Breast Tumor Exosomes Mediated Immune Suppression of NK Cell Tumor Cytotoxicity. Biochim. Biophys. Acta - Mol. Cell Res. 2007, 1773 (7), 1116–1123. 10.1016/j.bbamcr.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (166).Ning Y; Shen K; Wu Q; Sun X; Bai Y; Xie Y; Pan J; Qi C Tumor Exosomes Block Dendritic Cells Maturation to Decrease the T Cell Immune Response. Immunol. Lett. 2018, 199, 36–43. 10.1016/j.imlet.2018.05.002. [DOI] [PubMed] [Google Scholar]
- (167).Piper RC; Luzio JP Late Endosomes: Sorting and Partitioning in Multivesicular Bodies. Traffic 2001, 2 (9), 612–621. 10.1034/j.1600-0854.2001.20904.x. [DOI] [PubMed] [Google Scholar]
- (168).Anand PK Exosomal Membrane Molecules Are Potent Immune Response Modulators. Commun. Integr. Biol. 2010, 3 (5), 405–408. 10.4161/cib.3.5.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (169).Zhu M; Tian X; Song X; Li Y; Tian Y; Zhao Y; Nie G Nanoparticle-Induced Exosomes Target Antigen-Presenting Cells to Initiate Th1-Type Immune Activation. Small 2012, 8 (18), 2841–2848. 10.1002/smll.201200381. [DOI] [PubMed] [Google Scholar]
- (170).Brinton LT; Sloane HS; Kester M; Kelly KA Formation and Role of Exosomes in Cancer. Cell. Mol. Life Sci. 2015, 72 (4), 659–671. 10.1007/s00018-014-1764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (171).Alexander M; Hu R; Runtsch MC; Kagele DA; Mosbruger TL; Tolmachova T; Seabra MC; Round JL; Ward DM; O’Connell RM Exosome-Delivered MicroRNAs Modulate the Inflammatory Response to Endotoxin. Nat. Commun. 2015, 6 (1), 7321. 10.1038/ncomms8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (172).Tran T-H; Mattheolabakis G; Aldawsari H; Amiji M Exosomes as Nanocarriers for Immunotherapy of Cancer and Inflammatory Diseases. Clin. Immunol. 2015, 160 (1), 46–58. 10.1016/j.clim.2015.03.021. [DOI] [PubMed] [Google Scholar]
- (173).Nojehdehi S; Soudi S; Hesampour A; Rasouli S; Soleimani M; Hashemi SM Immunomodulatory Effects of Mesenchymal Stem Cell–Derived Exosomes on Experimental Type-1 Autoimmune Diabetes. J. Cell. Biochem. 2018, 119 (11), 9433–9443. 10.1002/jcb.27260. [DOI] [PubMed] [Google Scholar]
- (174).Zhao H; Shang Q; Pan Z; Bai Y; Li Z; Zhang H; Zhang Q; Guo C; Zhang L; Wang Q Exosomes From Adipose-Derived Stem Cells Attenuate Adipose Inflammation and Obesity Through Polarizing M2 Macrophages and Beiging in White Adipose Tissue. Diabetes 2018, 67 (2), 235 LP – 247. 10.2337/db17-0356. [DOI] [PubMed] [Google Scholar]
- (175).Xiao T; Zhang W; Jiao B; Pan C-Z; Liu X; Shen L The Role of Exosomes in the Pathogenesis of Alzheimer’ Disease. Transl. Neurodegener. 2017, 6, 3. 10.1186/s40035-017-0072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (176).Yáñez-Mó M; Siljander PRM; Andreu Z; Bedina Zavec A; Borràs FE; Buzas EI; Buzas K; Casal E; Cappello F; Carvalho J; Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles 2015, 4 (1), 27066. 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (177).Yuan Y; Du W; Liu J; Ma W; Zhang L; Du Z; Cai B Stem Cell-Derived Exosome in Cardiovascular Diseases: Macro Roles of Micro Particles. Front. Pharmacol. 2018, 9, 547. 10.3389/fphar.2018.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (178).Wang L; Jia Q; Xinnong C; Xie Y; Yang Y; Zhang A; Liu R; Zhuo Y; Zhang J Role of Cardiac Progenitor Cell-derived Exosome-mediated MicroRNA-210 in Cardiovascular Disease. J. Cell. Mol. Med. 2019, 23 (11), 7124–7131. 10.1111/jcmm.14562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (179).Wu R; Gao W; Yao K; Ge J Roles of Exosomes Derived From Immune Cells in Cardiovascular Diseases. Front. Immunol. 2019, 10, 648. 10.3389/fimmu.2019.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (180).Hough KP; Chanda D; Duncan SR; Thannickal VJ; Deshane JS Exosomes in Immunoregulation of Chronic Lung Diseases. Allergy 2017, 72 (4), 534–544. 10.1111/all.13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (181).Robbins PD; Morelli AE Regulation of Immune Responses by Extracellular Vesicles. Nat. Rev. Immunol. 2014, 14 (3), 195–208. 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (182).Johnstone RM Exosomes Biological Significance: A Concise Review. Blood Cells, Mol. Dis. 2006, 36 (2), 315–321. 10.1016/j.bcmd.2005.12.001. [DOI] [PubMed] [Google Scholar]
- (183).Chen G; Huang AC; Zhang W; Zhang G; Wu M; Xu W; Yu Z; Yang J; Wang B; Sun H; Exosomal PD-L1 Contributes to Immunosuppression and Is Associated with Anti-PD-1 Response. Nature 2018, 560 (7718), 382–386. 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (184).Porto-Carreiro I; Février B; Paquet S; Vilette D; Raposo G Prions and Exosomes: From PrPc Trafficking to PrPsc Propagation. Blood Cells, Mol. Dis. 2005, 35 (2), 143–148. 10.1016/j.bcmd.2005.06.013. [DOI] [PubMed] [Google Scholar]
- (185).Taylor DD; Akyol S; Gercel-Taylor C Pregnancy-Associated Exosomes and Their Modulation of T Cell Signaling. J. Immunol. 2006, 176 (3), 1534 LP – 1542. 10.4049/jimmunol.176.3.1534. [DOI] [PubMed] [Google Scholar]
- (186).Taylor DD; Gerçel-Taylor C Tumour-Derived Exosomes and Their Role in Cancer-Associated T-Cell Signalling Defects. Br. J. Cancer 2005, 92 (2), 305–311. 10.1038/sj.bjc.6602316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (187).Whiteside TL Immune Modulation of T-Cell and NK (Natural Killer) Cell Activities by TEXs (Tumour-Derived Exosomes). Biochem. Soc. Trans. 2013, 41 (1), 245–251. 10.1042/BST20120265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (188).Dong H; Strome SE; Salomao DR; Tamura H; Hirano F; Flies DB; Roche PC; Lu J; Zhu G; Tamada K; Tumor-Associated B7-H1 Promotes T-Cell Apoptosis: A Potential Mechanism of Immune Evasion. Nat. Med. 2002, 8 (8), 793–800. 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- (189).Chen L; Han X Anti-PD-1/PD-L1 Therapy of Human Cancer: Past, Present, and Future. J. Clin. Invest. 2015, 125 (9), 3384–3391. 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (190).Akinleye A; Rasool Z Immune Checkpoint Inhibitors of PD-L1 as Cancer Therapeutics. J. Hematol. Oncol. 2019, 12 (1), 92. 10.1186/s13045-019-0779-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (191).Arellano B; Graber DJ; Sentman CL Regulatory T Cell-Based Therapies for Autoimmunity. Discov. Med. 2016, 22 (119), 73–80. [PMC free article] [PubMed] [Google Scholar]
- (192).Dejaco C; Duftner C; Grubeck-Loebenstein B; Schirmer M Imbalance of Regulatory T Cells in Human Autoimmune Diseases. Immunology 2006, 117 (3), 289–300. 10.1111/j.1365-2567.2005.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (193).Anel A; Gallego-Lleyda A; de Miguel D; Naval J; Martínez-Lostao L Role of Exosomes in the Regulation of T-Cell Mediated Immune Responses and in Autoimmune Disease. Cells 2019, 8 (2). 10.3390/cells8020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (194).Huang Y; Li R; Ye S; Lin S; Yin G; Xie Q Recent Advances in the Use of Exosomes in Sjögren’s Syndrome. Frontiers in Immunology. 2020, p 1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (195).Zhang B; Yeo RWY; Lai RC; Sim EWK; Chin KC; Lim SK Mesenchymal Stromal Cell Exosome-Enhanced Regulatory T-Cell Production through an Antigen-Presenting Cell-Mediated Pathway. Cytotherapy 2018, 20 (5), 687–696. 10.1016/j.jcyt.2018.02.372. [DOI] [PubMed] [Google Scholar]
- (196).Daneman R; Prat A The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7 (1), a020412. 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (197).Griep LM; Wolbers F; de Wagenaar B; ter Braak PM; Weksler BB; Romero IA; Couraud PO; Vermes I; van der Meer AD; van den Berg A BBB ON CHIP: Microfluidic Platform to Mechanically and Biochemically Modulate Blood-Brain Barrier Function. Biomed. Microdevices 2013, 15 (1), 145–150. 10.1007/s10544-012-9699-7. [DOI] [PubMed] [Google Scholar]
- (198).Kabat-Zinn J; Lipworth L; Burney R The Clinical Use of Mindfulness Meditation for the Self-Regulation of Chronic Pain. J. Behav. Med. 1985, 8 (2), 163–190. 10.1007/BF00845519. [DOI] [PubMed] [Google Scholar]
- (199).Pardridge WM Transport of Small Molecules through the Blood-Brain Barrier: Biology and Methodology. Adv. Drug Deliv. Rev. 1995, 15 (1), 5–36. 10.1016/0169-409X(95)00003-P. [DOI] [PubMed] [Google Scholar]
- (200).Fischer H; Gottschlich R; Seelig A Blood-Brain Barrier Permeation: Molecular Parameters Governing Passive Diffusion. J. Membr. Biol. 1998, 165 (3), 201–211. 10.1007/s002329900434. [DOI] [PubMed] [Google Scholar]
- (201).Yang T; Martin P; Fogarty B; Brown A; Schurman K; Phipps R; Yin VP; Lockman P; Bai S Exosome Delivered Anticancer Drugs Across the Blood-Brain Barrier for Brain Cancer Therapy in Danio Rerio. Pharm. Res. 2015, 32 (6), 2003–2014. 10.1007/s11095-014-1593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (202).Wood MJA; O’Loughlin AJ; Lakhal S Exosomes and the Blood–Brain Barrier: Implications for Neurological Diseases. Ther. Deliv. 2011, 2 (9), 1095–1099. 10.4155/tde.11.83. [DOI] [PubMed] [Google Scholar]
- (203).Chen CC; Liu L; Ma F; Wong CW; Guo XE; Chacko JV; Farhoodi HP; Zhang SX; Zimak J; Ségaliny A; Elucidation of Exosome Migration Across the Blood–Brain Barrier Model In Vitro. Cell. Mol. Bioeng. 2016, 9 (4), 509–529. 10.1007/s12195-016-0458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (204).Zhao C; Wang H; Xiong C; Liu Y Hypoxic Glioblastoma Release Exosomal VEGF-A Induce the Permeability of Blood-Brain Barrier. Biochem. Biophys. Res. Commun. 2018, 502 (3), 324–331. 10.1016/j.bbrc.2018.05.140. [DOI] [PubMed] [Google Scholar]
- (205).O’Kane RL; Martínez-López I; DeJoseph MR; Viña JR; Hawkins RA Na + -Dependent Glutamate Transporters (EAAT1, EAAT2, and EAAT3) of the Blood-Brain Barrier. J. Biol. Chem. 1999, 274 (45), 31891–31895. 10.1074/jbc.274.45.31891. [DOI] [PubMed] [Google Scholar]
- (206).Barar J; Rafi MA; Pourseif MM; Omidi Y Blood-Brain Barrier Transport Machineries and Targeted Therapy of Brain Diseases. Bioimpacts 2016, 6 (4), 225–248. 10.15171/bi.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (207).Upadhyay RK Transendothelial Transport and Its Role in Therapeutics. Int. Sch. Res. Not. 2014, 2014, 309404. 10.1155/2014/309404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (208).Wu X; Xie C; Zhang Y; Fan Z; Yin Y; Blachier F Glutamate–Glutamine Cycle and Exchange in the Placenta–Fetus Unit during Late Pregnancy. Amino Acids 2015, 47 (1), 45–53. 10.1007/s00726-014-1861-5. [DOI] [PubMed] [Google Scholar]
- (209).van Dommelen SM; Vader P; Lakhal S; Kooijmans SAA; van Solinge WW; Wood MJA; Schiffelers RM Microvesicles and Exosomes: Opportunities for Cell-Derived Membrane Vesicles in Drug Delivery. J. Control. Release 2012, 161 (2), 635–644. 10.1016/j.jconrel.2011.11.021. [DOI] [PubMed] [Google Scholar]
- (210).Aryani A; Denecke B Exosomes as a Nanodelivery System: A Key to the Future of Neuromedicine? Mol. Neurobiol. 2016, 53 (2), 818–834. 10.1007/s12035-014-9054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (211).Saiz ML; Rocha-Perugini V; Sánchez-Madrid F Tetraspanins as Organizers of Antigen-Presenting Cell Function. Front. Immunol. 2018, 9, 1074. 10.3389/fimmu.2018.01074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (212).Haqqani AS; Delaney CE; Tremblay T-L; Sodja C; Sandhu JK; Stanimirovic DB Method for Isolation and Molecular Characterization of Extracellular Microvesicles Released from Brain Endothelial Cells. Fluids Barriers CNS 2013, 10 (1), 4. 10.1186/2045-8118-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (213).Balusu S; Van Wonterghem E; De Rycke R; Raemdonck K; Stremersch S; Gevaert K; Brkic M; Demeestere D; Vanhooren V; Hendrix A; Identification of a Novel Mechanism of Blood–Brain Communication during Peripheral Inflammation via Choroid Plexus-Derived Extracellular Vesicles. EMBO Mol. Med. 2016, 8 (10), 1162–1183. 10.15252/emmm.201606271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (214).András IE; Leda A; Contreras MG; Bertrand L; Park M; Skowronska M; Toborek M Extracellular Vesicles of the Blood-Brain Barrier: Role in the HIV-1 Associated Amyloid Beta Pathology. Mol. Cell. Neurosci. 2017, 79, 12–22. 10.1016/j.mcn.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (215).Tanzi RE; Gusella JF; Watkins PC; Bruns GA; St George-Hyslop P; Van Keuren ML; Patterson D; Pagan S; Kurnit DM; Neve RL Amyloid Beta Protein Gene: CDNA, MRNA Distribution, and Genetic Linkage near the Alzheimer Locus. Science (80-. ). 1987, 235 (4791), 880 LP – 884. 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- (216).Badhwar A; Haqqani AS Biomarker Potential of Brain-Secreted Extracellular Vesicles in Blood in Alzheimer’s Disease. Alzheimer’s Dement. (Amsterdam, Netherlands) 2020, 12 (1), e12001. 10.1002/dad2.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (217).Qu M; Lin Q; Huang L; Fu Y; Wang L; He S; Fu Y; Yang S; Zhang Z; Zhang L; Dopamine-Loaded Blood Exosomes Targeted to Brain for Better Treatment of Parkinson’s Disease. J. Control. Release 2018, 287, 156–166. 10.1016/j.jconrel.2018.08.035. [DOI] [PubMed] [Google Scholar]
- (218).Sharma A Liposomes in Drug Delivery: Progress and Limitations. Int. J. Pharm. 1997, 154 (2), 123–140. 10.1016/S0378-5173(97)00135-X. [DOI] [Google Scholar]
- (219).Kumari A; Yadav SK; Yadav SC Biodegradable Polymeric Nanoparticles Based Drug Delivery Systems. Colloids Surfaces B Biointerfaces 2010, 75 (1), 1–18. 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- (220).Tang BC; Dawson M; Lai SK; Wang Y-Y; Suk JS; Yang M; Zeitlin P; Boyle MP; Fu J; Hanes J Biodegradable Polymer Nanoparticles That Rapidly Penetrate the Human Mucus Barrier. Proc. Natl. Acad. Sci. 2009, 106 (46), 19268 LP – 19273. 10.1073/pnas.0905998106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (221).Musyanovych A; Schmitz-Wienke J; Mailänder V; Walther P; Landfester K Preparation of Biodegradable Polymer Nanoparticles by Miniemulsion Technique and Their Cell Interactions. Macromol. Biosci. 2008, 8 (2), 127–139. 10.1002/mabi.200700241. [DOI] [PubMed] [Google Scholar]
- (222).Chen C; Johnston TD; Jeon H; Gedaly R; McHugh PP; Burke TG; Ranjan D An in Vitro Study of Liposomal Curcumin: Stability, Toxicity and Biological Activity in Human Lymphocytes and Epstein-Barr Virus-Transformed Human B-Cells. Int. J. Pharm. 2009. 10.1016/j.ijpharm.2008.09.009. [DOI] [PubMed] [Google Scholar]
- (223).Feng S-S; Mu L; Win KY; Huang G Nanoparticles of Biodegradable Polymers for Clinical Administration of Paclitaxel. Curr. Med. Chem. 2004, 11 (4), 413–424. 10.2174/0929867043455909. [DOI] [PubMed] [Google Scholar]
- (224).Cheng Q; Feng J; Chen J; Zhu X; Li F Brain Transport of Neurotoxin-I with PLA Nanoparticles through Intranasal Administration in Rats: A Microdialysis Study. Biopharm. Drug Dispos. 2008, 29 (8), 431–439. 10.1002/bdd.621. [DOI] [PubMed] [Google Scholar]
- (225).GOMEZGAETE C; TSAPIS N; BESNARD M; BOCHOT A; FATTAL E Encapsulation of Dexamethasone into Biodegradable Polymeric Nanoparticles. Int. J. Pharm. 2007, 331 (2), 153–159. 10.1016/j.ijpharm.2006.11.028. [DOI] [PubMed] [Google Scholar]
- (226).Mu L; Feng S. A Novel Controlled Release Formulation for the Anticancer Drug Paclitaxel (Taxol®): PLGA Nanoparticles Containing Vitamin E TPGS. J. Control. Release 2003, 86 (1), 33–48. 10.1016/S0168-3659(02)00320-6. [DOI] [PubMed] [Google Scholar]
- (227).Vázquez-Ríos AJ; Molina-Crespo Á; Bouzo BL; López-López R; Moreno-Bueno G; de la Fuente M Exosome-Mimetic Nanoplatforms for Targeted Cancer Drug Delivery. J. Nanobiotechnology 2019, 17 (1), 85. 10.1186/s12951-019-0517-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (228).Damgé C; Maincent P; Ubrich N Oral Delivery of Insulin Associated to Polymeric Nanoparticles in Diabetic Rats. J. Control. Release 2007, 117 (2), 163–170. 10.1016/j.jconrel.2006.10.023. [DOI] [PubMed] [Google Scholar]
- (229).Goetzl EJ; Boxer A; Schwartz JB; Abner EL; Petersen RC; Miller BL; Kapogiannis D Altered Lysosomal Proteins in Neural-Derived Plasma Exosomes in Preclinical Alzheimer Disease. Neurology 2015, 85 (1), 40–47. 10.1212/WNL.0000000000001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (230).Calvo P; Gouritin B; Brigger I; Lasmezas C; Deslys J-P; Williams A; Andreux JP; Dormont D; Couvreur P PEGylated Polycyanoacrylate Nanoparticles as Vector for Drug Delivery in Prion Diseases. J. Neurosci. Methods 2001, 111 (2), 151–155. 10.1016/S0165-0270(01)00450-2. [DOI] [PubMed] [Google Scholar]
- (231).Lee KS; Chung HC; Im SA; Park YH; Kim CS; Kim S-B; Rha SY; Lee MY; Ro J Multicenter Phase II Trial of Genexol-PM, a Cremophor-Free, Polymeric Micelle Formulation of Paclitaxel, in Patients with Metastatic Breast Cancer. Breast Cancer Res. Treat. 2008, 108 (2), 241–250. 10.1007/s10549-007-9591-y. [DOI] [PubMed] [Google Scholar]
- (232).Sercombe L; Veerati T; Moheimani F; Wu SY; Sood AK; Hua S Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (233).Gabizon A; Goren D; Horowitz AT; Tzemach D; Lossos A; Siegal T Long-Circulating Liposomes for Drug Delivery in Cancer Therapy: A Review of Biodistribution Studies in Tumor-Bearing Animals. Adv. Drug Deliv. Rev. 1997, 24 (2), 337–344. 10.1016/S0169-409X(96)00476-0. [DOI] [Google Scholar]
- (234).Hines DJ; Kaplan DL Poly(Lactic-Co-Glycolic) Acid-Controlled-Release Systems: Experimental and Modeling Insights. Crit. Rev. Ther. Drug Carrier Syst. 2013, 30 (3), 257–276. 10.1615/critrevtherdrugcarriersyst.2013006475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (235).Lü J-M; Wang X; Marin-Muller C; Wang H; Lin PH; Yao Q; Chen C Current Advances in Research and Clinical Applications of PLGA-Based Nanotechnology. Expert Rev. Mol. Diagn. 2009, 9 (4), 325–341. 10.1586/erm.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (236).Green AE; Rose PG Pegylated Liposomal Doxorubicin in Ovarian Cancer. Int. J. Nanomedicine 2006, 1 (3), 229–239. [PMC free article] [PubMed] [Google Scholar]
- (237).Piccaluga PP; Visani G; Martinelli G; Isidori A; Malagola M; Rondoni M; Baccarani M; Tura S Liposomal Daunorubicin (DaunoXome) for Treatment of Relapsed Meningeal Acute Myeloid Leukemia. Leukemia 2002, 16 (9), 1880–1881. 10.1038/sj.leu.2402617. [DOI] [PubMed] [Google Scholar]
- (238).Bayever Eliel, Dhindsa Navreet, Fitzgerald Jonathan Basil, Laivins Peter, Moyo Victor, Niyikiza Clet, J. K. METHODS FORTREATING PANCREATIC CANCERUSING COMBINATION THERAPES COMPRISING LIPOSOMAL RINOTECAN. US 9,339,497 B2. [Google Scholar]
- (239).Chambers CD; Johnson DL Emerging Data on the Use of Anti-Tumor Necrosis Factor-Alpha Medications in Pregnancy. Birth Defects Res. Part A Clin. Mol. Teratol. 2012, 94 (8), 607–611. 10.1002/bdra.23033. [DOI] [PubMed] [Google Scholar]
- (240).Molineux G The Design and Development of Pegfilgrastim (PEG-RmetHuG-CSF, Neulasta). Curr. Pharm. Des 2004, 10 (11), 1235–1244. 10.2174/1381612043452613. [DOI] [PubMed] [Google Scholar]
- (241).Pettinati HM; Anton RF; Willenbring ML The COMBINE Study-: An Overview of the Largest Pharmacotherapy Study to Date for Treating Alcohol Dependence. Psychiatry (Edgmont). 2006, 3 (10), 36–39. [PMC free article] [PubMed] [Google Scholar]
- (242).Gordon MS; Kinlock TW; Vocci FJ; Fitzgerald TT; Memisoglu A; Silverman B A Phase 4, Pilot, Open-Label Study of VIVITROL® (Extended-Release Naltrexone XR-NTX) for Prisoners. J. Subst. Abuse Treat. 2015, 59, 52–58. 10.1016/j.jsat.2015.07.005. [DOI] [PubMed] [Google Scholar]
- (243).Samson SL Management of Hyperglycemia in Patients With Acromegaly Treated With Pasireotide LAR. Drugs 2016, 76 (13), 1235–1243. 10.1007/s40265-016-0615-y. [DOI] [PubMed] [Google Scholar]
- (244).McKeage K Pasireotide in Acromegaly: A Review. Drugs 2015, 75 (9), 1039–1048. 10.1007/s40265-015-0413-y. [DOI] [PubMed] [Google Scholar]
- (245).de Smet M; Heijman E; Langereis S; Hijnen NM; Grüll H Magnetic Resonance Imaging of High Intensity Focused Ultrasound Mediated Drug Delivery from Temperature-Sensitive Liposomes: An in Vivo Proof-of-Concept Study. J. Control. Release 2011, 150 (1), 102–110. 10.1016/j.jconrel.2010.10.036. [DOI] [PubMed] [Google Scholar]
- (246).Blanco E; Shen H; Ferrari M Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33 (9), 941–951. 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (247).Szebeni J; Barenholz Y Complement Activation, Immunogenicity and Immune Suppression as Potential Side Effects of Liposomes; 2012; pp 309–334. 10.1201/b11620-12. [DOI] [Google Scholar]
- (248).Ishida T; Harashima H; Kiwada H Liposome Clearance. Biosci. Rep. 2002, 22, 197–224. 10.1023/A:1020134521778. [DOI] [PubMed] [Google Scholar]
- (249).Cullis PR; Chonn A; Semple SC Interactions of Liposomes and Lipid-Based Carrier Systems with Blood Proteins: Relation to Clearance Behaviour in Vivo. Adv. Drug Deliv. Rev. 1998, 32 (1–2), 3–17. 10.1016/s0169-409x(97)00128-2. [DOI] [PubMed] [Google Scholar]
- (250).Inglut CT; Sorrin AJ; Kuruppu T; Vig S; Cicalo J; Ahmad H; Huang H-C Immunological and Toxicological Considerations for the Design of Liposomes. Nanomater. (Basel, Switzerland) 2020, 10 (2), 190. 10.3390/nano10020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (251).Saadati R; Dadashzadeh S; Abbasian Z; Soleimanjahi H Accelerated Blood Clearance of PEGylated PLGA Nanoparticles Following Repeated Injections: Effects of Polymer Dose, PEG Coating, and Encapsulated Anticancer Drug. Pharm. Res. 2013, 30 (4), 985–995. 10.1007/s11095-012-0934-y. [DOI] [PubMed] [Google Scholar]
- (252).Fornaguera C; Calderó G; Mitjans M; Vinardell MP; Solans C; Vauthier C Interactions of PLGA Nanoparticles with Blood Components: Protein Adsorption, Coagulation, Activation of the Complement System and Hemolysis Studies. Nanoscale 2015, 7 (14), 6045–6058. 10.1039/C5NR00733J. [DOI] [PubMed] [Google Scholar]
- (253).Dams ETM; Laverman P; Oyen WJG; Storm G; Scherphof GL; Van Der Meer JWM; Corstens FHM; Boerman OC Accelerated Blood Clearance and Altered Biodistribution of Repeated Injections of Sterically Stabilized Liposomes. J. Pharmacol. Exp. Ther. 2000. [PubMed] [Google Scholar]
- (254).Moghimi SM; Hunter AC Capture of Stealth Nanoparticles by the Body’s Defences. Crit. Rev. Ther. Drug Carrier Syst. 2001, 18 (6), 527–550. [PubMed] [Google Scholar]
- (255).Szebeni J Complement Activation-Related Pseudoallergy: A New Class of Drug-Induced Acute Immune Toxicity. Toxicology 2005, 216 (2–3), 106–121. 10.1016/j.tox.2005.07.023. [DOI] [PubMed] [Google Scholar]
- (256).Prabhakar U; Maeda H; Jain RK; Sevick-Muraca EM; Zamboni W; Farokhzad OC; Barry ST; Gabizon A; Grodzinski P; Blakey DC Challenges and Key Considerations of the Enhanced Permeability and Retention Effect for Nanomedicine Drug Delivery in Oncology. Cancer research. April 2013, pp 2412–2417. 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (257).Eetezadi S; Ekdawi SN; Allen C The Challenges Facing Block Copolymer Micelles for Cancer Therapy: In Vivo Barriers and Clinical Translation. Adv. Drug Deliv. Rev. 2015, 91, 7–22. 10.1016/j.addr.2014.10.001. [DOI] [PubMed] [Google Scholar]
- (258).LI C; ZHANG J; ZU Y-J; NIE S-F; CAO J; WANG Q; NIE S-P; DENG Z-Y; XIE M-Y; WANG S Biocompatible and Biodegradable Nanoparticles for Enhancement of Anti-Cancer Activities of Phytochemicals. Chin. J. Nat. Med. 2015, 13 (9), 641–652. 10.1016/S1875-5364(15)30061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (259).Rani S; Ritter T The Exosome - A Naturally Secreted Nanoparticle and Its Application to Wound Healing. Adv. Mater. 2016, 28 (27), 5542–5552. 10.1002/adma.201504009. [DOI] [PubMed] [Google Scholar]
- (260).Göran Ronquist K Extracellular Vesicles and Energy Metabolism. Clin. Chim. Acta 2019, 488, 116–121. 10.1016/j.cca.2018.10.044. [DOI] [PubMed] [Google Scholar]
- (261).Baquir B; Hancock REW Exosomes, Your Body’s Answer to Immune Health. Ann. Transl. Med. 2017, 5 (4), 81. 10.21037/atm.2017.01.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (262).Fu W; Lei C; Liu S; Cui Y; Wang C; Qian K; Li T; Shen Y; Fan X; Lin F; CAR Exosomes Derived from Effector CAR-T Cells Have Potent Antitumour Effects and Low Toxicity. Nat. Commun. 2019, 10 (1), 4355. 10.1038/s41467-019-12321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (263).Agrawal AK; Aqil F; Jeyabalan J; Spencer WA; Beck J; Gachuki BW; Alhakeem SS; Oben K; Munagala R; Bondada S; Milk-Derived Exosomes for Oral Delivery of Paclitaxel. Nanomedicine Nanotechnology, Biol. Med. 2017, 13 (5), 1627–1636. 10.1016/j.nano.2017.03.001. [DOI] [PubMed] [Google Scholar]
- (264).Turturici G; Tinnirello R; Sconzo G; Geraci F Extracellular Membrane Vesicles as a Mechanism of Cell-to-Cell Communication: Advantages and Disadvantages. Am. J. Physiol. Physiol. 2014, 306 (7), C621–C633. 10.1152/ajpcell.00228.2013. [DOI] [PubMed] [Google Scholar]
- (265).Kalimuthu S; Gangadaran P; Rajendran RL; Zhu L; Oh JM; Lee HW; Gopal A; Baek SH; Jeong SY; Lee S-W; A New Approach for Loading Anticancer Drugs Into Mesenchymal Stem Cell-Derived Exosome Mimetics for Cancer Therapy. Frontiers in Pharmacology. 2018, p 1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (266).Rani S; Ryan AE; Griffin MD; Ritter T Mesenchymal Stem Cell-Derived Extracellular Vesicles: Toward Cell-Free Therapeutic Applications. Mol. Ther. 2015, 23 (5), 812–823. 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (267).Gomzikova MO; James V; Rizvanov AA Therapeutic Application of Mesenchymal Stem Cells Derived Extracellular Vesicles for Immunomodulation. Frontiers in Immunology. 2019, p 2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (268).van den Boorn JG; Schlee M; Coch C; Hartmann G SiRNA Delivery with Exosome Nanoparticles. Nat. Biotechnol. 2011, 29 (4), 325–326. 10.1038/nbt.1830. [DOI] [PubMed] [Google Scholar]
- (269).Lin Y; Wu J; Gu W; Huang Y; Tong Z; Huang L; Tan J Exosome–Liposome Hybrid Nanoparticles Deliver CRISPR/Cas9 System in MSCs. Adv. Sci. 2018, 5 (4), 1700611. 10.1002/advs.201700611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (270).Sun D; Zhuang X; Xiang X; Liu Y; Zhang S; Liu C; Barnes S; Grizzle W; Miller D; Zhang H-G A Novel Nanoparticle Drug Delivery System: The Anti-Inflammatory Activity of Curcumin Is Enhanced When Encapsulated in Exosomes. Mol. Ther. 2010, 18 (9), 1606–1614. 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (271).Ravindran J; Prasad S; Aggarwal BB Curcumin and Cancer Cells: How Many Ways Can Curry Kill Tumor Cells Selectively? AAPS J. 2009, 11 (3), 495–510. 10.1208/s12248-009-9128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (272).Aggarwal BB; Harikumar KB Potential Therapeutic Effects of Curcumin, the Anti-Inflammatory Agent, against Neurodegenerative, Cardiovascular, Pulmonary, Metabolic, Autoimmune and Neoplastic Diseases. Int. J. Biochem. Cell Biol. 2009, 41 (1), 40–59. 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (273).Anand P; Sundaram C; Jhurani S; Kunnumakkara AB; Aggarwal BB Curcumin and Cancer: An “Old-Age” Disease with an “Age-Old” Solution. Cancer Lett. 2008, 267 (1), 133–164. 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- (274).Lv M; Zhu X; Chen W; Zhong S; Hu Q; Ma T; Zhang J; Chen L; Tang J; Zhao J Exosomes Mediate Drug Resistance Transfer in MCF-7 Breast Cancer Cells and a Probable Mechanism Is Delivery of P-Glycoprotein. Tumor Biol. 2014, 35 (11), 10773–10779. 10.1007/s13277-014-2377-z. [DOI] [PubMed] [Google Scholar]
- (275).Raposo G; Nijman HW; Stoorvogel W; Leijendekker R; Harding CV; Melief CJM; Geuze HJ B Lymphocytes Secrete Antigen-Presenting Vesicles. J. Exp. Med. 1996. 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (276).Chaput N; Théry C Exosomes: Immune Properties and Potential Clinical Implementations. Semin. Immunopathol. 2010, 33, 419–440. 10.1007/s00281-010-0233-9. [DOI] [PubMed] [Google Scholar]
- (277).Bianco NR; Kim S-H; Morelli AE; Robbins PD Modulation of the Immune Response Using Dendritic Cell-Derived Exosomes. Methods Mol. Biol. 2007, 380, 443–455. 10.1007/978-1-59745-395-0_28. [DOI] [PubMed] [Google Scholar]
- (278).Chen W; Huang Y; Han J; Yu L; Li Y; Lu Z; Li H; Liu Z; Shi C; Duan F; Immunomodulatory Effects of Mesenchymal Stromal Cells-Derived Exosome. Immunol. Res. 2016, 64 (4), 831–840. 10.1007/s12026-016-8798-6. [DOI] [PubMed] [Google Scholar]
- (279).Chen W; Wang J; Shao C; Liu S; Yu Y; Wang Q; Cao X Efficient Induction of Antitumor T Cell Immunity by Exosomes Derived from Heat-Shocked Lymphoma Cells. Eur. J. Immunol. 2006, 36 (6), 1598–1607. 10.1002/eji.200535501. [DOI] [PubMed] [Google Scholar]
- (280).Zitvogel L; Regnault A; Lozier A; Wolfers J; Flament C; Tenza D; Ricciardi-Castagnoli P; Raposo G; Amigorena S Eradication of Established Murine Tumors Using a Novel Cell-Free Vaccine: Dendritic Cell Derived Exosomes. Nat. Med. 1998, 4 (5), 594–600. 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- (281).Kaur S; Singh SP; Elkahloun AG; Wu W; Abu-Asab MS; Roberts DD CD47-Dependent Immunomodulatory and Angiogenic Activities of Extracellular Vesicles Produced by T Cells. Matrix Biol. 2014, 37, 49–59. 10.1016/j.matbio.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (282).Aucher A; Rudnicka D; Davis DM MicroRNAs Transfer from Human Macrophages to Hepato-Carcinoma Cells and Inhibit Proliferation. J. Immunol. 2013, 191 (12), 6250–6260. 10.4049/jimmunol.1301728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (283).Pascucci L; Coccè V; Bonomi A; Ami D; Ceccarelli P; Ciusani E; Viganò L; Locatelli A; Sisto F; Doglia SM; Paclitaxel Is Incorporated by Mesenchymal Stromal Cells and Released in Exosomes That Inhibit in Vitro Tumor Growth: A New Approach for Drug Delivery. J. Control. Release 2014, 192, 262–270. 10.1016/j.jconrel.2014.07.042. [DOI] [PubMed] [Google Scholar]
- (284).Yan L; Shen J; Wang J; Yang X; Dong S; Lu S Nanoparticle-Based Drug Delivery System: A Patient-Friendly Chemotherapy for Oncology. Dose. Response. 2020, 18 (3), 1559325820936161–1559325820936161. 10.1177/1559325820936161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (285).Zhuang X; Xiang X; Grizzle W; Sun D; Zhang S; Axtell RC; Ju S; Mu J; Zhang L; Steinman L; Treatment of Brain Inflammatory Diseases by Delivering Exosome Encapsulated Anti-Inflammatory Drugs from the Nasal Region to the Brain. Mol. Ther. 2011, 19 (10), 1769–1779. 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (286).Dhillon N; Aggarwal BB; Newman RA; Wolff RA; Kunnumakkara AB; Abbruzzese JL; Ng CS; Badmaev V; Kurzrock R Phase II Trial of Curcumin in Patients with Advanced Pancreatic Cancer. Clin. Cancer Res. 2008, 14 (14), 4491–4499. 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- (287).Lai RC; Arslan F; Lee MM; Sze NSK; Choo A; Chen TS; Salto-Tellez M; Timmers L; Lee CN; El Oakley RM; Exosome Secreted by MSC Reduces Myocardial Ischemia/Reperfusion Injury. Stem Cell Res. 2010, 4 (3), 214–222. 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- (288).Arslan F; Lai RC; Smeets MB; Akeroyd L; Choo A; Aguor ENE; Timmers L; van Rijen HV; Doevendans PA; Pasterkamp G; Mesenchymal Stem Cell-Derived Exosomes Increase ATP Levels, Decrease Oxidative Stress and Activate PI3K/Akt Pathway to Enhance Myocardial Viability and Prevent Adverse Remodeling after Myocardial Ischemia/Reperfusion Injury. Stem Cell Res. 2013, 10 (3), 301–312. 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- (289).Katsuda T; Tsuchiya R; Kosaka N; Yoshioka Y; Takagaki K; Oki K; Takeshita F; Sakai Y; Kuroda M; Ochiya T Human Adipose Tissue-Derived Mesenchymal Stem Cells Secrete Functional Neprilysin-Bound Exosomes. Sci. Rep. 2013, 3, 1197. 10.1038/srep01197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (290).Huang L; Ma W; Ma Y; Feng D; Chen H; Cai B Exosomes in Mesenchymal Stem Cells, a New Therapeutic Strategy for Cardiovascular Diseases? Int. J. Biol. Sci. 2015, 11 (2), 238–245. 10.7150/ijbs.10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (291).Susmita S; W. LD Exosomes and Cardiac Repair After Myocardial Infarction. Circ. Res. 2014, 114 (2), 333–344. 10.1161/CIRCRESAHA.114.300639. [DOI] [PubMed] [Google Scholar]
- (292).El-Andaloussi S; Lee Y; Lakhal-Littleton S; Li J; Seow Y; Gardiner C; Alvarez-Erviti L; Sargent IL; Wood MJA Exosome-Mediated Delivery of SiRNA in Vitro and in Vivo. Nat. Protoc. 2012, 7 (12), 2112–2126. 10.1038/nprot.2012.131. [DOI] [PubMed] [Google Scholar]
- (293).Lee Y; EL Andaloussi S; Wood MJA Exosomes and Microvesicles: Extracellular Vesicles for Genetic Information Transfer and Gene Therapy. Hum. Mol. Genet. 2012, 21 (R1), R125–R134. 10.1093/hmg/dds317. [DOI] [PubMed] [Google Scholar]
- (294).Shtam TA; Kovalev RA; Varfolomeeva EY; Makarov EM; Kil YV; Filatov MV Exosomes Are Natural Carriers of Exogenous SiRNA to Human Cells in Vitro. Cell Commun. Signal. 2013, 11, 88. 10.1186/1478-811X-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (295).Wahlgren J; De L Karlson T; Brisslert M; Vaziri Sani F; Telemo E; Sunnerhagen P; Valadi H Plasma Exosomes Can Deliver Exogenous Short Interfering RNA to Monocytes and Lymphocytes. Nucleic Acids Res. 2012, 40 (17), e130–e130. 10.1093/nar/gks463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (296).Alvarez-Erviti L; Seow Y; Yin H; Betts C; Lakhal S; Wood MJA Delivery of SiRNA to the Mouse Brain by Systemic Injection of Targeted Exosomes. Nat. Biotechnol. 2011, 29 (4), 341–345. 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- (297).Yerneni SS; Lathwal S; Shrestha P; Shirwan H; Matyjaszewski K; Weiss L; Yolcu ES; Campbell PG; Das SR Rapid On-Demand Extracellular Vesicle Augmentation with Versatile Oligonucleotide Tethers. ACS Nano 2019, 13 (9), 10555–10565. 10.1021/acsnano.9b04651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (298).Li J; Liu K; Liu Y; Xu Y; Zhang F; Yang H; Liu J; Pan T; Chen J; Wu M; Exosomes Mediate the Cell-to-Cell Transmission of IFN-α-Induced Antiviral Activity. Nat. Immunol. 2013, 14 (8), 793–803. 10.1038/ni.2647. [DOI] [PubMed] [Google Scholar]
- (299).Pitt JM; Charrier M; Viaud S; André F; Besse B; Chaput N; Zitvogel L Dendritic Cell–Derived Exosomes as Immunotherapies in the Fight against Cancer. J. Immunol. 2014, 193 (3), 1006 LP – 1011. 10.4049/jimmunol.1400703. [DOI] [PubMed] [Google Scholar]
- (300).Colino J; Snapper CM Exosomes from Bone Marrow Dendritic Cells Pulsed with Diphtheria Toxoid Preferentially Induce Type 1 Antigen-Specific IgG Responses in Naive Recipients in the Absence of Free Antigen. J. Immunol. 2006, 177 (6), 3757–3762. 10.4049/jimmunol.177.6.3757. [DOI] [PubMed] [Google Scholar]
- (301).Théry C; Regnault A; Garin J; Wolfers J; Zitvogel L; Ricciardi-Castagnoli P; Raposo G; Amigorena S Molecular Characterization of Dendritic Cell-Derived Exosomes. Selective Accumulation of the Heat Shock Protein Hsc73. J. Cell Biol. 1999, 147 (3), 599–610. 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (302).Rosenberg SA; Restifo NP Adoptive Cell Transfer as Personalized Immunotherapy for Human Cancer. Science 2015, 348 (6230), 62–68. 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (303).Lu Z; Zuo B; Jing R; Gao X; Rao Q; Liu Z; Qi H; Guo H; Yin H Dendritic Cell-Derived Exosomes Elicit Tumor Regression in Autochthonous Hepatocellular Carcinoma Mouse Models. J. Hepatol. 2017, 67 (4), 739–748. 10.1016/j.jhep.2017.05.019. [DOI] [PubMed] [Google Scholar]
- (304).Geis-Asteggiante L; Belew AT; Clements VK; Edwards NJ; Ostrand-Rosenberg S; El-Sayed NM; Fenselau C Differential Content of Proteins, MRNAs, and MiRNAs Suggests That MDSC and Their Exosomes May Mediate Distinct Immune Suppressive Functions. J. Proteome Res. 2018, 17 (1), 486–498. 10.1021/acs.jproteome.7b00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (305).Di Bonito P; Accardi L; Galati L; Ferrantelli F; Federico M Anti-Cancer Vaccine for HPV-Associated Neoplasms: Focus on a Therapeutic HPV Vaccine Based on a Novel Tumor Antigen Delivery Method Using Endogenously Engineered Exosomes. Cancers. Department of Infectious Diseases, Viral Hepatitis, Oncoviruses and Retroviruses (EVOR) unit, Istituto Superiore di Sanità, Viale Regina Elena 299, 00161 Rome, Italy. paola.dibonito@iss.it. 2019. 10.3390/cancers11020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (306).McElroy AK; Akondy RS; Davis CW; Ellebedy AH; Mehta AK; Kraft CS; Lyon GM; Ribner BS; Varkey J; Sidney J; Human Ebola Virus Infection Results in Substantial Immune Activation. Proc. Natl. Acad. Sci. U. S. A. 2015, 112 (15), 4719–4724. 10.1073/pnas.1502619112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (307).Wu X-M; Liao Y-W; Wang H-Y; Ji K-Q; Li G-F; Zang B Integrin Alphavbeta6 Is Involved in Measles Protein-Induced Airway Immune Suppression. Cytokine 2012, 59 (1), 59–64. 10.1016/j.cyto.2012.04.005. [DOI] [PubMed] [Google Scholar]
- (308).Li X; Li J-J; Yang J-Y; Wang D-S; Zhao W; Song W-J; Li W-M; Wang J-F; Han W; Zhang Z-C; Tolerance Induction by Exosomes from Immature Dendritic Cells and Rapamycin in a Mouse Cardiac Allograft Model. PLoS One 2012, 7 (8), e44045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (309).Song J; Huang J; Chen X; Teng X; Song Z; Xing Y; Wang M; Chen K; Wang Z; Yang P; Donor-Derived Exosomes Induce Specific Regulatory T Cells to Suppress Immune Inflammation in the Allograft Heart. Sci. Rep. 2016, 6 (1), 20077. 10.1038/srep20077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (310).Califf RM Biomarker Definitions and Their Applications. Exp. Biol. Med. (Maywood). 2018, 243 (3), 213–221. 10.1177/1535370217750088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (311).Mirzakhani M; Shahbazi M; Oliaei F; Mohammadnia-Afrouzi M Immunological Biomarkers of Tolerance in Human Kidney Transplantation: An Updated Literature Review. J. Cell. Physiol. 2019, 234 (5), 5762–5774. 10.1002/jcp.27480. [DOI] [PubMed] [Google Scholar]
- (312).Kennel PJ; Saha A; Maldonado DA; Givens R; Brunjes DL; Castillero E; Zhang X; Ji R; Yahi A; George I; Serum Exosomal Protein Profiling for the Non-Invasive Detection of Cardiac Allograft Rejection. J. Hear. Lung Transplant. 2018, 37 (3), 409–417. 10.1016/j.healun.2017.07.012. [DOI] [PubMed] [Google Scholar]
- (313).Stickney Z; Losacco J; McDevitt S; Zhang Z; Lu B Development of Exosome Surface Display Technology in Living Human Cells. Biochem. Biophys. Res. Commun. 2016, 472 (1), 53–59. 10.1016/j.bbrc.2016.02.058. [DOI] [PubMed] [Google Scholar]
- (314).Jeyaram A; Jay SM Preservation and Storage Stability of Extracellular Vesicles for Therapeutic Applications. AAPS J. 2017, 20 (1), 1. 10.1208/s12248-017-0160-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (315).Logozzi M; De Milito A; Lugini L; Borghi M; Calabrò L; Spada M; Perdicchio M; Marino ML; Federici C; Iessi E; High Levels of Exosomes Expressing CD63 and Caveolin-1 in Plasma of Melanoma Patients. PLoS One 2009, 4 (4), e5219–e5219. 10.1371/journal.pone.0005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (316).Lu J; Li J; Liu S; Wang T; Ianni A; Bober E; Braun T; Xiang R; Yue S Exosomal Tetraspanins Mediate Cancer Metastasis by Altering Host Microenvironment. Oncotarget 2017, 8 (37), 62803–62815. 10.18632/oncotarget.19119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (317).Welker M-W; Reichert D; Susser S; Sarrazin C; Martinez Y; Herrmann E; Zeuzem S; Piiper A; Kronenberger B Soluble Serum CD81 Is Elevated in Patients with Chronic Hepatitis C and Correlates with Alanine Aminotransferase Serum Activity. PLoS One 2012, 7 (2), e30796–e30796. 10.1371/journal.pone.0030796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (318).Ashraf Malik M; Ishtiyaq Ali Mirza J; Umar M; Manzoor S CD81(+) Exosomes Play a Pivotal Role in the Establishment of Hepatitis C Persistent Infection and Contribute Toward the Progression of Hepatocellular Carcinoma. Viral Immunol. 2019, 32 (10), 453–462. 10.1089/vim.2019.0077. [DOI] [PubMed] [Google Scholar]
- (319).Chettimada S; Lorenz DR; Misra V; Dillon ST; Reeves RK; Manickam C; Morgello S; Kirk GD; Mehta SH; Gabuzda D Exosome Markers Associated with Immune Activation and Oxidative Stress in HIV Patients on Antiretroviral Therapy. Sci. Rep. 2018, 8 (1), 7227. 10.1038/s41598-018-25515-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (320).Sandfeld-Paulsen B; Jakobsen KR; Bæk R; Folkersen BH; Rasmussen TR; Meldgaard P; Varming K; Jørgensen MM; Sorensen BS Exosomal Proteins as Diagnostic Biomarkers in Lung Cancer. J. Thorac. Oncol. 2016, 11 (10), 1701–1710. 10.1016/j.jtho.2016.05.034. [DOI] [PubMed] [Google Scholar]
- (321).Melo SA; Luecke LB; Kahlert C; Fernandez AF; Gammon ST; Kaye J; LeBleu VS; Mittendorf EA; Weitz J; Rahbari N; Glypican-1 Identifies Cancer Exosomes and Detects Early Pancreatic Cancer. Nature 2015, 523 (7559), 177–182. 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (322).Azmi AS; Bao B; Sarkar FH Exosomes in Cancer Development, Metastasis, and Drug Resistance: A Comprehensive Review. Cancer Metastasis Rev. 2013, 32 (3–4), 623–642. 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (323).Zhou H; Yuen PST; Pisitkun T; Gonzales PA; Yasuda H; Dear JW; Gross P; Knepper MA; Star RA Collection, Storage, Preservation, and Normalization of Human Urinary Exosomes for Biomarker Discovery. Kidney Int. 2006, 69 (8), 1471–1476. 10.1038/sj.ki.5000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (324).Knepper MA Common Sense Approaches to Urinary Biomarker Study Design. J. Am. Soc. Nephrol. 2009, 20 (6), 1175 LP – 1178. 10.1681/ASN.2009030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (325).Gonzales PA; Pisitkun T; Hoffert JD; Tchapyjnikov D; Star RA; Kleta R; Wang NS; Knepper MA Large-Scale Proteomics and Phosphoproteomics of Urinary Exosomes. J. Am. Soc. Nephrol. 2009, 20 (2), 363 LP – 379. 10.1681/ASN.2008040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (326).Alvarez ML; Khosroheidari M; Kanchi Ravi R; DiStefano JK Comparison of Protein, MicroRNA, and MRNA Yields Using Different Methods of Urinary Exosome Isolation for the Discovery of Kidney Disease Biomarkers. Kidney Int. 2012, 82 (9), 1024–1032. 10.1038/ki.2012.256. [DOI] [PubMed] [Google Scholar]
- (327).Zhou H; Cheruvanky A; Hu X; Matsumoto T; Hiramatsu N; Cho ME; Berger A; Leelahavanichkul A; Doi K; Chawla LS; Urinary Exosomal Transcription Factors, a New Class of Biomarkers for Renal Disease. Kidney Int. 2008, 74 (5), 613–621. 10.1038/ki.2008.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (328).Wang Z; Hill S; Luther JM; Hachey DL; Schey KL Proteomic Analysis of Urine Exosomes by Multidimensional Protein Identification Technology (MudPIT). Proteomics 2012, 12 (2), 329–338. 10.1002/pmic.201100477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (329).Miranda KC; Bond DT; McKee M; Skog J; Păunescu TG; Da Silva N; Brown D; Russo LM Nucleic Acids within Urinary Exosomes/Microvesicles Are Potential Biomarkers for Renal Disease. Kidney Int. 2010, 78 (2), 191–199. 10.1038/ki.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (330).Ferlay J; Steliarova-Foucher E; Lortet-Tieulent J; Rosso S; Coebergh JWW; Comber H; Forman D; Bray F Cancer Incidence and Mortality Patterns in Europe: Estimates for 40 Countries in 2012. Eur. J. Cancer 2013, 49 (6), 1374–1403. 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- (331).Wu L; Zhang X; Zhang B; Shi H; Yuan X; Sun Y; Pan Z; Qian H; Xu W Exosomes Derived from Gastric Cancer Cells Activate NF-ΚB Pathway in Macrophages to Promote Cancer Progression. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2016, 37 (9), 12169–12180. 10.1007/s13277-016-5071-5. [DOI] [PubMed] [Google Scholar]
- (332).Ohshima K; Inoue K; Fujiwara A; Hatakeyama K; Kanto K; Watanabe Y; Muramatsu K; Fukuda Y; Ogura S; Yamaguchi K; Let-7 MicroRNA Family Is Selectively Secreted into the Extracellular Environment via Exosomes in a Metastatic Gastric Cancer Cell Line. PLoS One 2010, 5 (10), e13247–e13247. 10.1371/journal.pone.0013247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (333).Manterola L; Guruceaga E; Gállego Pérez-Larraya J; González-Huarriz M; Jauregui P; Tejada S; Diez-Valle R; Segura V; Samprón N; Barrena C; A Small Noncoding RNA Signature Found in Exosomes of GBM Patient Serum as a Diagnostic Tool. Neuro. Oncol. 2014, 16 (4), 520–527. 10.1093/neuonc/not218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (334).Saadatpour L; Fadaee E; Fadaei S; Nassiri Mansour R; Mohammadi M; Mousavi SM; Goodarzi M; Verdi J; Mirzaei H Glioblastoma: Exosome and MicroRNA as Novel Diagnosis Biomarkers. Cancer Gene Ther. 2016, 23 (12), 415–418. 10.1038/cgt.2016.48. [DOI] [PubMed] [Google Scholar]
- (335).Masoudi MS; Mehrabian E; Mirzaei H MiR-21: A Key Player in Glioblastoma Pathogenesis. J. Cell. Biochem. 2018, 119 (2), 1285–1290. 10.1002/jcb.26300. [DOI] [PubMed] [Google Scholar]
- (336).Huang S-W; Ali N; Zhong L; Shi J MicroRNAs as Biomarkers for Human Glioblastoma: Progress and Potential. Acta Pharmacol. Sin. 2018, 39 (9), 1405–1413. 10.1038/aps.2017.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (337).Joyce DP; Kerin MJ; Dwyer RM Exosome-Encapsulated MicroRNAs as Circulating Biomarkers for Breast Cancer. Int. J. cancer 2016, 139 (7), 1443–1448. 10.1002/ijc.30179. [DOI] [PubMed] [Google Scholar]
- (338).Rodríguez-Martínez A; de Miguel-Pérez D; Ortega FG; García-Puche JL; Robles-Fernández I; Exposito J; Martorell-Marugan J; Carmona-Sáez P; Garrido-Navas MDC; Rolfo C; Exosomal MiRNA Profile as Complementary Tool in the Diagnostic and Prediction of Treatment Response in Localized Breast Cancer under Neoadjuvant Chemotherapy. Breast Cancer Res. 2019, 21 (1), 21. 10.1186/s13058-019-1109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (339).Zhu L; Liu J; Dao J; Lu K; Li H; Gu H; Liu J; Feng X; Cheng G Molecular Characterization of S. Japonicum Exosome-like Vesicles Reveals Their Regulatory Roles in Parasite-Host Interactions. Sci. Rep. 2016, 6 (1), 25885. 10.1038/srep25885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (340).Wang Y; Yuan W; Kimber M; Lu M; Dong L Rapid Differentiation of Host and Parasitic Exosome Vesicles Using Microfluidic Photonic Crystal Biosensor. ACS Sensors 2018, 3 (9), 1616–1621. 10.1021/acssensors.8b00360. [DOI] [PubMed] [Google Scholar]
- (341).Wu Z; Wang L; Li J; Wang L; Wu Z; Sun X Extracellular Vesicle-Mediated Communication Within Host-Parasite Interactions. Frontiers in Immunology. 2019, p 3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (342).Harding C; Heuser J; Stahl P Receptor-Mediated Endocytosis of Transferrin and Recycling of the Transferrin Receptor in Rat Reticulocytes. J. Cell Biol. 1983, 97 (2), 329–339. 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (343).Kamerkar S; LeBleu VS; Sugimoto H; Yang S; Ruivo CF; Melo SA; Lee JJ; Kalluri R Exosomes Facilitate Therapeutic Targeting of Oncogenic KRAS in Pancreatic Cancer. Nature 2017, 546 (7659), 498–503. 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (344).Betzer O; Barnoy E; Sadan T; Elbaz I; Braverman C; Liu Z; Popovtzer R Advances in Imaging Strategies for in Vivo Tracking of Exosomes. WIREs Nanomedicine and Nanobiotechnology 2020, 12 (2), e1594. 10.1002/wnan.1594. [DOI] [PubMed] [Google Scholar]
- (345).Gangadaran P; Hong CM; Ahn B-C An Update on in Vivo Imaging of Extracellular Vesicles as Drug Delivery Vehicles. Front. Pharmacol. 2018, 9, 169. 10.3389/fphar.2018.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (346).Stuker F; Ripoll J; Rudin M Fluorescence Molecular Tomography: Principles and Potential for Pharmaceutical Research. Pharmaceutics 2011, 3 (2), 229–274. 10.3390/pharmaceutics3020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (347).Gangadaran P; Hong CM; Ahn B-C Current Perspectives on In Vivo Noninvasive Tracking of Extracellular Vesicles with Molecular Imaging. Biomed Res. Int. 2017, 2017, 9158319. 10.1155/2017/9158319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (348).Chuo ST-Y; Chien JC-Y; Lai CP-K Imaging Extracellular Vesicles: Current and Emerging Methods. J. Biomed. Sci. 2018, 25 (1), 91. 10.1186/s12929-018-0494-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (349).Smyth T; Kullberg M; Malik N; Smith-Jones P; Graner MW; Anchordoquy TJ Biodistribution and Delivery Efficiency of Unmodified Tumor-Derived Exosomes. J. Control. Release 2015, 199, 145–155. 10.1016/j.jconrel.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (350).Close DM; Xu T; Sayler GS; Ripp S In Vivo Bioluminescent Imaging (BLI): Noninvasive Visualization and Interrogation of Biological Processes in Living Animals. Sensors (Basel). 2011, 11 (1), 180–206. 10.3390/s110100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (351).Takahashi Y; Nishikawa M; Shinotsuka H; Matsui Y; Ohara S; Imai T; Takakura Y Visualization and in Vivo Tracking of the Exosomes of Murine Melanoma B16-BL6 Cells in Mice after Intravenous Injection. J. Biotechnol. 2013, 165 (2), 77–84. 10.1016/j.jbiotec.2013.03.013. [DOI] [PubMed] [Google Scholar]
- (352).Kim T; Lee N; Arifin DR; Shats I; Janowski M; Walczak P; Hyeon T; Bulte JWM In Vivo Micro-CT Imaging of Human Mesenchymal Stem Cells Labeled with Gold-Poly- l</Scp> -Lysine Nanocomplexes. Adv. Funct. Mater. 2017, 27 (3), 1604213. 10.1002/adfm.201604213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (353).Perets N; Betzer O; Shapira R; Brenstein S; Angel A; Sadan T; Ashery U; Popovtzer R; Offen D Golden Exosomes Selectively Target Brain Pathologies in Neurodegenerative and Neurodevelopmental Disorders. Nano Lett. 2019, 19 (6), 3422–3431. 10.1021/acs.nanolett.8b04148. [DOI] [PubMed] [Google Scholar]
- (354).Betzer O; Perets N; Angel A; Motiei M; Sadan T; Yadid G; Offen D; Popovtzer R In Vivo Neuroimaging of Exosomes Using Gold Nanoparticles. ACS Nano 2017, 11 (11), 10883–10893. 10.1021/acsnano.7b04495. [DOI] [PubMed] [Google Scholar]
- (355).Weber J; Beard PC; Bohndiek SE Contrast Agents for Molecular Photoacoustic Imaging. Nat. Methods 2016, 13 (8), 639–650. 10.1038/nmeth.3929. [DOI] [PubMed] [Google Scholar]
- (356).Hu L; Wickline SA; Hood JL Magnetic Resonance Imaging of Melanoma Exosomes in Lymph Nodes. Magn. Reson. Med. 2015, 74 (1), 266–271. 10.1002/mrm.25376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (357).Jung KO; Jo H; Yu JH; Gambhir SS; Pratx G Development and MPI Tracking of Novel Hypoxia-Targeted Theranostic Exosomes. Biomaterials 2018, 177, 139–148. 10.1016/j.biomaterials.2018.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (358).Cao Y; Wu T; Zhang K; Meng X; Dai W; Wang D; Dong H; Zhang X Engineered Exosome-Mediated Near-Infrared-II Region V2C Quantum Dot Delivery for Nucleus-Target Low-Temperature Photothermal Therapy. ACS Nano 2019, 13 (2), 1499–1510. 10.1021/acsnano.8b07224. [DOI] [PubMed] [Google Scholar]
- (359).Srivastava P Interaction of Heat Shock Proteins with Peptides and Antigen Presenting Cells: Chaperoning of the Innate and Adaptive Immune Responses. Annu. Rev. Immunol. 2002, 20, 395–425. 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- (360).Gastpar R; Gehrmann M; Bausero MA; Asea A; Gross C; Schroeder JA; Multhoff G Heat Shock Protein 70 Surface-Positive Tumor Exosomes Stimulate Migratory and Cytolytic Activity of Natural Killer Cells. Cancer Res. 2005, 65 (12), 5238 LP – 5247. 10.1158/0008-5472.CAN-04-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (361).Perez-Hernandez D; Gutiérrez-Vázquez C; Jorge I; López-Martín S; Ursa A; Sánchez-Madrid F; Vázquez J; Yáñez-Mó M The Intracellular Interactome of Tetraspanin-Enriched Microdomains Reveals Their Function as Sorting Machineries toward Exosomes. J. Biol. Chem. 2013, 288 (17), 11649–11661. 10.1074/jbc.M112.445304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (362).Ahmed W; Philip PS; Attoub S; Khan G Epstein–Barr Virus-Infected Cells Release Fas Ligand in Exosomal Fractions and Induce Apoptosis in Recipient Cells via the Extrinsic Pathway. J. Gen. Virol. 2015, 96 (12), 3646–3659. 10.1099/jgv.0.000313. [DOI] [PubMed] [Google Scholar]
- (363).Gutiérrez-Vázquez C; Villarroya-Beltri C; Mittelbrunn M; Sánchez-Madrid F Transfer of Extracellular Vesicles during Immune Cell-Cell Interactions. Immunol. Rev. 2013, 251 (1), 125–142. 10.1111/imr.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (364).Wen C; Seeger RC; Fabbri M; Wang L; Wayne AS; Jong AY Biological Roles and Potential Applications of Immune Cell-Derived Extracellular Vesicles. J. Extracell. vesicles 2017, 6 (1), 1400370. 10.1080/20013078.2017.1400370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (365).Assil S; Webster B; Dreux M Regulation of the Host Antiviral State by Intercellular Communications. Viruses 2015, 7 (8), 4707–4733. 10.3390/v7082840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (366).Qian C; Cao X Dendritic Cells in the Regulation of Immunity and Inflammation. Semin. Immunol. 2018, 35, 3–11. 10.1016/j.smim.2017.12.002. [DOI] [PubMed] [Google Scholar]
- (367).Hezel M. E. van; Nieuwland R; Bruggen R. van; Juffermans NP The Ability of Extracellular Vesicles to Induce a Pro-Inflammatory Host Response. Int. J. Mol. Sci. 2017, 18 (6), 1285. 10.3390/ijms18061285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (368).Wang M; Zhao J; Zhang L; Wei F; Lian Y; Wu Y; Gong Z; Zhang S; Zhou J; Cao K; Role of Tumor Microenvironment in Tumorigenesis. J. Cancer 2017, 8 (5), 761–773. 10.7150/jca.17648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (369).Becker A; Thakur BK; Weiss JM; Kim HS; Peinado H; Lyden D Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell. 2016. 10.1016/j.ccell.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (370).Desrochers LM; Antonyak MA; Cerione RA Extracellular Vesicles: Satellites of Information Transfer in Cancer and Stem Cell Biology. Dev. Cell 2016, 37 (4), 301–309. 10.1016/j.devcel.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (371).McAllister SS; Weinberg RA The Tumour-Induced Systemic Environment as a Critical Regulator of Cancer Progression and Metastasis. Nat. Cell Biol. 2014, 16 (8), 717–727. 10.1038/ncb3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (372).Noy R; Pollard JW Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity 2014, 41 (1), 49–61. 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (373).Sisquella X; Ofir-Birin Y; Pimentel MA; Cheng L; Abou Karam P; Sampaio NG; Penington JS; Connolly D; Giladi T; Scicluna BJ; Malaria Parasite DNA-Harbouring Vesicles Activate Cytosolic Immune Sensors. Nature communications. The Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, VIC, 3052, Australia. 2017, p 1985. 10.1038/s41467-017-02083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (374).Schorey JS; Cheng Y; Singh PP; Smith VL Exosomes and Other Extracellular Vesicles in Host-Pathogen Interactions. EMBO Rep. 2015, 16 (1), 24–43. 10.15252/embr.201439363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (375).Pejawar-Gaddy S; Finn OJ Cancer Vaccines: Accomplishments and Challenges. Crit. Rev. Oncol. Hematol. 2008, 67 (2), 93–102. 10.1016/j.critrevonc.2008.02.010. [DOI] [PubMed] [Google Scholar]
- (376).Hartman ZC; Wei J; Glass OK; Guo H; Lei G; Yang X-Y; Osada T; Hobeika A; Delcayre A; Le Pecq J-B; Increasing Vaccine Potency through Exosome Antigen Targeting. Vaccine 2011, 29 (50), 9361–9367. 10.1016/j.vaccine.2011.09.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (377).Peters PJ; Geuze HJ; Van der Donk HA; Slot JW; Griffith JM; Stam NJ; Clevers HC; Borst J Molecules Relevant for T Cell-Target Cell Interaction Are Present in Cytolytic Granules of Human T Lymphocytes. Eur. J. Immunol. 1989, 19 (8), 1469–1475. 10.1002/eji.1830190819. [DOI] [PubMed] [Google Scholar]
- (378).Peters PJ; Borst J; Oorschot V; Fukuda M; Krähenbühl O; Tschopp J; Slot JW; Geuze HJ Cytotoxic T Lymphocyte Granules Are Secretory Lysosomes, Containing Both Perforin and Granzymes. J. Exp. Med. 1991, 173 (5), 1099–1109. 10.1084/jem.173.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (379).Li Y; Liu Y; Xiu F; Wang J; Cong H; He S; Shi Y; Wang X; Li X; Zhou H Characterization of Exosomes Derived from Toxoplasma Gondii and Their Functions in Modulating Immune Responses. Int. J. Nanomedicine 2018, 13, 467–477. 10.2147/IJN.S151110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (380).Roier S; Blume T; Klug L; Wagner GE; Elhenawy W; Zangger K; Prassl R; Reidl J; Daum G; Feldman MF; A Basis for Vaccine Development: Comparative Characterization of Haemophilus Influenzae Outer Membrane Vesicles. Int. J. Med. Microbiol. 2015, 305 (3), 298–309. 10.1016/j.ijmm.2014.12.005. [DOI] [PubMed] [Google Scholar]
- (381).Rajabi M; Mousa SA The Role of Angiogenesis in Cancer Treatment. Biomedicines 2017, 5 (2), 34. 10.3390/biomedicines5020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (382).Teleanu RI; Chircov C; Grumezescu AM; Teleanu DM Tumor Angiogenesis and Anti-Angiogenic Strategies for Cancer Treatment. J. Clin. Med. 2019, 9 (1), 84. 10.3390/jcm9010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (383).Zhang C; Ji Q; Yang Y; Li Q; Wang Z Exosome: Function and Role in Cancer Metastasis and Drug Resistance. Technol. Cancer Res. Treat. 2018, 17, 1533033818763450–1533033818763450. 10.1177/1533033818763450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (384).Manier S; Liu C-J; Avet-Loiseau H; Park J; Shi J; Campigotto F; Salem KZ; Huynh D; Glavey SV; Rivotto B; Prognostic Role of Circulating Exosomal MiRNAs in Multiple Myeloma. Blood 2017, 129 (17), 2429–2436. 10.1182/blood-2016-09-742296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (385).Yuwen D; Ma Y; Wang D; Gao J; Li X; Xue W; Fan M; Xu Q; Shen Y; Shu Y Prognostic Role of Circulating Exosomal MiR-425–3p for the Response of NSCLC to Platinum-Based Chemotherapy. Cancer Epidemiol. Biomarkers & Prev. 2019, 28 (1), 163 LP – 173. 10.1158/1055-9965.EPI-18-0569. [DOI] [PubMed] [Google Scholar]
- (386).Al-Nedawi K; Meehan B; Kerbel RS; Allison AC; Rak J Endothelial Expression of Autocrine VEGF upon the Uptake of Tumor-Derived Microvesicles Containing Oncogenic EGFR. Proc. Natl. Acad. Sci. U. S. A. 2009, 106 (10), 3794–3799. 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (387).Al-Nedawi K; Meehan B; Micallef J; Lhotak V; May L; Guha A; Rak J Intercellular Transfer of the Oncogenic Receptor EGFRvIII by Microvesicles Derived from Tumour Cells. Nat. Cell Biol. 2008, 10 (5), 619–624. 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- (388).Lucero R; Zappulli V; Sammarco A; Murillo OD; Cheah PS; Srinivasan S; Tai E; Ting DT; Wei Z; Roth ME; Glioma-Derived MiRNA-Containing Extracellular Vesicles Induce Angiogenesis by Reprogramming Brain Endothelial Cells. Cell Rep. 2020, 30 (7), 2065–2074.e4. 10.1016/j.celrep.2020.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (389).Todorova D; Simoncini S; Lacroix R; Sabatier F; Dignat-George F Extracellular Vesicles in Angiogenesis. Circ. Res. 2017, 120 (10), 1658–1673. 10.1161/CIRCRESAHA.117.309681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (390).Mamdani H; Ahmed S; Armstrong S; Mok T; Jalal SI Blood-Based Tumor Biomarkers in Lung Cancer for Detection and Treatment. Transl. lung cancer Res. 2017, 6 (6), 648–660. 10.21037/tlcr.2017.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (391).NCT03581435. A Study of Circulating Exosome Proteomics In Gallbladder Carcinoma Patients (EXOGBC001). [Google Scholar]
- (392).Shao Y; Shen Y; Chen T; Xu F; Chen X; Zheng S The Functions and Clinical Applications of Tumor-Derived Exosomes. Oncotarget 2016, 7 (37), 60736–60751. 10.18632/oncotarget.11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (393).Fu M; Gu J; Jiang P; Qian H; Xu W; Zhang X Exosomes in Gastric Cancer: Roles, Mechanisms, and Applications. Mol. Cancer 2019, 18 (1), 41. 10.1186/s12943-019-1001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (394).Sun Z; Shi K; Yang S; Liu J; Zhou Q; Wang G; Song J; Li Z; Zhang Z; Yuan W Effect of Exosomal MiRNA on Cancer Biology and Clinical Applications. Mol. Cancer 2018, 17 (1), 147. 10.1186/s12943-018-0897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (395).Wu Q; Wu X; Ying X; Zhu Q; Wang X; Jiang L; Chen X; Wu Y; Wang X Suppression of Endothelial Cell Migration by Tumor Associated Macrophage-Derived Exosomes Is Reversed by Epithelial Ovarian Cancer Exosomal LncRNA. Cancer Cell Int. 2017, 17 (1), 62. 10.1186/s12935-017-0430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (396).Mannavola F; Salerno T; Passarelli A; Tucci M; Internò V; Silvestris F Revisiting the Role of Exosomes in Colorectal Cancer: Where Are We Now? Front. Oncol. 2019, 9, 521. 10.3389/fonc.2019.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (397).Li T; Yan Y; Wang B; Qian H; Zhang X; Shen L; Wang M; Zhou Y; Zhu W; Li W; Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Liver Fibrosis. Stem Cells Dev. 2012, 22 (6), 845–854. 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (398).Nakamura Y; Miyaki S; Ishitobi H; Matsuyama S; Nakasa T; Kamei N; Akimoto T; Higashi Y; Ochi M Mesenchymal-Stem-Cell-Derived Exosomes Accelerate Skeletal Muscle Regeneration. FEBS Lett. 2015, 589 (11), 1257–1265. 10.1016/j.febslet.2015.03.031. [DOI] [PubMed] [Google Scholar]
- (399).Zhang B; Yeo RWY; Tan KH; Lim SK Focus on Extracellular Vesicles: Therapeutic Potential of Stem Cell-Derived Extracellular Vesicles. Int. J. Mol. Sci. 2016, 17 (2), 174. 10.3390/ijms17020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (400).Balbi C; Piccoli M; Barile L; Papait A; Armirotti A; Principi E; Reverberi D; Pascucci L; Becherini P; Varesio L; First Characterization of Human Amniotic Fluid Stem Cell Extracellular Vesicles as a Powerful Paracrine Tool Endowed with Regenerative Potential. Stem Cells Transl. Med. 2017, 6 (5), 1340–1355. 10.1002/sctm.16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (401).Andrews DW; Resnicoff M; Flanders AE; Kenyon L; Curtis M; Merli G; Baserga R; Iliakis G; Aiken RD Results of a Pilot Study Involving the Use of an Antisense Oligodeoxynucleotide Directed against the Insulin-like Growth Factor Type I Receptor in Malignant Astrocytomas. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2001, 19 (8), 2189–2200. 10.1200/JCO.2001.19.8.2189. [DOI] [PubMed] [Google Scholar]
- (402).Cage TA; Lamborn KR; Ware ML; Frankfurt A; Chakalian L; Berger MS; McDermott MW Adjuvant Enoxaparin Therapy May Decrease the Incidence of Postoperative Thrombotic Events Though Does Not Increase the Incidence of Postoperative Intracranial Hemorrhage in Patients with Meningiomas. J. Neurooncol. 2009, 93 (1), 151–156. 10.1007/s11060-009-9886-4. [DOI] [PubMed] [Google Scholar]
- (403).Ma T; Fu B; Yang X; Xiao Y; Pan M Adipose Mesenchymal Stem Cell-Derived Exosomes Promote Cell Proliferation, Migration, and Inhibit Cell Apoptosis via Wnt/β-Catenin Signaling in Cutaneous Wound Healing. J. Cell. Biochem. 2019, 120 (6), 10847–10854. 10.1002/jcb.28376. [DOI] [PubMed] [Google Scholar]
- (404).Wang X; Jiao Y; Pan Y; Zhang L; Gong H; Qi Y; Wang M; Gong H; Shao M; Wang X; Fetal Dermal Mesenchymal Stem Cell-Derived Exosomes Accelerate Cutaneous Wound Healing by Activating Notch Signaling. Stem Cells Int. 2019, 2019, 2402916. 10.1155/2019/2402916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (405).Ezquer F; Ezquer M; Contador D; Ricca M; Simon V; Conget P The Antidiabetic Effect of Mesenchymal Stem Cells Is Unrelated to Their Transdifferentiation Potential but to Their Capability to Restore Th1/Th2 Balance and to Modify the Pancreatic Microenvironment. Stem Cells 2012, 30 (8), 1664–1674. 10.1002/stem.1132. [DOI] [PubMed] [Google Scholar]
- (406).Lee HY; Yea K; Kim J; Lee BD; Chae YC; Kim HS; Lee D-W; Kim S-H; Cho J-H; Jin CJ; Epidermal Growth Factor Increases Insulin Secretion and Lowers Blood Glucose in Diabetic Mice. J. Cell. Mol. Med. 2008, 12 (5A), 1593–1604. 10.1111/j.1582-4934.2007.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (407).Xiao Y; Zheng L; Zou X; Wang J; Zhong J; Zhong T Extracellular Vesicles in Type 2 Diabetes Mellitus: Key Roles in Pathogenesis, Complications, and Therapy. J. Extracell. vesicles 2019, 8 (1), 1625677. 10.1080/20013078.2019.1625677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (408).Ge Q; Xie XX; Xiao X; Li X Exosome-Like Vesicles as New Mediators and Therapeutic Targets for Treating Insulin Resistance and β-Cell Mass Failure in Type 2 Diabetes Mellitus. J. Diabetes Res. 2019, 2019, 3256060. 10.1155/2019/3256060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (409).Chidester S; Livinski AA; Fish AF; Joseph PV The Role of Extracellular Vesicles in β-Cell Function and Viability: A Scoping Review. Frontiers in Endocrinology. 2020, p 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (410).Garcia-Contreras M; Brooks RW; Boccuzzi L; Robbins PD; Ricordi C Exosomes as Biomarkers and Therapeutic Tools for Type 1 Diabetes Mellitus. Eur. Rev. Med. Pharmacol. Sci. 2017, 21 (12), 2940–2956. [PubMed] [Google Scholar]
- (411).Czernek L; Düchler M Exosomes as Messengers Between Mother and Fetus in Pregnancy. Int. J. Mol. Sci. 2020, 21 (12), 4264. 10.3390/ijms21124264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (412).Pillay P; Moodley K; Moodley J; Mackraj I Placenta-Derived Exosomes: Potential Biomarkers of Preeclampsia. Int. J. Nanomedicine 2017, 12, 8009–8023. 10.2147/IJN.S142732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (413).Salomon C; Guanzon D; Scholz-Romero K; Longo S; Correa P; Illanes SE; Rice GE Placental Exosomes as Early Biomarker of Preeclampsia: Potential Role of Exosomal MicroRNAs Across Gestation. J. Clin. Endocrinol. Metab. 2017, 102 (9), 3182–3194. 10.1210/jc.2017-00672. [DOI] [PubMed] [Google Scholar]
- (414).Mitchell MD; Peiris HN; Kobayashi M; Koh YQ; Duncombe G; Illanes SE; Rice GE; Salomon C Placental Exosomes in Normal and Complicated Pregnancy. Am. J. Obstet. Gynecol. 2015, 213 (4, Supplement), S173–S181. 10.1016/j.ajog.2015.07.001. [DOI] [PubMed] [Google Scholar]
- (415).Hruby A; Hu FB The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33 (7), 673–689. 10.1007/s40273-014-0243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (416).Castaño C; Kalko S; Novials A; Párrizas M Obesity-Associated Exosomal MiRNAs Modulate Glucose and Lipid Metabolism in Mice. Proc. Natl. Acad. Sci. 2018, 115 (48), 12158 LP – 12163. 10.1073/pnas.1808855115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (417).Ji C; Guo X The Clinical Potential of Circulating MicroRNAs in Obesity. Nat. Rev. Endocrinol. 2019, 15 (12), 731–743. 10.1038/s41574-019-0260-0. [DOI] [PubMed] [Google Scholar]
- (418).Tan DBA; Armitage J; Teo T-H; Ong NE; Shin H; Moodley YP Elevated Levels of Circulating Exosome in COPD Patients Are Associated with Systemic Inflammation. Respir. Med. 2017, 132, 261–264. 10.1016/j.rmed.2017.04.014. [DOI] [PubMed] [Google Scholar]
- (419).Xu H; Ling M; Xue J; Dai X; Sun Q; Chen C; Liu Y; Zhou L; Liu J; Luo F; Exosomal MicroRNA-21 Derived from Bronchial Epithelial Cells Is Involved in Aberrant Epithelium-Fibroblast Cross-Talk in COPD Induced by Cigarette Smoking. Theranostics 2018, 8 (19), 5419–5433. 10.7150/thno.27876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (420).Abd El-Kader SM; Al-Shreef FM Inflammatory Cytokines and Immune System Modulation by Aerobic versus Resisted Exercise Training for Elderly. Afr. Health Sci. 2018, 18 (1), 120–131. 10.4314/ahs.v18i1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (421).Xu X; Gao X Immunological Responses against SARS-Coronavirus Infection in Humans. Cell. Mol. Immunol. 2004, 1 (2), 119–122. [PubMed] [Google Scholar]
- (422).Tufan A; Avanoğlu Güler A; Matucci-Cerinic M COVID-19, Immune System Response, Hyperinflammation and Repurposing Antirheumatic Drugs. Turkish J. Med. Sci. 2020, 50 (SI-1), 620–632. 10.3906/sag-2004-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (423).Wilk AJ; Rustagi A; Zhao NQ; Roque J; Martínez-Colón GJ; McKechnie JL; Ivison GT; Ranganath T; Vergara R; Hollis T; A Single-Cell Atlas of the Peripheral Immune Response in Patients with Severe COVID-19. Nat. Med. 2020, 26 (7), 1070–1076. 10.1038/s41591-020-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (424).Liu J; Li S; Liu J; Liang B; Wang X; Wang H; Li W; Tong Q; Yi J; Zhao L; Longitudinal Characteristics of Lymphocyte Responses and Cytokine Profiles in the Peripheral Blood of SARS-CoV-2 Infected Patients. EBioMedicine 2020, 55, 102763. 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (425).Blazquez R; Sanchez-Margallo FM; de la Rosa O; Dalemans W; Álvarez V; Tarazona R; Casado JG Immunomodulatory Potential of Human Adipose Mesenchymal Stem Cells Derived Exosomes on in Vitro Stimulated T Cells. Frontiers in Immunology. 2014, p 556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (426).Alzahrani FA; Saadeldin IM; Ahmad A; Kumar D; Azhar EI; Siddiqui AJ; Kurdi B; Sajini A; Alrefaei AF; Jahan S The Potential Use of Mesenchymal Stem Cells and Their Derived Exosomes as Immunomodulatory Agents for COVID-19 Patients. Stem Cells Int. 2020, 2020, 8835986. 10.1155/2020/8835986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (427).Maqsood M; Kang M; Wu X; Chen J; Teng L; Qiu L Adult Mesenchymal Stem Cells and Their Exosomes: Sources, Characteristics, and Application in RegeneraFtive Medicine. Life Sci. 2020, 256, 118002. 10.1016/j.lfs.2020.118002. [DOI] [PubMed] [Google Scholar]
- (428).Rosendahl Huber S; van Beek J; de Jonge J; Luytjes W; van Baarle D T Cell Responses to Viral Infections - Opportunities for Peptide Vaccination. Front. Immunol. 2014, 5, 171. 10.3389/fimmu.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (429).Cox MA; Kahan SM; Zajac AJ Anti-Viral CD8 T Cells and the Cytokines That They Love. Virology 2013, 435 (1), 157–169. 10.1016/j.virol.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (430).Vella LA; Herati RS; Wherry EJ CD4(+) T Cell Differentiation in Chronic Viral Infections: The Tfh Perspective. Trends Mol. Med. 2017, 23 (12), 1072–1087. 10.1016/j.molmed.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (431).Schmidt ME; Varga SM The CD8 T Cell Response to Respiratory Virus Infections. Frontiers in Immunology. 2018, p 678. [DOI] [PMC free article] [PubMed] [Google Scholar]


