Abstract
Objective:
The aim of the study was to investigate whether inhibition of Sonic Hedgehog (SHH) pathway would prevent progression of Barrett’s Esophagus (BE) to esophageal adenocarcinoma.
Background:
The hedgehog signaling pathway is a leading candidate as a molecular mediator of BE and esophageal adenocarcinoma (EAC). Repurposed use of existing off-patent, safe and tolerable drugs that can inhibit hedgehog, such as itraconazole, could prevent progression of BE to EAC.
Methods:
The efficacy of itraconazole was investigated using a surgical rat reflux model of Barrett’s Metaplasia (BM). Weekly intraperitoneal injections of saline (control group) or itraconazole (treatment group; 200mg/kg) were started at 24 weeks postsurgery. Esophageal tissue was harvested at 40 weeks. The role of the Hh pathway was also evaluated clinically. Esophageal tissue was harvested after 40 weeks for pathological examination and evaluation of the SHH pathway by immunohistochemistry.
Results:
BM was present in control animals 29 of 31 (93%) versus itraconazole 22 of 24 (91%). EAC was significantly lower in itraconazole 2 of 24 (8%) versus control 10 of 31 (32%), respectively (P = 0.033). Esophageal SHH levels were lower in itraconazole vs control (P = 0.12). In esophageal tissue from humans with recurrent or persistent dysplastic BE within 24 months of ablative treatment, strong SHH and Indian Hedgehog expression occurred in distal BE versus proximal squamous epithelium, odds ratio = 6.1 (95% confidence interval: 1.6, 23.4) and odds ratio = 6.4 (95% confidence interval: 1.2, 32.8), respectively.
Conclusion:
Itraconazole significantly decreases EAC development and SHH expression in a preclinical animal model of BM. In humans, BE tissue expresses higher SHH, Indian Hedgehog, and bone morphogenic protein levels than normal squamous esophageal epithelium.
Keywords: Barrett’s Metaplasia, esophageal adenocarcinoma, itraconazole, sonic hedgehog inhibitor, sonic hedgehog pathway
Two types of prevention are relevant in the premalignant condition, Barrett’s Esophagus (BE)*: (1) primary prevention of BE development and (2) prevention of BE progression to esophageal adenocarcinoma (EAC). The present status of chemoprevention with proton pump inhibitors (PPIs), statins, aspirin, and nonsteroidal anti-inflammatory agents remains uncertain. However, the recently published phase III ASPECT trial demonstrated that a high-dose PPI plus full dose aspirin significantly improves outcome compared with low-dose PPI alone.1 This study is clearly a step in the right direction, but more needs to be done to prevent progression to invasive EAC, which continues to exhibit a dismal 5-year overall survival rate of only 15% to 19%.2
It has been estimated that the prevalence of BE in the general population of the United States is 1% to 5%.3,4 Current clinical management of BE is, however, hampered by a relative paucity of targetable pathways for chemoprevention. The Sonic Hedgehog (SHH) pathway, as a stem cell differentiation and growth factor, is a key player in gastrointestinal tract development during embryonic organogenesis and later gastric epithelial maintenance in adult mammals. Although the hedgehog (Hh) pathway is highly expressed and functional at early stages of foregut development in embryonic life, its activity gradually decreases in adult differentiated epithelium. Reactivation of the pathway in adulthood has been described to be associated with several pathologies in the gastrointestinal tract and associated organs.5 The Hh target genes PTCH1 and bone morphogenic protein 4 (BMP4) induce the intestinal crypt transcription factor SOX9, which may trigger reprogramming of the normal squamous esophageal epithelium to a metaplastic columnar phenotype.6 In this context, upregulation of Hh ligand expression [SHH and Indian Hedgehog (IHH)] occurs in 98% of BE patient samples) as a result of bile or acid exposure, whereas normal squamous epithelium does not express SHH.6
Targeting of Hh expression by a smoothened (Smo) inhibitor has been shown to prevent neoplastic progression in an in vivo model of BE/EAC; however, application of this result to humans is problematic, given the known toxicities of Smo inhibitors, especially in BE patients who are generally medically healthy.7
In an effort to comply with the National Institutes of Health repurposing of existing molecules initiative, investigators in the Department of Pharmacology and Molecular Sciences at Johns Hopkins built and screened a library of more than 3000 drugs.8 Itraconazole, a commonly used, off-patent, antifungal drug with minimal side effects, was identified in this screen as a potent inhibitor of the Hh pathway.8 In preclinical experiments using the intracranial drug-resistant mouse model of medulloblastoma, SMO (D477G), itraconazole exerted comparable effects to the Hh (Smoothened) inhibitor, vismodegib, but itraconazole overcame all reported resistance-conferring smoothened mutants and GLI2 overexpression.9
In this study, we investigated whether inhibition of the Hh pathway by itraconazole prevented progression from BE to EAC in the Levrat animal model, which induces gastroduodenoesophageal reflux. The sequence of events occurring in these animals closely replicates the molecular and histological events that occur in humans who develop BE/EAC.10,11 In addition, we evaluated a set of Hh pathway–related proteins in patients with BE to determine whether this pathway should be evaluated in future studies as a potential chemopreventive strategy.
MATERIALS AND METHODS
In Vitro Experiments—Esophageal Cancer Cell Line Culture
The esophageal adenocarcinoma human cell line, SKGT4 (Sigma Chemical, St Louis, MO), was grown in culture medium containing 10% fetal bovine serum (Invitrogen, San Diego, CA). All cultures were maintained at 37°C in an incubator supplemented with 5% CO2. Itraconazole (Sinoway Industrial Co, Xiamen, China) was dissolved in dimethyl sulfoxide (DMSO) for invitro experiments and was delivered to cells to reach concentrations of 1, 5, and 10μM in 2mL of cell culture medium. Total RNA was extracted from SKGT4 cells using TRIzol (Invitrogen, Frederick, MD) according to the manufacturer’s protocol. Total RNA of 500 ng was used for reverse transcription with high-capacity cDNA Reverse Transcription Kits (Applied Biosystems, Waltham, MA). Real-time PCR was performed using iQ SYBR Green Supermix (BioRad, Hercules, CA). The primer sequences were as follows: BMP4, 5′-GGC TGG AAT GAC TGG ATT GT-3′ and 5′-TGG TTG AGT TGA GGT GGT CA-3′; GAPDH, 5′-CAG CCT CAA GAT CAT CAG CA- 3′and 5′-TGT CGT CAT GAG TCC TTC CA-3′. Fold change in expression of target mRNA (BMP4) relative to GAPDH mRNA was calculated based on the threshold cycle (CT) for amplification as 2Δ(ΔCT), where ΔCT =CT,target – CT,GAPDH.
Preclinical Experimental Design and Levrat Model
The Animal Care and Use Committee of Johns Hopkins University approved all procedures, and animals received humane care in compliance with the “Guide for the care and use of laboratory animals.”12 To create a reflux-induced esophageal reflux model, an esophagojejunal anastomosis (modified Levrat model) was performed on 8-week-old, male Sprague-Dawley rats as previously described (Fig. 1).10,11,13
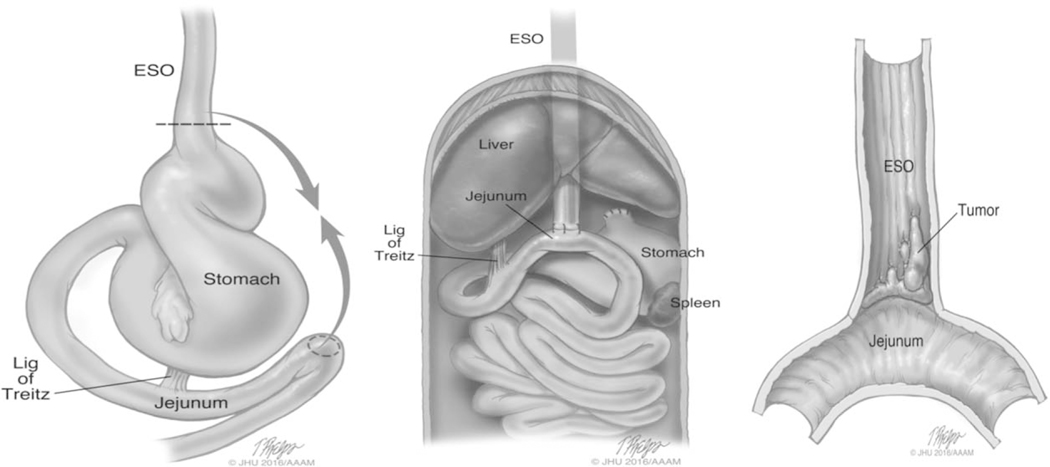
FIGURE 1.
Schematic illustration of esophagojejunostomy and appearance of adenocarcinoma at the site of anastomosis. After a midline abdominal incision, the esophagus was ligated and divided at the esophageal gastric junction. The esophagus end was then anastomosed to a 4mm wide jejunostomy located 4cm distal to the ligament of Treitz by 4 interrupted 6–0 nylon sutures in an end-to-side fashion.
A pilot pharmacokinetic experiment was conducted to determine appropriate itraconazole dosage prior to assessing efficacy of treatment. Animals received itraconazole intraperitoneally at a dose of 100 mg/kg twice a week. Dosing solutions were prepared as 2 g itraconazole added to 100 mL of 10% DMSO/Tricaprylin (1 part DMSO/9 parts Tricapylin). All rats were euthanized at 3 weeks. The esophagus was harvested and plasma was collected and stored at −70°C until analysis. Itraconazole and 4-hydroxyitraconazole concentrations were assessed using a validated liquid chromatography–mass spectrometry assay, over the range of 2 to 2000 ng/mL.14 A dose of 100 mg/kg twice a week resulted in itraconazole esophageal tissue concentrations of 0.7μM, which was 9.5-fold higher than plasma. In further support of our dosing selection, daily oral dosages of 100 mg of itraconazole for 3 weeks to humans resulted in itraconazole levels in the esophagus that were 3 times the concentration in the plasma.15 In addition, in a rat hepatotoxicity model, there was minimal hepatocellular degeneration and inflammation with no necrosis or bile duct hyperplasia at doses of 100 mg/kg/day.16 Finally, itraconazole was not associated with elevated LDH and significant liver toxicity at tissue concentrations of 1μM.17 Therefore, we opted to proceed with 200 mg/kg weekly for the efficacy study due to ease of administration.
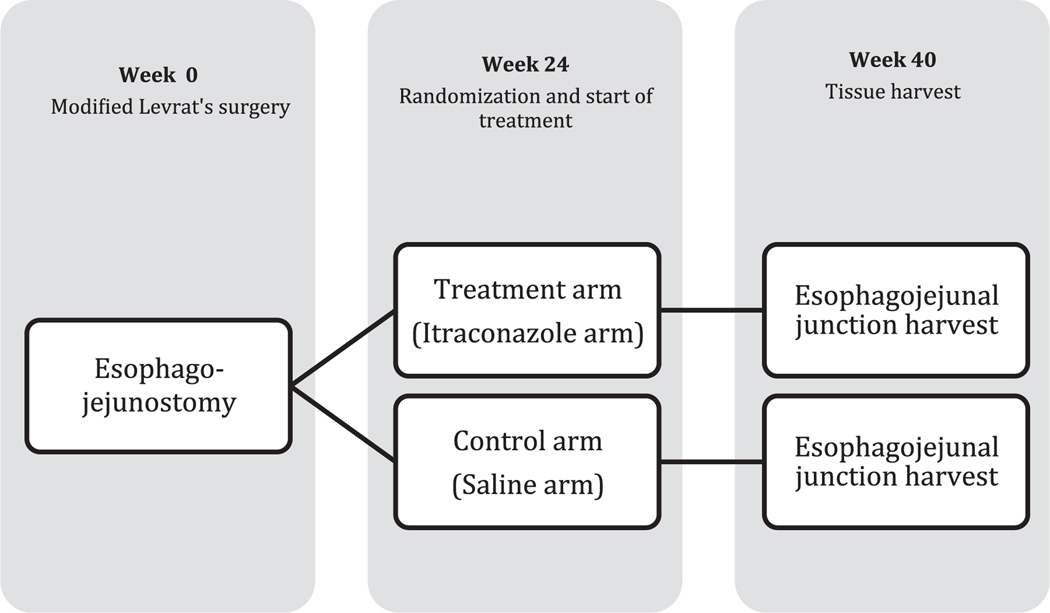
One hundred twenty male Sprague-Dawley rats underwent surgery to induce esophageal cancer. At 24 weeks postsurgery, when adenocarcinoma is typically not yet present in the model, 76 rats were randomly assigned to either treatment or control groups. The treatment group received 200 mg/kg itraconazole intraperitoneally weekly for 16 weeks, whereas the control group received equal amounts of normal saline as a placebo. All rats were euthanized at week 40, 2 days post-last dose (Fig. 2). Esophageal samples were harvested immediately after euthanasia and subsequently prepped for H&E and SHH pathway protein staining for pathological evaluation.
FIGURE 2.
Study design.
At necropsy, the esophagus with adjacent jejunum was harvested and cut longitudinally into 3 uniform slices, fixed in 10% formalin, and embedded in paraffin. Pathological assessment was carried out on 5-μm-thick H&E-stained sections from each paraffin block. Two pathologists, who were blinded to the groups, reviewed all slides for Barrett’s Metaplasia (BM), adenocarcinoma, and squamous cell carcinoma as previously described.18
Hedgehog Pathway Staining
Human and rat formalin-fixed, paraffin-embedded tissues were sectioned at 6 μm and mounted onto plus slides. Slides were then baked for 20 minutes at 60°C. High-temperature antigen retrieval and paraffin removal was performed by immersing slides in Trilogy (Cell Marque, Hot Springs, AR) in a pressure cooker until chamber conditions reached 126°C and 23 psi. Endogenous peroxidase and alkaline phosphatase activities were blocked for 5 minutes with Dual Endogenous Block (DAKO North America Inc, Carpenteria, CA). Slides were incubated with a rabbit primary antibody to either SHH (Novus Biologicals), IHH (Abcam, Cambridge, MA), BMP4 (Atlas Antibodies, Bromma, Sweden), or Sry-Box 9 (SOX9; Millipore-Sigma, St. Louis, MO) for 45 minutes followed by an incubation with PowerVision Poly-HRP anti-Rabbit IgG (Leica Biosystems, Chicago, IL) for 30 minutes.
Pancreas tissue was used as positive control for IHH and SHH. Tonsil tissue was used as positive control for BMP4 and SOX9. For negative control, the assay was performed without secondary antibody for all the immunohistochemistry (IHCs) slides. The antibody-HRP complex was visualized with Impact DAB (Vector Labs, Burlingame, CA). Slides were then counterstained with hematoxylin (Richard-Allen Scientific, Kalamazoo, MI).
Clinical Experiment
Thirteen patients were enrolled in this study. For inclusion in the study, patients had to have been treated endoscopically as per standard of care treatment for BE (including radiofrequency ablation or endoscopic mucosal resection) and had to have recurrent or persistent dysplastic BE within 24 months of ablative treatment. Patients in the study also had to have 2 blocks of tissue, 1 from the distal area with Barrett’s, and 1 from the proximal area with normal squamous epithelial (SE) tissue, available for IHC analysis. Four slides were prepared from each block of tissue, each stained for SHH, SOX9, BMP4, and IHH. Code 1 was given for negative to weak staining, 2 for moderate staining, and 3 for strong staining. IHC for SHH pathway peptides was then compared between human biopsies of proximal and distal esophagus.
STATISTICAL ANALYSIS
Preclinical Sample Size Calculation
This was a randomized, placebo-controlled preclinical experiment designed to investigate the efficacy of itraconazole in preventing progression from BE to EAC. Treatment consisted of itraconazole and control rats were given the same amount of saline at the same treatment intervals. The incidence of adenocarcinoma was evaluated by the study pathologists. Based on previous findings, we expected that 100% of the animals would develop BE 6 months after surgery.10 In addition, we hypothesized that following initial treatment, the proportion of control animals (pA) that would develop BE would be between 95% and 100%, whereas the proportion that would progress to cancer would be 80%. In the itraconazole group (pB), we hypothesized that BE development would be reduced to less than 60%, whereas cancer development would be less than 40%. Sample size was based on exact values given in Haseman.19 Thus, based on a one-sided 0.05 Fisher exact test, a sample size of 25 per group would have 90% power to detect a 35% difference (pA = 0.95 vs pB = 0.60) in the proportion of rats with BE and greater than 80% power to detect a 40% difference (pA = 0.80 vs pB = 0.40) in the proportion of rats with cancer. Because mortality from initial surgery is high with this rat model, sample size was increased substantially to ensure this minimum number would be obtained for each group. The association of treatment arm with adenocarcinoma was assessed with a Fisher exact test using a one-sided 0.05 significance level. Other secondary outcomes were assessed using two-sided 0.05 significance levels. Statistical analyses were performed using R version 3.00.
CLINICAL
In the clinical experiments, we analyzed Hh and downstream pathway expression levels in esophageal tissues from patients with BE identified from the Johns Hopkins BE registry. The objective of this study was to compare expression of 4 Hh proteins at the site of disease, distal BE, to distal SE, or proximal SE. SHH pathway gene expression was measured by IHC staining and qualitative scoring, as previously described.6 The hypothesis of the study was that the expression of Hh pathway proteins would be elevated in distal BE. It was also expected that expression in proximal SE would be the lowest, whereas increased expression would occur in distal SE. IHC scores were plotted with line plots by location and type. IHC expression levels of each of the 4 Hh proteins (SHH, SOX9, BMP4, and IHH) were qualitatively measured and compared among location and type categories: proximal SE, distal SE, and distal BE. To account for correlations among samples obtained from the same patient, generalized estimating equations (GEE) were used (assuming a compound symmetry correlation structure) for model estimation and hypothesis testing of differences. For analysis purposes, IHC scores were dichotomized as strong versus weak/moderate IHC expression. Both blocks of tissue (proximal and distal) taken from the same patient were assessed for IHC staining in both SE and BE areas when both were present; however, BE was so rarely seen proximally that expression for proximal BE could not be evaluated separately. In cases where strong IHC expression was seen for all BE segments, proportions of patients with SE/BE-concordant and SE/BE-discordant scores were reported.
RESULTS
Sonic Hedgehog Pathway Activity in Human Esophageal Cancer Cell Line
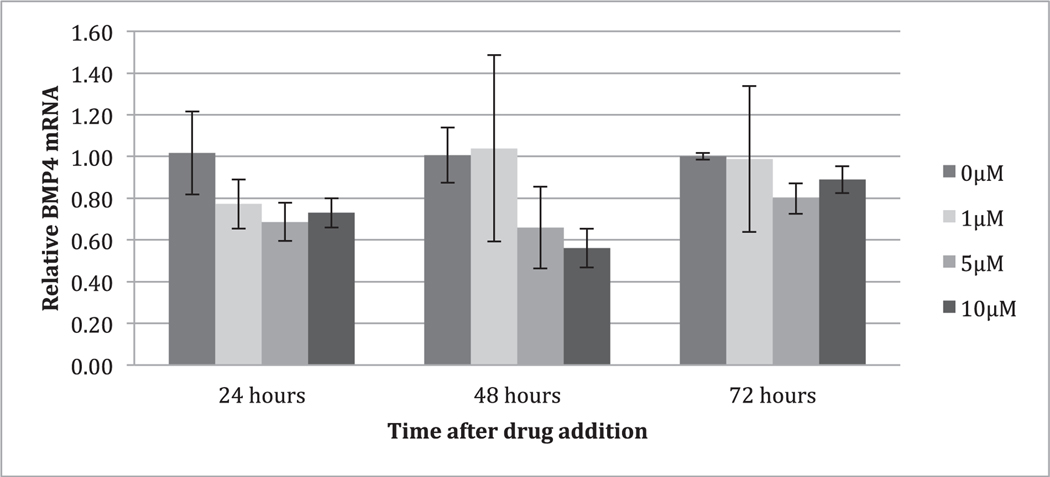
After treatment with itraconazole for 24, 48, and 72hours, mRNA levels of SHH-related genes were determined by qRT-PCR. Results revealed that treatment with itraconazole reduced expression of BMP4 (Fig. 3).
FIGURE 3.
Analysis of mRNA expression of SKGT4 cells following addition of multiple concentrations of itraconazole. Analysis of mRNA expression of SKGT4 cells following addition of multiple concentrations of itraconazole (0, 1, 5, 10μM). RNA was extracted at the indicated hour after itraconazole addition and analyzed by qRT-PCR for mRNAs encoding bone morphogenic protein 4 (BMP4). The results were normalized to 0μM of itraconazole at each time point. *P < 0.05 compared to 0μM, **P < 0.005 compared to 0μM, Student t test, mean ± SD, n = 6 independent cell culture for each condition at 24 hours and n = 4 independent cell culture for each condition at 48 and 72hours.
Preclinical Levrat Model
As in prior work with the Levrat model by our group and others10 mortality remained a problem in this study, which was designed in 2 phases with treatment beginning at 24 weeks. In the pretreatment phase, mortality was the result of anastomotic strictures, which were recognized on autopsy in 44 of the original 120 animals. At 24 weeks surviving animals were weight-matched and randomized to treatment versus saline control. Mortality continued from anastomotic strictures, but other animals without strictures also succumbed to peritonitis from intraperitoneal injections. Mortality was higher in the treatment group after week 24 with 14 deaths, versus 7 in saline-control animals. This increased mortality appeared to be due to the viscous carrier for itraconazole, which caused peritonitis in some of the animals. No tumors caused mortality in either group, as all animals were sacrificed before developing advanced bulky disease.
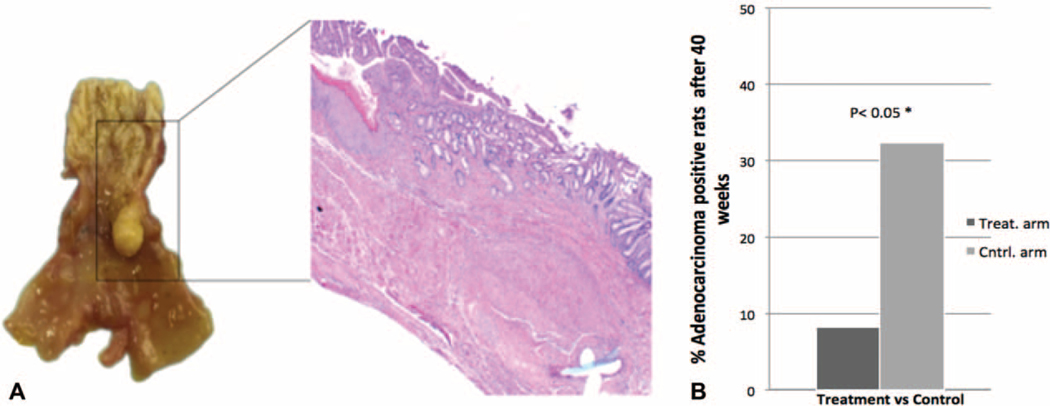
Frequencies of BE, adenocarcinoma, and other outcomes assessed in this experiment are given in Table 1. At the time of sacrifice, BE was essentially equal in both arms of the study, that is, 22 of 24 (91.7%) [95% confidence interval (CI): 73, 98.97%] in the itraconazole arm compared to 29 of 31 (93.5%) (95% CI: 78.58, 99.21%) in the control arm. That the proportion of animals developing BM remained high, greater than 90%, and equal between the 2 groups suggests that itraconazole did not impact BE development. However, the itraconazole arm had significantly fewer rats progressing to EAC, that is, 2 of 24 (8.3%) (95% CI: 1.03, 27%) compared to 10 of 31 (32.3%) (95% CI: 16.68, 51.37%) in the control arm; one-sided Fisher exact test, P = 0.033 (Fig. 4). Since not all the rats in the study had evidence of BE at the time of sacrifice, we performed a sensitivity analysis by excluding the 4 rats that did not have BE at the end of the study. In this analysis, the conclusions remained unchanged: 2 of 22 in the treatment arm progressed to EAC (9.1%) (95% CI: 1.12, 29.16%) compared to 10 of 29 in the control arm (34.5%) (95% CI: 17.94, 54.33%), one-sided Fisher exact test, P = 0.034. We also considered the effect of treatment on a composite outcome of either dysplasia or EAC. These results also indicated that itraconazole reduced neoplastic progression: 3 of 24 in the treatment arm progressed to dysplasia or adenocarcinoma (12.5%) (95% CI: 2.66, 32.36%) compared to 12 of 31 in the control arm (38.7%) (95% CI: 21.85, 57.81%); one-sided Fisher exact test, P = 0.029.
TABLE 1.
Histopathological Outcome Analysis of Rat Reflux Experiment, Two-Sided P Values of Fisher Exact Test Added
| Variable | Levels | N Control | % Control | N Itraconazole | % Itraconazole | N all | % All |
|---|---|---|---|---|---|---|---|
| Barrett’s Metaplasia P = 1.00 | Negative | 2 | 6.4 | 2 | 8.3 | 4 | 7.3 |
| Positive | 29 | 93.5 | 22 | 91.7 | 51 | 92.7 | |
| All | 31 | 100.0 | 24 | 100.0 | 55 | 100.0 | |
| Ba.dysplasia* P = 1.00 | Negative | 29 | 93.5 | 23 | 95.8 | 52 | 94.5 |
| Positive | 2 | 6.4 | 1 | 4.2 | 3 | 5.4 | |
| All | 31 | 100.0 | 24 | 100.0 | 55 | 100.0 | |
| Adenocarcinoma or dysplasia P = 0.029 | Negative | 19 | 61.3 | 21 | 87.5 | 40 | 72.7 |
| Positive | 12 | 38.7 | 3 | 12.5 | 15 | 27.3 | |
| All | 31 | 100.0 | 24 | 100.0 | 55 | 100.0 | |
| Adenocarcinoma† P = 0.033 | Negative | 21 | 67.7 | 22 | 91.7 | 53 | 96.4 |
| Positive | 10 | 32.3 | 2 | 8.3 | 2 | 3.6 | |
| All | 31 | 100.0 | 24 | 100.0 | 55 | 100.0 | |
| Squamous hyperplasia P = 0.37 | Negative | 4 | 12.9 | 1 | 4.2 | 5 | 9.1 |
| Positive | 27 | 87.1 | 23 | 95.8 | 50 | 90.9 | |
| All | 31 | 100.0 | 24 | 100.0 | 55 | 100.0 | |
| SqCC‡ P = 1.00 | Negative | 30 | 96.8 | 23 | 95.8 | 53 | 96.4 |
| Positive | 1 | 3.2 | 1 | 4.2 | 2 | 3.6 | |
| All | 31 | 100.0 | 24 | 100.0 | 55 | 100.0 |
Barrett’s dysplasia.
Adenocarcinoma.
Squamous cell carcinoma.
FIGURE 4.
Esophageal Adenocarcinoma at 40 weeks in the itraconazole versus control groups. A, Gross section of distal esophageal adenocarcinoma with hematoxylin and eosin staining showing a well-differentiated adenocarcinoma. B, Percentage of the adenocarcinoma positive rats in the treatment arm versus control arm at week 40 after surgery. 8.3% (2/24) versus 32.3% (10/31), P = 0.033.
The treatment group exhibited a trend toward lower SHH expression. In the control group, SHH expression was strong in 24 (80%) and moderate in 6 (20%) of 30 animals; in the treated group of 24 rats, SHH expression was strong in 15 (62.5%), moderate in 7 (29.2%), weak in 1 (4.2%), and negative in 1 (4.2%), (P = 0.12, CA trend test) (Fig. 5).
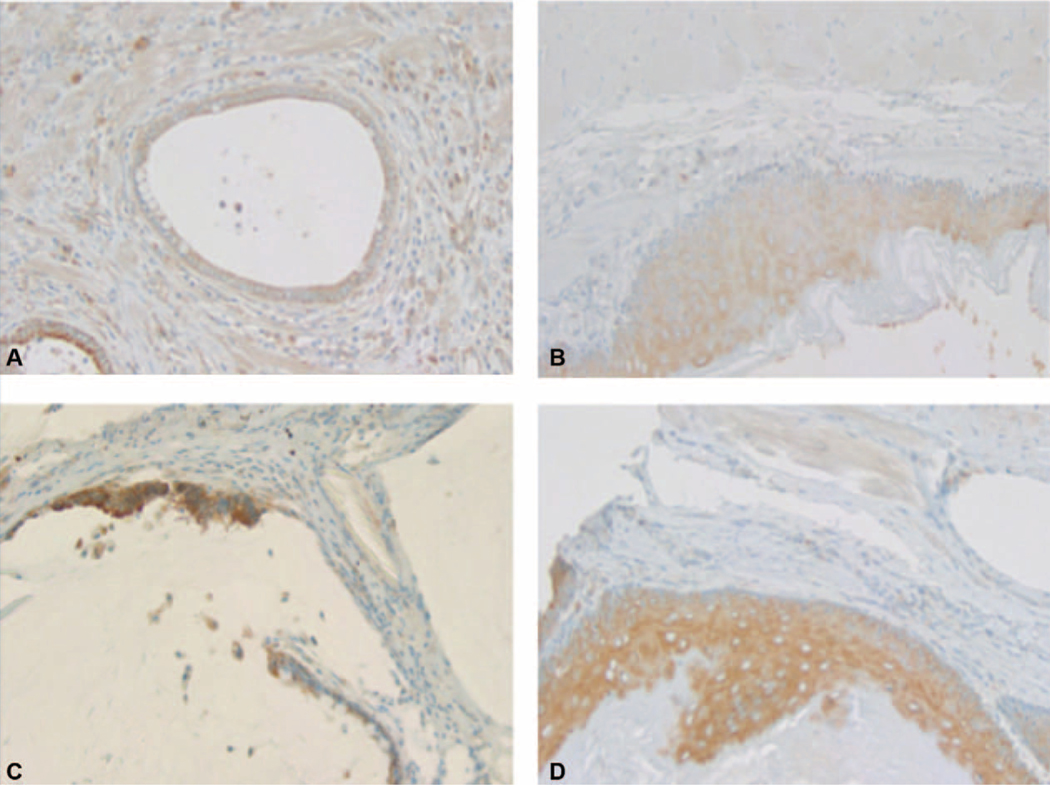
FIGURE 5.
Immunohistochemistry (IHC) stain for Sonic Hedgehog (SHH) in normal squamous and adenocarcinoma cells in treatment and control groups. A, Weak SHH expression in adenocarcinoma cells in the treatment group. B, Weak SHH expression in squamous cells in the treatment group. C, Strong SHH expression in adenocarcinoma cells in the control group. D, Strong SHH expression in squamous cells in the control group.
Clinical Experiments
Points have been offset a small random amount by location and IHC score to prevent overlapping caused by the categorical data. As both categories of IHC expression were not present for BMP and SOX for all tissue types and location combinations, comparisons for these proteins were performed separately, first proximal versus distal and then SE versus BE.
For SHH and IHH, there was a higher probability of strong expression in distal BE compared to proximal SE, odds ratio (OR) = 6.1 (95% CI: 1.6, 23.4, N = 13) and OR = 6.4 (95% CI: 1.2, 32.8, N = 12), respectively. Distal SE, adjacent to BE, was also more likely to exhibit strong expression compared to proximal SE (ORs >1.0); however, these differences were not significant. For SOX and BMP, there were no trends in expression by location. Comparing BMP expression between SE and BE, however, there was a higher probability of strong expression in BE, with OR = 12.1 (95% CI: 1.86, 79.0, N = 13), P = 0.009. SOX expression was moderate or strong in 100% of slides, and in BE, it always coded strong. GEE analysis was therefore not possible for this protein. Of the 10 patients with paired proximal SE and distal BE SOX expression data, 6 (60%) were SE/BE-concordant, with strong expression for both types, whereas 4 (40%) were SE/BE-discordant with moderate staining in SE and strong staining in BE.
DISCUSSION
At the present time, endoscopic therapy is the accepted first-line treatment for high-grade dysplasia (HGD) and intramucosal cancer of the esophagus. However, a significant number of patients have recurrent or persistent dysplastic BE within 24 months of endoscopic treatment, or progress to neoplasia. In patients in whom endotherapy is failing, there is a concern that with persistent endoscopic treatment, a local curable disease may progress to invasive cancer. Options for these patients include esophagectomy, repeated endotherapy, and definitive chemoradiotherapy. Chemopreventive strategies to prevent a debilitating esophagectomy are promising; with recent publication of the phase III ASPECT study showing long-term efficacy of the high-dose PPI esomeprazole and/or high-dose aspirin on progression to HGD or EAC in more than 2500 patients.1 Certain considerations, however, diminish enthusiasm for this approach. High-dose aspirin has known side effects and in the ASPECT study low-dose aspirin in combination with PPIs or without was not studied and therefore its effect on the progression of HGD to EAC remains unclear. Furthermore high-dose PPI is associated with higher risk of side effects such as Clostridium difficile infection, decreased bone density and vitamin B12 deficiency, etc. There remains, therefore, a clear need for alternative chemopreventive strategies above and beyond combining a PPI with aspirin.
In this context, activation of Hh signaling is an attractive target in resistant BE. It has previously been demonstrated that this pathway is reactivated in adult esophageal epithelium that has been injured by acid and bile as a result of gastroesophageal reflux disease.6 Downstream effects of Hh include activation of mesenchymal BMP4, which ultimately signals the epithelium to activate SOX9, which may initiate columnar transcriptional reprogramming. In esophageal epithelial tissue, it is believed that SHH released into the extracellular matrix is targeted at stromal fibroblasts inducing secretion of BMP4, which feeds back to the epithelium causing SOX9 upregulation; SOX9 appears sufficient to drive columnar differentiation of squamous epithelium and expression of intestinal markers.6,20,21 Our observed decrease in BMP4 expression in primary EAC cells suggests that itraconazole has an inhibitory effect on this key step in columnar differentiation.
This proposed molecular cascade, linking acid and bile injury to columnar metaplasia and subsequent BE via the Hh system, provides an ideal opportunity to evaluate the chemopreventive effects of novel Hh inhibitors. A number of authors have expressed a desire to study Hh, Sox, or BMP4 antagonists as therapeutic agents in BE; however, one substantial challenge has been that these medications have significant toxicities and are expensive to study in cohorts of patients who are generally medically well and who do not have cancer. Previously, the Smoothened inhibitor BMS 833923 showed a highly significant risk reduction for development of both BE and EAC in animals, suggesting that this molecule has the potential to prevent carcinogenesis in the context of gastroesophageal reflux disease.7 Patients receiving the Hh inhibitors vismodegib or saridegib for other indications, however, have developed muscle spasms, dysgeusia, alopecia, weight loss, fatigue, diarrhea, and liver dys-function.22,23
In this study, we provided evidence that the Hh pathway is a viable target in patients who have been treated for advanced BE with endoscopic therapy and have resistant BE. SHH and IHH levels were higher in distal BE compared to the proximal squamous epithelium. Prior groups have demonstrated that itraconazole is a potent Hh inhibitor8,9,24; we therefore sought to test the hypothesis that itraconazole could prevent the progression to invasive cancer in a well-established surgical rat reflux model of BM created by end-to-side esophagojejunostomy. We established a statistically significant lower incidence of EAC in the treatment versus control group, and that esophageal SHH protein levels were lower in the preclinical itraconazole than the control group.
One potential weakness of this study is that itraconazole also targets vascular endothelial growth factor signaling and is not a pure Hh inhibitor.25 The benefits seen in this animal model may thus be due to multiple pathway inhibition, above and beyond Hh targeting alone. This fact, however, may be an advantage in humans, as clinical trials have informed us that in the absence of an oncogenic driver mutation, multiple pathway inhibition may be superior to targeting a single pathway. Additional limitations of our study include the relatively small sample size of the human and animal cohorts and the limitations on the number of procedural interventions that can be performed in the Levrat model, for example, intraperitoneal drug administration can increase toxicity compared with controls. Interestingly, however, this model does demonstrate chromosomal abnormalities similar to human EAC and closely replicates human pathophysiology, albeit at an accelerated rate.10,11
In conclusion, we have demonstrated that the activated Hh pathway may play an important role in resistant BE in animals and humans, and that the Hh inhibitory properties of itraconazole can prevent progression from BE to EAC in a significant number of animals in the preclinical setting. These preliminary results support the need for a detailed evaluation of the Hh pathway in esophageal cancer, notably in patients considered at high risk for progression from BE to EAC. Repurposing existing well-characterized drugs for new indications is a highly attractive strategy in premalignant diseases, none more so than BE, which can result in debilitating cancers as a result of current surgical and chemoradiation options, and the inherent likelihood of treatment failure and death.
Acknowledgments
This study was supported by a Prevent Cancer Foundation Young Investigator Award and GI SPORE career development award to Dr Kelly. The project described was supported by the Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins [NIH grants P30CA006973 and UL1TR001079, and the Shared Instrument Grant (S10OD020091)]. The project described was supported by grant number UL1 TR001079 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCATS or NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Jankowski JAZ, De Caestecker J, Love SB, et al. Esomeprazole and aspirin in Barrett’s oesophagus (AspECT): a randomised factorial trial. Lancet. 2018;392:400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Njei B, McCarty TR, Birk JW. Trends in esophageal cancer survival in United States adults from 1973 to 2009: a SEER database analysis. J Gastroenterol Hepatol. 2016;31:1141–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rex DK, Cummings OW, Shaw M, et al. Screening for Barrett’s esophagus in colonoscopy patients with and without heartburn. Gastroenterology. 2003;125:1670–1677. [DOI] [PubMed] [Google Scholar]
- 4.Hayeck TJ, Kong CY, Spechler SJ, et al. The prevalence of Barrett’s esophagus in the US: estimates from a simulation model confirmed by SEER data. Dis Esophagus. 2010;23:451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22:2454–2472. [DOI] [PubMed] [Google Scholar]
- 6.Wang DH, Clemons NJ, Miyashita T, et al. Aberrant epithelial-mesenchymal Hedgehog signaling characterizes Barrett’s metaplasia. Gastroenterology. 2010;138:1810–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibson MK, Zaidi AH, Davison JM, et al. Prevention of Barrett esophagus and esophageal adenocarcinoma by smoothened inhibitor in a rat model of gastroesophageal reflux disease. Ann Surg. 2013;258:82–88. [DOI] [PubMed] [Google Scholar]
- 8.Kim J, Tang JY, Gong R, et al. Itraconazole, a commonly used antifungal that inhibits Hedgehog pathway activity and cancer growth. Cancer Cell. 2010;17:388–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J, Aftab BT, Tang JY, et al. Itraconazole and arsenic trioxide inhibit Hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists. Cancer Cell. 2013;23:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonde P, Gao D, Chen L, et al. Duodenal reflux leads to down regulation of DNA mismatch repair pathway in an animal model of esophageal cancer. Ann Thorac Surg. 2007;83:433–440. discussion 40. [DOI] [PubMed] [Google Scholar]
- 11.Bonde P, Sui G, Dhara S, et al. Cytogenetic characterization and gene expression profiling in the rat reflux-induced esophageal tumor model. J Thorac Cardiovasc Surg. 2007;133:763–769. [DOI] [PubMed] [Google Scholar]
- 12.National Research Council. Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington, DC: The National Academies Press; 2011, 10.17226/12910. [DOI] [Google Scholar]
- 13.Buttar NS, Wang KK, Leontovich O, et al. Chemoprevention of esophageal adenocarcinoma by COX-2 inhibitors in an animal model of Barrett’s esophagus. Gastroenterology. 2002;122:1101–1112. [DOI] [PubMed] [Google Scholar]
- 14.Antonarakis ES, Heath EI, Smith DC, et al. Repurposing itraconazole as a treatment for advanced prostate cancer: a noncomparative randomized phase II trial in men with metastatic castration-resistant prostate cancer. Oncologist. 2013;18:163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darouiche RO, Setoodeh A, Anaissie EJ. Potential use of a simplified method for determination of itraconazole levels in plasma and esophageal tissue by using high-performance liquid chromatography. Antimicrob Agents Chemother. 1995;39:757–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Somchit N, Norshahida AR, Hasiah AH, et al. Hepatotoxicity induced by antifungal drugs itraconazole and fluconazole in rats: a comparative in vivo study. Hum Exp Toxicol. 2004;23:519–525. [DOI] [PubMed] [Google Scholar]
- 17.Somchit N, Hassim SM, Samsudin SH. Itraconazole- and fluconazole-induced toxicity in rat hepatocytes: a comparative in vitro study. Hum Exp Toxicol. 2002;21:43–48. [DOI] [PubMed] [Google Scholar]
- 18.Miyashita T, Shah FA, Marti GP, et al. Rabeprazole impedes the development of reflux-induced esophageal cancer in a surgical rat model. Dig Dis Sci. 2011;56:1309–1314. [DOI] [PubMed] [Google Scholar]
- 19.Haseman JK. Exact sample sizes for the use with the Fisher-Irwin test for 2×2 tables. Biometrics. 1978;34:106–109. [Google Scholar]
- 20.Milano F, Van Baal JW, Buttar NS, et al. Bone morphogenetic protein 4 expressed in esophagitis induces a columnar phenotype in esophageal squamous cells. Gastroenterology. 2007;132:2412–2421. [DOI] [PubMed] [Google Scholar]
- 21.Mari L, Milano F, Parikh K, et al. A pSMAD/CDX2 complex is essential for the intestinalization of epithelial metaplasia. Cell Rep. 2014;7:1197–1210. [DOI] [PubMed] [Google Scholar]
- 22.Sekulic A, Migden MR, Lewis K, et al. Pivotal ERIVANCE basal cell carcinoma (BCC) study: 12-month update of efficacy and safety of vismodegib in advanced BCC. J Am Acad Dermatol. 2015;72:1021.e8–1026.e8. [DOI] [PubMed] [Google Scholar]
- 23.Jimeno A, Weiss GJ, Miller WH Jr, et al. Phase I study of the Hedgehog pathway inhibitor IPI-926 in adult patients with solid tumors. Clin Cancer Res. 2013;19:2766–2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi W, Nacev BA, Aftab BT, et al. Itraconazole side chain analogues: structure-activity relationship studies for inhibition of endothelial cell proliferation, vascular endothelial growth factor receptor 2 (VEGFR2) glycosylation, and hedgehog signaling. J Med Chem. 2011;54:7363–7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aftab BT, Dobromilskaya I, Liu JO, et al. Itraconazole inhibits angiogenesis and tumor growth in non-small cell lung cancer. Cancer Res. 2011;71:6764–6772. [DOI] [PMC free article] [PubMed] [Google Scholar]