Abstract
Electron beam (E-beam) irradiation is an attractive and efficient method for sterilizing clinically implantable medical devices made of natural and/or synthetic materials such as poly(methyl methacrylate) (PMMA). As ionizing irradiation can affect the physicochemical properties of PMMA, understanding the consequences of E-beam sterilization on the intrinsic properties of PMMA is vital for clinical implementation. A detailed assessment of the chemical, optical, mechanical, morphological, and biological properties of medical-grade PMMA after E-beam sterilization at 25 and 50 kiloGray (kGy) is reported. Fourier transform infrared spectroscopy, thermogravimetric analysis, and differential scanning calorimetry studies indicate that E-beam irradiation has minimal effect on the chemical properties of the PMMA at these doses. While 25 kGy irradiation does not alter the mechanical and optical properties of the PMMA, 50 kGy reduces the flexural strength and transparency by 10% and 2%, respectively. Atomic force microscopy demonstrates that E-beam irradiation reduces the surface roughness of PMMA in a dose dependent manner. Live-Dead, AlamarBlue, immunocytochemistry, and complement activation studies show that E-beam irradiation up to 50 kGy has no adverse effect on the biocompatibility of the PMMA. These findings suggest that E-beam irradiation at 25 kGy may be a safe and efficient alternative for PMMA sterilization.
Keywords: biocompatibility, electron beam irradiation, poly(methyl methacrylate), sterilization
1. Introduction
The irradiation of organic polymers intended for medical use with ionizing irradiation such as E-beam often cause the formation of reactive intermediates such as free radicals, ions, and atoms in their excited states.[1] These intermediates can undergo various chemical reactions including but not limited to disproportionation, hydrogen abstraction, rearrangements, and breaking and/or formation of new chemical bonds. This is beneficial when it alters the macromolecular structures present in contaminating pathogens, as it eradicates any bio-burden, and sterilizes medical packaging.[2] However, such chemical reactions can also modify polymer structures and their properties, depending on the structure of the polymer, exposure dose, duration time, and the irradiation conditions.[1,3] One of the most widely used polymers in medical technologies and implants is poly(methyl methacrylate) (PMMA). This is due to its excellent biocompatibility, reliability, relative ease of manipulation, and low toxicity.[4] Some of the most common application of PMMA in medical technologies are i) keratoprosthesis,[5] ii) contact and intraocular lens,[6] iii) orthopedic,[7] iv) dental,[4] and v) plastic surgery.[8] PMMA-based implants typically come into direct contact with the blood stream or are contained in avascular body compartments, and must be sterilized prior to their clinical application.
There are numerous sterilization methods in use including treatment with steam, dry heat, pressured vapor, ethylene oxide (EtO), H2O2 gas plasma, exposing peracetic acid, as well as irradiation – either gamma or E-beam. Effective sterilization must eliminate all infectious agents, including bacteria, viruses, fungi, and parasites, without damaging the intrinsic properties of the material.[9] EtO is preferred for sensitive materials that cannot tolerate heat, and thus it is a commonly applied method for sterilization of PMMA-made medical devices,[10] for example the Boston keratoprosthesis, a device used to replace a diseased cornea when corneal allograft surgery fails.[11] However, EtO sterilization is a slow and expensive process and requires careful handling of EtO due to its toxicity and flammability. Thus, ventilation and aeration are necessary to purge the gas which adds to the cost.[9] In contrast, gamma irradiation has been shown to be a feasible and tractable method to sterilize PMMA devices.[10b,12] However, concerns remain around PMMA yellowing when the device is for optical purposes.
E-beam irradiation is an attractive, emission free, and faster sterilization alternative for medical devices, compatible with low temperature requirements for plastics.[13] E-beam irradiation allows full control of the dose and temperature during sterilization.[9] It has been shown that PMMA can reasonably tolerate a single irradiation sterilization dose, although not repeated sterilizations.[14] It was also shown that the E-beam irradiation can affect mechanical and optical properties of industrial PMMA.[15] However, whether the level of E-beam irradiation sufficient to achieve sterilization to regulatory standards induces any clinically relevant effects on the properties of medical-grade PMMA has not been fully studied. The recommended dose for terminal sterilization of medical products is 25 kGy, and guarantees a Sterility Assurance Level (SAL) of 10–6.[9] Herein, we irradiated medical grade PMMA at 25 and 50 kGy and assessed for alterations in chemical, mechanical, morphological, optical, and biological properties of the PMMA, to determine the feasibility of using E-beam irradiation for sterilization.
2. Results and Discussion
2.1. Chemical Characterization
The FT-IR spectra of the PMMA specimens are shown in Figure 1a. The FT-IR absorbance spectra of non-irradiated and 25 kGy irradiated PMMA samples are almost superimposable. Yet, upon closer inspection, it is apparent that the C=C stretching vibration at around 1637 cm−1 grows after irradiation. This coincides with reduction in the intensity of C–O, C=O bonds groups at 1149 and 1731 cm−1, respectively. Such changes in intensities are more predominant with 50 kGy and suggest that as the E-beam dose increases, the rate of chain scission increases to generate free radicals that recombine to form C=C bonds.[16] Such reactions deplete the hydrogen from PMMA and transforms the polymer into a hydrogen depleted network, as previously suggested.[17] Moreover, FT-IR shows a broad low-intensity peak in the higher energy side of the spectra (3000–4000 cm−1) for PMMA samples after 50 kGy irradiation, suggesting the presence of an OH stretching bond. This may have originated from the oxidative degradation of PMMA during high energy electron bombardment. It was previously shown that electron bombardment generates carbon-centered radicals that can either react with each other to form a crosslinked network or react with atmospheric oxygen to produce peroxyl radicals, which can act as initiator to induce various reactions or can form stable products including hydroxyl bearing functional groups.[16,18]
Figure 1.
Chemical characterization of PMMA after E-beam irradiation. a) Fourier transform infrared (FT-IR) spectra, b) Thermal Gravimetric analysis (TGA), and c) Differential scanning calorimetry (DSC) plots of PMMA before and after 25 or 50 kGy irradiation. Increasing C=C and O–H and reducing C=O and C–O peaks intensities suggest that 50 kGy has a more prominent effect on the chemical structure of the PMMA. Irradiated samples have two decomposition stages compared to one in non-irradiated PMMA, with varying T10, T50, Tmax, (TGA) and Tg (DSC), suggesting the chain scission and crosslinking in the 25 and 50 kGy, respectively.
To confirm surface oxidation, we also performed X-ray photoelectron spectroscopy (XPS). Our data shows that E-beam irradiation decreases C/O atomic ratio from 2.62 (PMMA) to 2.49 (25 kGy) and 2.33 (50 kGy) as tabulated in Table S1. While 25 kGy irradiation decreases the C–O–C atomic ratio and maintains the O=C atomic ratio, 50 kGy decreases both C–O–C and O=C atomic ratios. The former suggests transformation of ester functionality to carboxylic acid, and the later suggests the loss of small gaseous molecules, such as CO and CO2, as also proposed by others after irradiation PMMA film with low energy electrons.[19] However, 50 kGy irradiation also increases the C–OH atomic ratio, indicating the addition of hydroxyl groups onto the surface of the PMMA (Figure S2–S3). These data suggest that the higher doses of irradiation have a more pronounced effect on the chemical structure of PMMA.
The TGA and TGA-derivative plots of non-irradiated and irradiated (25 and 50 kGy) PMMA are co-plotted in Figure 1b. As shown in Figure 1 and Table 1, the irradiation of PMMA with 25 and 50 kGy reduces the onset temperature (T10: temperature at 10% mass loss) from 273.4 to 236.4 and 238.4 °C, respectively. This coincides with the first decomposition stage of the PMMA as indicated by the appearance of the TGA derivative peak at 228.8 and 224.2 °C. This finding is believed to be originated from the presence of lower molecular weight and unstable fragments in irradiated PMMA that undergo degradation and evaporation at lower temperatures, as compared to non-irradiated PMMA, in which there was no such change.[20] Lower doses of irradiation (25 kGy) appeared to decrease the onset temperature more than those of higher doses (50 kGy). On the other hand, the midpoint temperature (T50: temperature at 50% mass loss) drops for 25 kGy but increases for 50 kGy. This suggests that at a lower dose of irradiation, chain scission dominates the degradation processes, while at a higher dose, crosslinking of the generated reactive species may occur. Such chain scission and crosslinking are also responsible for reducing and increasing Tmax (maximum weight loss temperature) in 25 kGy and 50 kGy irradiated PMMA, respectively. Although the thermal behavior of PMMA after E-beam irradiation with varying doses has not been previously studied, prior studies of gamma irradiated PMMA showed similar behavior.[21]
Table 1.
Data obtained from TGA and DSC thermograms.
| Samples | TGA | DCS | |||
|---|---|---|---|---|---|
| T10 [°C] | T50 [°C] | Tmax/°C [°C] | Tg [°C] | ||
| Stage 1 | Stage 2 | ||||
| PMMA | 273.4 | 340.0 | - | 349.l | 131.1 |
| 2S kGy | 236.4 | 337.8 | 228.8 | 347.9 | 128.2 |
| S0 kGy | 238.4 | 342.8 | 224.2 | 353.6 | 128.3 |
The DSC thermograms of non-irradiated, 25 and 50 kGy irradiated PMMA along with their corresponding glass transition temperature (Tg), are shown in Figure 1c. E-beam irradiation of PMMA shifted the Tg from 131.1 °C to 128.2 °C and 128.3 °C, for 25 and 50 kGy respectively, and altered the slope of heat flow in the DSC curves. The former suggests increase in the mobility of the polymer chains, and the latter indicates the presence of smaller molecular weight species which then can undergo degradation or evaporation.[22] This is in agreement with the TGA studies, validating the dominance of chain scission and crosslinking for 25 and 50 kGy, respectively. Although data suggest that E-beam irradiation affects the thermal properties of the PMMA, this is biologically insignificant, considering that PMMA implants reside at body temperature (37 °C).
2.2. Mechanical Characterization
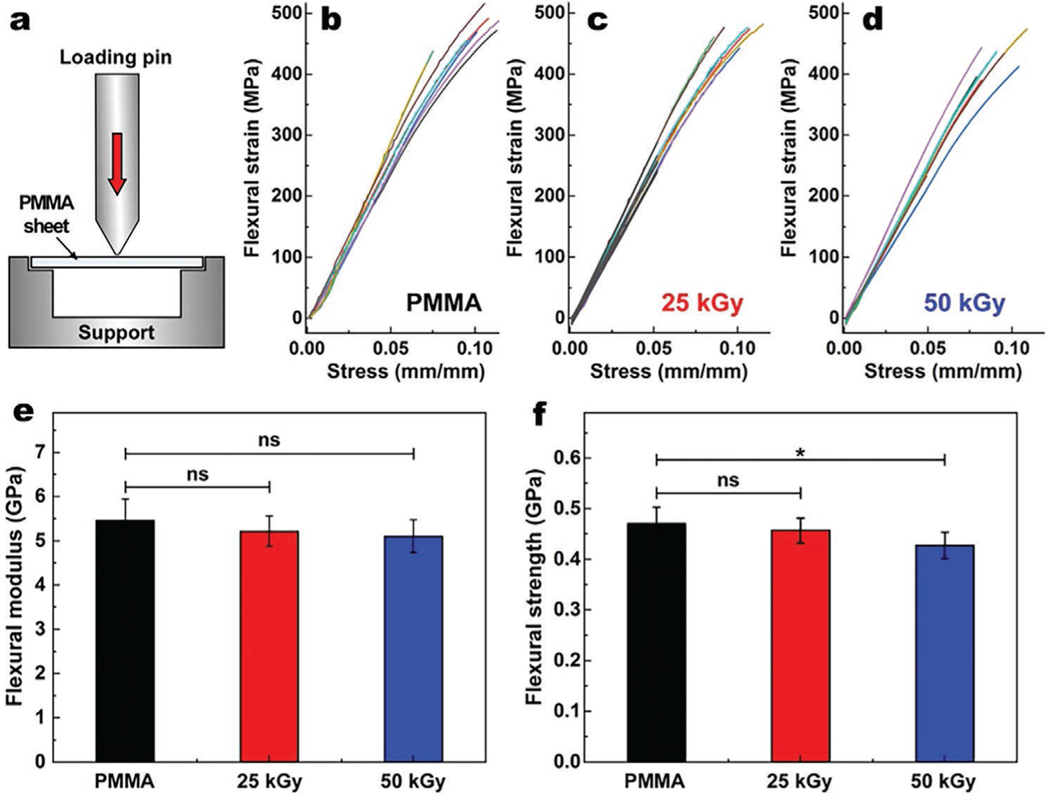
To determine whether the E-beam irradiation impacts the mechanical properties of the PMMA, we performed 3-point bending test (Figure 2a) and calculated flexural modulus and strength according to a previously described approach.[23] Figure 1b–d represents the flexural strains for non-irradiated, 25, and 50 kGy irradiated PMMA discs as function of stress. Our data shows that the flexural modulus of non-irradiated, 25, and 50 kGy irradiated PMMA is 5.46 ± 0.48, 5.22 ± 0.35 and 5.10 ± 0.37 GPa, respectively (Figure 1e). Although the apparent gradual decrease in the flexural modulus of PMMA is consistent with a prior study,[15] statistical analysis showed no significant difference between the irradiated and non-irradiated PMMA (p > 0.05 for both comparisons). Moreover, 25 and 50 kGy irradiated PMMA samples demonstrate a flexural strength of 0.456 ± 0.024 and 0.427 ± 0.025 GPa, respectively, as compared to 0.470 ± 0.033 GPa of non-irradiated PMMA (Figure 2f). These data suggest that only the 50 kGy dose was statistically different from non-irradiated PMMA (p = 0.012). Thus, irradiation at 25 kGy with E-beam did not significantly change the mechanical properties of PMMA.
Figure 2.
Schematic illustration of standard three-point bending flexure test a). Obtained flexural strain as function of stress for non-irradiated b), 25 kGy c) and 50 kGy d) irradiated PMMA and their corresponding flexural moduli e) and flexural strengths f). There was no significant difference in flexural modulus between non-irradiated and 25 and 50 kGy PMMA. ns, and * represent p > 0.05, p ≤ 0.05, respectively. Values are presented as mean ± SD; n = 8.
2.3. Optical Properties
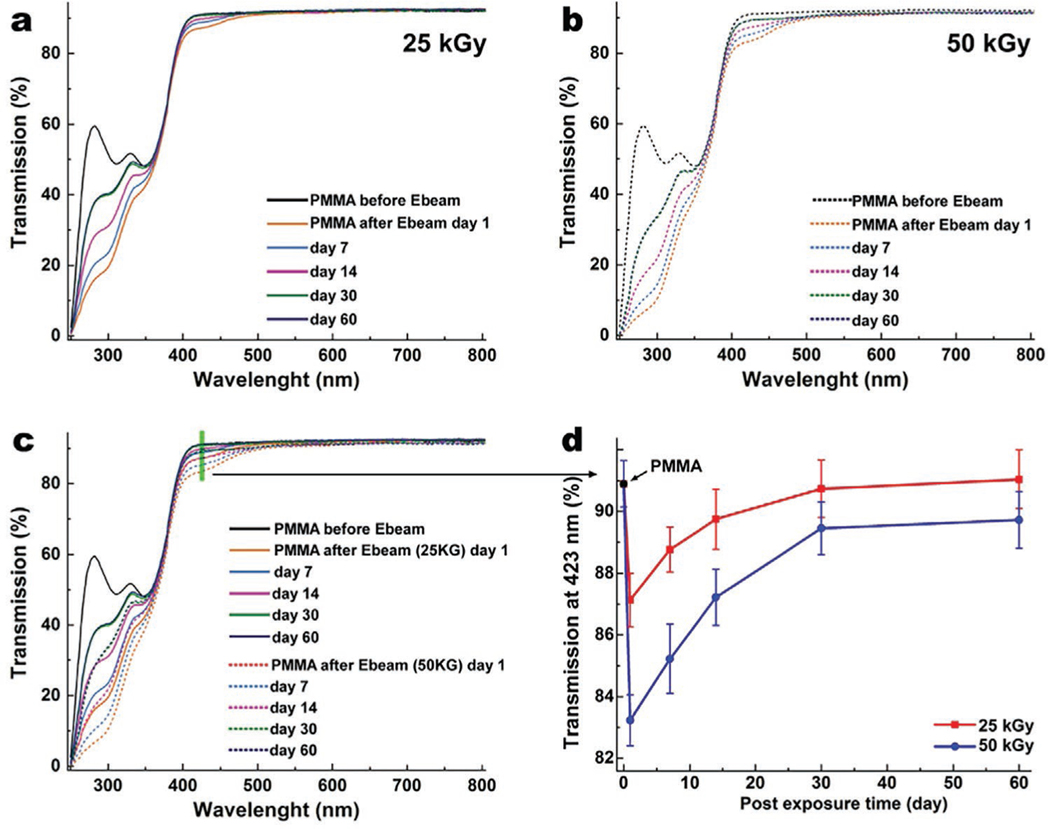
Optical transmission studies of PMMA discs before and after E-beam irradiation using UV-Vis spectroscopy show that the E-beam irradiation reduces the optical transmission of PMMA in a dose-dependent manner, as illustrated in Figure 3a–d. The reduction of transmission in the visible range (390–490 nm) was 2.7 ± 0.2% and 6.2 ± 0.3% for 25 and 50 kGy irradiated PMMA, respectively. However, those reductions were more pronounced: 52.7 ± 0.8% and 67.6 ± 1.1% in the ultraviolet (UV) region of spectra (250–350 nm) for 25 and 50 kGy, respectively. The E-beam treatment also results in reduction of light transmission in the visible range (390–490 nm) and consequently yellowing of the PMMA samples. However, the yellowing effect gradually fades away at room temperature within the first 30 days after irradiation (Figure 3), and then the color remains stable for both 25 and 50 kGy samples. While light transmission in the visible range recovered fully in the 25 kGy group, in the 50 kGy group it failed to recover (Figure 3d). The recovery rate in the UV range was dose-dependent, and neither of the two groups (25 and 50 kGy) achieved full recovery. The reduction in transmission originates from the changes in the chemical structure of the PMMA, as indicated by FT-IR, DSC, and TGA studies. While 25 kGy irradiation does not affect the transmission of visible light, it stably reduces the transmission of harmful UV light, which could be beneficial for device recipients. This is due to the fact that UV light elicits phototoxicity and was shown to induce reactive oxygen species (ROS) production in the aqueous humor, damage mitochondrial DNA (mtDNA) and nuclear DNA (nDNA) in the endothelial cells, and cause corneal endothelial cell loss, leading to corneal edema.[24] Moreover, UV light can cause photochemical damage in retinal cells through (i) direct reactions involving proton or electron transfers and (ii) reactions involving reactive oxygen species mechanisms.[25] The 50 kGy irradiation of PMMA, on the other hand, stably reduces the light transmission in the visible and UV range. In comparison, a similar dose of gamma irradiation (25 kGy) showed a significantly greater impact on the optical properties of PMMA, and reduced transmission in the visible range of 350–500 nm by 15.0 ± 0.5% with only 3.0 ± 0.1% recovery in 90 days.[10b]
Figure 3.
Graphs of optical transmission of PMMA before and after irradiation at 25 kGy a), 50 kGy b), and with both superimposed c) as a function of time to 60 days post treatment, in the range on 250–800 nm. d) The recovery of transmission plotted at 423 nm (extracted from (c) plot, as shown by green line) after 25 and 50 kGy irradiation. 25 kGy irradiated PMMA samples reach near full recovery after 1 month. The 50 kGy irradiated PMMA recovered 95% of transmission in the blue light area. Data are presented as mean ± SD; n = 4.
2.4. Surface Properties
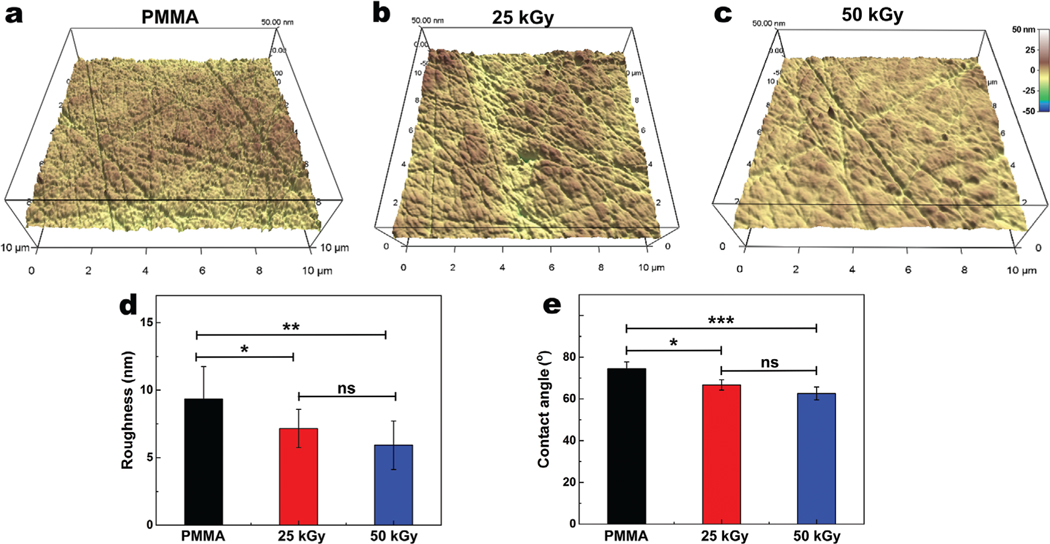
Bombardment of the PMMA surface with high energy E-beam irradiation has shown to alter the crosslinking density and chemical and morphologic characteristics of PMMA films, potentially leading to amorphization.[17,26] To investigate whether E-beam irradiation impacts the morphological properties of medical-grade PMMA, we employed an AFM study. Our data demonstrate a dose dependent smoothing of the surface after irradiation (Figure 4a–c). The surface roughness analysis showed Root Mean Square (RMS) values of 9.34 ± 2.39, 7.15 ± 1.42, and 5.92 ± 1.79 for non-irradiated, 25 kGy, and 50 kGy irradiated PMMA, respectively, demonstrating that irradiation reduced surface roughness (Figure 4d). We performed water contact angle measurements to determine whether E-beam irradiation has affected the wettability of the PMMA surface. This analysis showed reductions in the water contact angle from 74.5 ± 3.2° for non-irradiated PMMA to 66.7 ± 2.5°, and 62.6 ± 3.1° for 25 and 50 kGy irradiated samples, respectively (Figure 4e). These changes could originate from oxidation of the PMMA surface and formation of hydroxyl groups, as shown by XPS, along with the changes to morphological properties due to electron bombardment.[27]
Figure 4.
Morphological characterization of PMMA surface before a), after 25 kGy b), and 50 kGy c) irradiation with a scan size of 10 × 10 μm2 and their analyzed surface roughness d), using Atomic Force Microscopy (AFM). e) Contact angle values of PMMA surfaces before and after 25 kGy and 50 kGy irradiation. AFM and contact angle studies suggested that irradiation decreases the roughness and contact angle in a dose dependent manner. ns, *, and ** represent p > 0.05, p ≤ 0.05, and p ≤ 0.01, respectively. Data are presented as mean ± SD; n = 5.
2.5. Biocompatibility
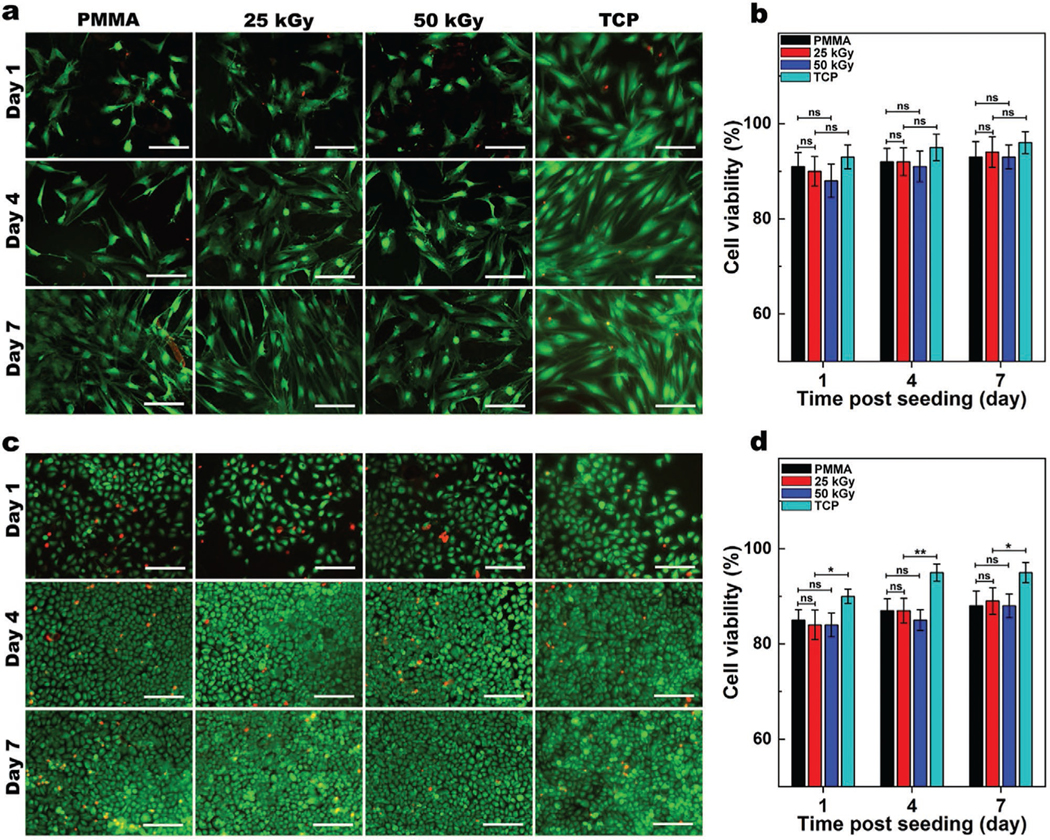
Since E-beam irradiation alters the structure and surface properties of PMMA, we assessed the effect of these changes on cytotoxicity and biocompatibility of the PMMA. After culturing human corneal fibroblasts (HCF) on non-irradiated or irradiated PMMA discs (25 and 50 kGy), we performed a Live-Dead assay and studied the cell viability (Figure 5a,b). In the Live-Dead assay, live cells stain with green-fluorescent calcein-AM on the basis of ongoing intracellular esterase activity, while dead cells stain with red-fluorescent ethidium homodimer-1 because of loss of plasma membrane integrity. Therefore, this assays enables estimation of cell viability within a cell population.[28] Live-Dead analysis (Figure 5b) showed no significant differences between cell viability for non-irradiated, 25, and 50 kGy PMMA discs during 7 days of cell culture (> 90%), suggesting that irradiation did not produce biologically significant cytotoxic products, which otherwise would affect cell viabilities. Moreover, cells cultured on all three PMMA groups had similar cell morphologies and spreading patterns indicating similar biocompatibility.[29] We also studied the interaction of human corneal epithelial cells (HCEp) with PMMA with and without E-beam irradiation (Figure 5c,d). HCEp seeded on non-irradiated, 25, and 50 kGy irradiated PMMA samples demonstrated similar morphology and viability (> 85%) over 7 days of culture (Figure 5d). These data suggest that E-beam irradiation of PMMA does not induce cytotoxicity or reduce its biocompatibility.
Figure 5.
Biocompatibility of PMMA with and without E-beam irradiation. Representative Live/Dead images of human corneal fibroblasts (HCF) a) and human corneal epithelial (HCEp) cells c) cultured on PMMA, with and without 25 or 50 kGy irradiation compared to those cultured on tissue culture plates (TCP), and their corresponding cell viability b,d) after 1, 4, and 7 days of cell culture (scale bar: 200 μm). Cells viability was quantified from Live/Dead images using ImageJ (Green [calcein AM]: lived cells; Red [ethidium homodimer-1]: dead cells). Values are presented as mean ± SD; n = 4. ns, *, **, ***, and **** represent p > 0.05, p ≤ 0.05, p ≤ 0.01, p ≤ 0.001 and p ≤ 0.0001.
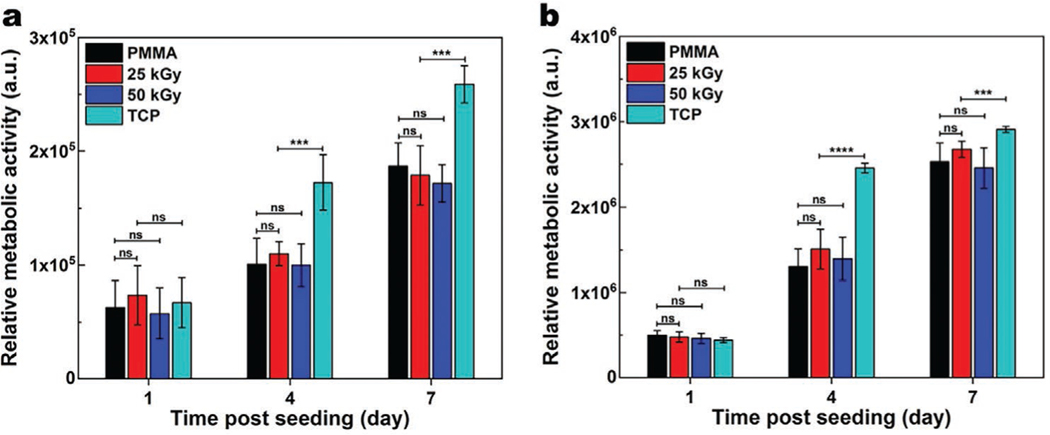
We further studied the metabolic activity of HCF and HCEp seeded on PMMA with or without irradiation using the AlamarBlue assay. A steady increase in the relative metabolic activity as a function of incubation time suggests cellular growth and proliferation over time on all samples. At days 1, 3, and 7, of cell culture, HCF and HCEp grown on 25 and 50 kGy PMMA discs had similar activity to those on non-irradiated PMMA (Figure 6). These results are consistent with the Live-Dead assays and indicate that 25 and 50 kGy E-beam irradiation do not adversely affect PMMA biocompatibility.
Figure 6.
Quantification of the metabolic activity of HCF a), and HCEp b) cultured on PMMA, with or without 25 and 50 kGy irradiation compared to those cultured on tissue culture plate (TCP), using AlamarBlue assay performed at 1, 4, and 7 days of cell culture. The cells seeded on all three surfaces demonstrated similar metabolic activity, suggesting that the irradiation did not diminish biocompatibility of the PMMA. Data are presented as mean ± SD; n = 12. ns, *, **, ***, and **** represent p > 0.05, p ≤ 0.05, p ≤ 0.01, p ≤ 0.001 and p ≤ 0.0001.
2.6. Immunocytochemistry (ICC)
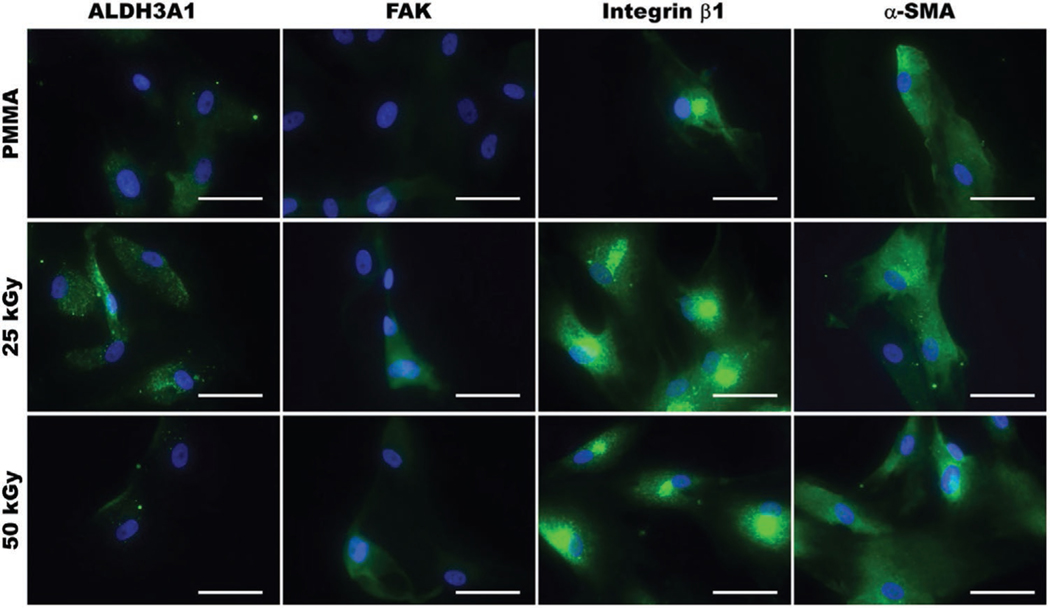
To assess the interaction of HCF with E-beam irradiated PMMA, we also studied the expression of specific cellular markers, including those indicative of adhesion, proliferation, and inflammation (Figure 7) using ICC. ALDH3A1 (keratocyte specific marker) expression was limited on both non-irradiated and irradiated (25 and 50 kGy) PMMA as previously shown.[30] Moreover, the expression of focal adhesion kinase (FAK), which is associated with cellular adhesion and spreading, was similar for cells grown on non-irradiated and irradiated PMMA. The expression of integrin β1, which is associated with the cellular adhesion and interaction with the surrounding extracellular matrix is also similar between groups. Thus, these expression patterns indicate that E-beam irradiation does not adversely impact cellular adhesion, or proliferation and spreading of HCF on PMMA. In addition, most HCF cultured on both non-irradiated and irradiated (25 and 50 kGy) PMMA expressed α−SMA, indicating a myofibroblast phenotype, which can be associated with fibrotic responses.[31]
Figure 7.
Representative immunofluorescence images of HCF cultured on non-irradiated or E-beam irradiated (25 and 50 kGy) PMMA for 7 days, and immunostained for ALDH3A1, focal adhesion kinase (FAK), integrin β1, and smooth muscle actin (α-SMA). Minimal variations were observed between cells grown on non-irradiated and irradiated PMMA. All cell nuclei were counterstained using DAPI (blue). Scale bar is 50 μm.
2.7. Complement Activation
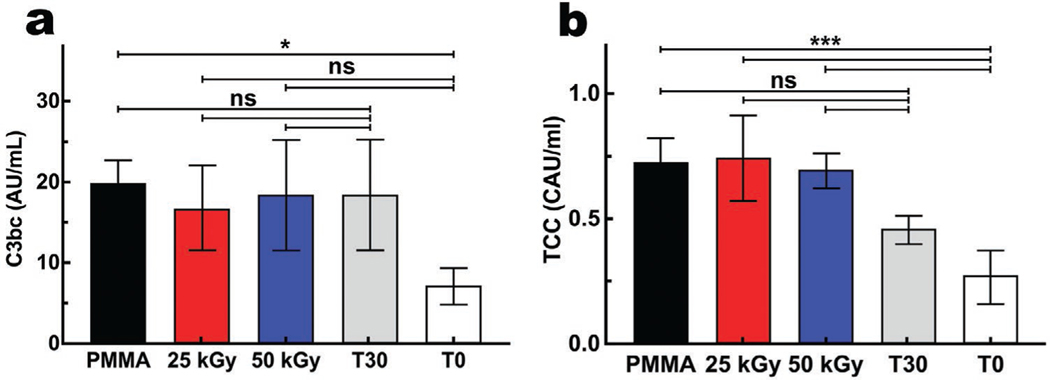
Implanted materials were exposed to recognition molecules of the innate immune system in plasma cascades, of which the complement system is a central component, playing a key role in homeostasis, regeneration, and inflammation.[32] To assess complement activation, two activation fragments of the complement system were studied, C3bc and TCC (Figure 8). C3bc levels were similar between non-irradiated, and irradiated (25 and 50 kGy) PMMA, and to the background activation after 30 min incubation without PMMA (T30), but higher in comparison to the plasma levels immediately after blood draw (T0) (p ≤ 0.05), as illustrated in Figure 8a. Soluble TCC levels were also similar between non-irradiated, irradiated (25 and 50 kGy) PMMA, and T30, and higher than at T0 (p ≤ 0.0001) (Figure 8b). These data suggest that although PMMA induces low-grade complement activation, E-beam irradiation has no impact on its complement activating properties.
Figure 8.
Complement activation of C3bc a) and soluble terminal complement complex (TCC)[b) with or without E-beam irradiation compared to the background activation without PMMA after 30 minutes (T30), and the level at the start of incubation (T0). The level of C3bc was statistically different only for T0; the latter represents the status of complement activation immediately after drawing blood from the donor. Values are presented as mean ± SD; n = 4. ns (p > 0.05), *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, and ****p ≤ 0.0001.
3. Conclusion
In summary, we have shown that 25 kGy E-beam irradiation has a minimal impact on the chemical, mechanical, and optical properties of PMMA and has no apparent adverse effect on its biocompatibility with human corneal cells. These findings suggest that 25 kGy E-beam irradiation may be a feasible sterilization alternative for PMMA used in medical implants. Future studies are required to determine the effect of E-beam sterilization in vivo.
4. Experimental Section
E-Beam Irradiation:
Medical grade PMMA (Rod number 2, PolyOne; Littleton, MA) discs with 0.5 mm thickness and 40.0 mm diameter were acquired from JG Machine Company (Wilmington, MA). The PMMA discs were placed and sealed in Medical Pouches (Steriking SS-T 4A; Helsinki, Finland), placed on an aluminum carrier tray, and irradiated using a Van de Graaff (Model K) electron accelerator (Electron Technology Company; South Windsor, CT) at 2.6 Mev with the dose rate of 5 kGy per pass (5, and 10 passes to reach 25 and 50 kGy irradiation, respectively).
Fourier Transform Infrared Spectroscopy (FT-IR):
FT-IR spectra of the PMMA discs (non-irradiated, 25 and 50 KGy irradiated) were collected in the range from 500 to 4000 cm−1 using a Nicolet iS50 FT-IR Spectrometer (Thermo Scientific; Waltham, MA) equipped with all-reflective diamond Attenuated Total Reflectance (ATR). Spectra were acquired and analyzed via OMNIC software (Thermo Scientific) with 64 scans and 0.5 cm−1 resolution after spectral correction with ambient atmosphere.
X-Ray Photoelectron Spectroscopy (XPS):
XPS spectra of the PMMA discs (non-irradiated, 25 and 50 KGy irradiated) were obtained in the range of 0 to 1300 eV with an energy step size of 1 eV using a Thermo Scientific K-Alpha (Thermo Scientific, Waltham, MA). The spectra were acquired and analyzed via Avantage software (Thermo Scientific) in both survey and high-resolution modes. The high-resolution XPS spectra of C and O were deconvoluted using Avantage software with Simplex fitting algorithm and Gaussian-Lorentzian product function.
Water Contact Angle Measurement:
The contact angle measurements were carried out by a Contact Angle and Surface Tension Measurement System (FTA100, First Ten Angstroms, Portsmouth, VA) using a static sessile drop technique. At room temperature, 5 μL size drop of distilled water was placed by a syringe, located above the sample surface, and then a high-resolution camera used to capture an image from the side. The contact angle was analyzed and recorded using FTA 2.1 software and averaged for each group [n = 4].
Optical Transmission:
The light transmission of the PMMA discs (0, 25 and 50 KGy irradiated) was assessed by a UV-Vis spectrometer (Molecular Devices SpectraMax 384 Plus Microplate Reader; Molecular Devices, San Jose, CA). Briefly, PMMA discs were cut to 6.0 mm diameter by a laser cutter (Helix 75W Laser Cutter; Epilog, Golden, CO), and placed in a 96-well quartz microplate to record their optical transmittance from 250–850 nm in a quartz microplate at 1-nm wavelength increments at varying time points (1–60 days) after irradiation. The transmittance of the samples [n = 4] was corrected with blank media (air) and the mean transmittance (%) for each group was calculated and plotted as a function of wavelength.
Atomic Force Microscopy (AFM):
The morphology of the PMMA discs (0, 25 and 50 KGy irradiated) was scanned by an AFM instrument (Asylum Research Cypher—Oxford Instruments, High Wycombe, UK) in the AC mode. The data was acquired in phase and height profiles with scan size of 10 × 10 μm2 and rate of 2.0 Hz.
Thermal Gravimetric Analysis (TGA):
TGA was performed using a TA Instruments TGA Q50 (New Castle, DE). Samples weighing between 2.3–3.5 mg, were initially heated in a platinum pan at a rate of 10°C/min to reach 100°C, and kept isothermal for 15 mins in argon with a flow rate of 80 mL/min. The temperature was then increased to 600°C at a rate of 20°C/min under argon.
Differential Scanning Calorimetry (DSC):
DSC measurements were performed using a TA Instruments DSC Q20 thermal analyser (New Castle, DE). The PMMA samples were cut into 4.0 mm diameter discs, weighed using a Sartorious balance (Göttingen, Germany), and encapsulated in crimped aluminium pans. An empty pan was used as a reference. The temperature system was allowed to equilibrate at 0°C and remained isothermal for 10 mins before being ramped to 250°C at 10°C/min under an argon flow of 80 ml/min.
Mechanical Properties:
The mechanical properties of PMMA before and after varying E-beam irradiation were assessed using standard three-point bending flexure test.[23] Briefly, PMMA discs (0, 25 and 50 KGy irradiated) were cut to rectangular shapes (15 × 4 mm) with the thickness of 0.5 mm using a Helix 75W laser cutter, then placed on the two-point holder and fixed on the stationary stage of a mechanical tester (Mark-10 ESM 303; Copiague, NY). A longitudinal downward force was applied using the flathead loading pin from the mobile stage with crosshead speed of 2 mm/min. The applied force as a function of displacement was recorded and used to calculate the flexural modulus and strength of the specimens [n = 8].
Live-Dead Assay:
To assess the biocompatibility of the PMMA before and after E-beam irradiation with respect to human corneal fibroblasts (HCF) and human corneal epithelial cells (HCEp), we performed a standard Live-Dead assay. After E-beam irradiation of 8.0 mm PMMA discs, they were placed in a 48- well cell culture plates and washed with sterile phosphate buffered saline (PBS). Next, HCF (10000) or HCEp (5000) were seeded on each disc suspended in 20 μL of respective media (i.e., HCF: low glucose DMEM media (Gibco; Gaithersburg, MD) supplemented 1mM of L-ascorbic acid 2-phosphate (Sigma-Aldrich, St. Louis, MO), 1x Insulin-Transferrin-Selenium Supplement (ITS; Sigma-Aldrich), 1% Glutamax (Gibco), 1x penicillin/streptomycin (Gibco), 1g/L of D-glucose (Sigma-Aldrich), and 2.5 g/L of D-mannitol (Sigma-Aldrich); and HCEp: serum-free medium (KSFM) supplemented with 50 μg/ml bovine pituitary extract and 5 ng/ml epidermal growth factor (Gibco)) and allowed to adhere for 30 min prior to addition of 500 μL of extra media,[10b,33] followed by incubation at 37°C and 5% CO2 for up to 7 days. The respective culture media was changed every other day. Following 1, 4, and 7 days of cell culture, Live-Dead staining was performed according to the manufacturer’s instructions (Life Technologies Carlsbad, CA) and imaged using an inverted fluorescent microscope (Zeiss Axio Observer Z1; Thornwood, NY). Four samples per group (25 and 50 kGy) were examined and compared to those of tissue culture well plate (TCP) and non-irradiated PMMA discs as controls. Cellular viability was analyzed using ImageJ software (NIH, Bethesda, Maryland) from images obtained from each sample (n = 4), as described previously.[30,34]
AlamarBlue Assay:
Standard AlamarBlue assay was performed to evaluate the metabolic activity of the HCF and HCEp seeded on the PMMA discs (0, 25 and 50 kGy irradiated). After culturing the cells on the discs, as explained above, the AlamarBlue study was carried out at days 1, 4, and 7 post-seeding. At each time point, the discs were transfer to a new well and 300μL cell culture media containing 0.0004% resazurin sodium salt (Sigma-Aldrich) was added and incubated for 4 h at 37 °C. Next, 300 μL of the same media was transferred to a new 96 well plate (100 μL in each well) and read on a BioTek plate reader (Synergy 2, BioTek Instruments) at 530/25 nm for excitation and 600/25 nm for emission, and corrected for the fluorescence of discs incubated without cells. Twelve samples per group (25 and 50 kGy) were tested and compared to those of TCP and non-irradiated PMMA discs as controls. Discs were discarded after each time point.
Immunocytochemistry (ICC):
Specific markers (ALDH3A1, integrin β1, FAK and α -SMA) expressed by HCF seeded on PMMA substrates (non-irradiated, 25 and 50 KGy) were assessed by fluorescence ICC. After culturing cells on the PMMA discs for 6 days as above, the discs were removed from the media, gently washed with PBS, and fixed with 4% paraformaldehyde. Fixed cells were permeabilized with 0.25% Triton X-100 and treated with 5% Fetal bovine serum in PBS with 0.05% Tween-20 (PBST), followed by incubation with primary antibodies overnight at 4 °C in humidifying conditions. The specific antibodies included: (i) mouse monoclonal antibody against ALDH3A1 (clone 1B6; GTX84889, dilution 1:100, GenTex); (ii) rabbit polyclonal antibody against Integrin β1 (GTX112971, dilution 1:250, GenTex); (iii) rabbit monoclonal antibody against FAK (clone EP695Y; ab40794, dilution 1:250, Abcam); and (iv) mouse monoclonal antibody against α-SMA (clone 1A4; ab7817, dilution 1:200, Abcam). Subsequently, the specimens were incubated with FITC-conjugated anti-mouse antibody (ab6785, dilution 1:1000, Abcam), or FITC-conjugated anti-rabbit antibody (ab6717, dilution 1:1000, Abcam) for 1h at room temperature, mounted in VectaShield mounting media containing DAPI (Vector Laboratories) and imaged by an inverted fluorescent microscope (Zeiss Axio Observer Z1).
Ex Vivo Complement Activation:
Activation of complement by PMMA before and after irradiation was assessed as previously described, using blood from human donors.[35] The ethical committee at Oslo University Hospital approved the study (REK SØR S-04114), and the research conformed to the Declaration of Helsinki. Informed written consent was obtained from each donor. Human blood was drawn from healthy volunteers into Vacutainer tubes (Becton, Dickinson and Co., Plymouth, UK) containing a specific thrombin inhibitor, lepirudin (Refludan, Celgene, Uxbridge, UK) at a final concentration of 50 μg/mL. For each set of experiments, 300 μL of the blood was aliquoted into five cryogenic vials (Thermofisher, MA). In one vial, EDTA was added immediately at 10 mM final concentration to measure the complement activation status at time zero (T0). The other four vials containing whole blood were incubated at 37 °C as follows: non-irradiated, 25, and 50 kGy irradiated PMMA discs were each placed in a vial. The fourth vial was left with only whole blood. After incubation, EDTA was added to stop further complement activation, and plasma was separated for preservation. The incubated whole blood without an immersed specimen served as a negative control for complement activation. A total of five independent experiments were performed at 30 minutes incubation. Collected plasma was preserved at −70 °C. Complement activation was assessed by measuring soluble C3bc fragments, and the soluble terminal complement complex sC5b-9 (TCC), both using ELISA as described previously.[36] The C3bc content was determined using monoclonal antibody bH6 for capture and polyclonal rabbit anti-C3c (Behringwerke AG, Marburg, Germany) and peroxidase-labeled anti-rabbit immunoglobulin (GE Healthcare, Chicago, IL) for detection. Levels of sC5b-9 were determined with anti-neo C9 monoclonal antibody aE11 for capture,[37] and biotinylated monoclonal anti-C6 (clone 9C4) as previously described.[36–37] Streptavidin-HRP conjugate (GE Healthcare, Chicago, IL) was added for detection.
Statistical Analysis:
One-way ANOVA with Tukey comparison test was used to compare flexural strain and modulus surface roughness, contact angle, viability, metabolic activity, and TCC between groups. A value of p ≤ 0.05 was considered statistically significant. n.s., *, **, ***, and **** represent p > 0.05, p ≤ 0.05, p ≤ 0.01, p ≤ 0.001 and p ≤ 0.0001, respectively. GraphPad Prism Software (GraphPad Software version 8.3.0, CA, USA) was used to analyze the data.
Supplementary Material
Acknowledgements
This work was supported by a Barbara L. Crow Investigator-Concept Grant from Lions VisionGift, Portland, Oregon, and Boston Keratoprosthesis. R.S. was supported in part by EBAA/Richard Lindstrom Research Grant (530720), and by a grant from NIH, K99 EY030553. T.N.H. acknowledges funding from the EPSRC Centre for Doctoral Training in Graphene Technology (No. EP/L016087/1) and the Aziz Foundation. This work was performed in part at the Center for Nanoscale Systems (CNS), Harvard University, a member of the National Nanotechnology Coordinated Infrastructure Network (NNCI), which is supported by the National Science Foundation under NSF award no. 1541959.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Data Availability Statement
Research data are not shared.
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Sina Sharifi, Disruptive Technology Laboratory Massachusetts Eye and Ear and Schepens Eye Research Institute Department of Ophthalmology Harvard Medical School Boston, MA 02114, USA.
Mohammad Mirazul Islam, Disruptive Technology Laboratory Massachusetts Eye and Ear and Schepens Eye Research Institute Department of Ophthalmology Harvard Medical School Boston, MA 02114, USA.
Hannah Sharifi, Disruptive Technology Laboratory Massachusetts Eye and Ear and Schepens Eye Research Institute Department of Ophthalmology Harvard Medical School Boston, MA 02114, USA.
Rakibul Islam, Department of Immunology Oslo University Hospital Rikshospitalet University of Oslo Oslo 0424, Norway.
Tahmida N. Huq, Department of Materials Science and Metallurgy University of Cambridge Cambridge CB3 0FS, UK
Per H. Nilsson, Department of Immunology Oslo University Hospital Rikshospitalet University of Oslo Oslo 0424, Norway Linnaeus Center for Biomaterials Chemistry Linnaeus University Kalmar 45027, Sweden.
Tom E. Mollnes, Department of Immunology Oslo University Hospital Rikshospitalet University of Oslo Oslo 0424, Norway Research Laboratory, Nordland Hospital, Bodø, and Faculty of Health Sciences, K.G. Jebsen TREC University of Tromsø Tromsø 9019, Norway; Centre of Molecular Inflammation Research Norwegian University of Science and Technology Trondheim 7491, Norway.
Khoa D. Tran, Vision Research Laboratory Lions VisionGift Portland, OR 97214, USA
Corrina Patzer, Vision Research Laboratory Lions VisionGift Portland, OR 97214, USA.
Claes H. Dohlman, Disruptive Technology Laboratory Massachusetts Eye and Ear and Schepens Eye Research Institute Department of Ophthalmology Harvard Medical School Boston, MA 02114, USA
Hirak K. Patra, Department of Chemical Engineering and Biotechnology Cambridge University Cambridge CB3 0AS, UK
Eleftherios I. Paschalis, Disruptive Technology Laboratory Massachusetts Eye and Ear and Schepens Eye Research Institute Department of Ophthalmology Harvard Medical School Boston, MA 02114, USA
Miguel Gonzalez-Andrades, Disruptive Technology Laboratory Massachusetts Eye and Ear and Schepens Eye Research Institute Department of Ophthalmology Harvard Medical School Boston, MA 02114, USA; Maimonides Biomedical Research Institute of Cordoba (IMIBIC) Department of Ophthalmology Reina Sofia University Hospital and University of Cordoba Cordoba 14004, Spain.
James Chodosh, Disruptive Technology Laboratory Massachusetts Eye and Ear and Schepens Eye Research Institute Department of Ophthalmology Harvard Medical School Boston, MA 02114, USA.
References
- [1].Haji-Saeid M, Sampa MHO, Chmielewski AG, Radiat. Phys. Chem. 2007, 76, 1535. [Google Scholar]
- [2].Woo L, Sandford CL, Radiat. Phys. Chem. 2002, 63, 845. [Google Scholar]
- [3].a) Drobny JG, Ionizing Radiation and Polymers, William Andrew Publishing, Norwich, NY: 2013, p. 11; [Google Scholar]; b) Chapiro A, Nucl. Instrum. Methods Phys. Res., Sect. B 1988, 32, 111. [Google Scholar]
- [4].Hill RG, in Biomaterials, Artificial organs and Tissue Engineering (Eds: Hench LL, Jones JR), Woodhead Publishing, England: 2005, Ch. 10. [Google Scholar]
- [5].a) Khan BF, Harissi-Dagher M, Khan DM, Dohlman CH, Int. Ophthalmol. Clin. 2007, 47, 61; [DOI] [PubMed] [Google Scholar]; b) Pujari S, Siddique SS, Dohlman CH, Chodosh J, Cornea 2011, 30, 1298. [DOI] [PubMed] [Google Scholar]
- [6].Atchison DA, J. Cataract Refractive Surg. 1990, 16, 178. [DOI] [PubMed] [Google Scholar]
- [7].a) Lewis G, Biomed J . Mater. Res., Part B 2017, 105, 1260; [DOI] [PubMed] [Google Scholar]; b) Gulec A, Acar MA, Aydin BK, Demir T, Ozkaya M, Proc. Inst. Mech. Eng., Part H 2018, 232, 1025; [DOI] [PubMed] [Google Scholar]; c) Bourell D, Stucker B, Espalin D, Arcaute K, Rodriguez D, Medina F, Posner M, Wicker R, Rapid Prototyping J. 2010, 16, 164; [Google Scholar]; d) Winking M, Stahl JP, Oertel M, Schnettler R, Boker DK, Ger. Med. Sci. 2003, 1, Doc08. [PMC free article] [PubMed] [Google Scholar]
- [8].U. S. F. A. D. Administration, Filling in wrinkles safely, https://www.fda.gov/consumers/consumer-updates/filling-wrinkles-safely (accessed: January 2020).
- [9].Gunay MS, Ozer Y, FABAD J. Pharm. Sci. 2009, 34, 43. [Google Scholar]
- [10].a) Lewis G, Mladsi S, Biomaterials 1998, 19, 117; [DOI] [PubMed] [Google Scholar]; b) Gonzalez-Andrades M, Sharifi R, Islam MM, Divoux T, Haist M, Paschalis EI, Gelfand L, Mamodaly S, Di Cecilia L, Cruzat A, Ulm FJ, Chodosh J, Delori F, Dohlman CH, Ocul. Surf. 2018, 16, 322. [DOI] [PubMed] [Google Scholar]
- [11].Traish AS, Chodosh J, Semin. Ophthalmol. 2010, 25, 239. [DOI] [PubMed] [Google Scholar]
- [12].a) Munker T, van de Vijfeijken S, Mulder CS, Vespasiano V, Becking AG, Kleverlaan CJ, CranioSafe G, CranioSafe G, Becking AG, Dubois L, Karssemakers LHE, Milstein DMJ, van de Vijfeijken S, Depauw P, Hoefnagels FWA, Vandertop WP, Kleverlaan CJ, Munker T, Maal TJJ, Nout E, Riool M, Zaat SAJ, J. Mech. Behav. Biomed. Mater. 2018, 81, 168; [DOI] [PubMed] [Google Scholar]; b) Brinston RM, Wilson BK, Med. Device Technol. 1993, 4, 18; [PubMed] [Google Scholar]; c) Meltzer DW, Am. Intra-Ocul. Implant Soc. J. 1981, 7, 126. [DOI] [PubMed] [Google Scholar]
- [13].Urano S, Wakamoto I, Yamakawa T, Tech. Rev. - Mitsubishi Heavy Ind. 2003, 40, 282. [Google Scholar]
- [14].Plester DW, Effects of Radiation Sterilization on Plastics, Duke University Press, Durham, NC: 1973. [Google Scholar]
- [15].Massey LK, in The Effect of Sterilization Methods on Plastics and Elastomers, 2nd ed. (Ed: Massey LK), William Andrew Publishing, Norwich, NY: 2005, Ch. 3. [Google Scholar]
- [16].Ashfaq A, Clochard M-C, Coqueret X, Dispenza C, Driscoll MS, Ulański P, Al-Sheikhly M, Polymers 2020, 12, 2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tiwari P, Srivastava AK, Khattak BQ, Verma S, Upadhyay A, Sinha AK, Ganguli T, Lodha GS, Deb SK, AIP Conf. Proc. 2012, 1447, 587. [Google Scholar]
- [18].Wach RA, Mitomo H, Yoshii F, Kume T, Macromol. Mater. Eng. 2002, 287, 285. [Google Scholar]
- [19].a) Nathawat R, Kumar A, Acharya NK, Vijay YK, Surf. Coat. Technol. 2009, 203, 2600; [Google Scholar]; b) Lazare S, Srinivasan R, Phys J. Chem. 1986, 90, 2124. [Google Scholar]
- [20].a) Żenkiewicz M, Rauchfleisz M, Czupryńska J, Polański J, Karasiewicz T, Engelgard W, Appl. Surf. Sci. 2007, 253, 8992; [Google Scholar]; b) Clough RL, Gillen K, Radiation damage to organic materials in nuclear reactors and radiation environments (Eds: Markovic V), International Atomic Energy Agency, Vienna, Austria: 1990, Ch. 1; [Google Scholar]; c) Elshereksi NW, Mohamed SH, Arifin A, Z. A. M. Ishak, 2014, 25. [Google Scholar]
- [21].a) Barton S, Foot PJS, Tate P, Kishi M, Ghatora B, Polym. Polym. Compos. 2013, 21, 1; [Google Scholar]; b) Kohnen T, J Cataract Refract. Surg. 1996, 22, 1255; [DOI] [PubMed] [Google Scholar]; c) El-Salmawi K, Abu Zeid MM, El-Naggar AM, Mamdouh M, J. Appl. Polym. Sci. 1999, 72, 509. [Google Scholar]
- [22].a) Sperlin LH, in Introduction to Physical Polymer Science, Ch. 8, p. 349; [Google Scholar]; b) Derbil S, Khemici MW, Doulache N, Gourari A, Haine N, Int. J. Polym. Anal. Charact. 2017, 22, 622. [Google Scholar]
- [23].ASTM, in ASTM D790–17, West Conshohocken, PA: 2017. [Google Scholar]
- [24].Liu C, Miyajima T, Melangath G, Miyai T, Vasanth S, Deshpande N, Kumar V, Ong Tone S, Gupta R, Zhu S, Vojnovic D, Chen Y, Rogan EG, Mondal B, Zahid M, Jurkunas UV, Proc. Natl. Acad. Sci. USA 2020, 117, 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Glickman RD, Eye Contact Lens 2011, 37, 196. [DOI] [PubMed] [Google Scholar]
- [26].Rashi N, Anil K, Vijay YK, presented at 2007 IEEE Particle Accelerator Conference (PAC), U.S, 25–29 June 2007, 2007. [Google Scholar]
- [27].a) Mouaci S, Saidi M, Saidi-Amroun N, in Micro & Nano Letters, Vol. 12, Institution of Engineering and Technology, 2017, p. 478; [Google Scholar]; b) Dorati R, Patrini M, Perugini P, Pavanetto F, Stella A, Modena T, Genta I, Conti B, J. Microencapsulation 2006, 23, 123. [DOI] [PubMed] [Google Scholar]
- [28].a) Somodi S, Guthoff R, Ophthalmology 1995, 92, 452; [PubMed] [Google Scholar]; b) Sharifi S, Islam MM, Sharifi H, Islam R, Nilsson PH, Dohlman CH, Mollnes TE, Paschalis EI, Chodosh J, Transl. Vis. Sci. Technol. 2020, 9, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].a) M’Hamdi L, M’Hamdi N, M’Hamdi N, 10.5772/53542 2013; [DOI] [Google Scholar]; b) Altankov G, Grinnell F, Groth T, J. Biomed. Mater. Res. 1996, 30, 385; [DOI] [PubMed] [Google Scholar]; c) Marchant RE, J. Adhes. 1986, 20, 211. [Google Scholar]
- [30].Sharifi R, Mahmoudzadeh S, Islam MM, Koza D, Dohlman CH, Chodosh J, Gonzalez-Andrades M, Adv. Mater. Interfaces 2020, 7, 1900767. [Google Scholar]
- [31].Gonzalez-Andrades M, de la Cruz Cardona J, Ionescu AM, Campos A, Del Mar Perez M, Alaminos M, Invest. Ophthalmol. Vis. Sci. 2011, 52, 215. [DOI] [PubMed] [Google Scholar]
- [32].a) Ekdahl KN, Lambris JD, Elwing H, Ricklin D, Nilsson PH, Teramura Y, Nicholls IA, Nilsson B, Adv. Drug Deliv. Rev. 2011, 63, 1042; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Schoengraf P, Lambris JD, Recknagel S, Kreja L, Liedert A, Brenner RE, Huber-Lang M, Ignatius A, Immunobiology 2013, 218, 1. [DOI] [PubMed] [Google Scholar]
- [33].a) Hackett JM, Ferguson C, Dare E, McLaughlin CR, Griffith, Toxicol. In Vitro 2010, 24, 567; [DOI] [PubMed] [Google Scholar]; b) Chen KH, Azar D, Joyce C, Cornea 2001, 20, 731. [DOI] [PubMed] [Google Scholar]
- [34].a) Busschots S, O’Toole S, O’Leary JJ, Stordal B, MethodsX 2015, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Madden PW, Lai JN, George KA, Giovenco T, Harkin DG, Chirila TV, Biomaterials 2011, 32, 4076; [DOI] [PubMed] [Google Scholar]; c) Ivanova SI, Chakarov S, Momchilova A, Pankov R, Mater. Sci. Eng. C 2017, 78, 230. [DOI] [PubMed] [Google Scholar]
- [35].Islam MM, Sharifi R, Mamodaly S, Islam R, Nahra D, Abusamra DB, Hui PC, Adibnia Y, Goulamaly M, Paschalis EI, Cruzat A, Kong J, Nilsson PH, Argueso P, Mollnes TE, Chodosh J, Dohlman CH, Gonzalez-Andrades M, Acta Biomater. 2019, 96, 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bergseth G, Ludviksen JK, Kirschfink M, Giclas PC, Nilsson B, Mollnes TE, Mol. Immunol. 2013, 56, 232. [DOI] [PubMed] [Google Scholar]
- [37].Mollnes TE, Lea T, Frøland SS, Harboe M, Scand. J. Immunol. 1985, 22, 197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.