Abstract
A questionnaire on COVID-19-related thrombosis in patients hospitalized before Aug 31, 2020, was sent to 399 hospitals throughout Japan. Responses were received from 111 (27.8%) with information on 6,202 COVID-19 patients. Of these, 333 and 56 required ventilation or extracorporeal membrane oxygenation (ECMO), respectively, and 212 died (3.4%). D-dimer levels were measured in 75.0% of the patients, revealing that 9.2% and 7.6% exhibited D-dimer increases of 3–8-fold and ≥ 8-fold the reference value, respectively. Thrombotic events occurred in 108 patients (1.86% of the 5,807 patients with available data) including symptomatic cerebral infarction in 24, myocardial infarction in 7, deep vein thrombosis in 41, pulmonary thromboembolism in 30, and other thrombotic events in 22. Some patients developed multiple thrombotic events. Thrombosis occurred in 32 patients with mild or moderate COVID-19 severity (0.59% of those with data available) and in 52 patients on ventilation or ECMO (13.5% of severe patients for whom data were available). Thrombosis occurred in 67 patients during worsening clinical condition and in 26 during recovery. Anticoagulant therapy was provided to 893 patients (14.6% of the 6,119 patients with available data), the main reasons being provided as elevated D-dimer levels and worsening clinical condition.
Keywords: COVID-19, Thrombosis, Venous thromboembolism, Cerebral infarction, D-dimer
Introduction
In 2020, SARS-CoV2 infection causing COVID-19 became pandemic. Many reports from Western countries and China suggest that thrombosis is intimately involved in clinical deterioration of COVID-19 patients. For example, it has been reported from the Netherlands that of 184 COVID-19 patients in the ICU, thrombosis occurred in 75 (40.8%) during a median observation time of 14 days. Events included pulmonary thromboembolism in 65 patients, other venous thromboses in 3, cerebral infarction in 5 and peripheral arterial thrombosis in 2 patients1). However, data on COVID-19-related thrombosis in Japan are limited. Thus, in order to gather data on this topic and elucidate how COVID-19-related thrombosis is diagnosed and treated in Japan, a Joint Team of the Japanese Society of Thrombosis and Hemostasis, the Japanese Atherosclerosis Society, and the Research Study Team for Intractable Disease (Blood Coagulation Abnormalities) supported by the Ministry of Health, Labour and Welfare of Japan carried out a questionnaire-based survey on COVID-19-related thrombosis.
Methods
With approval by the relevant Ethics Committees, a set of questionnaire sheets was sent to 399 hospitals distributed all over Japan. This was focused on COVID-19 patients hospitalized by August 31, 2020, around the end of the so-called second peak of COVID-19 in Japan; a total of 67,865 individuals had been diagnosed as having COVID-19 by that date in Japan, according to the publicly-available data from the Ministry of Health, Labour and Welfare, Japan.
Results
Study Population
Responses to the questionnaire were returned from 111 hospitals (27.8%) with clinical information on 6,202 patients. The hospitals were distributed throughout Japan, with 10 in Hokkaido and Tohoku district, 35 in Tokyo and Kanto, 28 in Chubu, Hokuriku and Koshinetsu, 18 in Kinki, 9 in Chugoku and Shikoku, and 11 hospitals in the Kyushu district. The number of severely ill patients (defined as requiring mechanical ventilation or ECMO) treated in each hospital was also various: 51 hospitals treated no severe COVID-19 patients, 24 hospitals treated only one or two, 21 treated 3–10 and 15 hospitals treated 11–26 severely ill patients.
Some returns omitted responses to some of the questions. For example, the replies from 4 hospitals did not include answers to the questions concerning development of thrombotic events. Finally, 5,807 patients' data were evaluable for thrombosis development. Some responses required interpretation and in such cases, data handling was decided on discussion by the survey team members.
Of the 6,202 patients from 111 hospitals, 108 (1.74%) were transferred to other hospitals due to deterioration of their clinical state, and 212 patients died (3.4%). We had information concerning severity of COVID-19 of 6,188 patients. Patients with mild (defined as without oxygen inhalation) and moderate COVID-19 severity (defined as with oxygen inhalation) numbered 5,786 (93.5%). Thirteen patients (0.21%) required only noninvasive positive pressure ventilation (NPPV), whereas those requiring mechanical ventilation or ECMO therapy numbered 333 (5.4%), and 56 (0.90%), respectively (Fig. 1). Hemodialysis was required for 82 patients (1.33%). In the following, severe COVID-19 was defined by either a requirement for ventilation or ECMO therapy during at least part of the hospitalization period.
Fig. 1.
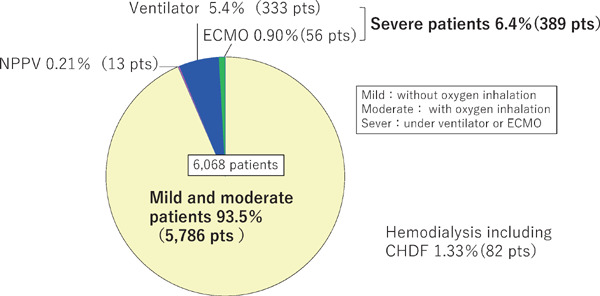
Severity of COVID-19 in 6,068 patients according to the response to the questionnaire on COVID-19-related thrombosis
D-dimer Levels
D-dimer is a product of the degradation of cross-linked fibrin fibers generated by a fibrinolytic protease, plasmin, while the fibrin(ogen)-degradation product (FDP) includes products of the degradation of fibrinogen and fibrin before and after cross-linking. Both values are known to be elevated during thrombus formation and its lysis. Strictly, however, the plasma FDP levels reflect fibrinolytic activity more closely, while the plasma D-dimer levels reflect both fibrinolytic and coagulation activity as indicators of secondary fibrinolysis. In the interim guidelines issued by the International Society of Thrombosis and Hemostasis on COVID-19 coagulopathy, anticoagulant therapy is recommended even for asymptomatic patients with D-dimer levels > 3–4-fold the reference value2). It has also been suggested that D-dimer values reflect not only thrombus formation but also exacerbation of COVID-19 itself3).
The questionnaire contained a query on whether D-dimer levels or FDP levels were emphasized more, a response to which was returned from 108 hospitals. Three hospitals described multiple emphases, whereas D-dimer was focused on by 85 hospitals (78.7%), FDP by only one (0.93%), and an emphasis on both D-dimer and FDP by 20 (18.5%), and on other factors by 5 (4.6%).
Responses to the questions on D-dimer and/or FDP levels were returned for 6,028 patients with D-dimer levels measured in 4,523 (75.0%) (Fig. 2A).
Fig. 2.
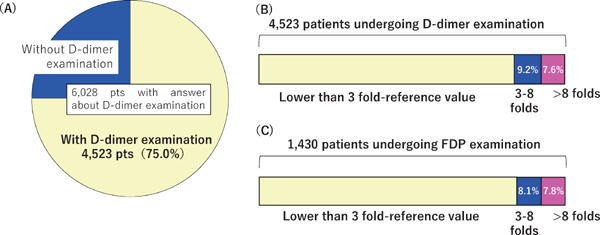
Distribution of D-dimer and FDP values
The proportion of patients with D-dimer assessments (A) and maximal values of D-dimer (B) and FDP (C) during hospitalization.
Methods for quantifying D-dimer and FDP values have not yet been standardized. Because the reference values vary among hospitals, the question was framed in terms of the reference values, but not the absolute plasma concentrations. Concerning maximal D-dimer levels during hospitalization, 418 patients (9.2% of the 4,523 patients) exhibited a 3–8-fold elevation over the reference values and 343 patients (7.6%) > 8-fold (Fig. 2B). FDP levels were measured in 1,430 patients (23.7%) of whom 116 (8.1%) exhibited maximal levels of 3–8-fold the reference values and 112 (7.8%) > 8-fold (Fig. 2C).
During hospitalization, a > 100-fold elevation of D-dimer or FDP levels was observed in 39 patients (Fig. 3). Of these, 8 patients did not have platelet counts decreased to < 100,000/µL or decreased oxygen partial pressure, and 17 did have decreased oxygen partial pressure, but not platelet counts. Two patients had decreased platelet counts, but not oxygen partial pressure, and finally, 12 had both decreased platelet counts and oxygen partial pressure.
Fig. 3.
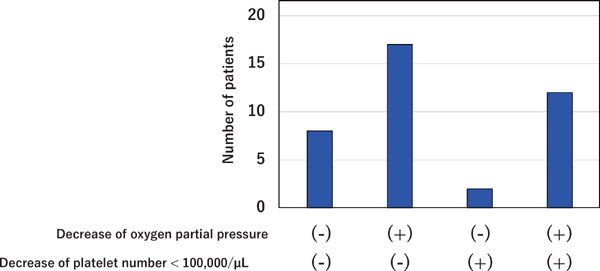
Patients with D-dimer or FDP levels > 100-fold the reference value
Thirty-nine patients had D-dimer or FDP levels > 100 times the reference value
Development of Thrombotic Events
Responses to questions on the development of thrombotic events were returned for 5,807 patients of whom 108 (1.86%) had developed thrombotic disease. Twenty-four patients (22.2% of the 108) developed symptomatic cerebral infarction, 7 (6.5%) had myocardial infarction, 41 (38.0%) had deep vein thrombosis, 30 (27.8%) suffered from pulmonary thromboembolism and 22 (20.4%) had other thrombotic issues such as peripheral artery disease and splenic infarction (Fig. 4). It is to be noted that multiple thrombotic events occurred in several patients.
Fig. 4.
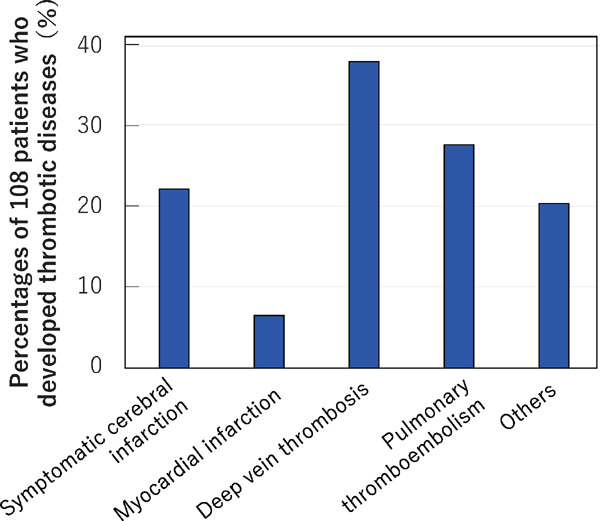
Numbers of patients developing each thrombotic disease
Thrombotic disease developed in 108 patients (1.86% of the 5,807 patients for whom responses to questions about thrombosis were provided). Multiple answers were permitted when one patient developed multiple thrombotic events.
Next, we asked the clinical conditions at the time of the development of thrombotic events. This revealed that they occurred in 31 mild and moderate COVID-19 patients (0.59% of the 5,409 objective mild and moderate COVID-19 patients), while this was the case for 52 (13.5%) during mechanical ventilation or ECMO therapy of the 385 patients with severe COVID-19 (p < 0.0001; Chi-square test) (Fig. 5).
Fig. 5.
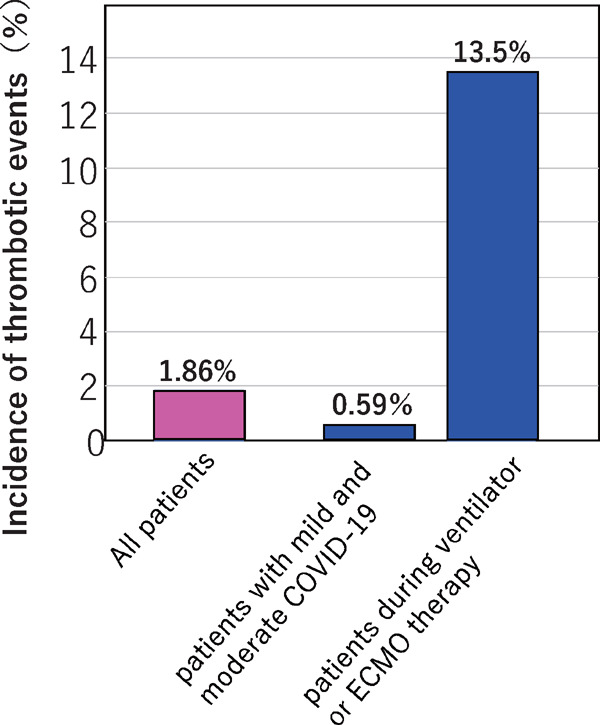
Incidence of thrombotic events in patients with mild and moderate COVID-19 and in those during ventilation and ECMO therapy
Of the 108 patients with thrombosis, 67 (62.0%) developed thrombosis during periods of worsening general condition, while 26 patients (24.1%) did so during the recovery period (Fig. 6). There were 42 patients ≥ 70 years of age (38.9%) and 65 were male (60.2%). Concerning renal function, 14 (13.0%) of the 108 patients with thrombosis had a serum creatinine level ≥ 2 mg/dL on admission, and 27 patients (25.0%) had elevation of serum creatinine level ≥ 2 mg/dL during hospitalization. Thirteen patients (12.0%) were on hemodialysis therapy such as continuous hemodiafiltration (CHDF).
Fig. 6.
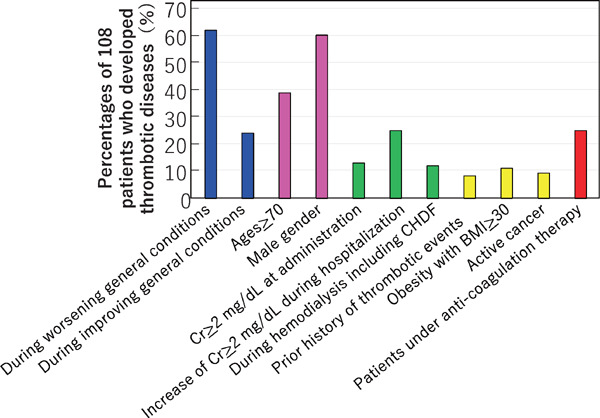
Clinical conditions of the 108 patients developing thrombosis
Multiple answers were permitted
Furthermore, 9 patients (8.3%) had a prior history of thrombotic events, 12 (11.1%) were obese with a body mass index > 30, and 10 (9.3%) had active cancer. None of the 108 patients was pregnant or using contraceptives. Notably, 27 of the patients (25.0%) who developed thrombosis were under anticoagulant therapy.
The last set of questions on thrombosis development was directed to hospitals that provided information on deep vein thrombosis and pulmonary thromboembolism patients. These questions concerned how they were diagnosed separately in mild/moderate COVID-19 patients and in severe patients (multiple answers were permitted). Concerning diagnostic methods for deep vein thrombosis in the mild/moderate COVID-19 patients, of 18 responding hospitals, 6 relied on clinical symptoms such as unilateral swelling of the lower extremities, whereas 12 relied on elevation of D-dimer levels, 5 used ultrasonography of the lower extremities, and 10 used contrast CT. For severe patients, of 11 hospitals providing information, one relied on clinical symptoms, 6 on elevation of D-dimer levels, 5 on ultrasonography of the lower extremities, and 7 on contrast CT.
Concerning diagnostic methods for pulmonary thromboembolism in mild/moderate COVID-19 patients, 12 hospitals provided information (Fig. 7). Five of them relied on clinical symptoms such as decreased arterial oxygen concentration, 8 used elevation of D-dimer levels, one recorded changes in the electrocardiogram and 9 used contrast CT. For severe COVID-19 patients, of 9 hospitals providing information, 3 relied on clinical symptoms, 5 on elevation of D-dimer levels, one on echocardiogram, and 8 on contrast CT.
Fig. 7.
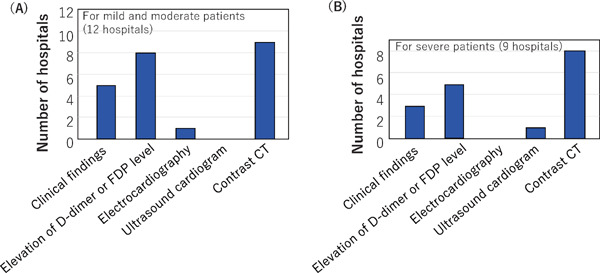
Methods used for the diagnosis of pulmonary thromboembolism
Methods used for the diagnosis of pulmonary thromboembolism were requested to hospitals that made this diagnosis for mild and moderate COVID-19 patients, and separately for severe patients. Responses were received from 12 hospitals for mild and moderate COVID-19 patients and 9 hospitals for severe patients. Multiple answers were permitted.
Anticoagulant Therapy
Anticoagulant therapy was provided to 893 patients (14.6%) of the 6,119 patients with data on this topic (Fig. 8A). We asked which drugs were used for it, with multiple answers permissible. Unfractionated heparin was used in 604 patients (67.6%), low molecular weight heparin in 114 (12.8%), nafamostat in 234 (26.2%), recombinant thrombomodulin 42 (4.7%), and combinations of the above drugs in 138 (15.5%), as well as direct oral anticoagulant (DOAC) in 91 patients (10.2%), and other drugs in 42 (4.7%) (Fig. 8B).
Fig. 8.
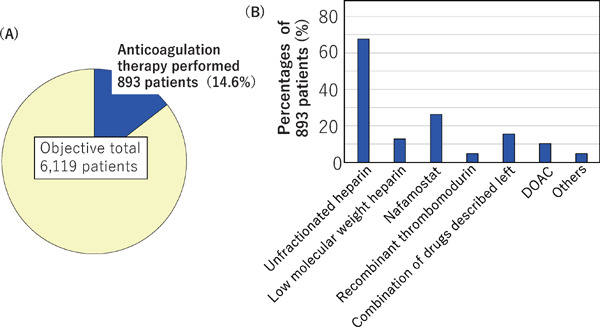
Incidence of (A) and drugs used (B) for anticoagulant therapy
(A) Anticoagulant therapy was given to 893 of the 6,119 patients with available data. (B) Drugs used for the anticoagulant therapy. Multiple answers were permitted.
The reasons for initiating anticoagulant therapy were development of thrombotic disease in 45 patients (5.0% of the 893 patients undergoing anticoagulant therapy), elevation of D-dimer levels in 253 (28.3%), elevation of FDP levels in 41 (4.6%), worsened clinical condition such as decrease of blood oxygen concentration in 226 patients (25.3%), prophylactic treatment in 444 (49.7%), and other reasons in 64 patients (7.2%) (Fig. 9). As reported by multiple hospitals, some of the ‘other reasons’ were development of atrial fibrillation.
Fig. 9.
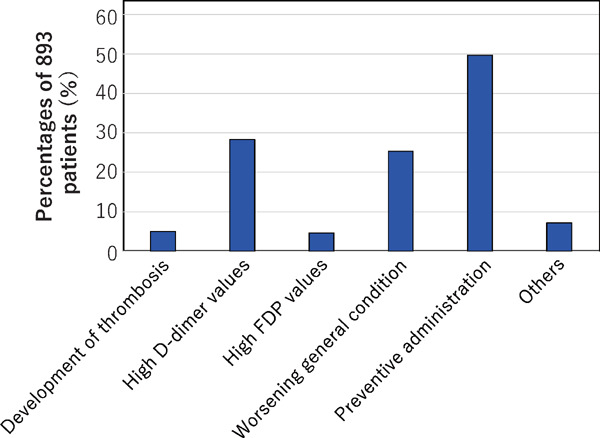
The reasons for initiating anticoagulant therapy in the 893 COVID-19 patients
Multiple answers were permitted.
We asked what the reasons were for prophylactic anticoagulant therapy with multiple answers permissible; 51 hospitals provided information (Fig. 10) with 24 (47.1%) reporting that it was due to high D-dimer levels, 20 (39.2%) that it was due to NPPV/ventilator therapy, 16 (31.4%) because of oxygen administration, 10 (19.6%) due to a prior history of thrombotic disease and 13 (25.5%) for other reasons. All hospitalized COVID-19 patients were given anticoagulant therapy in 9 hospitals (17.6%), most of which were specialized for treating severe patients. Some hospitals reported their criteria for setting D-dimer level thresholds, which varied ranging from the reference value to 10-fold the reference value of the respective hospital. Some hospitals specified the ‘other reasons’ as including a high risk of developing ‘severe’ states (7 hospitals, 13.7%) or administration of steroid hormone (2 hospitals, 3.9%).
Fig. 10.
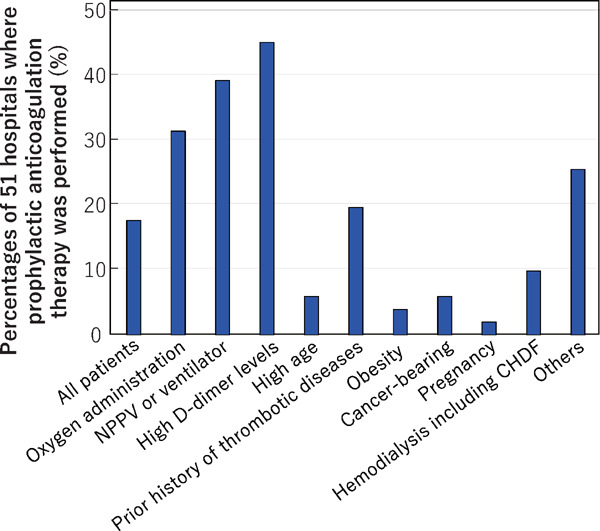
The reasons for initiating prophylactic anticoagulant therapy
Answers were obtained from 51 hospitals that performed prophylactic anticoagulant therapy. Multiple answers were permitted.
We then asked what was responsible for the discontinuation of anticoagulant therapy, where multiple answers were permissible. Responses were received from 50 hospitals, at which 606 patients had been treated with anticoagulant therapy. This was discontinued due to improvement of thrombosis in 36 patients (5.9% of the 606), decreased D-dimer levels in 197 (32.5%), improvement of clinical symptoms in 287 patients (47.3%), discharge or death in 124 (20.5%) and for other reasons in 22 patients (3.6%) (Fig. 11). Bleeding was also a reason in 21 patients (3.5%), with the sites being described for several patients, namely, intracranial bleeding in 3, intrailiopsoas muscle bleeding in 4, upper gastrointestinal tract bleeding in one patient, melena in one patient, and hematuria in one patient.
Fig. 11.
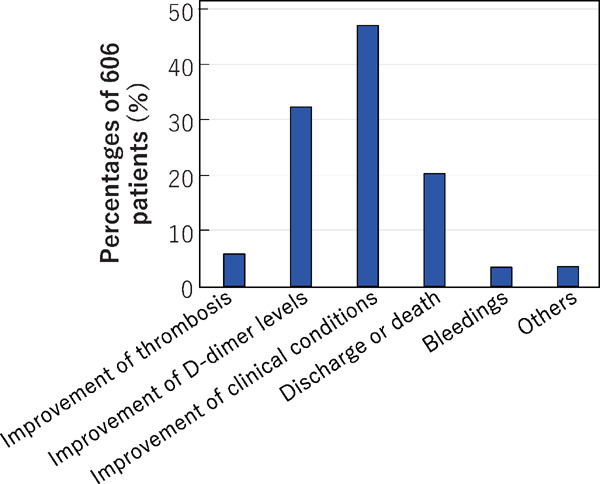
The reasons for discontinuation of anticoagulant therapy
Information on the reasons for discontinuation of anticoagulant therapy was provided for 606 patients in 50 hospitals. Multiple answers were permitted.
Of the 108 patients who developed thrombosis, DOAC and antiplatelet drugs were prescribed at discharge to 40 (37.0%) and 2 patients (1.9%), respectively (patients taking antithrombotic drugs on admission and continuing at discharge are not included here). Thus, > 37% of the patients who developed thrombosis during hospitalization required DOAC at discharge. In response to the question about development of thrombotic disease after discharge, only two patients were reported. Many hospitals commented that they did not follow the patients after discharge.
Discussion
We performed a questionnaire-based survey on COVID-19-related thrombosis and obtained responses from 111 hospitals (27.8% of the 399 hospitals invited to participate). Information on > 6,000 patients was collated. Thus, the results would reflect the clinical situation of COVID-19 related thrombosis in Japan to a considerable degree.
D-dimer levels were measured in 75% of the hospitalized COVID-19 patients, suggesting that many clinicians treated COVID-19 patients using D-dimer levels as a valuable reference tool. It is noted that, together with D-dimer, FDP, reflecting fibrinolytic activity more closely than D-dimer levels, was also emphasized and measured in a quarter of these Japanese patients. Although thrombotic disease developed in 1.86% of patients overall, as many as 9.2% and 7.6% of patients exhibited elevation of D-dimer levels to 3–8-fold the reference values and > 8-fold, respectively, suggesting a rather high rate of COVID-19 patients had prothrombotic states The discrepancy between low incidence of development of thrombosis and high incidence of high D-dimer levels, may reflect the occurrence of so-called ‘microthrombus’4).
D-dimer or FDP levels rarely exceed 100 times the reference level, except for the hyperfibrinolytic type of disseminated intravascular coagulation (DIC). On the other hand, several papers have reported elevation of D-dimer levels > 100-fold in COVID-19 patients5). In the present study, 39 patients were reported with such large increases. Approximately 60% of these patients did not exhibit a decrease of platelet counts to < 100,000/µL, a typical laboratory finding in DIC. This phenotype might reflect an atypical hyperfibrinolytic type of DIC, or otherwise a peculiar pathophysiology of coagulopathy with hyperfibrinolytic activity, specific to COVID-19.
Thrombosis occurred in 108 patients (1.86%) among the 5,807 COVID-19-hospitalized patients surveyed. Of these 108, 38.0% had deep vein thrombosis and 27.8% had pulmonary thromboembolisms, both showing the highest incidence. On the other hand, these frequencies are relatively much lower than those that have been reported from Western countries. For example, the rate of pulmonary embolism was much higher (86.7%) in the report from the Netherlands cited above1). It is difficult to exclude the possibility that not all pulmonary thromboembolism events were diagnosed in the present study, because of some difficulties in examining COVID-19 patients by contrast CT due to issues of infection control. While the incidence of myocardial infarction was low, symptomatic cerebral infarction frequently occurred (in 24 patients, 22.2% of the 108 patients with thrombosis and 0.41% of the total 5,807 patients). The high rate of cerebral infarction among all thrombotic events could be a more remarkable feature in Japanese than Western patients.
The first report from Mt. Sinai Hospital in the USA on the development of cerebral infarction in young COVID-19 patients6) was greeted with surprise. Thereafter, many studies focusing on COVID-19-related cerebral infarction were carried out. It was reported that cerebral infarction developed in 32 patients (0.90%) among the 3,556 COVID-19 patients hospitalized in New York City7). Another retrospective study reported acute cerebral infarction in 1.6% of patients visiting the emergency room or who were hospitalized8). Thus, compared to the incidence reported in Western countries, the absolute incidence for Japanese patients in this survey (0.41%) seems lower, although for many reasons we may not simply compare these figures.
The incidence of thrombosis including arterial and venous thromboses in mild and moderate COVID-19 hospitalized patients in our survey was 0.59%, whereas it was 13.5% during ventilation or ECMO treatment. Some severe patients such as those developing thrombosis before and after ventilation were not included in the ‘13.5%’ (the percentage of patients developing thrombosis during ventilation or ECMO treatment). If we assume that the rest of the mild and moderate patients are all ‘severe patients’, the incidence becomes 19.7%. Thus, the incidence in the ‘severe patients’ in the survey is assumed to be 13.5–19.7%.
Arterial and venous thrombosis developed in 2.6% of 229 patients hospitalized in a general ward and in 35.3% of 170 patients in the intensive care unit (ICU) in a registry study, reported from the USA9). Recently, several meta-analyses of data on venous thromboembolism (combinations of deep vein thrombosis and pulmonary thromboembolism) have been published. Jimenez et al. reported that venous thromboembolism developed in 7.1% of patients on the general ward and 27.9% of ICU patients10). Nopp et al. reported that it occurred in 5.5% on the general ward and 18.7% in the ICU (without screening by ultrasonography) 11). Because the incidence of thrombosis in the mild and moderate COVID-19 patients in our survey was 0.59%, this would be much less than in Western countries, if the assumption may be allowed that patients in general wards were mild and moderate COVID-19 patients and those in the ICU were severe patients, in the studies described above performed in Western countries. On the other hand, the incidence of 13.5–19.7% in severe COVID-19 patients in our survey would be similar or slightly lower compared to those in Western countries. In any case, thrombosis incidence in the severe patients was so high that it is considered that anticoagulant therapy should be performed for all severe patients, if not contraindicated.
In the present survey, thrombosis developed during worsening of their general condition in the 62.0% of the 108 patients with thrombosis. This suggests that thrombosis might be contributing to the development of severe COVID-19. On the other hand, it developed in 24.1% of patients during the recovery phase, indicating that one needs to remain alert to the possibility of thrombosis even when the clinical condition has passed peak severity.
It is well known that COVID-19 causes renal dysfunction12). In this survey, 25% of patients with thrombosis exhibited elevation of serum creatinine levels > 2 mg/dL after admission. Furthermore, 82 patients (1.33%) were treated with hemodialysis. Additionally, 4 patients were reported to be undergoing maintenance dialysis on admission. According to the results of the present survey, 13 patients developed thrombosis during hemodialysis. Taken together, the thrombosis rate during hemodialysis could be 15.1% (13 patients with thrombosis among the total of 86 patients). Thus, hemodialysis might be a thrombosis risk in COVID-19 patients, comparable to the ‘severe’ condition requiring ventilation and ECMO. It is noted, however, that it could not be determined whether renal dysfunction is associated with severity of COVID-19 or is an independent risk factor for thrombosis, because the difference between mild/moderate and severe COVID-19 patients in thrombosis development was so large.
Of the 108 patients with thrombosis, 38.9% were > 70 years of age and 60.2% were male; patients with a history of thrombotic disease, obesity (BMI > 30), or with cancer made up 8.3%, 11.1%, and 9.3% of these, respectively. Thus, these factors could be risks for COVID-19-associated thrombosis, although it is difficult to exclude the possibility that these factors are not independent risks for thrombosis, but rather risk for becoming severely ill with COVID-19.
Of the 108 patients with thrombosis, 25% were on anticoagulant therapy. Although all the patients were treated, 40.8% suffered from thrombosis in the cohort from the Netherlands1). Thus, special attention is required for thrombosis development even under anticoagulant therapy. On the other hand, from a different point of view, 75% of patients might develop thrombosis without anticoagulant therapy in the survey. It would be important to consider whether to initiate anticoagulant therapy for high-risk patients on the basis of observing their clinical condition, D-dimer levels, etc.
We enquired about the methods used for the diagnosis of deep vein thrombosis and pulmonary thromboembolism to the hospitals that diagnosed these patients. However, the number of hospitals that responded was fewer than expected and thus it is difficult to reach any conclusions. Nevertheless, for the diagnosis of pulmonary thromboembolism, contrast CT was frequently used in 9 of the 12 hospitals reporting on diagnosis in mild and moderate COVID-19 patients and in 8 of 9 hospitals for severe. Contrast CT is the gold standard for the diagnosis, although performing CT in COVID-19 patients is associated with some difficulty due to infection control. Pulmonary thromboembolism might have been diagnosed only in patients definitely diagnosed by contrast CT, so it is possible that more patients might develop pulmonary thromboembolism than identified here.
In this survey, unfractionated heparin was the most frequently used anticoagulant in COVID-19 patients, because low molecular weight heparin is not permitted by health insurance agreements for the prevention or therapy of thrombotic diseases in Japan. Nafamostat is rather often used in this survey. As reported from multiple hospitals, the purpose of the use of nafamostat could be due to expectation of the control of COVID-19 itself rather than of its anticoagulant effects because it has been shown to inhibit SARS-CoV2 infection of cells in vitro13).
The reasons for performing anticoagulant therapy were mainly high D-dimer values, worsened clinical condition including decrease in arterial oxygen concentration, and prophylactic administration, in addition to development of thrombotic disease. The reasons for prophylactic administration of anticoagulants were also similar, mainly high D-dimer values and worsened clinical conditions. Thus, responses to both questions overlapped. In any case, it was the patients with high D-dimer values, and/or worsened clinical conditions who were treated with anticoagulants.
Anticoagulant therapy was discontinued in many patients because clinical conditions improved and D-dimer levels decreased, and because patients were discharged (or died). Bleedings were the reason for the discontinuation in 21 patients. One of possible reasons for bleeding is anticoagulant therapy that increase bleeding risk. On the other hand, DIC associated severe COVID-19 may also cause bleedings, since DIC is associated with decrease of platelet counts and fibrinogen levels. Furthermore, ECMO therapy is also known to cause bleeding due to the robust anticoagulant therapy required for the prevention of thrombosis in the apparatus, the decreased platelet counts and platelet function, and acquired von Willebrand syndrome caused by excessively strong high shear stress in the apparatus14, 15). Because the presence of DIC or ECMO therapy in patients with bleedings was not questioned in this survey, we cannot speculate the degree of its contribution to bleeding. Nevertheless, we should always be aware of anticoagulant therapy-associated bleeding. Thus, especially for mild and moderate COVID-19 patients with a low incidence of thrombosis (0.59% in this study), anticoagulant therapy should be considered selectively only for patients at high risk of thrombosis.
Of the 108 patients with thrombosis, 40 patients not receiving DOAC treatment on admission were prescribed DOAC at discharge. Although we presented the rate of patients with newly prescribed DOAC as 37% (40/108), it may be more because the number of patients prescribed DOAC on admission or how many patients with thrombosis died are not known. The actual fraction of patients given DOAC therapy at discharge would be rather higher than 37%. Thus, probably rather high rate of COVID-19 patients with thrombosis during hospitalization would be prescribed with DOAC at discharge.
In summary,
(1) Thrombosis occurred in 1.86% of all hospitalized COVID-19 patients in this survey performed in Japan; there was a low incidence of 0.59% in mild/moderate patients, but a high incidence > 13.5% in patients with severe COVID-19.
(2) D-dimer levels were frequently measured (with data returned on 75.0% of patients), and 9.2% and 7.6% of patients exhibited 3–8-fold and > 8-fold increases, respectively, suggesting that many COVID-19 patients are in a prothrombotic state.
(3) Of the thromboses, although deep vein thrombosis and pulmonary thromboembolism were most frequent, symptomatic cerebral infarction was also frequent, occurring as 22% of thromboses.
(4) While 62% of patients developed thrombosis in the worsening clinical periods, 24% did so in the recovery period.
(5) Anticoagulant therapy was provided to 14.6% of patients mainly due to high D-dimer levels and worsened general condition.
Acknowledgements
We gratefully thank the following hospitals for providing valuable information in response to the questionnaire: Japanese Red Cross Kitami Hospital, Hakodate Municipal Hospital, Otaru General Hospital, Tohoku University Hospital, Nihonkai General Hospital, Japanese Red Cross Fukushima Hospital, Ohara General Hospital, Aizu Medical Center Fukushima Medical University, Iwaki City Medical Center, Japanese Red Cross Mito Hospital, Ibaraki Prefectural Central Hospital • Ibaraki Cancer Center, Tsuchiura Kyodo General Hospital, Saiseikai Utsunomiya Hospital, Haga Red Cross Hospital, International University of Health and Welfare Narita Hospital, Kameda Medical Center, Isumi Medical Center, Chiba Central Medical Center, Nippon Medical School Chiba Hokusoh Hospital, National Hospital Organization Takasaki General Medical Center, Isesaki Municipal Hospital, Public Tomioka General Hospital, Saitama City Hospital, Saitama Medical Center, Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital, Musashino Red Cross Hospital, Flowers & Forest Tokyo Hospital, Tokyo Teishin Hospital, International University of Health and Welfare, Tokyo Metropolitan Geriatric Medical Center, Tokyo Saiseikai Mukojima Hospital, Moriyama Memorial Hospital, Machida Municipal Hospital, Ome Municipal General Hospital, St. Marianna University Yokohama Seibu Hospital, Tokai University Hospital, Hiratsuka City Hospital, Hokuto City Koyo Hospital, Niigata City General Hospital, Niigata Prefectural Shibata Hospital, Toyama Prefectural Central Hospital, Toyama Red Cross Hospital, Kanazawa University Hospital, National Hospital Organization Kanazawa Medical Center, Japan Community Healthcare Organization Kanazawa Hospital, Komatsu Municipal Hospital, Kaga Medical Center, Fukui Prefectural Hospital, Hamamatsu Medical Center, Nagoya University Hospital, Nagoya City University Hospital, Fujita Health University Hospital, Fujita Health University Okazaki Medical Center, Kasugai Municipal Hospital, Kariya Toyota General Hospital, Tokoname Municipal Hospital, Mie University Hospital, Shinshu University Hospital, Iida Municipal Hospital, Chuno Kosei Hospital, Gifu Municipal Hospital, Ogaki Municipal Hospital, Shiga University of Medical Science Hospital, Otsu City Hospital, Omihachiman Community Medical Center, Kyoto City Hospital, Kyoto Kizugawa Hospital, Uji-Tokushukai Medical Center, National Hospital Organization Osaka National Hospital, Osaka City Juso Hospital, Kindai Universitiy Hospital, Kobe City Medical Center General Hospital, Hyogo Prefectural Awaji Medical Center, Japanese Red Cross Society Himeji Hospital, Shinko Hospital, Arida City Hospital, Okayama University Hospital, Hiroshima City Funairi Citizens Hospital, Fukuyama City Hospital, National Hospital Organization Higashihiroshima Medical Center, Shobara Red Cross Hospital, Matsuyama Red Cross Hospital, Ehime Prefectural Niihama Hospital, Fukuoka University Hospital, Fukuoka Tokushukai Hospital, Kurume University Hospital, Karatsu Red Cross Hospital, National Hospital Organization Oita Medical Center, University of the Ryukyus Hospital, Okinawa Prefectural Nanbu Medical Center & Children's Medical Center, Chubu Tokushukai Hospital, and 20 other hospitals.
We also thank Dr. Tadashi Matsushita, President of the Japanese Society of Thrombosis and Hemostasis, Nagoya University, Dr. Midori Shima, former President of the Japanese Society of Thrombosis and Hemostasis, Nara Medical University, and Dr. Kenichi Hirata, President of the Japan Atherosclerosis Society, Kobe University, for encouraging the survey.
The survey was financially supported by the Japanese Society of Thrombosis and Hemostasis, the Japanese Atherosclerosis Society, and a Research Grant from the Ministry of Health, Labour and Welfare of Japan.
The first report of the survey, including some of the results described here, has been available since December 8, 2020, in the Japanese language on the homepages of the Japanese Society of Thrombosis and Hemostasis, the Japan Atherosclerosis Society, and the Research Study Team for Intractable Disease (Blood Coagulation Abnormalities) that conducted this survey. This final report of the survey was made by addition of the data from two hospitals which arrived later, and data not referred to in the first report.
COI Discosure
All authors have no conflict of interest in the survey.
References
- 1). Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals HAM, Huisman MV, Endeman H: Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res, 2020; 191: 148-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, Clark C, Iba T: ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost, 2020; 18: 1023-1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, Zhang Z: D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost, 2020; 18: 1324-1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Ackermann M, Verleden S, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D: Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med, 2020; 383: 120-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Hayakawa M, Takano K, Kayashima M, Kasahara K, Fukushima H, Matsumoto M: Management of a COVID-19 Patient during ECMO: Paying Attention to Acquired von Willebrand Syndrome. J Atheroscler Thromb, 2021; 28: 396-401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah, H, Singh IP, De Leacy RA, Shigematsu T, Ladner TR, Yaeger KA, Skliut M, Weinberger J, Dangayach NS, Bederson JB, Tuhrim S, Fifi JT: Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med, 2020; 382: e60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Yaghi S, Ishida K, Torres J, Mac Grory B, Raz E, Humbert K, Henninger N, Trivedi T, Lillemoe K, Alam S, Sanger M, Kim S, Scher E, Dehkharghani S, Wachs M, Tanweer O, Volpicelli F, Bosworth B, Lord A, Frontera J: SARSCoV-2 and stroke in a New York Healthcare System. Stroke, 2020; 51: 2002-2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Merkler AE, Parikh NS, Mir S, Gupta A, Kamel H, Lin E, Lantos J, Schenck EJ, Goyal P, Bruce SS, Kahan J, Lansdale KN, LeMoss NM, Murthy SB, Stieg PE, Fink ME, Iadecola C, Segal AZ, Cusick M, Campion TR Jr, Diaz I, Zhang C, Navi BB: Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol, 2020; 77: 1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Piazza G, Campia U, Hurwitz S, Snyder JE, Rizzo SM, Pfeferman MB, Morrison RB, Leiva O, Fanikos J, Nauffal V, Almarzooq Z, Goldhaber SZ: Registry of arterial and venous thromboembolic complications in patients with COVID-19. J Am Coll Cardiol, 2020; 76: 2060-2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Jiménez D, García-Sanchez A, Rali P, MurielA, Bikdeli B, Ruiz-Artacho P, Le Mao R, Rodríguez C, Hunt BJ, Monreal M: Incidence of venous thromboembolism and bleeding among hospitalized patients with COVID-19: a systematic review and meta-analysis. Chest, 2021; in press (S0012-3692(20)35146-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Nopp S, Moik F, Jilma B, Pabinger I, Ay C: Risk of venous thromboembolism in patients with COVID-19: A systematic review and meta-analysis. Res Pract Thromb Haemost, 2020; 4: 1178-1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Ronco C, Reis T, Husain-Syed F: Management of acute kidney injury in patients with COVID-19. Lancet Respir Med, 2020; 8: 738-742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Yamamoto M, Kiso M, Sakai-Tagawa Y, Iwatsuki-Horimoto K, Imai M, Takeda M, Kinoshita N, Ohmagari N, Gohda J, Semba K, Matsuda Z, Kawaguchi Y, Kawaoka Y, Inoue JI: The anticoagulant nafamostat potently inhibits SARS-CoV-2 S protein-mediated fusion in a cell fusion assay system and viral infection in vitro in a cell-type-dependent manner. Viruses, 2020; 12: 629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Thomas J, Kostousov V, Teruya J: Bleeding and thrombotic complications in the use of extracorporeal membrane oxygenation. Semin Thromb Hemost, 2018; 44, 20-22 [DOI] [PubMed] [Google Scholar]
- 15). Horiuchi H, Doman T, Kokame K, Saiki Y, Matsumoto M: Acquired von Willebrand syndrome associated with cardiovascular diseases. J Atheroscler Thromb, 2019; 26: 303-314 [DOI] [PMC free article] [PubMed] [Google Scholar]


