Abstract
Near-infrared (NIR) laser-induced phototherapy through NIR agents has demonstrated the great potential for cancer therapy. However, insufficient tumor killing due to the nonuniform heat or cytotoxic singlet oxygen (1O2) distribution over tumors from phototherapy results in tumor recurrence and inferior outcomes. To achieve high tumor killing efficacy, one of the solutions is to employ the combinational treatment of phototherapy with other modalities, especially with chemotherapeutic agents. In this paper, a simple and effective multimodal therapeutic system was designed via combining chemotherapy, photothermal therapy (PTT), and photodynamic therapy (PDT) to achieve the polytherapy of malignant glioma which is one of the most aggressive tumors in the brain. IR-780 (IR780) dye-labeled tube-forming peptoids (PepIR) were synthesized and self-assembled into crystalline nanotubes (PepIR nanotubes). These PepIR nanotubes showed an excellent efficacy for PDT/PTT because the IR780 photosensitizers were effectively packed and separated from each other within crystalline nanotubes by tuning IR780 density; thus, a self-quenching of these IR780 molecules was significantly reduced. Moreover, the efficient DOX loading achieved due to the nanotube large surface area contributed to an efficient and synergistic chemotherapy against glioma cells. Given the unique properties of peptoids and peptoid nanotubes, we believe that the developed multimodal DOX-loaded PepIR nanotubes in this work offer great promises for future glioma therapy in clinic.
1. Introduction
Photobased therapies such as photothermal therapy (PTT) and photodynamic therapy (PDT) have recently been developed as promising therapeutic strategies for antitumor therapy because of their minimal invasiveness, low side effects, and high specificity [1–6]. For PTT and PDT, they usually utilize the near-infrared (NIR) laser-induced capability of photothermal agents and photosensitizers (PSs) to generate heat or cytotoxic singlet oxygen (1O2), which subsequently causes cell apoptosis [7–10]. However, because of the nonuniform distribution of the near-infrared agents at the tumor site, the heat or cytotoxic 1O2 distribution over tumors is also nonuniform. It leads to the insufficient tumor cell killing, incomplete ablation, tumor recurrence, and inferior outcomes [11, 12]. Recently, the combination of phototherapy and chemotherapy (chemophototherapy, CPT) has been designed and proved for a synergistic cancer treatment to enhance the cancer treatment efficacy and reduce the toxicities and drug resistance during the chemotherapy [13–15]. For example, a tumor microenvironment- (TME-) responsive system PCPTSS/IR820 was designed by Shi et al.'s group for synergistic CPT [16]. Pan et al.'s group reported a biodegradable PRDCuS@AG nanocomposite to load doxorubicin and copper sulfide (CuS) for deep tumor CPT [17]. The phototherapy mainly utilizes the NIR light in the wavelength range from 700 to 1100 nm which can efficiently penetrate into biological tissues and selectively act on tumor for an irreversible cellular damage [11]. The chemotherapy, more like the traditional anticancer drug delivery, can be used to kill the cancer cells at the biomolecular level [18].
IR-780 iodide (IR780) photosensitizer is a popular NIR PTT or PDT agent for cancer therapy which holds the suitable fluorescence, good stability, strong absorption in the NIR region, and efficient light conversion [19–21]. Moreover, unlike some synergistic PDT/PTT systems that generally need two lasers with different wavelengths, IR780 dyes can simultaneously produce singlet oxygen and generate heat under one laser irradiation (808 nm), which can avoid the sequential irradiation and shorten treatment time [22, 23]. However, the poor aqueous solubility and concentration-dependent aggregation of IR780 monomers will largely compromise its therapeutic efficacy and should be addressed before their use in biomedical applications [24]. To extend the therapeutic window for IR780, many nanoparticles (NPs), including gold nanostructures [23], carbon nanomaterials [25], and silica NPs [26], were designed to arrange IR780 molecules for PDT and PTT. Compared to others, IR780-doped nanomicelles or self-assembling NPs exhibit advantages in phototherapy [27]. Importantly, these NPs are fabricated using biodegradable polymers, such as poly(D,L-lactide-co-glycolide) (PLGA), which have been extensively studied for many years as therapeutic platforms [28]. Although the strategies of PS-doped nanoplatform have been widely reported, the main challenge in the design of IR780-doped NPs is to confine the PSs at a high concentration inside NPs without a self-quenching because IR780 PSs tend to stack to form H-aggregates in polar solvents such as phosphate-buffered saline (PBS) buffer [29]. Unfortunately, all previously developed carries including self-assembled NPs are limited to precisely control the arrangement and ordering of PSs at a high concentration [30]. Therefore, the key to design highly fluorescent PDT/PTT NPs is to precisely place and align IR780 PSs within crystalline nanomaterials with programmable interfluorophore distance and orientation.
We recently developed a new type of highly stable and dynamic single-walled nanotubes from sequence-defined peptoids (poly-N-substituted glycines) [31]. We have showed that these nanotubes can be programmed for various applications, such as water decontamination and cellular imaging. By taking advantage of easy synthesis of peptoids and robustness of nanotube self-assembly, we have demonstrated the introduction of various functional groups, including fluorescent dye, macrocyclic compound, biomolecule, and peptides, as peptoid side chains within peptoid nanotubes. In addition, because peptoids are highly stable and biocompatible and exhibit protein-like high selectivity [32], these peptoid nanotubes are expected to provide an effective platform for precisely engineering IR780 dyes with well-controlled interfluorophore distance and orientation, developing biocompatible NIR nanomaterials for CPT applications [33–35].
Malignant gliomas are the most common brain tumors that are highly aggressive and show very high morbidity and mortality [36–38]. They exhibit invasive growth into surrounding normal brain tissues. Chemotherapy remains the main treatment, but the single therapy result is very limited due to its inability to address the highly invasive nature of gliomas [39]. Recently, phototherapy showed the great potential for malignant glioma therapy by using a laser to selectively cause tumor cell apoptosis [40, 41]. For the above reasons, herein, we developed a simple and effective multimodal therapeutic system against gliomas by using the CPT strategy, in which highly stable and crystalline peptoid nanotubes were used as the biocompatible scaffold to precisely display and align IR780 PSs and to simultaneously load chemotherapeutic drug doxorubicin (DOX). Owing to the precise adjustment of IR780 intermolecular distance as a result of nanotube high crystallinity, nanotubes assembled from IR780-conjugated peptoids (PepIR) exhibited a simple, safe, and effective platform for simultaneous PDT/PTT, in which DOX drug molecules were loaded within PepIR nanotubes for a synergistic treatment. The combined CPT strategy (Scheme 1) resulted in significantly higher therapeutic efficiency than individual phototherapy or chemotherapy did.
Scheme 1.
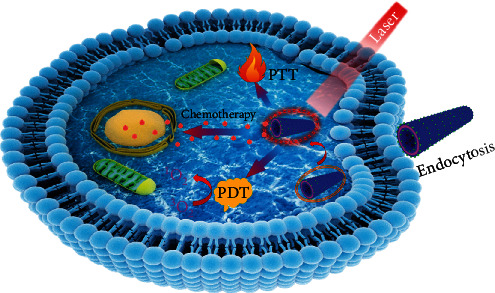
Schematic illustration of DOX-loaded sc-PepIR-40 nanotubes for trimodal chemo-PDT/PTT synergistic therapy of glioma cancer cells, in which the loaded IR780 dyes can simultaneously produce singlet oxygen and generate heat under 808 nm laser irradiation to facilitate the effective PDT/PTT against tumor cells. Furthermore, DOX drug molecules were loaded within PepIR nanotubes for a synergistic chemotherapy treatment.
2. Results and Discussion
2.1. Synthesis, Assembly, and Characterization of PepIR Nanotubes
According to a previously developed solid-phase submonomer synthesis method [31], tube-forming peptoids with and without IR780 were designed and synthesized. As shown in Figure 1(a), these peptoid sequences have six N-(2-carboxyethyl) glycine (Nce) groups as the polar domain and six N-[(4-bromophenyl)methyl] glycine (Nbrpm) groups as the hydrophobic region. The IR780 PS was conjugated at the N-terminus adjacent to the polar domain. The detailed preparation, purification, and characterizations of these two peptoids (Pep: Nbrpm6Nce6; PepIR: Nbrpm6Nce6Nc6IR780) are described in the Materials and Methods and supporting information (Figure S1). Peptoid nanotubes with a tunable density of IR780 PSs were synthesized by coassembling PepIR with Pep in a variable ratio using a similar evaporation-induced crystallization approach described previously (see Materials and Methods) [31]. As shown in Figure 1, both transmission electron microscopy (TEM) and atomic force microscopy (AFM) data showed that nanotubes assembled from 100% PepIR (PepIR-100), 40% PepIR (PepIR-40), or 20% PepIR (PepIR-20) all have similar structure. TEM images showed that PepIR-20 nanotubes exhibited a diameter of 32.66 ± 1.90 nm (Figure S3a) and a wall thickness of 3.34 ± 0.34 nm (Figure 1(b)). A high-resolution image of these nanotubes is shown in Figure 1(f), and the increased tube wall thickness is due to the introduction of IR780 PSs within peptoid nanotubes [31]. TEM results showed that PepIR-40 nanotubes exhibited a wall thickness of 3.43 ± 0.34 nm (Figure 1(d) and S2c) and a diameter of 33.71 ± 2.7 nm (Figure S3b), and PepIR-100 nanotubes had a wall thickness of 3.45 ± 0.23 nm (Figure 1(g) and S2e) and a diameter of 35.67 ± 2.04 nm (Figure S3c). The heights of PepIR-20, PepIR-40, and PepIR-100 nanotubes from ex situ AFM results are 6.72 ± 0.33 nm, 6.91 ± 0.31 nm, and 6.94 ± 0.26 nm, respectively (Figures 1(c), 1(e), and 1(h)), which are approximately two times the wall thickness, showing the deformation of PepIR nanotubes under dry conditions [26]. The X-ray diffraction (XRD) data proved that these PepIR nanotubes have crystalline nanostructures and PepIR-20, PepIR-40, and PepIR-100 nanotubes all exhibited similar structures by having similar XRD patterns (Figure 1(i), Figure S4a and b). As shown in Figure 1(i), the first low q peak (d = 3.34 nm) indicates the wall thickness of PepIR-20 nanotube, which is consistent with the data analyzed by TEM tests (Figures 1(b) and 1(f)). The peak at 1.65 nm is the distance between two peptoid backbones in the direction of the hydrophobic Nbrpm groups facing each other [31]. The spacing of 5.7 Å is attributed to the ordered packing of aromatic side chains Nbrpm6. The 4.6 Å spacing corresponds to the alignment of lipid-like peptoid chains. The peak at 3.36 Å indicates the interresidue distance along the chain direction. And the peaks at 4.3 Å, 3.8 Å, and 2.9 Å indicate the presence of extensive π-stacking. As the model we proposed in previous work [31], the hydrophobic Nbrpm groups are packed with each other and embedded in the center of the nanotube wall. As for the polar Nce groups, they are located on both surfaces of the nanotube and exhibit a similar packing to Pep nanotubes that we reported previously [31]. These results showed that IR780 dye molecules could be precisely displayed within crystalline nanotubes with a tunable density while retaining the similar tubular framework structure.
Figure 1.
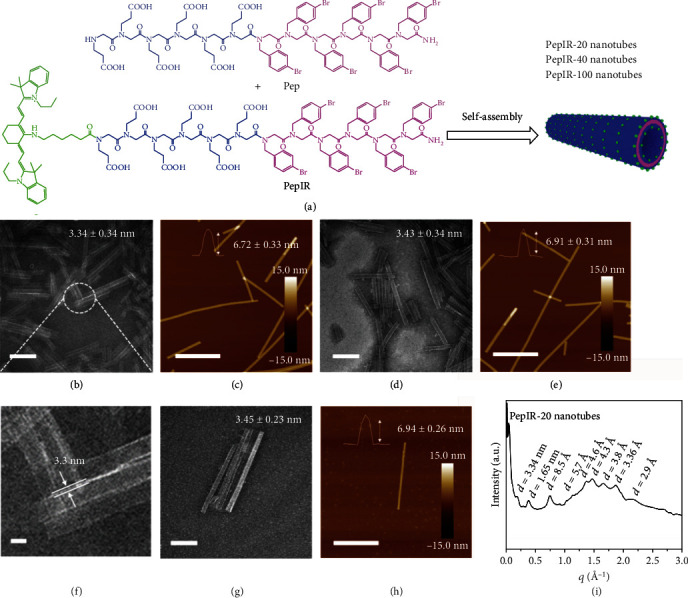
Characterizations of PepIR nanotubes: (a) structures of Pep and PepIR and the scheme showing the self- or coassembly into PepIR nanotubes with a tunable density of IR780 dyes. (b) TEM image of PepIR-20 nanotubes coassembled from 20% PepIR and 80% Pep (scale bar, 200 nm). (c) Ex situ AFM image of PepIR-20 nanotubes (scale bar, 1.0 μm). The top inset is the height of PepIR-20 nanotube. 20 nanotubes were analyzed for obtaining the height distribution. (d) TEM image of PepIR-40 nanotubes coassembled from 40% PepIR and 60% Pep (scale bar, 200 nm). (e) Ex situ AFM image of PepIR-40 nanotubes (scale bar, 1.0 μm). The inset is the height of PepIR-40 nanotube. 20 nanotubes were analyzed for obtaining the height distribution. (f) The high-resolution TEM image showing the wall thickness of nanotubes in (b) (scale bar, 10 nm). (g) TEM image of PepIR-100 nanotubes assembled from 100% PepIR (scale bar, 200 nm). (h) Ex situ AFM image of PepIR-100 nanotubes (scale bar, 1.0 μm). The inset is the height of PepIR-100 nanotube. 20 nanotubes were analyzed for obtaining the height distribution. (i) XRD spectrum of PepIR-20 nanotubes. The formula of d = 2π/q was used to calculate the values above each peak.
The main challenge in the design of PS-doped nanoparticles for phototherapy is to address the issue of self-quenching of the PSs [27, 42]. Indeed, as flat aromatic structures, IR780 PSs tend to form π-stacking aggregates (H-aggregates) that exhibit poor fluorescent signals [29]. Crystalline nanotubes exhibit well-defined orientations and distances of functional groups [31], which is an obvious advantage for engineering IR780 PSs at a high concentration for phototherapy. To demonstrate that, we analyzed the fluorescence properties of these PepIR-20, PepIR-40, and PepIR-100 nanotubes. As shown in Figure 2(a), PepIR nanotubes gradually increased their fluorescence emission intensity as the percentage of PepIR increased from 20% to 40%, while the fluorescence emission intensity of PepIR-100 nanotubes significantly decreased in contrast to the PepIR-40 nanotubes which might be due to the self-quenching of some IR780 dyes. These results indicated that the PepIR-40 nanotube is the best candidate for the phototherapy among these three types of nanotubes. Besides, the UV-vis absorption of PepIR-40 nanotubes in water is significantly higher than those free IR780 PSs in methanol or water (Figure 2(b)), which further indicated that the high crystallinity of peptoid nanotubes facilitated the fluorescence emission intensity of IR780 PSs by reducing the self-quenching effect in aqueous solution. These PepIR-40 nanotubes are good candidates for biological applications. Furthermore, the high loading molar ratio of IR780 PSs within PepIR-40 nanotubes indicates that peptoid-based coassembly strategy is an effective way for precisely controlling the loading and ordering of PSs at high concentrations.
Figure 2.
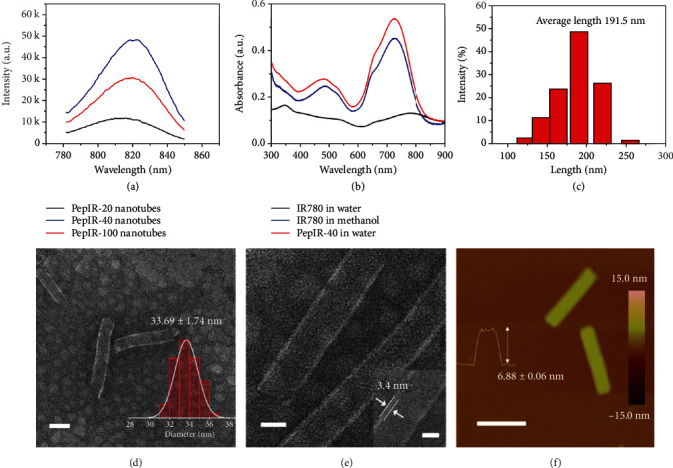
(a) Fluorescence spectra of PepIR-20, PepIR-40, and PepIR-100 nanotubes. (b) UV-vis data of IR780 dye in water/methanol and PepIR-40 nanotubes in water. (c) Dynamic light scattering (DLS) data of sc-PepIR-40 nanotubes showing that the average tube length is about 191.5 nm. (d) TEM image of sc-PepIR-40 nanotubes (scale bar, 50 nm). The right inset is the statistical tube diameter distribution measured from the TEM results. The average tubular diameter is shown above the histogram. (e) Magnified TEM image of sc-PepIR-40 nanotubes (scale bar, 20 nm). The right inset is the high-resolution TEM image showing the tube wall thickness of 3.4 nm (scale bar, 10 nm). (f) Ex situ AFM image of two sc-PepIR-40 nanotubes (scale bar, 200 nm). The inset is the AFM height measurement showing the sc-PepIR-40 nanotube height. 20 nanotubes were analyzed for obtaining the height distribution.
2.2. Synthesis and Characterization of Colloidal PepIR-40 Nanotubes
To improve cellular uptake of PepIR-40 nanotubes, the prepared nanotubes were sonication-cut (see Materials and Methods section), and the dynamic light scattering (DLS) data showed that the obtained colloidal PepIR-40 nanotubes (denoted as sc-PepIR-40 nanotubes) have an average of length of ~191.5 nm (Figure 2(c)). TEM images (Figure 2(d), Figure S5a and b) showed that these sc-PepIR-40 nanotubes retained the tubular morphology with a diameter of ~33.69 ± 1.74 nm (inset in Figure 2(d)), which is consistent with that of presonicated PepIR-40 nanotubes. Moreover, as shown in the high-resolution TEM image in Figure 2(e), the wall thickness of these sonication-cut nanotubes (3.4 nm) is also comparable to presonicated PepIR-40 nanotubes. The height measured from ex situ AFM image of sonication-cut nanotubes is around 6.88 ± 0.06 nm (Figure 2(f)), similar to the height obtained from presonicated PepIR-40 nanotubes (Figure 1(e)). All these results indicate that sonication is an effective way to cut PepIR nanotubes into a short length without changing other structural parameters.
2.3. The Photothermal Behavior and 1O2 Generation of sc-PepIR-40 Nanotubes
After the sonication cutting and characterizations of these PepIR nanotubes, we then studied their photothermal behaviors (Figure 3(a)). The temperature of the nanotube aqueous solution increased to 49.2°C in 5 min under the 808 nm laser irradiation, and the maximum temperature was 50.1°C. It has been reported that hyperthermia can kill tumor cells directly at >45°C [14], and the above temperatures are high enough to cause significant hyperthermia damage to cancer cells while peptoid nanotubes are stable in such condition (Figure S6). These results showed that sc-PepIR-40 nanotubes proved the sufficient photothermal conversion capability suitable for a PTT application.
Figure 3.
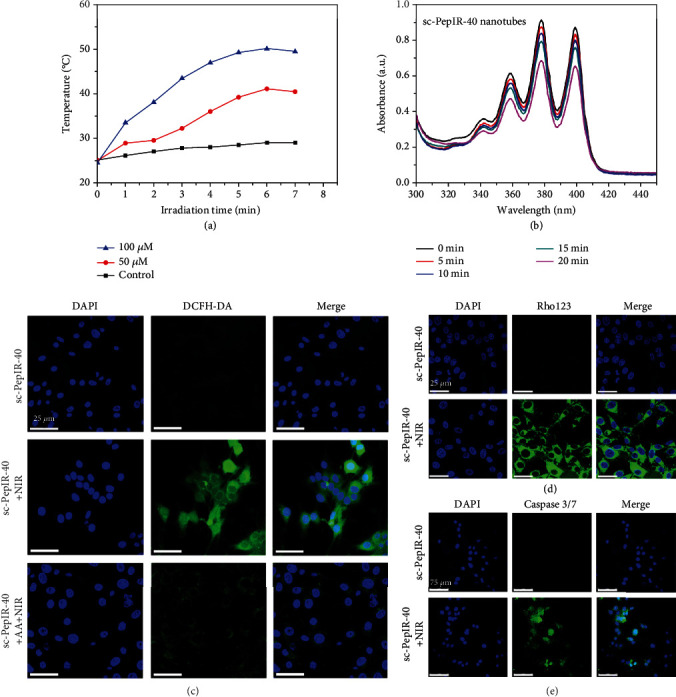
(a) Photothermal effect of different concentrations (50 μM and 100 μM) of sc-PepIR-40 nanotubes under 808 nm laser irradiation, and PBS buffer was used as the control. (b) UV-vis absorption spectra of ABDA under 808 nm laser irradiation for different times in the presence of sc-PepIR-40 nanotubes. Intracellular 1O2 generation of sc-PepIR-40 nanotubes in U87MG cells with/without NIR laser irradiation detected by DCFH-DA (c), Rho123 (d), and caspase 3/7 kit (e). Cell nuclei were stained with DAPI in blue color. All laser irradiation was 5 min (808 nm, 1.8 W cm−2).
To verify the photodynamic effects of these sc-PepIR-40 nanotubes, they were evaluated for 1O2 generation using 9,10-anthracenediyl-bis (methylene) dimalonic acid (ABDA) as an indicator. ABDA could undergo oxidation to yield an endoperoxide once 1O2 exist, resulting in the decreased absorption of ABDA. As shown in Figure S7, in the absence of sc-PepIR-40 nanotubes, there was no obvious decrease of ABDA absorption under 808 nm laser irradiation, while after adding sc-PepIR-40 nanotubes, the ABDA absorption was gradually decreased under 808 nm laser irradiation (Figure 3(b)), proving that 1O2 was generated and sc-PepIR-40 nanotubes can be used for cancer PDT.
To investigate whether sc-PepIR-40 nanotubes could be used for effective intracellular PDT, 2′,7′-dichlorofluorescin diacetate (DCFH-DA) was used along with sc-PepIR-40 nanotubes to detect intracellular 1O2 generation of these nanotubes under NIR laser (Figure 3(c)). After cellular uptake, DCFH-DA is converted to nonfluorescent 2′,7′-dichlorodihydrofluorescein (DCFH), and the nanotube-induced 1O2 generation can oxidize DCFH to generate green fluorescence. As shown in Figure 3(c), a fluorescence signal was barely observed in the U87MG cells treated with sc-PepIR-40 nanotubes without 808 nm laser irradiation. In contrast, the nanotube-treated cells under 808 nm laser irradiation exhibited a strong green fluorescence signal, suggesting that 1O2 were generated from sc-PepIR-40 nanotubes within U87MG cells upon NIR laser irradiation. When the 1O2 scavenger ascorbic acid (AA) was premixed with sc-PepIR-40 nanotubes before laser irradiation, the green fluorescence signal significantly decreased, which indicated that the appearance of green fluorescence signal was a result of 1O2 generation [43]. In addition, a burst of 1O2 can disrupt the normal physiological redox state, resulting in the destruction of mitochondrial membrane potential (MMP), as indicated by using rhodamine 123 (Rho 123) [44, 45]. As shown in Figure 3(d), compared to the control group without NIR laser irradiation, a bright green fluorescence signal was observed within U87MG cells when they were incubated with sc-PepIR-40 nanotubes for 4 h and treated with 808 nm laser irradiation, indicating the destruction of mitochondria. These results showed that sc-PepIR-40 nanotubes under 808 nm laser irradiation could generate 1O2 within U87MG cells. PDT-induced apoptosis of cancer cells can be analyzed using a caspase 3/7 kit, a novel fluorogenic substrate for activated caspase-3/7, which is an essential event during apoptosis [46]. As shown in Figure 3(e), a treatment of U87MG cells with sc-PepIR-40 nanotubes without 808 nm laser irradiation showed no effect on caspase activity, while an obvious green fluorescence signal of activated caspase 3/7 was observed when these cells were incubated with sc-PepIR-40 nanotubes under 808 nm laser irradiation, further confirming that sc-PepIR-40 nanotubes under 808 laser irradiation can induce effective cell apoptosis and be used for PDT.
2.4. Drug Loading, Release, and Cellular Uptake of sc-PepIR-40 Nanotubes
DOX, a well-known anticancer drug, was chosen as a model drug that was loaded in these sc-PepIR-40 nanotubes with a loading content up to ~27.6% (calculated by the loading experiments described in Materials and Methods section). While the UV-vis spectra of the sc-PepIR-40 nanotubes showed the characteristic peak of IR780 at ∼800 nm (Figure 4(a)), the DOX-loaded sc-PepIR-40 nanotubes had the characteristic peaks of both DOX and IR780 in the UV-vis spectra (Figure 4(a)). The successful loading of DOX within sc-PepIR-40 nanotubes was further confirmed by the fluorescent measurements (Figure 4(b)). Compared to that of free DOX, the fluorescence emission of DOX-loaded sc-PepIR-40 nanotubes was significantly quenched. The cellular uptake and drug release behavior of the DOX-loaded sc-PepIR-40 nanotubes in U87MG cells were analyzed by confocal laser scanning microscopy (CLSM). The fluorescence emission of DOX was quenched after being loaded in the sc-PepIR-40 nanotubes. However, the recovery of DOX fluorescence within cancer cells was observed when the DOX was released from nanotubes. Therefore, we used the red fluorescence signal of DOX to track the intracellular drug release (Figure 4(c)). After 2 h of incubation, compared with the cells in the control group, the glioma cancer cells treated with DOX-loaded sc-PepIR-40 nanotubes showed an obvious red color in the cytoplasm. Furthermore, DOX was gradually distributed in the nuclear area after 4 h of incubation. These results indicated that DOX was successfully released from sc-PepIR-40 nanotubes within glioma cancer cells.
Figure 4.
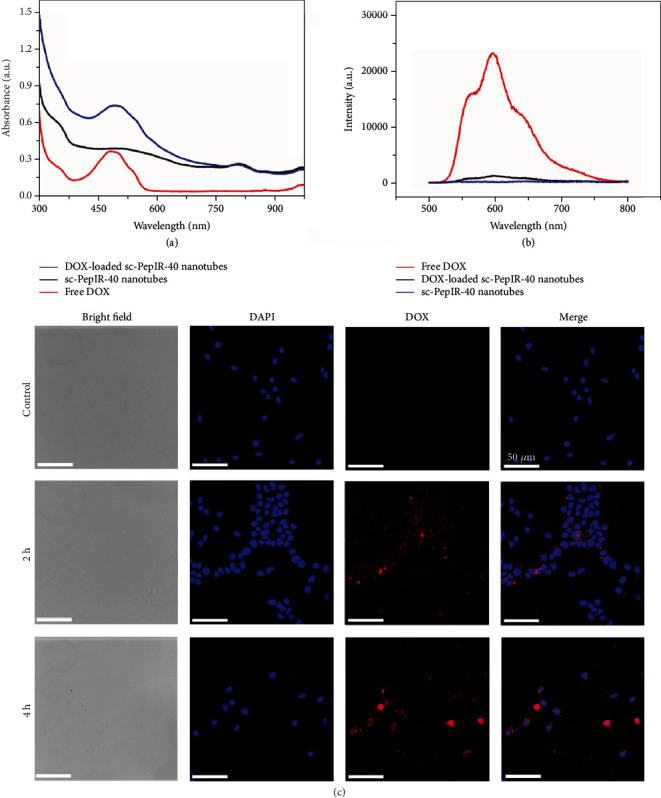
UV-vis absorbance spectra (a) and fluorescence spectra (b) of sc-PepIR-40 nanotubes, free DOX, and DOX-loaded sc-PepIR-40 nanotubes. (c) CLSM images of U87MG cells incubated with DOX-loaded sc-PepIR-40 nanotubes for 2 h and 4 h. Blue color comes from cell nuclei stained with DAPI.
2.5. In Vitro Cytotoxicity Assay of sc-PepIR-40 Nanotube-Based System
The synergistic effect of DOX-loaded sc-PepIR-40 nanotubes on the apoptosis of U87MG cells was investigated by the (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (MTT) assay. Figure 5 described the MTT data; sc-PepIR-40 nanotubes without (Figure 5(a)) and with a loading of DOX (Figure 5(b)) both exhibited an increased cytotoxicity in a concentration-dependent manner after 48 h incubation under 808 nm laser irradiation. For the DOX-loaded sc-PepIR-40 nanotubes treated with laser irradiation (808 nm), over 70% of the cells were killed at peptoid tube concentration of 12.5 μM, indicating that this combined treatment had a significantly high therapeutic efficiency against glioma cell growth. Moreover, the half-maximal inhibitory concentrations (IC50) of these treatments are shown in Table S1, and the combination index (CI) was calculated to be 0.323 (<1), confirming a high synergistic therapeutic effect [47].
Figure 5.
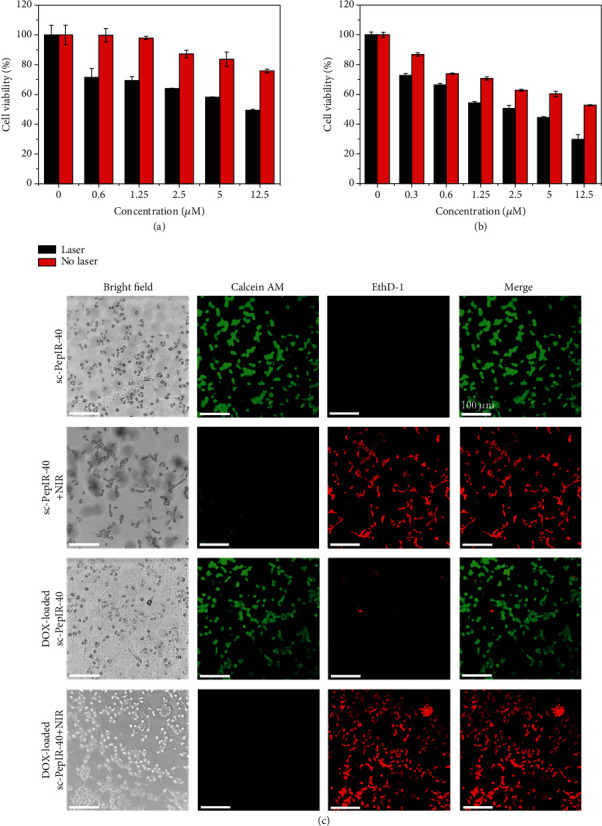
MTT assay of U87MG cells incubated with sc-PepIR-40 nanotubes with/without 808 nm laser irradiation (a) and DOX-loaded sc-PepIR-40 nanotubes with/without 808 nm laser irradiation (b). (c) CLSM images of cell viability assay after incubating with sc-PepIR-40 nanotubes or with DOX-loaded sc-PepIR-40 nanotubes with/without NIR laser irradiation. The cells were stained with live/dead viability kit (live cells were labeled with calcein AM with green fluorescence, and the dead cells were shown in red fluorescence after being labeled with EthD-1). All laser irradiations were performed using an 808 nm laser with a power intensity of 1.8 W cm−2 for 5 minutes.
To visually estimate the in vitro therapeutic effect of these sc-PepIR-40 nanotubes, we used a live/dead cell viability assay kit to evaluate the influence of the combined treatment in killing glioma cells. Live cells were labeled with calcein AM with green fluorescence, and the dead cells were shown in red fluorescence after being labeled with ethidium homodimer-1 (EthD-1). Figure 5(c) showed the confocal images of the live/dead cells marked with calcein-AM and EthD-1 after incubating U87MG cells with sc-PepIR-40 nanotubes or DOX-loaded sc-PepIR-40 nanotubes for 4 h, respectively. Before 808 nm laser irradiation, while DOX-loaded nanotubes induced an obvious cell death, almost no dead cells were induced by sc-PepIR-40 nanotubes. Interestingly, upon 808 nm laser irradiation, even sc-PepIR-40 nanotubes induced a significant population of dead cells (Figure 5(c)), while DOX-loaded sc-PepIR-40 nanotubes induced a much higher population of dead cells as sc-PepIR-40 nanotubes did. These results further confirmed the potent phototherapeutic efficacy of sc-PepIR-40 nanotubes and the synergistic effect of DOX-loaded sc-PepIR-40 nanotubes on the treatment of malignant glioma as a result of simultaneous chemo-PDT/PTT polytherapy. This result is also consistent with the conclusion obtained from the MTT assay above (Figures 5(a) and 5(b)).
3. Conclusion
In summary, PS-doped peptoid-based crystalline nanotubes, in which IR780 was attached at the N-terminus of tube-forming peptoid sequences, were coassembled and developed as an efficient platform to deliver DOX for the simultaneous chemo-PDT/PTT trimodal treatment of glioma. In this versatile system, the IR780 PSs were effectively packed and separated from each other within crystalline nanotubes; thus, a self-quenching of these IR780 molecules was significantly reduced. The precise placement of IR780 dyes within peptoid nanotubes enabled the high stability of IR780 for an excellent 1O2 production and photothermal conversion within glioma cancer cells. Moreover, efficient DOX-loading was achieved because of the large surface area of the nanotubes, contributing to an efficient CPT strategy against the glioma cells. The enhanced antitumor efficiency of DOX-loaded sc-PepIR-40 nanotubes was confirmed by the CLSM and MTT assays. Given the above results and the unique properties of peptoids and peptoid nanotubes, the developed multimodal DOX-loaded sc-PepIR-40 nanotubes in this work offer great promises for future glioma therapy in clinic.
4. Materials and Methods
4.1. Methods for Synthesizing Pep (Nbrpm6Nce6) Using a Solid-Phase Synthesizer
The relative peptoids were synthesized on a commercial Aapptec Apex 396 robotic synthesizer using a solid-phase submonomer cycle according to a previously developed solid-phase submonomer synthesis method [31].
4.2. Synthesis of Aminohexanoic Acid-Modified Peptoids (PepNc6, Nbrpm6Nce6Nc6)
The resins containing Nbrpm6Nce6Nc6 were obtained through the previously reported method [31]. Briefly, the Nc6 group was conjugated on the peptoid manually. Then, the resin containing Nbrpm6Nce6 obtained from automated solid-phase synthesis was mixed with a DMF solution of Fmoc-6-aminohexanoic acid (1.5 mL, 0.9 mmol) and 0.50 mL of 50% (v/v) DIC/DMF. After agitating the mixture at room temperature for overnight, the resin was filtered and washed with DMF. The terminal Fmoc group was removed by an addition of 2 mL of 20% (v/v) 4-methylpiperidine/DMF. The mixture was agitated for 1 h; then, the resin was filtered and washed well with DMF.
4.3. Synthesis of IR780-Modified Peptoids (PepIR, Nbrpm6Nce6Nc6IR780)
The resins (0.09 mmol) containing PepNc6 were mixed with 6.0 mL CH3CN solution of IR780 dye (0.9 mmol) and DIPEA (diisopropylethylamine, 0.99 mmol). The mixture was agitated for 24 hours at 80°C (reflux), filtered, and washed well with CH3CN and DMF. The resins containing PepIR were synthesized.
4.4. Peptoid Cleavage and HPLC Purification
The final crude products (Pep or PepIR) were cleaved from the corresponding resins by using 95% trifluoroacetic acid (TFA) in water, which was conducted following the previously reported method [31]. Finally, the peptoid powder was stored at -80°C.
4.5. Assembly of PepIR Nanotubes
Pep and PepIR were coassembled in the mixture of H2O/CH3CN (v/v = 3 : 7). For coassembly, peptoids in a relative ratio were dissolved in the mixture of H2O and CH3CN (v/v = 3 : 7) with peptoid concentration which was 5.0 mM. Then, the solutions were put in a refrigerator at 4°C to achieve slow evaporation. After 48 h, the cloudy solution containing crystalline nanotubes was prepared.
4.6. Synthesis of sc-PepIR-40 Nanotubes by Sonication
The cloudy aqueous solution that contains diluted peptoid nanotubes (250 μM) was sonicated using an ultrasonic processor (FS-450N, 150 W) for 5 min; ice water was used to keep the nanotube solution temperature consistent during the sonication-cutting process.
4.7. Photothermal Heating Effect of Sonicated sc-PepIR-40 Nanotubes
Different concentrations of sc-PepIR-40 nanotubes (50 μM and 100 μM) were treated under 808 nm laser irradiation. The PBS solution containing no sc-PepIR-40 nanotubes was also investigated as the control. The temperatures at different time points were recorded using a thermometer.
4.8. Loading DOX to sc-PepIR-40 Nanotubes
150 μL aqueous solution of DOX (1.0 mg/mL) was mixed with 800 μL aqueous solution of sc-PepIR-40 nanotubes (250 μM), and the resulting mixture was stirred under the dark condition for 24 h. After removing all unloaded DOX through centrifugation, the obtained DOX-loaded sc-PepIR-40 nanotubes were redispersed in 800 μL H2O and diluted to the corresponding assay by checking the UV-vis absorbance at 490 nm. Based on a calibration curve obtained from four DOX solutions at different concentrations (Figure S8), the amount of loaded DOX within sc-PepIR-40 nanotubes was determined. The drug loading content was determined by the following equation:
| (1) |
4.9. Cellular Uptake of DOX-Loaded sc-PepIR-40 Nanotubes
U87MG cells were incubated in DMEM and seeded in a 12-well culture plate for 24 h. After washing with PBS, the cells were incubated with DOX-loaded sc-PepIR-40 nanotubes (2.5 μM) for 2 h and 4 h, respectively. Cells without nanotube treatment were used as the control. 4% paraformaldehyde was used to fix cells, and cell nuclei were stained with DAPI in blue color for CLSM observation (excitation at 405 nm and 488 nm).
4.10. Detection of 1O2 Generation
The extracellular 1O2 generation was monitored with ABDA, a 1O2 monitor via its decreased fluorescence absorption due to the reaction with 1O2. In the experiment, 400 μM ABDA with and without sc-PepIR-40 nanotubes (25 μM) were under the 808 nm laser irradiation (1.8 W cm−2). Their ABDA absorbance changes at different irradiation time intervals (0-20 min) were tested using a microplate reader.
The intracellular 1O2 generation was monitored with DCFH-DA, a probe that turned to highly green fluorescent 2′,7′-dichlorofluorescein in the presence of 1O2. U87MG cells were seeded in a 12-well culture plate for 24 h and incubated 4 h with sc-PepIR-40 nanotubes. Then, 1.0 mL DMEM containing 10 μM DCFH-DA was added to each well with incubation at 37°C for 20 min. In addition, 10 μM DCFH − DA + 100 μM AA were added as the control. Cells without the laser irradiation were also added as the control. After washing with PBS, the cells were irradiated by 808 nm laser (1.8 W cm−2, 5 min) and cell nuclei were stained with DAPI for CLSM observation.
The rupture of mitochondrial membrane potential (MMP) and mitochondrial-mediated apoptosis were measured using rhodamine 123 (Rho 123) and caspase 3/7 kit, respectively. In these experiments, U87MG cells were seeded in the culture dishes at a density of 1 × 105 cells per dish. After 24 h incubation at 37°C, cells were incubated with sc-PepIR-40 nanotubes for 4 h and treated with a 5 min laser irradiation (808 nm, 1.8 W cm−2). After a further 4 h incubation, Rho 123 (10 μM, 30 min) and caspase 3/7 kits (2 drops/mL, 30 min) were used to stain cells, respectively. CLSM observation was conducted to obtain fluorescence images.
4.11. Therapy Efficacy
U87MG cells were seeded in a 12-well culture plate for 24 h and incubated with sc-PepIR-40 nanotubes and DOX-loaded sc-PepIR-40 nanotubes for 4 h. Then, a 5 min laser irradiation (808 nm, 1.8 W cm−2) was conducted. As a control, cells were incubated with these nanotubes for 4 h without a laser irradiation. After that, calcein AM (2.0 μM) and EthD-1 (4.0 μM) solutions were added to each well to costain cells at 37°C for 15 min. Finally, CLSM observations were conducted to visualize assay cell viability. To investigate cell viability by MTT assay, the incubation time between U87MG cells and samples was extended to 24 h before the irradiation by 808 nm laser (1.8 W cm−2, 5 min). Then, the cells were further incubated for 24 h and the viability was detected by a microplate reader. Each well was obtained by following the MTT kit instruction.
Acknowledgments
This work was supported by Washington State University (WSU) start-up fund. Peptoid synthesis work was supported by the Materials Synthesis and Simulation Across Scales (MS3) Initiative through the LDRD fund at Pacific Northwest National Laboratory (PNNL). Assembly of peptoid nanotubes and their structural characterizations were supported by the U.S. Department of Energy, Office of Basic Energy Sciences, Biomolecular Materials Program at PNNL. XRD work was conducted at the Advanced Light Source with support from the Molecular Foundry, at Lawrence Berkeley National Laboratory, both of which are supported by the Office of Science, under Contract No. DE-AC02-05CH11231. PNNL is a multiprogram national laboratory operated for Department of Energy by Battelle under Contract No. DE-AC05-76RL01830.
Contributor Information
Yang Song, Email: yang.song@wsu.edu.
Chun-Long Chen, Email: chunlong.chen@pnnl.gov.
Yuehe Lin, Email: yuehe.lin@wsu.edu.
Data Availability
All data are available in the manuscript, supplementary materials, or from the author.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Authors' Contributions
Xiaoli Cai and Mingming Wang contributed equally to this work.
Supplementary Materials
Figure S1: ultraperformance liquid chromatography (UPLC) and mass spectrometry (MS) data of peptoids: (a) Nbrpm6Nce6 (Pep) and (b) Nbrpm6Nce6Nc6IR780 (PepIR). Figure S2: (a) TEM image of PepIR-20 nanotubes (scale bar, 500 nm). (b) Ex situ AFM image of PepIR-20 nanotubes (scale bar, 2.0 μm). (c) TEM image of PepIR-40 nanotubes (scale bar, 500 nm). (d) Ex situ AFM image of PepIR-40 nanotubes (scale bar, 2.0 μm). (e) TEM image of PepIR-100 nanotubes (scale bar, 200 nm). Figure S3: statistical diameter distribution of PepIR-20 nanotubes (a), PepIR-40 nanotubes (b), and PepIR-100 nanotubes (c) measured from the TEM results. The number above the histogram is the average tubular diameter; 50 nanotubes were analyzed for each size distribution. Figure S4: XRD data of PepIR-40 nanotubes (a) and PepIR-100 nanotubes (b). The values above each peak were calculated according to the formula of d = 2π/q, and these numbers are similar to those of Pep nanotubes, indicating that PepIR-20, PepIR-40, and PepIR-100 nanotubes all exhibited similar structures to those of Pep nanotubes. Figure S5: TEM images ((a) scale bar, 50 nm; (b) scale bar, 100 nm) and ex situ AFM image ((c) scale bar, 500 nm) of sc-PepIR-40 nanotubes as a result of ultrasonication. Figure S6: TEM images ((a, b) scale bar, 50 nm; (c) scale bar, 20 nm) and ex situ AFM images ((d) scale bar, 200 nm; (e, f) scale bar, 500 nm) of sc-PepIR-40 nanotubes after being treated at 50°C. These results showed the high stability of these sc-PepIR-40 nanotubes, which remained to have the same tubular morphology after being incubated in aqueous solution at 50°C for 6 h. Figure S7: UV-vis absorption spectra of ABDA under 808 nm laser irradiation for different times. There was no obvious change in ABDA absorption after exposure to 808 nm laser irradiation. Figure S8: the calibration curve obtained from four DOX solutions at different concentrations. Table S1: the half-maximal inhibitory concentration (IC50) (μM) of U87MG cells under different treatments. The first row shows the different treatments of U87MG cells; the second row shows the targets that we calculated IC50; the third row shows the values of the corresponding IC50 (μM). The IC50 of DOX in chemotherapy and the IC50 of sc-PepIR-40 nanotubes in phototherapy are 17.37131 μM and 10.954 μM, respectively. A significant decrease of IC50 in combined therapy was observed (2.1078 μM). Moreover, the combination index (CI) was calculated to be 0.323 (<1), confirming a high synergistic therapeutic effect of chemotherapy and phototherapy.
References
- 1.Ma Z., Zhang M., Jia X., et al. FeIII-doped two-dimensional C3N4 nanofusiform: a new O2-evolving and mitochondria-targeting photodynamic agent for MRI and enhanced antitumor therapy. Small. 2016;12(39):5477–5487. doi: 10.1002/smll.201601681. [DOI] [PubMed] [Google Scholar]
- 2.Bharathiraja S., Manivasagan P., Santha Moorthy M., et al. Photo-based PDT/PTT dual model killing and imaging of cancer cells using phycocyanin-polypyrrole nanoparticles. European Journal of Pharmaceutics and Biopharmaceutics. 2018;123:20–30. doi: 10.1016/j.ejpb.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Oh J., Yoon H., Park J. Nanoparticle platforms for combined photothermal and photodynamic therapy. Biomedical Engineering Letters. 2013;3(2):67–73. doi: 10.1007/s13534-013-0097-8. [DOI] [Google Scholar]
- 4.Sahu A., Choi W., Lee J., Tae G. Graphene oxide mediated delivery of methylene blue for combined photodynamic and photothermal therapy. Biomaterials. 2013;34(26):6239–6248. doi: 10.1016/j.biomaterials.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 5.Duan S., Yang Y., Zhang C., Zhao N., Xu F. NIR-responsive polycationic gatekeeper-cloaked hetero-nanoparticles for multimodal imaging-guided triple-combination therapy of cancer. Small. 2017;13(9):p. 1603133. doi: 10.1002/smll.201603133. [DOI] [PubMed] [Google Scholar]
- 6.Xiao X., Chen G., Libanori A., Chen J. Wearable Triboelectric Nanogenerators for Therapeutics. Trends in Chemistry. 2021;3(4):279–290. doi: 10.1016/j.trechm.2021.01.001. [DOI] [Google Scholar]
- 7.Deng W., Wu Q., Sun P., et al. Zwitterionic diketopyrrolopyrrole for fluorescence/photoacoustic imaging guided photodynamic/photothermal therapy. Polymer Chemistry. 2018;9(20):2805–2812. doi: 10.1039/C8PY00244D. [DOI] [Google Scholar]
- 8.Dong W., Li Y., Niu D., et al. Facile synthesis of monodisperse superparamagnetic Fe3O4 core@hybrid@Au shell nanocomposite for bimodal imaging and photothermal therapy. Advanced Materials. 2011;23(45):5392–5397. doi: 10.1002/adma.201103521. [DOI] [PubMed] [Google Scholar]
- 9.Cai X., Ding S., Shi Q., et al. Eyeball-like yolk-shell bimetallic nanoparticles for synergistic photodynamic-photothermal therapy. ACS Applied Bio Materials. 2020;3(9):5922–5929. doi: 10.1021/acsabm.0c00624. [DOI] [PubMed] [Google Scholar]
- 10.He Z., Zhao L., Zhang Q., et al. An acceptor-donor-acceptor structured small molecule for effective NIR triggered dual phototherapy of cancer. Advanced Functional Materials. 2020;30(16, article 1910301) [Google Scholar]
- 11.Wang Y., Huang Q., He X., et al. Multifunctional melanin-like nanoparticles for bone-targeted chemo- photothermal therapy of malignant bone tumors and osteolysis. Biomaterials. 2018;183:10–19. doi: 10.1016/j.biomaterials.2018.08.033. [DOI] [PubMed] [Google Scholar]
- 12.Ai X., Mu J., Xing B. Recent advances of light-mediated theranostics. Theranostics. 2016;6(13):2439–2457. doi: 10.7150/thno.16088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu N., Huang P., Fan W., et al. Tri-stimuli-responsive biodegradable theranostics for mild hyperthermia enhanced chemotherapy. Biomaterials. 2017;126:39–48. doi: 10.1016/j.biomaterials.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Z., Wang J., Chen C. Near-infrared light-mediated nanoplatforms for cancer thermo-chemotherapy and optical imaging. Advanced Materials. 2013;25(28):3869–3880. doi: 10.1002/adma.201301890. [DOI] [PubMed] [Google Scholar]
- 15.Zhu X., Feng W., Chang J., et al. Temperature-feedback upconversion nanocomposite for accurate photothermal therapy at facile temperature. Nature Communications. 2016;7(1):p. 10437. doi: 10.1038/ncomms10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi X., Ma X., Ren E., et al. Tumor-microenvironment-activatable nanoreactor based on a polyprodrug for multimodal-imaging-medicated enhanced cancer chemo/phototherapy. ACS Applied Materials & Interfaces. 2019;11(43):40704–40715. doi: 10.1021/acsami.9b16054. [DOI] [PubMed] [Google Scholar]
- 17.Pan X., Li P., Bai L., et al. Biodegradable nanocomposite with dual cell-tissue penetration for deep tumor chemo-phototherapy. Small. 2020;16(22, article 2000809) doi: 10.1002/smll.202000809. [DOI] [PubMed] [Google Scholar]
- 18.Hauck T., Jennings T., Yatsenko T., Kumaradas J., Chan W. Enhancing the toxicity of cancer chemotherapeutics with gold nanorod hyperthermia. Advanced Materials. 2008;20(20):3832–3838. doi: 10.1002/adma.200800921. [DOI] [Google Scholar]
- 19.Zhang E., Luo S., Tan X., Shi C. Mechanistic study of IR-780 dye as a potential tumor targeting and drug delivery agent. Biomaterials. 2014;35(2):771–778. doi: 10.1016/j.biomaterials.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y., Liu T., Zhang E., Luo S., Tan X., Shi C. Preferential accumulation of the near infrared heptamethine dye IR-780 in the mitochondria of drug-resistant lung cancer cells. Biomaterials. 2014;35(13):4116–4124. doi: 10.1016/j.biomaterials.2014.01.061. [DOI] [PubMed] [Google Scholar]
- 21.Yue C., Liu P., Zheng M., et al. IR-780 dye loaded tumor targeting theranostic nanoparticles for NIR imaging and photothermal therapy. Biomaterials. 2013;34(28):6853–6861. doi: 10.1016/j.biomaterials.2013.05.071. [DOI] [PubMed] [Google Scholar]
- 22.Wang K., Zhang Y., Wang J., et al. Self-assembled IR780-loaded transferrin nanoparticles as an imaging, targeting and PDT/PTT agent for cancer therapy. Scientific reports. 2016;6(1, article 27421) doi: 10.1038/srep27421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagy-Simon T., Potara M., Craciun A., Licarete E., Astilean S. IR780-dye loaded gold nanoparticles as new near infrared activatable nanotheranostic agents for simultaneous photodynamic and photothermal therapy and intracellular tracking by surface enhanced resonant Raman scattering imaging. Journal of Colloid and Interface Science. 2018;517:239–250. doi: 10.1016/j.jcis.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Jiang C., Cheng H., Yuan A., Tang X., Wu J., Hu Y. Hydrophobic IR780 encapsulated in biodegradable human serum albumin nanoparticles for photothermal and photodynamic therapy. Acta Biomaterialia. 2015;14:61–69. doi: 10.1016/j.actbio.2014.11.041. [DOI] [PubMed] [Google Scholar]
- 25.Li S., Zhou S., Li Y., et al. Exceptionally high payload of the IR780 iodide on folic acid-functionalized graphene quantum dots for targeted photothermal therapy. ACS Applied Materials & Interfaces. 2017;9(27):22332–22341. doi: 10.1021/acsami.7b07267. [DOI] [PubMed] [Google Scholar]
- 26.Singh A., Hahn M., Gutwein L., et al. Multi-dye theranostic nanoparticle platform for bioimaging and cancer therapy. International Journal of Nanomedicine. 2012;7:2739–2750. doi: 10.2147/IJN.S28357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng C. L., Shih Y. H., Lee P. C., Hsieh T. M., Luo T. Y., Shieh M. J. Multimodal image-guided photothermal therapy mediated by 188Re-labeled micelles containing a cyanine-type photosensitizer. ACS Nano. 2011;5(7):5594–5607. doi: 10.1021/nn201100m. [DOI] [PubMed] [Google Scholar]
- 28.Zhou J., Patel T. R., Sirianni R. W., et al. Highly penetrative, drug-loaded nanocarriers improve treatment of glioblastoma. PNAS. 2013;110(29):11751–11756. doi: 10.1073/pnas.1304504110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levitz A., Marmarchi F., Henary M. Synthesis and optical properties of near-infrared meso-phenyl-substituted symmetric heptamethine cyanine dyes. Molecules. 2018;23(2):p. 226. doi: 10.3390/molecules23020226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abbas M., Zou Q., Li S., Yan X. Self-assembled peptide- and protein-based nanomaterials for antitumor photodynamic and photothermal therapy. Advanced materials. 2017;29(12, article 1605021) doi: 10.1002/adma.201605021. [DOI] [PubMed] [Google Scholar]
- 31.Jin H., Ding Y. H., Wang M., et al. Designable and dynamic single-walled stiff nanotubes assembled from sequence-defined peptoids. Nature communications. 2018;9(1):p. 270. doi: 10.1038/s41467-017-02059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun J., Zuckermann R. Peptoid polymers: a highly designable bioinspired material. ACS Nano. 2013;7(6):4715–4732. doi: 10.1021/nn4015714. [DOI] [PubMed] [Google Scholar]
- 33.Wang M., Song Y., Mu P., Cai X., Lin Y., Chen C. L. Peptoid-based programmable 2D nanomaterial sensor for selective and sensitive detection of H2S in live cells. ACS Applied Bio Materials. 2020;3(9):6039–6048. doi: 10.1021/acsabm.0c00657. [DOI] [PubMed] [Google Scholar]
- 34.Luo Y., Song Y., Wang M., et al. Bioinspired peptoid nanotubes for targeted tumor cell imaging and chemo-photodynamic therapy. Small. 2019;15(43, article 1902485) doi: 10.1002/smll.201902485. [DOI] [PubMed] [Google Scholar]
- 35.Song Y., Wang M., Li S., et al. Efficient cytosolic delivery using crystalline nanoflowers assembled from fluorinated peptoids. Small. 2018;14(52, article 1803544) doi: 10.1002/smll.201803544. [DOI] [PubMed] [Google Scholar]
- 36.Huse J., Holland E. Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nature Reviews Cancer. 2010;10(5):319–331. doi: 10.1038/nrc2818. [DOI] [PubMed] [Google Scholar]
- 37.Singh D., Chan J. M., Zoppoli P., et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science. 2012;337(6099):1231–1235. doi: 10.1126/science.1220834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tao Y., Ning M., Dou H. A novel therapeutic system for malignant glioma: nanoformulation, pharmacokinetic, and anticancer properties of cell-nano-drug delivery. Nanomedicine: Nanotechnology, Biology and Medicine. 2013;9(2):222–232. doi: 10.1016/j.nano.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Guo J., Gao X., Su L., et al. Aptamer-functionalized PEG-PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials. 2011;32(31):8010–8020. doi: 10.1016/j.biomaterials.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Akimoto J., Haraoka J., Aizawa K. Preliminary clinical report on safety and efficacy of photodynamic therapy using talaporfin sodium for malignant gliomas. Photodiagnosis and Photodynamic Therapy. 2012;9(2):91–99. doi: 10.1016/j.pdpdt.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Baek S. K., Makkouk A. R., Krasieva T., Sun C. H., Madsen S. J., Hirschberg H. Photothermal treatment of glioma; an in vitro study of macrophage-mediated delivery of gold nanoshells. Journal of Neuro-Oncology. 2011;104(2):439–448. doi: 10.1007/s11060-010-0511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan A., Qiu X., Tang X., Liu W., Wu J., Hu Y. Self-assembled PEG-IR-780-C13 micelle as a targeting, safe and highly-effective photothermal agent for in vivo imaging and cancer therapy. Biomaterials. 2015;51:184–193. doi: 10.1016/j.biomaterials.2015.01.069. [DOI] [PubMed] [Google Scholar]
- 43.Cheng H., Zhu J. Y., Li S. Y., et al. An O2 self-sufficient biomimetic nanoplatform for highly specific and efficient photodynamic therapy. Advanced Functional Materials. 2016;26(43):7847–7860. doi: 10.1002/adfm.201603212. [DOI] [Google Scholar]
- 44.Cai X., Luo Y., Song Y., et al. Integrating in situ formation of nanozymes with three-dimensional dendritic mesoporous silica nanospheres for hypoxia-overcoming photodynamic therapy. Nanoscale. 2018;10(48):22937–22945. doi: 10.1039/C8NR07679K. [DOI] [PubMed] [Google Scholar]
- 45.Song Y., Shi Q., Zhu C., et al. Mitochondrial-targeted multifunctional mesoporous Au@Pt nanoparticles for dual-mode photodynamic and photothermal therapy of cancers. Nanoscale. 2019;9:15813–15824. doi: 10.1039/c7nr04881e. [DOI] [PubMed] [Google Scholar]
- 46.Wang H., Feng Z., Wang Y., Zhou R., Yang Z., Xu B. Integrating enzymatic self-assembly and mitochondria targeting for selectively killing cancer cells without acquired drug resistance. Journal of the American Chemical Society. 2016;138(49):16046–16055. doi: 10.1021/jacs.6b09783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chou T. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacological Reviews. 2006;58(3):621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: ultraperformance liquid chromatography (UPLC) and mass spectrometry (MS) data of peptoids: (a) Nbrpm6Nce6 (Pep) and (b) Nbrpm6Nce6Nc6IR780 (PepIR). Figure S2: (a) TEM image of PepIR-20 nanotubes (scale bar, 500 nm). (b) Ex situ AFM image of PepIR-20 nanotubes (scale bar, 2.0 μm). (c) TEM image of PepIR-40 nanotubes (scale bar, 500 nm). (d) Ex situ AFM image of PepIR-40 nanotubes (scale bar, 2.0 μm). (e) TEM image of PepIR-100 nanotubes (scale bar, 200 nm). Figure S3: statistical diameter distribution of PepIR-20 nanotubes (a), PepIR-40 nanotubes (b), and PepIR-100 nanotubes (c) measured from the TEM results. The number above the histogram is the average tubular diameter; 50 nanotubes were analyzed for each size distribution. Figure S4: XRD data of PepIR-40 nanotubes (a) and PepIR-100 nanotubes (b). The values above each peak were calculated according to the formula of d = 2π/q, and these numbers are similar to those of Pep nanotubes, indicating that PepIR-20, PepIR-40, and PepIR-100 nanotubes all exhibited similar structures to those of Pep nanotubes. Figure S5: TEM images ((a) scale bar, 50 nm; (b) scale bar, 100 nm) and ex situ AFM image ((c) scale bar, 500 nm) of sc-PepIR-40 nanotubes as a result of ultrasonication. Figure S6: TEM images ((a, b) scale bar, 50 nm; (c) scale bar, 20 nm) and ex situ AFM images ((d) scale bar, 200 nm; (e, f) scale bar, 500 nm) of sc-PepIR-40 nanotubes after being treated at 50°C. These results showed the high stability of these sc-PepIR-40 nanotubes, which remained to have the same tubular morphology after being incubated in aqueous solution at 50°C for 6 h. Figure S7: UV-vis absorption spectra of ABDA under 808 nm laser irradiation for different times. There was no obvious change in ABDA absorption after exposure to 808 nm laser irradiation. Figure S8: the calibration curve obtained from four DOX solutions at different concentrations. Table S1: the half-maximal inhibitory concentration (IC50) (μM) of U87MG cells under different treatments. The first row shows the different treatments of U87MG cells; the second row shows the targets that we calculated IC50; the third row shows the values of the corresponding IC50 (μM). The IC50 of DOX in chemotherapy and the IC50 of sc-PepIR-40 nanotubes in phototherapy are 17.37131 μM and 10.954 μM, respectively. A significant decrease of IC50 in combined therapy was observed (2.1078 μM). Moreover, the combination index (CI) was calculated to be 0.323 (<1), confirming a high synergistic therapeutic effect of chemotherapy and phototherapy.
Data Availability Statement
All data are available in the manuscript, supplementary materials, or from the author.


