Supplemental Digital Content is available in the text.
Keywords: body mass index, cardiovascular diseases, inflammation, stem cells
Objective:
The underlying pathobiology of the paradoxical relationship between obesity and adverse outcomes in coronary artery disease (CAD) is unclear. Our objective was to determine the association between obesity and circulating progenitor cell (CPC) counts—a measure of intrinsic regenerative capacity—in asymptomatic individuals and patients with CAD and its impact on the obesity paradox.
Approach and Results:
CPCs were enumerated by flow cytometry as CD45med+ cells expressing CD34+, CD133+, and CXCR4+ epitopes in 672 asymptomatic individuals (50 years of age; 28% obese) and 1277 patients with CAD (66 years of age; 39% obese). The association between obesity and CPCs was analyzed using linear regression models. The association of obesity and CPCs with cardiovascular death/myocardial infarction events over 3.5-year follow-up in patients with CAD was studied using Cox models. Obesity was independently associated with 16% to 34% higher CPC counts (CD34+, CD34+/CD133+, and CD34+/CXCR4+) in asymptomatic individuals. This association was not attenuated by systemic inflammation, insulin resistance, or secretion but partly attenuated by cardiorespiratory fitness and body composition. In patients with CAD, obesity was associated with 8% to 12% higher CPC counts and 30% lower risk of adverse outcomes. Compared with nonobese patients, only obese patients with high CPC counts (CD34+ cells ≥median, 1806 cells/mL) were at a lower risk (hazard ratio, 0.52 [95% CI, 0.31–0.88]), whereas those with low counts (<median) were at a similar risk (hazard ratio, 0.75 [95% CI, 0.48–1.15]).
Conclusions:
Obesity is associated with higher CPC counts. The obesity paradox of improved outcomes with obesity in CAD is limited to patients with intact regenerative capacity who have high CPC counts.
Highlights.
Obesity is paradoxically associated with favorable outcomes in patients with coronary artery disease (CAD), but the underlying pathobiology is unclear.
High circulating progenitor cell counts—a measure of endogenous regenerative capacity—are associated with improved outcomes in patients with CAD.
Obesity was independently associated with 16% to 34% higher circulating progenitor cell (CD34+, CD34+/CD133+, and CD34+/CXCR4+) counts in asymptomatic individuals and with 8% to 12% higher circulating progenitor cell counts in patients with CAD.
The paradoxical association of obesity with improved outcomes in patients with CAD was limited to those with high, but not low, circulating progenitor cell counts.
These findings provide novel insight into the pathobiology of the obesity paradox in CAD.
Obesity is a complex, multifactorial disease characterized by abnormal or excessive accumulation of body fat.1 A body mass index (BMI) of ≥30 kg/m2 is used to define obesity.2 The incidence and prevalence of obesity has increased dramatically in the past few decades, and this epidemic is a leading public health concern.3 In addition to the associated metabolic risks, obesity is a major risk factor for development of cardiovascular disease.4 However, in patients with established cardiovascular disease, obesity appears to portend a favorable prognosis,5 which is referred to as the obesity paradox. The obesity paradox has been observed in several patient populations,5–8 but the pathophysiologic mechanisms underlying it are incompletely understood.
Recent studies have demonstrated that obesity is directly associated with higher circulating progenitor cell (CPC) counts in the peripheral blood.9–12 CPCs are mononuclear cells primarily derived from the bone marrow that contribute to vascular repair and regeneration through direct and paracrine mechanisms.13–15 CPC counts in the peripheral blood can be considered an index of endogenous vascular regenerative capacity.16 CPCs have the potential to differentiate into endothelial, hematopoietic, and nonhematopoietic phenotypes and can be identified in the peripheral circulation as cells expressing the CD (cluster of differentiation) 34 epitope.17 CD133 is a 5-transmembrane antigen observed on primitive stem cells, which is lost during cellular maturation, and coexpression of CD133 with CD34 (CD34+/CD133+) identifies a hematopoietic CPC-enriched subpopulation.18 Coexpression of chemokine CXCR4 (C-X-C motif receptor 4) with CD34 (CD34+/CXCR4+) characterizes cells with the capacity for tissue repair that is mediated by homing of CPCs to stromal-derived factor-1α–enriched hypoxic environments.19 Our group has reported that the presence of cardiovascular risk factors like diabetes, hypertension, dyslipidemia, and smoking early in life is associated with higher CPC counts.16,20 This finding is in contrast to older studies that have suggested an inverse association between risk factors and CPCs.21–24 Our recent findings suggest that progenitor cells are mobilized from the bone marrow in response to risk factor–mediated vascular injury, and this response represents activation of an endogenous regenerative or reparative system.16 Notably, a murine model of atherosclerosis suggested that continuous exposure to risk factors across the life span leads to depletion in CPC counts at an older age.25
In this context, the association of obesity, a cardiovascular risk factor that is reaching epidemic proportions, with vascular regenerative capacity, measured using CPC counts, in large cohorts has not been thoroughly evaluated. Furthermore, the potential role of CPCs in providing pathophysiologic insights into the obesity paradox has not been studied to date. Therefore, in this study, we sought to investigate (1) the association between obesity and CPC counts in 2 separate cohorts of individuals with and without coronary artery disease (CAD) and (2) the impact of CPC counts on the association between obesity and adverse outcomes in patients with CAD along with their ability to provide insights into the obesity paradox. We hypothesized that obesity will be associated with higher CPC counts and higher CPC counts will be associated with favorable outcomes in patients with obesity and CAD.
Methods
Study Populations
The study population comprised of participants of the Emory and Georgia Tech Center for Health Discovery and Well Being (CHDWB) and Emory Cardiovascular Biobank (EmCAB) cohorts. The CHDWB was established as an initiative aimed toward the prevention of the chronic diseases through promotion of a healthy lifestyle in employees of the Emory University and Georgia Institute of Technology in Atlanta, GA.26 Our analysis included 672 unique participants without known CAD who were recruited between 2007 and 2010 and had CPC counts measured at the time of enrollment. Subjects with an acute illness, hospitalization within a year before enrollment, pregnant women, and individuals with poorly controlled medical comorbidities were excluded.26
The EmCAB is a prospective registry of patients undergoing cardiac catheterization for evaluation of CAD at 3 Emory Healthcare–affiliated hospitals.27 Our analysis included 1277 stable participants enrolled between 2008 and 2014. Participants with acute myocardial infarction (MI), severe valvular heart disease, organ transplantation, immunosuppressive medication use, leukocytosis (defined as white blood cell [WBC] count >11 000 cells/μL), and active infection, inflammatory disorder, or cancer were excluded. CHDWB and EmCAB participants provided written informed consent at enrollment, and these studies were approved by the Emory University Institutional Review Board. This study complies with the Declaration of Helsinki. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Participant Characteristics
Participants in both cohorts were interviewed to obtain information about demographic characteristics, medical history, and behavioral habits. BMI was calculated as weight (in kilograms) divided by height (in meters) squared, and obesity status was defined using the cutoff of 30 kg/m2. WBC count and serum creatinine were measured at enrollment in both cohorts, and estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.28
In CHDWB, hypertension, dyslipidemia, and diabetes were defined according to the Joint National Committee, Adult Treatment Panel III, and American Diabetes Association criteria, respectively.26 Fasting blood samples were collected for measuring high-sensitivity C-reactive protein (hsCRP), insulin, and glucose levels.26 Fasting insulin and glucose levels were used for calculating Homeostasis Model Assessment (HOMA) 2 indices of insulin resistance (IR; HOMA2 IR) and pancreatic β-cell function (HOMA2 β).29 Cardiorespiratory fitness was measured using a submaximal, modified Balke treadmill exercise protocol and quantified as maximal oxygen consumption.30 Body fat composition was measured using dual-energy x-ray absorptiometry (iDXA, GE Lunar Densitometry; General Electric Company, Boston, MA).31 The android region included an area from the top of the iliac crest to 20% of the distance from the iliac crest to the bottom of the subject’s head.32 The gynoid region extended from the top of the greater trochanter down a distance twice the height of the android region.32 Total body fat percentage, gynoid fat percentage (GFP), and android fat percentage (AFP) were calculated by dividing the respective fat mass by total body weight. Lean mass percentage was calculated by dividing the lean mass by total body weight.
In the EmCAB cohort, the presence of diabetes, hypertension, and dyslipidemia was determined by physician diagnosis or treatment.27 History of coronary artery bypass grafting, peripheral artery disease, heart failure, and cardiovascular medication (ACE [angiotensin-converting enzyme] inhibitor or angiotensin-II receptor blocker, aspirin, blocker, clopidogrel, and statin) use was also recorded.27 Medical records and International Classification of Diseases, Ninth Revision, codes were reviewed to confirm participant-reported medical history.27
CPC Assays
In both cohorts, CPC counts were measured in blood samples collected in EDTA tubes after an overnight fast.20 Blood samples were prepared within 4 hours of collection and incubated with fluorochrome-labeled monoclonal antihuman mouse antibodies to identify surface markers expressed on mononuclear cells before quantification using flow cytometry.20 Three hundred microliters of peripheral blood was incubated with 7 μL of FITC-CD34 (BD Biosciences), 7 μL PerCP-CD45 (BD Biosciences), 5 μL APC-CD133 (Miltenyi), and 3 μL PE-Cy7–conjugated anti-CXCR4 (EBioscience; clone 12G5) in the dark for 15 minutes.33 Then 1.5 mL ammonium chloride lysing buffer was added to lyse red blood cells, following which 1.5 mL staining medium (PBS with 3% heat-inactivated serum and 0.1% sodium azide) was added to stop the lysing reaction.33 Before flow cytometry, 100 μL of AccuCheck Counting Beads (Invitrogen, catalog No. PCB100) were added to act as an internal standard for direct estimation of the concentration of target cell subsets.33 At least 2.5 million events were acquired from the cytometer. Flow cytometry data were analyzed with the Flowjo software (Treestar, Inc) with its filter set at CD45med+ cells. This selection excludes CD45bright (lymphoblasts) and CD45− (nonhematopoietic progenitor) cells. CPC populations (CD34+, CD34+/CD133+, and CD34+/CXCR4+) were measured after using the CD45med+ filter and are reported as cell counts per milliliter.33 Interobserver variability was tested in 20 samples that were analyzed on 2 occasions by 2 technicians. Percentage repeatability coefficients (%) were calculated as the SD of differences between pairs of measurements/mean of measurements×100. The repeatability coefficients was 2.9%, 4.8%, and 6.5% for CD34+, CD34+/CD133+, and CD34+/CXCR4+, respectively.20
Follow-Up and Adverse Outcomes
EmCAB participants were prospectively followed for 2 outcomes of interest, a composite of cardiovascular death and nonfatal MI events, and all-cause death. Follow-up data were available for 1249 participants and were obtained by annual phone contact, electronic medical record review, and the social security death index and state records.27 The cause of death was determined from medical record review or by direct contact with the participants’ family member(s). Cardiovascular death and nonfatal MI events were adjudicated by 2 independent cardiologists blinded to study data.27 Cardiovascular death was defined as death attributable to an ischemic cardiovascular cause such as fatal MI, stroke, or sudden death secondary to a presumed cardiovascular cause in this high-risk population.27 Nonfatal MI events were adjudicated using the third universal definition of MI.34
Statistical Analysis
Characteristics of participants in CHDWB and EmCAB cohorts were reported as frequencies (proportion) for categorical variables and as medians (25th to 75th percentile) for continuous variables. Differences between participants with and without obesity were studied using the χ2 test for categorical variables and the Mann-Whitney U test for continuous variables. CPC counts were non-normally distributed and were plotted against BMI in both cohorts. The correlation between CPC counts and BMI in both cohorts was determined using Spearman tests. CPC counts were log-transformed (log2[cell count+0.0001]), and the association of BMI and obesity status with CPC counts was determined using linear regression models. β-Coefficient estimates were exponentiated to back-transform CPC counts to allow interpretation in the original scale. Models were adjusted for demographics, risk factors, and WBC count, followed by further adjustment for hsCRP, HOMA2 IR, HOMA2 β, maximal oxygen consumption, GFP, AFP, and lean mass percentage to explore residual confounding. The multiplicative interaction of obesity with age and diabetes for association with CPC counts was tested. Moreover, the predictors of CPC counts in participants with obesity were determined.
EmCAB participants were stratified by obesity status, and the differences in survival from cardiovascular death/MI and all-cause death events were determined using Kaplan-Meier survival analyses. The association of obesity with adverse outcomes was determined using Cox proportional hazards regression models adjusted for demographics, risk factors, and established cardiovascular disease. The impact of adding log2-transformed CPC counts (translating to a 50% relative decrease in count) to these Cox models was studied. Following this, obese by log2-CPC count interactions were tested, and the association of CPC counts with outcomes in the subset of participants with obesity was evaluated. Participants with and without obesity were stratified by the respective median CPC counts to create 4 mutually exclusive groups in EmCAB: (1) obese with higher CPC count, (2) obese with lower CPC count, (3) nonobese with higher CPC count, and (4) nonobese with lower CPC count. The association of these categories with outcomes was analyzed using Kaplan-Meier survival analyses and multivariable-adjusted Cox models. All analyses were performed using IBM SPSS Statistics, version 25 (Armonk, NY), and R 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). A 2-tailed P<0.05 was considered statistically significant.
Results
Association of Obesity With CPC Counts
The baseline characteristics of CHDWB participants are described in Table 1. Among 672 participants, 28.4% were obese, and these individuals were more frequently women, Black, and had a higher prevalence of diabetes, hypertension, and dyslipidemia (Table 1). Participants with obesity, on average, had higher levels of hsCRP, HOMA2 IR, HOMA2 β, body fat percentage, AFP, GFP, WBC count, and lower lean mass percentage and maximal oxygen consumption as compared with participants without obesity (Table 1). Median CD34+, CD34+/CD133+, and CD34+/CXCR4+ counts were significantly higher in participants with obesity as well (Table 1). In the overall cohort, BMI correlated significantly with circulating CD34+, CD34+/CD133+, and CD34+/CXCR4+ counts (Figure 1A through 1C). The association between BMI and CPC counts was linear, and each 1-kg/m2 increase in BMI was independently associated with higher CD34+ (1.9% [95% CI, 1.1%–2.8%]; P<0.001), CD34+/CD133+ (2.5% [95% CI, 1.5%–3.5%]; P<0.001), and CD34+/CXCR4+ (1.4% [95% CI, 0.4%–2.3%]; P=0.004) counts.
Table 1.
Baseline Characteristics of Center for Health Discovery and Well Being Participants Stratified by Obesity Status
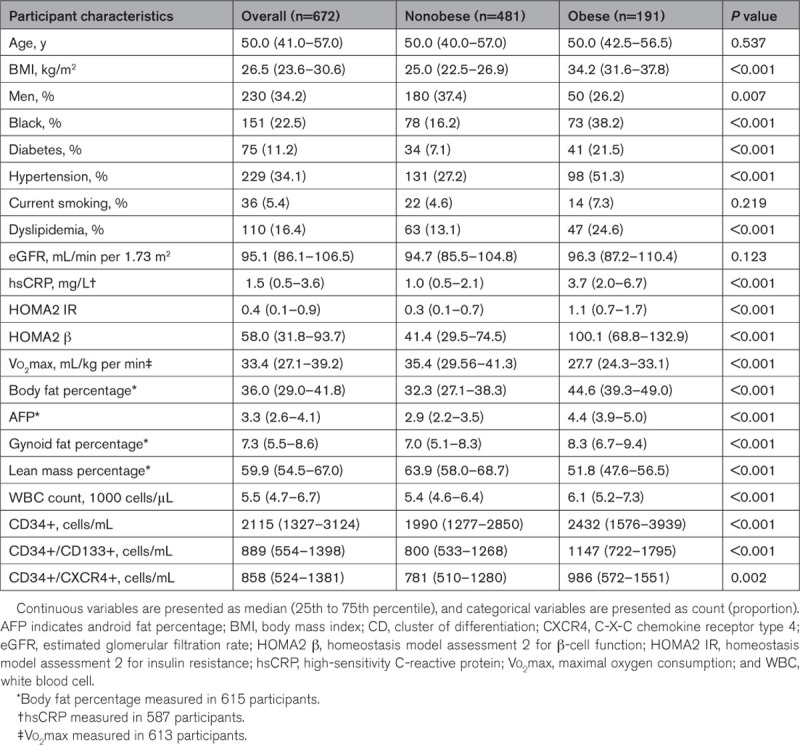
Figure 1.
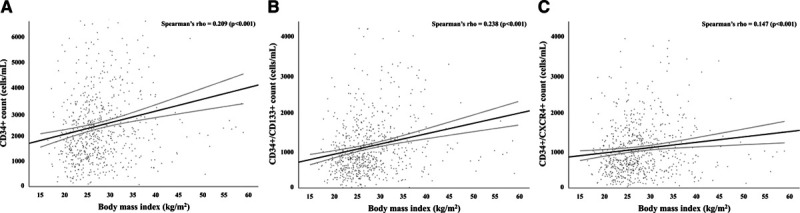
Correlation of body mass index with circulating progenitor cell counts among participants of the Center for Health and Well Being cohort. Correlation of body mass index with CD34+ (A), CD34+/CD133+ (B), and CD34+/CXCR4+ (C) counts among participants of the Center for Health and Well Being cohort. Body mass index significantly correlated with CD34+ (ρ=0.209, P<0.001), CD34+/CD133+ (ρ=0.238, P<0.001), and CD34+/CXCR4+ (ρ=0.147, P<0.001) counts. CD indicates cluster of differentiation; and CXCR4, C-X-C motif receptor 4.
Obesity (BMI≥30 kg/m2) was associated with higher CPC counts in linear regression models (Table 2). This association remained significant after adjustment for demographics, risk factors, and WBC count (Table 2, Clinical Model), such that participants with obesity had 16% to 34% higher CPCs as compared with participants without obesity. Further adjustment for hsCRP, HOMA2 IR, HOMA2 β, or GFP did not attenuate this association, and none of these markers were associated with CPC counts. Adding maximal oxygen consumption, AFP, or lean mass percentage into the model attenuated the association of obesity with CD34+ and CD34+/CXCR4+ counts (Table 2, Models 4, 6, and 7). The relationship of GFP and AFP with CPC counts was not changed after further adjustment for body fat percentage. The association of obesity with CPC counts was not modified by age (all P interaction, >0.15), sex (all P interaction, >0.05), or diabetes (all P interaction, >0.20). Among participants with obesity, male sex and WBC counts were associated with higher CPC counts, while age was inversely associated with CD34+ and CD34+/CD133+ counts (Table I in the Data Supplement).
Table 2.
Association Between Obesity and Circulating Progenitor Cell Counts in the Center for Health Discovery and Well Being Cohort
The baseline characteristics of EmCAB participants are described in Table II in the Data Supplement. Among 1277 participants, 39.2% were obese, and these individuals were younger, more frequently Black, and had a higher prevalence of diabetes, hypertension, and dyslipidemia but lower prevalence of smoking and peripheral artery disease (Table II in the Data Supplement). Participants with obesity had higher WBC and CPC counts as compared with participants without obesity (Table II in the Data Supplement), and BMI significantly correlated with CPC counts (Figure IA through IC in the Data Supplement). The association between BMI and CPC counts was linear, and each 1-kg/m2 increase in BMI was independently associated with higher CD34+ (1.1% [95% CI, 0.4%–1.7%]; P=0.001) and CD34+/CD133+ (1.4% [95% CI, 0.6%–2.1%]; P<0.001) counts but not with CD34+/CXCR4+ (0.6% [95% CI, −0.1% to 1.3%]; P=0.117) counts. Obesity was associated with higher CD34+ (11.7% [95% CI, 3.5%–20.6%]; P=0.005), CD34+/CD133+ (11.6% [95% CI, 1.6%–22.6%]; P=0.021), and CD34+/CXCR4+ (8.6% [95% CI, −1.1% to 19.3%]; P=0.083) counts in linear regression models adjusted for demographics, risk factors, history of coronary artery bypass grafting, heart failure, and peripheral artery disease. The association of obesity with CD34+ (P interaction, 0.049) and CD34+/CD133+ counts (P interaction, 0.039) was modified by age, but this interaction was not seen with CD34+/CXCR4+ counts (P interaction, 0.262). The direct association of obesity with CPC counts was stronger in younger patients with CAD and was attenuated with aging (Figure IIA through IIC in the Data Supplement). An obesity-by-diabetes interaction was not observed for CPC counts (all P interactions, >0.07). Among participants with obesity, male sex and WBC counts were associated with higher CPC counts (Table III in the Data Supplement). Age was associated with lower CPC counts, and diabetes was associated with lower CD34+ and CD34+/CD133+ counts (Table III in the Data Supplement).
Obesity Paradox in Patients With CAD
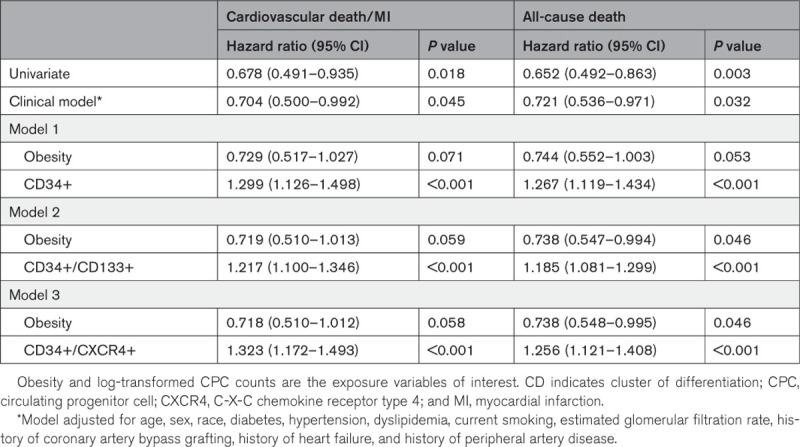
EmCAB participants experienced 173 cardiovascular death/MI (54 in obese) and 233 all-cause death (70 in obese) events during a median follow-up of 3.5 (1.5–5.2) years. Patients with obesity experienced a lower incidence of cardiovascular death/MI and all-cause death as compared with patients without obesity in Kaplan-Meier survival analyses (Figure 2A and 2B). Obesity status was associated with a 30% and 28% lower risk of cardiovascular death/MI and all-cause death, respectively, in multivariable-adjusted Cox regression analyses (Table 3, Clinical Model), and no multiplicative interaction between obesity and sex was observed (all P interactions, >0.05). The addition of CD34+, CD34+/CD133+, or CD34+/CXCR4+ counts to this clinical model revealed that each CPC subtype count was independently associated with adverse outcomes (Table 3, Models 1–3). Addition of CPC counts to the Cox models partly attenuated the association between obesity and cardiovascular outcomes (Table 3, Models 1–3). There was no interaction between CPC counts and obesity or between CPC counts and sex for the association between obesity and outcomes (all P interactions, >0.05), the former indicating that CPC counts are associated with outcomes in participants with and without obesity. Indeed, CPC counts were independently associated with cardiovascular death/MI and all-cause death in Cox regression analyses limited to obese EmCAB participants (Table IV in the Data Supplement).
Figure 2.
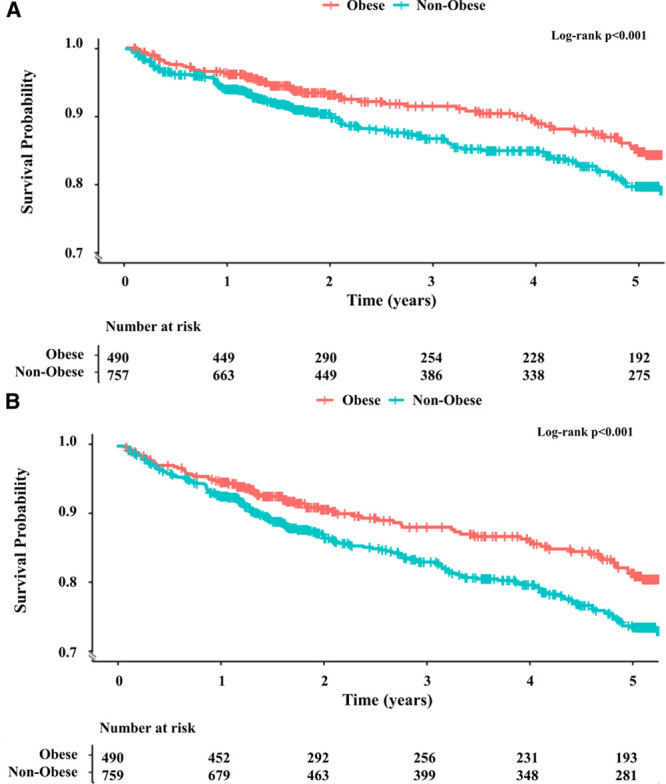
Kaplan-Meier survival curves for adverse outcomes among obese and nonobese participants of the Emory Cardiovascular Biobank cohort. Kaplan-Meier survival curves for cardiovascular death/myocardial infarction (A) and all-cause death (B) among participants with and without obesity of the Emory Cardiovascular Biobank cohort. Participants with obesity had a lower incidence of cardiovascular death/myocardial infarction (A) and all-cause death (B) as compared with participants without obesity.
Table 3.
Association Between Cardiovascular Outcomes, Obesity and CPC Counts in the Emory Cardiovascular Biobank Cohort
CPC Counts and the Obesity Paradox
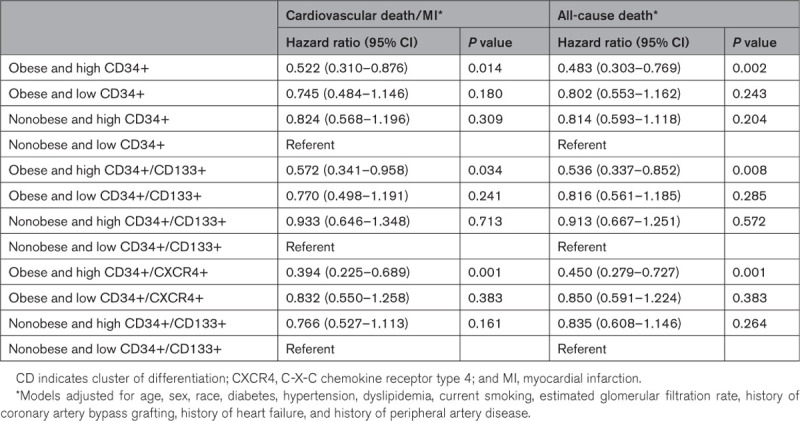
Participants of the EmCAB cohort were divided into 4 mutually exclusive groups based on obesity status (obese/nonobese) and CPC cutoffs (above or below the respective median). Participants with obesity and high CD34+ counts were at the lowest risk, while participants without obesity and low counts were at the highest risk of adverse outcomes (Figure 3A and 3B). The incidence of cardiovascular death/MI and all-cause death was similar for participants with obesity and low CD34+ counts and participants without obesity and high CD34+ counts (Figure 3A and 3B). Similar associations were observed when participants were stratified based on CD34+/CD133+ (Figure IIIA and IIIB in the Data Supplement) and CD34+/CXCR4+ counts (Figure IVA and IVB in the Data Supplement). In multivariable-adjusted Cox regression analyses, participants with obesity and high CPC counts were at a 43% to 61% lower risk of cardiovascular death/MI and 46% to 55% lower risk of all-cause death as compared with participants without obesity and low counts (Table 4). In contrast, participants with obesity and low counts and participants without obesity and high counts had a similar risk of adverse outcomes as participants without obesity and low counts (Table 4).
Figure 3.
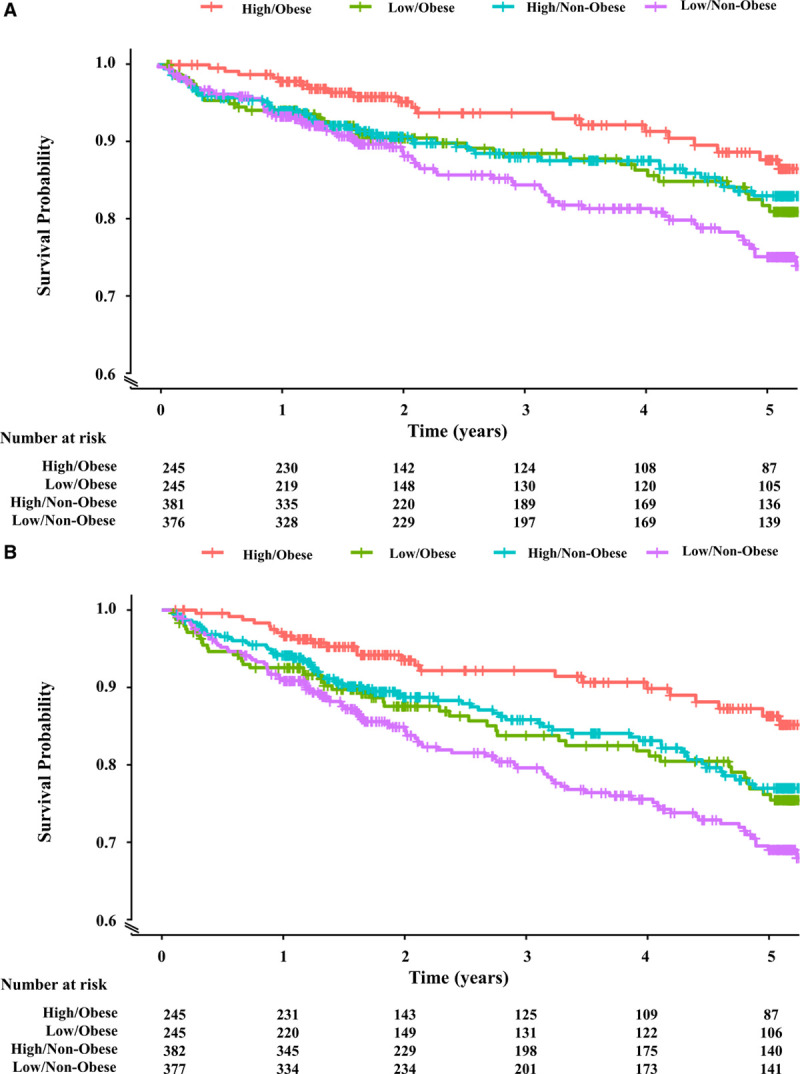
Kaplan-Meier survival curves for adverse outcomes among Emory Cardiovascular Biobank participants stratified by obesity status and CD34+ count. Kaplan-Meier survival curves for cardiovascular death/myocardial infarction (A) and all-cause death (B) among the Emory Cardiovascular Biobank participants stratified by obesity status and CD34+ count. Participants with obesity and high CD34+ counts were at the lowest risk; participants without obesity and low counts were at the highest risk; and the incidence of cardiovascular death/myocardial infarction (A) and all-cause death (B) was similar for participants with obesity and low CD34+ counts and participants without obesity and high CD34+ counts. CD indicates cluster of differentiation.
Table 4.
Cardiovascular Outcomes in the Emory Cardiovascular Biobank Cohort Participants Stratified by Obesity and Circulating Progenitor Cell Counts
Discussion
We report 2 key findings in this study. First, obesity is associated with higher CPC counts (CD34+, CD34+/CD133+, and CD34+/CXCR4+) in cohorts of individuals without and with CAD (Figure 4). Second, we observed that patients with CAD and obesity are at a lower risk of adverse outcomes compared with those without obesity. Importantly, the paradoxical relationship of obesity with favorable outcomes compared with patients without obesity is limited to those with high CPC counts, that is, preserved endogenous regenerative capacity, and is not observed in patients with low CPC counts or impaired regenerative capacity (Figure 4).
Figure 4.
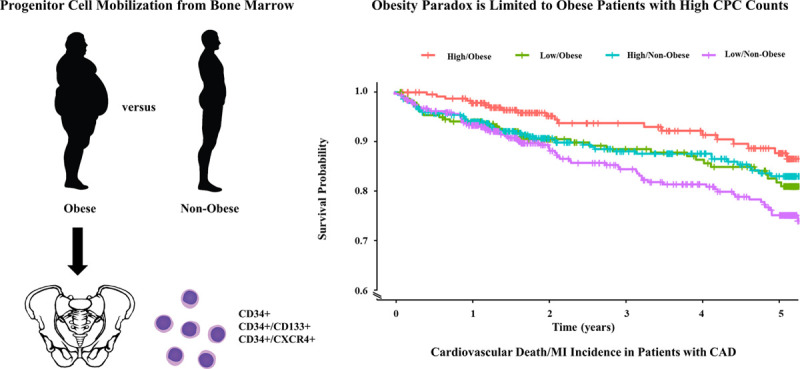
Obesity is associated with higher circulating progenitor cell (CPC) counts (CD34+, CD34+/CD133+, and CD34+/CXCR4+) in asymptomatic individuals and in patients with coronary artery disease (CAD). The decreased risk of cardiovascular outcomes in patients with obesity and CAD is limited to those with higher CPC counts. CD indicates cluster of differentiation; CXCR4, C-X-C motif receptor 4; and MI, myocardial infarction.
Obesity and CPC Counts
Several studies have evaluated the association of BMI and obesity with CPC counts over the past 2 decades. These studies have yielded inconsistent results with a few early reports of small cohorts revealing an inverse relationship between higher BMI and CPC counts or function.35–39 In contrast, more recent and relatively larger studies indicate that obesity is associated with higher CPC counts.9–12 In this context, we have systematically studied the relationship between obesity and CPC counts in 2 large independent cohorts of individuals without and with CAD. We observed that CPC counts were 16% to 34% higher in middle-aged asymptomatic individuals with obesity as compared with those without obesity. This association was independent of demographics, risk factors, and importantly, WBC counts.
Asymptomatic participants of CHDWB underwent extensive phenotyping for markers of systemic inflammation, IR, pancreatic β-cell function, cardiorespiratory fitness, and visceral adiposity. Our findings indicate that although individuals with and without obesity had significant differences in hsCRP, HOMA2 IR, HOMA2 β, and GFP, the association of BMI and obesity with CPC counts was independent of these factors. Furthermore, we observed that cardiorespiratory fitness, lean body mass, and android fat depot—a harbinger of future cardiometabolic risk40—only slightly attenuated the association of obesity with CD34+ and CD34+/CXCR4+ counts.
Relatively few studies provide insights regarding the mechanistic basis of the counterintuitive obesity-CPC relationship. In a seminal report, Nagareddy et al41 showed that adipose tissue macrophages in murine obesity models promoted proliferation and expansion of myeloid progenitors in the bone marrow via the NLRP3 inflammasome-dependent interleukin-1β pathway. Other hypotheses include adipose tissue acting as an extramedullary progenitor cell reservoir capable of releasing progenitor cells into the circulation,42 and stimulation of CPC release from the bone marrow in response to hypoxia associated with obesity and obstructive sleep apnea.43 Taken together, these findings suggest that obesity stimulates hematopoiesis in the bone marrow and mobilizes progenitor cells into the peripheral circulation.
Whereas the asymptomatic, middle-aged (mean age, 50 years) participants with obesity had 16% to 34% higher CPC counts compared with participants without obesity, the relative difference in the older (mean age, 66 years) patients with CAD was lower (only 8%–12% higher). Additionally, the association of obesity with CD34+ and CD34+/CD133+ counts in patients with CAD was modified by age, such that the obesity was associated with higher counts at a younger age and lower counts at an older age. These findings are consistent with our current understanding of the impact of risk factors and aging on progenitor cell pathobiology.16 In this model, exposure to cardiovascular risk factors at a young age might stimulate the release of progenitor cells from the bone marrow.16 This likely compensatory response gets exhausted after continuous risk factor exposure during aging.16 Thus, individuals with a high cardiovascular risk factor burden would have a higher CPC count at a young age followed by depleted vascular regenerative capacity manifesting as lower CPC counts at an older age.16
CPC Counts and the Obesity Paradox in Patients With CAD
Patients with CAD and obesity are at a reduced short-term risk of adverse outcomes compared with those without obesity—a paradox that has been observed in several heterogenous cohorts5–8—and also confirmed in our study population where patients were followed for a median duration of 3.5 years. Explanations for this counterintuitive phenomenon have included the role of physical activity, cardiorespiratory fitness, role of lean body mass,5 the imperfect nature of BMI as an obesity metric that may be confounded by age and disease severity,5 along with confounding due to lead-time bias and collider stratification bias.44
Herein, we have demonstrated the crucial contribution of endogenous regenerative capacity, measured using CPC counts, for providing pathobiologic insights into in the obesity paradox observed in patients with CAD. To the best of our knowledge, we are the first to demonstrate that the inverse association of obesity with adverse outcomes in CAD is limited to those with preserved endogenous regenerative capacity. We also observed that participants with obesity and lower CPC counts were at a similar risk as participants without obesity and high or low CPC counts in multivariable-adjusted survival analyses. Thus, obesity, that is accompanied with higher CPC counts, confers short-term protection from adverse cardiovascular outcomes possibly due to preserved regenerative capacity. However, when CPC counts in patients with obesity fall and their regenerative capacity is exhausted with aging or the presence of concomitant diabetes, their risk is similar to that of patients without obesity.
Strengths and Limitations
Our study has several notable strengths. We have studied the association of obesity with CPC counts in 2 large cohorts of individuals without and with CAD. Asymptomatic participants underwent extensive phenotyping, which helped address the issue of confounding due to systemic inflammation, IR, pancreatic β-cell function, cardiorespiratory fitness, and fat distribution. Participants with CAD were prospectively followed for adjudicated cardiovascular outcomes, which helped us determine the role of CPC counts in explaining the obesity paradox. Limitations include our inability to exclude residual confounding given the observational nature of these cohorts. Second, the duration of obesity and longitudinal changes in body composition or metabolic rate with aging were not available in our cohorts. Thus, we are not able to study their impact on CPC counts and the obesity paradox. Last, we have not evaluated the role of adipocytokines in affecting CPC counts, and participants in our CAD cohort did not undergo extensive phenotyping with body composition and cardiorespiratory fitness measurement. These measurements might help provide further insights into the pathobiologic mechanisms underlying the association between vascular regenerative capacity and the obesity paradox in patients with CAD.
Conclusions
Obesity, defined as BMI ≥30 kg/m2, is associated with higher CD34+, CD34+/CD133+, and CD34+/CXCR4+ counts in asymptomatic individuals and in patients with CAD. The paradoxical association of obesity with decreased risk of adverse outcomes in patients with CAD is limited to those with high CPC counts that are reflective of preserved regenerative capacity.
Acknowledgments
We would like to acknowledge Shruti Nain, MDes, for assistance with illustrations and the Emory Cardiovascular Biobank participants and study coordinators.
Sources of Funding
A. Mehta is supported by the American Heart Association postdoctoral fellowship award 19POST34400057. A.A. Quyyumi is supported by the National Institutes of Health grants 1P20HL113451-01, 1R61HL138657-02, 1P30DK111024-03S1, 5R01HL095479-08, 3RF1AG051633-01S2, 5R01AG042127-06, 2P01HL086773-08, U54AG062334-01, 1R01HL141205-01, 5P01HL101398-02, 1P20HL113451-01, 5P01HL086773-09, 1RF1AG051633-01, R01 NS064162-01, R01 HL89650-01, HL095479-01, and 1DP3DK094346-01 and the American Heart Association grant 15SFCRN23910003.
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ACE
- angiotensin-converting enzyme
- AFP
- android fat percentage
- BMI
- body mass index
- CAD
- coronary artery disease
- CD
- cluster of differentiation
- CHDWB
- Center for Health Discovery and Well Being
- CPC
- circulating progenitor cell
- CXCR4
- C-X-C motif receptor 4
- EmCAB
- Emory Cardiovascular Biobank
- GFP
- gynoid fat percentage
- HOMA
- homeostasis model assessment
- hsCRP
- high-sensitivity C-reactive protein
- IR
- insulin resistance
- MI
- myocardial infarction
- WBC
- white blood cell
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/ATVBAHA.120.315703.
For Sources of Funding and Disclosures, see page 2107.
Contributor Information
Anurag Mehta, Email: anurag.mehta@emory.edu.
Qi Meng, Email: mengqee@gmail.com.
Xiaona Li, Email: xiaona.li@emory.edu.
Annie H. Ho, Email: annie.hang.ho@emory.edu.
Shabatun J. Islam, Email: sjisla2@emory.edu.
Devinder S. Dhindsa, Email: ddhinds@emory.edu.
Zakaria Almuwaqqat, Email: zalmuwa@emory.edu.
Aditi Nayak, Email: aditi.nayak@emory.edu.
Ayman A. Alkhoder, Email: ayman.alkhoder@emory.edu.
Ananya Hooda, Email: ahooda2@emory.edu.
Anil Varughese, Email: anil.varughese@emory.edu.
Syed F. Ahmad, Email: syed.faisal.ahmad@emory.edu.
Ali Mokhtari, Email: ali.mokhtari@emory.edu.
Iraj Hesaroieh, Email: iraj.ghaini.hesaroieh@emory.edu.
Laurence S. Sperling, Email: lsperli@emory.edu.
Yi-An Ko, Email: yi-an.ko@emory.edu.
Edmund K. Waller, Email: ewaller@emory.edu.
References
- 1.Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15:288–298. doi: 10.1038/s41574-019-0176-8 [DOI] [PubMed] [Google Scholar]
- 2.Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics. 2015;33:673–689. doi: 10.1007/s40273-014-0243-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedrich MJ. Global obesity epidemic worsening. JAMA. 2017;318:603. doi: 10.1001/jama.2017.10693 [DOI] [PubMed] [Google Scholar]
- 4.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RHAmerican Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016 [DOI] [PubMed] [Google Scholar]
- 5.Elagizi A, Kachur S, Lavie CJ, Carbone S, Pandey A, Ortega FB, Milani RV. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis. 2018;61:142–150. doi: 10.1016/j.pcad.2018.07.003 [DOI] [PubMed] [Google Scholar]
- 6.Sharma A, Vallakati A, Einstein AJ, Lavie CJ, Arbab-Zadeh A, Lopez-Jimenez F, Mukherjee D, Lichstein E. Relationship of body mass index with total mortality, cardiovascular mortality, and myocardial infarction after coronary revascularization: evidence from a meta-analysis. Mayo Clin Proc. 2014;89:1080–1100. doi: 10.1016/j.mayocp.2014.04.020 [DOI] [PubMed] [Google Scholar]
- 7.Wang ZJ, Zhou YJ, Galper BZ, Gao F, Yeh RW, Mauri L. Association of body mass index with mortality and cardiovascular events for patients with coronary artery disease: a systematic review and meta-analysis. Heart. 2015;101:1631–1638. doi: 10.1136/heartjnl-2014-307119 [DOI] [PubMed] [Google Scholar]
- 8.Lavie CJ, Oktay AA, Milani RV. The obesity paradox and obesity severity in elderly STEMI patients. Eur Heart J Qual Care Clin Outcomes. 2017;3:166–167. doi: 10.1093/ehjqcco/qcx018 [DOI] [PubMed] [Google Scholar]
- 9.Bellows CF, Zhang Y, Simmons PJ, Khalsa AS, Kolonin MG. Influence of BMI on level of circulating progenitor cells. Obesity (Silver Spring). 2011;19:1722–1726. doi: 10.1038/oby.2010.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fadini GP, Bonora BM, Marcuzzo G, Marescotti MC, Cappellari R, Pantano G, Sanzari MC, Duran X, Vendrell J, Plebani M, et al. Circulating stem cells associate with adiposity and future metabolic deterioration in healthy subjects. J Clin Endocrinol Metab. 2015;100:4570–4578. doi: 10.1210/jc.2015-2867 [DOI] [PubMed] [Google Scholar]
- 11.Pires A, Martins P, Paiva A, Pereira AM, Marques M, Castela E, Sena C, Seiça R. Circulating endothelial progenitor cells in obese children and adolescents. J Pediatr (Rio J). 2015;91:560–566. doi: 10.1016/j.jped.2015.01.011 [DOI] [PubMed] [Google Scholar]
- 12.Graziani F, Leone AM, Basile E, Cialdella P, Tritarelli A, Bona RD, Liuzzo G, Nanni G, Iaconelli A, Iaconelli A, et al. Endothelial progenitor cells in morbid obesity. Circ J. 2014;78:977–985. doi: 10.1253/circj.cj-13-0976 [DOI] [PubMed] [Google Scholar]
- 13.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964 [DOI] [PubMed] [Google Scholar]
- 14.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221 [DOI] [PubMed] [Google Scholar]
- 15.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78 [DOI] [PubMed] [Google Scholar]
- 16.Fadini GP, Mehta A, Dhindsa DS, Bonora BM, Sreejit G, Nagareddy P, Quyyumi AA. Circulating stem cells and cardiovascular outcomes: from basic science to the clinic. Eur Heart J. 2021;41:4271–4282. doi: 10.1093/eurheartj/ehz923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110:624–637. doi: 10.1161/CIRCRESAHA.111.243386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 19.Seeger FH, Rasper T, Koyanagi M, Fox H, Zeiher AM, Dimmeler S. CXCR4 expression determines functional activity of bone marrow-derived mononuclear cells for therapeutic neovascularization in acute ischemia. Arterioscler Thromb Vasc Biol. 2009;29:1802–1809. doi: 10.1161/ATVBAHA.109.194688 [DOI] [PubMed] [Google Scholar]
- 20.Al Mheid I, Hayek SS, Ko YA, Akbik F, Li Q, Ghasemzadeh N, Martin GS, Long Q, Hammadah M, Maziar Zafari A, et al. Age and human regenerative capacity impact of cardiovascular risk factors. Circ Res. 2016;119:801–809. doi: 10.1161/CIRCRESAHA.116.308461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fadini GP, Sartore S, Albiero M, Baesso I, Murphy E, Menegolo M, Grego F, Vigili de Kreutzenberg S, Tiengo A, Agostini C, et al. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol. 2006;26:2140–2146. doi: 10.1161/01.ATV.0000237750.44469.88 [DOI] [PubMed] [Google Scholar]
- 22.Imanishi T, Moriwaki C, Hano T, Nishio I. Endothelial progenitor cell senescence is accelerated in both experimental hypertensive rats and patients with essential hypertension. J Hypertens. 2005;23:1831–1837. doi: 10.1097/01.hjh.0000183524.73746.1b [DOI] [PubMed] [Google Scholar]
- 23.Imanishi T, Hano T, Sawamura T, Nishio I. Oxidized low-density lipoprotein induces endothelial progenitor cell senescence, leading to cellular dysfunction. Clin Exp Pharmacol Physiol. 2004;31:407–413. doi: 10.1111/j.1440-1681.2004.04022.x [DOI] [PubMed] [Google Scholar]
- 24.Michaud SE, Dussault S, Haddad P, Groleau J, Rivard A. Circulating endothelial progenitor cells from healthy smokers exhibit impaired functional activities. Atherosclerosis. 2006;187:423–432. doi: 10.1016/j.atherosclerosis.2005.10.009 [DOI] [PubMed] [Google Scholar]
- 25.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, Wang T, Gregg D, Ramaswami P, Pippen AM, Annex BH, Dong C, Taylor DA. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108:457–463. doi: 10.1161/01.CIR.0000082924.75945.48 [DOI] [PubMed] [Google Scholar]
- 26.Tabassum R, Cunningham L, Stephens EH, Sturdivant K, Martin GS, Brigham KL, Gibson G. A Longitudinal Study of Health Improvement in the Atlanta CHDWB Wellness Cohort. J Pers Med. 2014;4:489–507. doi: 10.3390/jpm4040489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ko YA, Hayek S, Sandesara P, Samman Tahhan A, Quyyumi A. Cohort profile: the Emory Cardiovascular Biobank (EmCAB). BMJ Open. 2017;7:e018753. doi: 10.1136/bmjopen-2017-018753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2192. doi: 10.2337/diacare.21.12.2191 [DOI] [PubMed] [Google Scholar]
- 30.Brigham KL. Predictive health: the imminent revolution in health care. J Am Geriatr Soc. 2010;58(suppl 2):S298–S302. doi: 10.1111/j.1532-5415.2010.03107.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelli HM, Corrigan FE, 3rd, Heinl RE, Dhindsa DS, Hammadah M, Samman-Tahhan A, Sandesara P, O’Neal WT, Al Mheid I, Ko YA, et al. Relation of changes in body fat distribution to oxidative stress. Am J Cardiol. 2017;120:2289–2293. doi: 10.1016/j.amjcard.2017.08.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu W, Chafi H, Guo B, Heymsfield SB, Murray KB, Zheng J, Jia G. Quantitative comparison of 2 dual-energy X-ray absorptiometry systems in assessing body composition and bone mineral measurements. J Clin Densitom. 2016;19:298–304. doi: 10.1016/j.jocd.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 33.Patel RS, Li Q, Ghasemzadeh N, Eapen DJ, Moss LD, Janjua AU, Manocha P, Kassem HA, Veledar E, Samady H, et al. Circulating CD34+ progenitor cells and risk of mortality in a population with coronary artery disease. Circ Res. 2015;116:289–297. doi: 10.1161/CIRCRESAHA.116.304187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, et al. ; Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1161/CIR.0b013e31826e105822923432 [Google Scholar]
- 35.Müller-Ehmsen J, Braun D, Schneider T, Pfister R, Worm N, Wielckens K, Scheid C, Frommolt P, Flesch M. Decreased number of circulating progenitor cells in obesity: beneficial effects of weight reduction. Eur Heart J. 2008;29:1560–1568. doi: 10.1093/eurheartj/ehn213 [DOI] [PubMed] [Google Scholar]
- 36.MacEneaney OJ, Kushner EJ, Van Guilder GP, Greiner JJ, Stauffer BL, DeSouza CA. Endothelial progenitor cell number and colony-forming capacity in overweight and obese adults. Int J Obes (Lond). 2009;33:219–225. doi: 10.1038/ijo.2008.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heida NM, Müller JP, Cheng IF, Leifheit-Nestler M, Faustin V, Riggert J, Hasenfuss G, Konstantinides S, Schäfer K. Effects of obesity and weight loss on the functional properties of early outgrowth endothelial progenitor cells. J Am Coll Cardiol. 2010;55:357–367. doi: 10.1016/j.jacc.2009.09.031 [DOI] [PubMed] [Google Scholar]
- 38.MacEneaney OJ, Kushner EJ, Westby CM, Cech JN, Greiner JJ, Stauffer BL, DeSouza CA. Endothelial progenitor cell function, apoptosis, and telomere length in overweight/obese humans. Obesity (Silver Spring). 2010;18:1677–1682. doi: 10.1038/oby.2009.494 [DOI] [PubMed] [Google Scholar]
- 39.Tobler K, Freudenthaler A, Baumgartner-Parzer SM, Wolzt M, Ludvik B, Nansalmaa E, Nowotny PJ, Seidinger D, Steiner S, Luger A, et al. Reduction of both number and proliferative activity of human endothelial progenitor cells in obesity. Int J Obes (Lond). 2010;34:687–700. doi: 10.1038/ijo.2009.280 [DOI] [PubMed] [Google Scholar]
- 40.Després JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126:1301–1313. doi: 10.1161/CIRCULATIONAHA.111.067264 [DOI] [PubMed] [Google Scholar]
- 41.Nagareddy PR, Kraakman M, Masters SL, Stirzaker RA, Gorman DJ, Grant RW, Dragoljevic D, Hong ES, Abdel-Latif A, Smyth SS, et al. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 2014;19:821–835. doi: 10.1016/j.cmet.2014.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han J, Koh YJ, Moon HR, Ryoo HG, Cho CH, Kim I, Koh GY. Adipose tissue is an extramedullary reservoir for functional hematopoietic stem and progenitor cells. Blood. 2010;115:957–964. doi: 10.1182/blood-2009-05-219923 [DOI] [PubMed] [Google Scholar]
- 43.Lui MM, Tse HF, Mak JC, Lam JC, Lam DC, Tan KC, Ip MS. Altered profile of circulating endothelial progenitor cells in obstructive sleep apnea. Sleep Breath. 2013;17:937–942. doi: 10.1007/s11325-012-0781-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sperrin M, Candlish J, Badrick E, Renehan A, Buchan I. Collider bias is only a partial explanation for the obesity paradox. Epidemiology. 2016;27:525–530. doi: 10.1097/EDE.0000000000000493 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


