Abstract
Circulating lipoproteins as risk factors or prognostic indicators for various cancers have been investigated previously; however, no clear consensus has been reached. In this study, we aimed at evaluating the impact of serum lipoproteins on the prognosis of patients with squamous cell carcinoma of the head and neck (SCCHN). Levels of total cholesterol, low-density lipoproteins (LDL), high-density lipoproteins (HDL), triglycerides and lipoprotein(a) were measured in serum samples from 106 patients and 28 healthy controls. We found that HDL was the only lipoprotein exhibiting a significant difference in concentration between healthy controls and patients (p = 0.012). Kaplan–Meier survival curves indicated that patients with high levels of total cholesterol or LDL had better overall survival than patients with normal levels (p = 0.028 and p = 0.007, respectively). Looking at patients without lipid medication (n = 89) and adjusting for the effects of TNM stage and weight change, multivariate Cox regression models indicated that LDL was an independent prognostic factor for both overall (p = 0.005) and disease-free survival (p = 0.013). In summary, our study revealed that high LDL level is beneficial for survival outcome in patients with SCCHN. Use of cholesterol-lowering medicines for prevention or management of SCCHN needs to be evaluated carefully.
Keywords: lipoprotein, SCCHN, prognosis
1. Introduction
Squamous cell carcinoma of the head and neck (SCCHN) is a common tumor type and a significant cause of death worldwide [1]. The most common intraoral site for SCCHN is the tongue and squamous cell carcinoma of the oral tongue (SCCOT) is a serious public health problem with significant morbidity and mortality [2,3].
Several studies have shown that blood lipids are involved in the initiation and development of different types of cancer, including oral cancer [4]. Among blood lipids, triglycerides (TGs) and cholesterol are the most abundant, with cholesterol being essential for cell membrane biogenesis, proliferation and differentiation [5] and also being involved in the production of vitamin D and steroid hormones [6]. In humans, cholesterol is synthesized mainly by the liver, but is also provided in the diet. Low-density lipoproteins (LDL) transport cholesterol from the liver to cells, and high-density lipoproteins (HDL) transport cholesterol from cells to the liver [6].
It is well-known that chronically sick people have lower circulating levels of total cholesterol and that the levels of LDL and the LDL/HDL ratio are predictors of different diseases and outcomes [7,8,9,10].
A major topic of interest is the potential association between cholesterol and cancer. The exact role of cholesterol in the development of SCCHN is not clear and studies have shown contradictory results, where development of SCCHN is related to either a decrease or an increase in serum cholesterol, HDL and LDL [4,11,12,13]. A variant of LDL is lipoprotein(a), which is a risk factor for atherosclerosis and related diseases. Higher levels of lipoprotein(a) have been associated with poor prognosis in SCCHN [14].
Obesity, defined as body mass index (BMI) ≥ 30 kg/m2, has become a worldwide health problem and a risk factor for several malignancies [15]. Lipid alterations associated with obesity are decreased circulating HDL, and/or increased LDL, total cholesterol and TGs [16]. A recent study showed obesity to be an independent predictor of poor prognosis for patients with oral squamous cell carcinoma (OSCC) [17].
The aim of this study was to analyze blood samples from patients with SCCOT and other subtypes of SCCHN and map lipoprotein profiles and BMI in correlation to clinicopathological features to evaluate the potential of blood lipids as prognostic markers.
2. Materials and Methods
2.1. Patient Cohort
A cohort of 106 patients with clinically and histopathologically confirmed primary SCCHN were included. Of these, 28 had tumors in the oral mobile tongue (SCCOT), 9—on the floor of the mouth, 21—in gingivae, and 48—in the oropharynx (tonsils and/or base of the tongue). A group of 28 healthy volunteers without pre-cancerous lesions or any history of malignancies was included for control. Informed consent had been given by all the participants, and the study was approved by the local ethics committee (Dnr 08–003M). For SCCHN patients, a thorough clinical history including weight change judged as “No change”, “Increase of 1–5 kg”, “Decrease of 1–5 kg” and “Decrease of more than 5 kg” during the three months before diagnosis was taken [18]. For clinical data, see Table 1.
Table 1.
Clinical features of patients with SCCHN.
| Clinical Features | Number of Patients | |
|---|---|---|
| Tumor Localization | Tongue | 28 |
| Floor of the mouth | 9 | |
| Gingivae | 21 | |
| Oropharynx (tonsils and/or base of the tongue) | 48 | |
| Age at Diagnosis | ≤40 years | 8 |
| 41 to 65 years | 50 | |
| >65 years | 48 | |
| Gender | Female | 36 |
| Male | 70 | |
| Smoking | Non-smoker | 38 |
| Previous smoker | 31 | |
| Smoker | 37 | |
| Alcohol | No | 24 |
| Yes | 82 | |
| HPV Status | Negative | 67 |
| Positive | 39 | |
| BMI | Underweight (<18.5) | 4 |
| Normal (18.5 to 24) | 46 | |
| Overweight (25 to 30) | 40 | |
| Obese (>30) | 16 | |
| Weight change | No change | 64 |
| Increase of 1 to 5 kg | 4 | |
| Decrease of 1 to 5 kg | 20 | |
| Decrease > 5 kg | 18 | |
| Cholesterol-lowering Medication | No | 89 |
| Yes | 17 | |
| T Stage | T1 | 22 |
| T2 | 35 | |
| T3 | 5 | |
| T4 | 44 | |
| Lymph Node Metastasis | No | 50 |
| Yes | 56 | |
| Distant Metastasis | No | 104 |
| Yes | 2 | |
| Clinical TNM Stage (8th edition) | I | 35 |
| II | 16 | |
| III | 19 | |
| IV | 1 | |
| IVa | 30 | |
| IVb | 4 | |
| IVc | 1 | |
| Treatment | Surgery alone | 12 |
| Surgery with postoperative radiotherapy | 27 | |
| Surgery with postoperative radiotherapy and chemotherapy | 2 | |
| Radiotherapy alone | 38 | |
| Preoperative radiotherapy and surgery | 15 | |
| Radiotherapy in combination with chemotherapy or pharmacotherapy | 12 | |
| Status | Alive | 76 |
| Dead | 30 | |
| Total | 106 | |
2.2. Blood Collection and Lipoprotein Analysis
Blood was collected from SCCHN patients in connection with diagnostic examination/surgical procedure before initiation of treatment. From both SCCHN patients and controls, peripheral blood (3 mL) was collected into vacutainers (SST™ II; cat. No. 368498; BD Biosciences, San Jose, CA, USA) using standardized venepuncture procedures. Tubes contained a serum separator, an acrylic-based gel forming a barrier between the clot and the serum after centrifugation, but no anticoagulant. After at least 30 min at room temperature, the tubes were centrifuged at 1300× g for 10 min at room temperature, followed by collection of the serum layer which was then stored at −80 °C until further use.
Levels of total cholesterol, HDL, LDL, TGs and lipoprotein(a) were analyzed at the accredited laboratory at Clinical Chemistry, Umeå University Hospital, NUS. Total cholesterol, HDL, TGs and lipoprotein(a) were measured directly, and the Friedewald equation was used to calculate LDL levels [19]. The unit for total cholesterol, HDL, LDL and TGs was mmol/L, for lipoprotein(a)—nmol/L.
2.3. Statistical Analysis
Comparison of lipoprotein levels between controls and patients was performed using the non-parametric Mann–Whitney U-test. Associations between categorized clinicopathological variables and categorized lipoprotein levels were determined by the chi-squared test. Associations between continuous variables were determined by the Spearman correlation analysis. The Kaplan–Meier method with log-rank test was used for survival analysis. The following cut-off levels were used for classification into high-/low-risk or normal: for cholesterol, 5.0 mmol/L; for LDL, 3.0 mmol/L; for HDL, 1.0 mmol/L for males and 1.2 mmol/L for females; for TGs, 1.7 mmol/L; for lipoprotein(a), 75 nmol/L [20,21]. Follow-up time for overall survival was calculated from time of diagnosis. Disease-free survival was defined as the time from completion of treatment to recurrence or death. In the Cox’s regression model, levels of cholesterol, LDL, age at diagnosis, BMI and weight change were treated as continuous variables. Gender, alcohol, smoking, HPV status, T stage, lymph node metastasis and TNM stage were categorical variables. For the multivariate Cox regression analysis, TNM stage and weight change were considered covariates. All statistical tests were conducted in IBM SPSS Statistics 25. A two-sided p-value < 0.05 was considered significant.
3. Results
3.1. Serum Lipid Levels in SCCHN Patients Compared to Controls
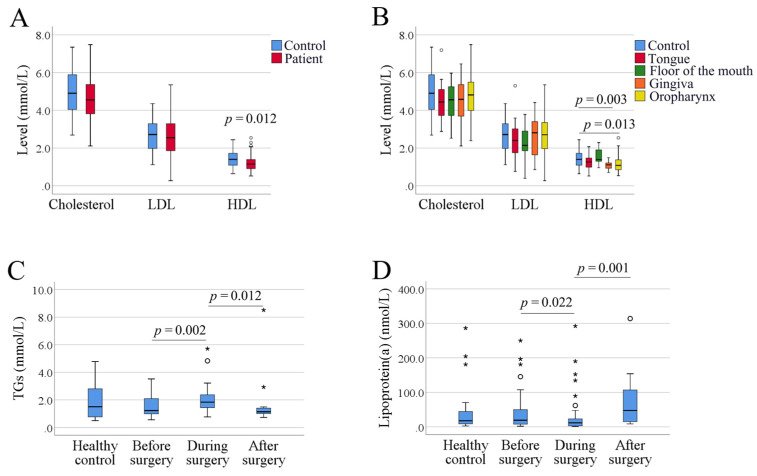
There was no significant difference between total cholesterol levels in SCCHN patients and controls (p = 0.208). Similarly, no significant difference in LDL levels was found between patients and controls (p = 0.554), whereas HDL levels were lower in patients (p = 0.012) (Figure 1A). As the Friedwald equation cannot be used for calculation of LDL levels if TG levels are too high (> 4.5 mmol/L), six samples (four SCCHN patients and two controls) lacked values for LDL. Since tumors in different sublocations of the head and neck area vary in characteristics [22,23], patients were divided according to sublocation of the tumor, exhibiting significant differences only for HDL in patients with gingival and oropharyngeal SCC (p = 0.003 and 0.013, respectively) (Figure 1B).
Figure 1.
Box plots of serum lipoprotein levels in healthy controls and patients with SCCHN. (A) Levels of total cholesterol, low-density lipoproteins (LDL) and high-density lipoproteins (HDL). (B) Patients were divided into four subgroups according to tumor locations. (C) Levels of triglycerides (TGs) in healthy controls and patients with different timepoints of blood collection. (D) Levels of lipoprotein(a) in healthy controls and patients with different timepoints of blood collection. Small circles indicate outliers and asterisks indicate extreme outliers.
For analysis of TGs and lipoprotein(a), it is recommended to use serum samples collected at fasting stage. Our samples were taken before, at or after diagnostic examination/surgery, thus, the fasting stage was variable. All the patients were required to fast before surgery, while during surgery, they received an infusion drip containing glucose, and after surgery, the fasting status was not known. Dividing results into three different timepoints of collection showed a difference in levels of both TGs and lipoprotein(a) (p < 0.05, Figure 1C,D).
3.2. Clinicopathological Data in Correlation to Levels of Lipoproteins
When analyzing clinicopathological data in correlation to levels of lipoproteins, correlations between overall survival and cholesterol (p = 0.048), overall survival and LDL (p = 0.017) and disease-free survival and LDL (p = 0.030) were found (Table 2). For levels of cholesterol and BMI, we further analyzed correlations using the Spearman correlation analysis of non-categorized values. The results showed a positive correlation between LDL and BMI (correlation coefficient = 0.217, p = 0.029), whereas a negative correlation between HDL and BMI was observed (correlation coefficient = −0.380, p < 0.0001) (Figure S1).
Table 2.
Correlations between clinical variables and levels of cholesterol/LDL/HDL.
| Clinical Variables | Total Cholesterol | LDL | HDL | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal | High | p-Value | Normal | High | p-Value | Normal | Low | p-Value | ||
| Age at diagnosis (years) | 20–40 | 5 | 3 | 0.066 | 6 | 2 | 0.092 | 3 | 5 | 0.302 |
| 41–65 | 25 | 25 | 25 | 21 | 33 | 17 | ||||
| > 65 | 35 | 13 | 36 | 12 | 29 | 19 | ||||
| Gender | Female | 23 | 13 | 0.834 | 26 | 10 | 0.384 | 24 | 12 | 0.528 |
| Male | 42 | 28 | 41 | 25 | 41 | 29 | ||||
| Alcohol | Yes | 49 | 33 | 0.637 | 52 | 26 | 0.807 | 51 | 31 | 0.813 |
| No | 16 | 8 | 15 | 9 | 14 | 10 | ||||
| Smoking | Smoker | 19 | 18 | 0.244 | 23 | 12 | 0.233 | 25 | 12 | 0.558 |
| Previous smoker | 22 | 9 | 23 | 7 | 17 | 14 | ||||
| Non-smoker | 24 | 14 | 21 | 16 | 23 | 15 | ||||
| BMI | Underweight | 3 | 1 | 0.374 | 4 | 0 | 0.351 | 3 | 1 | 0.093 |
| Normal | 32 | 14 | 32 | 14 | 34 | 12 | ||||
| Overweight | 21 | 19 | 23 | 16 | 20 | 20 | ||||
| Obese | 9 | 7 | 8 | 5 | 8 | 8 | ||||
| Cholesterol-lowering medication | No | 50 | 39 | 0.014 | 51 | 34 | 0.005 | 54 | 35 | 1.000 |
| Yes | 15 | 2 | 16 | 1 | 11 | 6 | ||||
| T stage | T1, T2 | 36 | 21 | 0.694 | 39 | 16 | 0.296 | 38 | 19 | 0.237 |
| T3, T4 | 29 | 20 | 28 | 19 | 27 | 22 | ||||
| Lymph node metastasis | Negative | 28 | 22 | 0.322 | 29 | 19 | 0.305 | 32 | 18 | 0.690 |
| Positive | 37 | 19 | 38 | 16 | 33 | 23 | ||||
| Clinical TNM stage | I, II | 33 | 18 | 0.552 | 34 | 16 | 0.680 | 34 | 17 | 0.321 |
| III, IV | 32 | 23 | 33 | 19 | 31 | 24 | ||||
| HPV status | Negative | 42 | 25 | 0.836 | 44 | 20 | 0.518 | 46 | 21 | 0.062 |
| Positive | 23 | 16 | 23 | 15 | 19 | 20 | ||||
| Weight change (kg) | No change | 37 | 27 | 0.454 | 40 | 23 | 0.144 | 41 | 23 | 0.121 |
| Increase of 1–5 | 2 | 2 | 2 | 1 | 2 | 2 | ||||
| Decrease of 1–5 | 12 | 8 | 10 | 9 | 15 | 5 | ||||
| Decrease > 5 | 14 | 4 | 15 | 2 | 7 | 11 | ||||
| Overall survival status | Alive | 42 | 34 | 0.048 | 44 | 31 | 0.017 | 46 | 30 | 0.828 |
| Dead | 23 | 7 | 23 | 4 | 19 | 11 | ||||
| Disease-free status | Disease-free | 44 | 34 | 0.113 | 46 | 31 | 0.030 | 50 | 28 | 0.370 |
| With disease | 21 | 7 | 21 | 4 | 15 | 13 | ||||
It is known that carcinogens in tobacco can damage lipids and other cell membrane components [24]; however, in contrast to other studies, we observed no correlation between the lipid profile and smoking.
3.3. Levels of Lipoproteins in Correlation to Survival
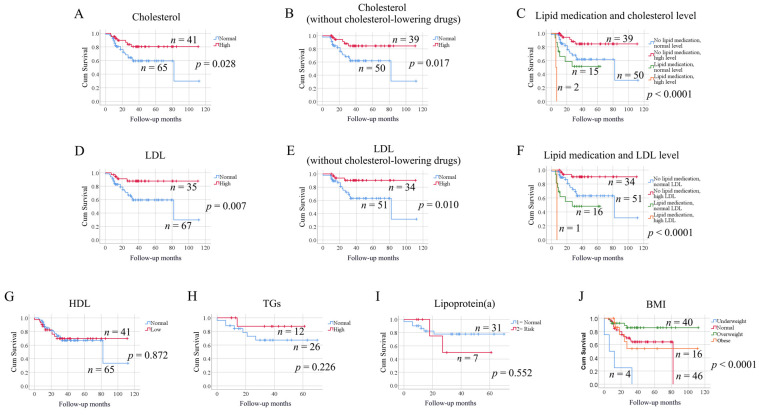
Next, we analyzed the impact of serum lipoprotein levels on patient survival using the Kaplan–Meier method. SCCHN patients with high levels of total cholesterol exhibited longer overall survival than patients with normal levels (p = 0.028) (Figure 2A). As 17 patients were on anti-lipid medication, we next analyzed patients without lipid medication only (n = 89), and the results still showed high levels of cholesterol to be beneficial for survival (p = 0.017) (Figure 2B). Significant difference in overall survival was also observed when dividing patients into four groups according to both lipid medication status and cholesterol levels (Figure 2C, p < 0.0001). Patients without lipid medication but high cholesterol level had the best survival outcome. In general, overall survival in patients without lipid medication was better than in those with lipid medication (p = 0.007).
Figure 2.
Kaplan–Meier curves showing the impact of lipoprotein levels on the overall survival of patients with SCCHN. (A) Comparison between cholesterol-high and cholesterol-normal patients. (B) The impact of cholesterol levels on the overall survival was investigated only for patients without cholesterol-lowering drugs. (C) Patients were grouped according to the lipid medication status and cholesterol levels. (D) Comparison between LDL-high and LDL-normal patients. (E) The impact of LDL levels on the overall survival was investigated only for patients without cholesterol-lowering drugs. (F) Patients were grouped according to the lipid medication status and LDL levels. (G) Comparison between HDL-low and HDL-normal patients. (H) Comparison between TGs-high and TGs-normal patients (only the samples taken at fasting were analyzed). (I) Comparison between lipoprotein(a)-risk and lipoprotein(a)-normal patients (only the samples taken at fasting were analyzed). (J). Comparison of patients with different BMI levels.
Levels of LDL also correlated to overall survival for SCCHN patients, where high levels of LDL were advantageous (p = 0.007) (Figure 2D). When selecting only patients without lipid medication (n = 85), high levels of LDL remained significantly correlated to longer survival in patients (p = 0.010) (Figure 2E). Dividing patients into four groups according to both lipid medication status and cholesterol levels, we found that patients without lipid medication but high LDL level had the best survival outcome (p < 0.0001) (Figure 2F).
HDL levels did not correlate with overall survival (p = 0.872) (Figure 2G). As levels of TGs and lipoprotein(a) were influenced by the fasting status, only the samples taken at fasting were analyzed. No significant differences in overall survival were observed for either of these two factors (Figure 2H,I).
We further analyzed the impact of BMI on overall survival. The group of overweight patients (defined as having a BMI of 25–30) exhibited better survival compared to normal-weight (BMI 18.5–24), obese (BMI > 30) and underweight patients (BMI < 18.5) (p < 0.001) (Figure 2J). The overall survival for oropharyngeal cancer in relation to the HPV status was also analyzed; however, no significant differences were found (p = 0.177, Figure S2).
The Kaplan–Meier survival analysis of disease-free survival according to levels of lipoproteins, BMI and HPV status also indicated that total cholesterol, LDL and BMI were significantly correlated with disease-free survival (data not shown).
Using the univariate Cox regression model for patients without lipid medication, impacts of total cholesterol, LDL, T stage, TNM stage and weight change on patient survival were observed (Table 3). Adjusting for the effects of TNM stage and weight change, the multivariate Cox regression indicated that LDL levels remained an independent prognostic factor for both overall (p = 0.005) and disease-free survival (p = 0.013), whereas total cholesterol was an independent prognostic factor only for overall survival (p = 0.033).
Table 3.
Cox regression analysis for patient survival (patients without lipid medication).
| Group | Overall Survival | Disease-Free Survival | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p-Value | Hazard Ratio | 95% CI for Hazard Ratio | p-Value | Hazard Ratio | 95% CI for Hazard Ratio | |||||
| Lower | Upper | Lower | Upper | |||||||
| Univariate Cox regression analysis | Cholesterol | 0.021 | 0.599 | 0.387 | 0.926 | 0.068 | 0.676 | 0.444 | 1.029 | |
| LDL | 0.006 | 0.514 | 0.319 | 0.827 | 0.015 | 0.551 | 0.341 | 0.890 | ||
| Age | 0.113 | 1.031 | 0.993 | 1.071 | 0.115 | 1.030 | 0.993 | 1.068 | ||
| Gender | 0.559 | 0.763 | 0.308 | 1.891 | 0.355 | 0.664 | 0.278 | 1.583 | ||
| Alcohol | 0.971 | 0.980 | 0.327 | 2.935 | 0.817 | 1.126 | 0.412 | 3.077 | ||
| Smoking | 0.730 | 1.087 | 0.677 | 1.744 | 0.964 | 1.011 | 0.628 | 1.627 | ||
| BMI | 0.080 | 0.901 | 0.801 | 1.013 | 0.073 | 0.907 | 0.816 | 1.009 | ||
| HPV status | 0.568 | 0.757 | 0.291 | 1.971 | 0.300 | 0.606 | 0.235 | 1.563 | ||
| T stage | 0.065 | 1.460 | 0.977 | 2.183 | 0.046 | 1.489 | 1.007 | 2.201 | ||
| Lymph node metastasis | 0.064 | 2.374 | 0.952 | 5.918 | 0.192 | 1.793 | 0.746 | 4.312 | ||
| TNM stage | 0.011 | 1.376 | 1.075 | 1.762 | 0.002 | 1.510 | 1.166 | 1.956 | ||
| Weight change | 0.011 | 0.903 | 0.834 | 0.977 | 0.024 | 0.915 | 0.846 | 0.988 | ||
| Multivariate Cox regression analysis |
Independent predictors | Cholesterol | 0.033 | 0.599 | 0.374 | 0.959 | 0.105 | 0.691 | 0.442 | 1.080 |
| TNM stage | 0.021 | 1.355 | 1.047 | 1.755 | 0.004 | 1.488 | 1.138 | 1.944 | ||
| Weight change | 0.088 | 0.931 | 0.858 | 1.011 | 0.232 | 0.951 | 0.875 | 1.033 | ||
| Independent predictors | LDL | 0.005 | 0.451 | 0.259 | 0.787 | 0.013 | 0.501 | 0.290 | 0.867 | |
| TNM stage | 0.034 | 1.357 | 1.024 | 1.800 | 0.006 | 1.494 | 1.122 | 1.991 | ||
| Weight change | 0.046 | 0.916 | 0.841 | 0.998 | 0.154 | 0.941 | 0.865 | 1.023 | ||
4. Discussion
The role of lipids in tumor development has been a focus of interest in many recent studies, but findings from different tumor types are often contradictive [25]. Previous studies have shown an association between blood cholesterol levels and different cancers [26] such as breast [27], colorectal [28,29] and lung cancer [30]. So far, there are only a few studies available on serum lipid profiles in SCCHN. In accordance with previous studies [4,11,30], we found levels of total cholesterol, LDL and HDL to be lower in SCCHN patients than in controls, although HDL was the only lipoprotein that was statistically significantly lower in the SCCHN patients in our study. Notably, 42% of the patients in our study had T4 tumors. Patients with tumors in advanced stages might have lower lipid levels due to food intake difficulties and malnutrition. However, no correlation between the T stage and lipid levels were observed in this study, excluding the possible link between tumor stages, malnutrition and low lipid levels in these patients. A reduction in HDL was observed in numerous previous studies and low HDL is believed to be an additional cancer predictor and is proposed to be caused by excess cholesterol utilization for membrane biogenesis by proliferating malignant cells [24].
LDL is composed of several proteins and lipids carrying cholesterol into peripheral tissues and also affecting the metabolism of fatty acids. Recent reports have indicated an emerging role of LDL in breast cancer, affecting cell proliferation and migration to facilitate disease progression [27].
In our group of SCCHN patients, high levels of LDL correlated with improved survival, whereas a previous study on SCCHN did not show a correlation between LDL and survival [14]. Apart from using different methods for detection of LDL, Li et al. excluded patients with a family history of lipidemia as well as obese persons, differences that could aid in explaining the different results achieved. In another study of 1081 patients with locoregionally advanced nasopharyngeal cancer, high LDL tended to be inferior for overall survival in comparison with low LDL [13]. Furthermore, a previous study of 601 patients with small-cell lung cancer showed lower LDL to be an independent prognostic factor for longer overall survival [31]. However, in patients with esophageal squamous cell carcinoma, low serum LDL levels were predictive factors for poor prognosis [32]. In ovarian cancer, longer overall survival has been observed for patients with normal compared to elevated levels of LDL [33]. However, it must be kept in mind that cholesterol is involved in formation of steroid hormones like estrogen and can thus have different effects in hormone-dependent cancer types (ovary, breast) compared to non-hormone-dependent cancers like SCCHN.
Cholesterol synthesis decreases in the liver with statin treatments which promote cholesterol uptake from plasma through upregulation of LDL receptors on the surface of hepatocytes, also increasing LDL degradation in the liver. The use of statins is now common and when excluding patients on statin medication (17 patients), we still obtained significantly better survival for patients with high levels of total cholesterol and LDL. As cholesterol levels were lower in SCCHN patients compared to healthy controls, it could be speculated that higher levels mimic the normal state.
Another interesting finding in our study was that the group of overweight, but not obese, patients (pre-treatment BMI of 25–30) exhibited better survival compared to normal weight, obese and underweight patients. This is in accordance with other studies that higher BMI is related to better survival and lower recurrence and distant metastasis rates in SCCHN [34,35]. Obesity, on the other hand, which is characterized by increased levels of LDL, cholesterol and TGs, as well as by decreased HDL [16], was previously identified as an independent adverse prognostic variable, where obese SCCOT patients had a five-fold increase in the risk of death compared to normal-weight patients [36].
Even if measuring a single pre-treatment LDL level may not adequately reflect the variance in lipoproteins over the clinical course of SCCHN, these data are among the first to examine LDL levels as a predictor of outcome in SCCHN. The results also suggest a mechanism by which cholesterol-altering drugs may be used to influence outcomes in the future. Based on the present results showing improved survival for patients with high LDL, it could also be speculated whether statin medication should be withdrawn for SCCHN patients being treated for their tumor.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biomedicines9050506/s1.
Author Contributions
Conceptualization, K.N.; data curation, T.W., L.B., X.G. and K.N.; formal analysis, T.W., L.B. and X.G.; funding acquisition, K.N. and P.J.C.; methodology, L.B. and K.N.; project administration, K.N.; supervision, K.N.; writing—original draft preparation, T.W., L.B., X.G. and K.N.; writing—review and editing, L.B., X.G., P.J.C., N.S. and K.N. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Lion’s Cancer Research Foundation, Umeå University; the Swedish Cancer Society (contract number 20 0754 PjF 01H); Umeå University; Region Västerbotten; the Ministry of Health of the Czech Republic, Conceptual Development of Research Organization (MMCI, 00209805).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the local ethics committee (Dnr 08–003M, 2008-09-24).
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Henderson B.E., Lee N.H., Seewaldt V., Shen H. The influence of race and ethnicity on the biology of cancer. Nat. Rev. Cancer. 2012;12:648–653. doi: 10.1038/nrc3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen Goldemberg D., de Araujo L.H.L., Antunes H.S., de Melo A.C., Santos Thuler L.C. Tongue cancer epidemiology in Brazil: Incidence, morbidity and mortality. Head Neck. 2018;40:1834–1844. doi: 10.1002/hed.25166. [DOI] [PubMed] [Google Scholar]
- 3.Miranda-Filho A., Bray F. Global patterns and trends in cancers of the lip, tongue and mouth. Oral Oncol. 2020;102:104551. doi: 10.1016/j.oraloncology.2019.104551. [DOI] [PubMed] [Google Scholar]
- 4.Lohe V.K., Degwekar S.S., Bhowate R.R., Kadu R.P., Dangore S.B. Evaluation of correlation of serum lipid profile in patients with oral cancer and precancer and its association with tobacco abuse. J. Oral Pathol. Med. 2010;39:141–148. doi: 10.1111/j.1600-0714.2009.00828.x. [DOI] [PubMed] [Google Scholar]
- 5.Silvente-Poirot S., Poirot M. Cholesterol and Cancer, in the Balance. Science. 2014;343:1445–1446. doi: 10.1126/science.1252787. [DOI] [PubMed] [Google Scholar]
- 6.Boretti A. Nutrition, lipidic parameters, and cancer risk and progress. Nutrition. 2020;69:110538. doi: 10.1016/j.nut.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Lemieux I., Lamarche B., Couillard C., Pascot A., Cantin B., Bergeron J., Dagenais G.R., Despres J.P. Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men: The Quebec Cardiovascular Study. Arch. Intern. Med. 2001;161:2685–2692. doi: 10.1001/archinte.161.22.2685. [DOI] [PubMed] [Google Scholar]
- 8.Weiss A., Beloosesky Y., Schmilovitz-Weiss H., Grossman E., Boaz M. Serum total cholesterol: A mortality predictor in elderly hospitalized patients. Clin. Nutr. 2013;32:533–537. doi: 10.1016/j.clnu.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Kawachi K., Kataoka H., Manabe S., Mochizuki T., Nitta K. Low HDL cholesterol as a predictor of chronic kidney disease progression: A cross-classification approach and matched cohort analysis. Heart Vessel. 2019;34:1440–1455. doi: 10.1007/s00380-019-01375-4. [DOI] [PubMed] [Google Scholar]
- 10.Li B., McIntyre M., Gandhi C., Halabi M., Long A., Van Hoof A., Afridi A., Schmidt M., Cole C., Santarelli J., et al. Low total cholesterol and high density lipoprotein are independent predictors of poor outcomes following aneurysmal subarachnoid hemorrhage: A preliminary report. Clin. Neurol. Neurosurg. 2020;197:106062. doi: 10.1016/j.clineuro.2020.106062. [DOI] [PubMed] [Google Scholar]
- 11.Kark J.D., Smith A.H., Hames C.G. Serum retinol and the inverse relationship between serum cholesterol and cancer. Br. Med. J. (Clin. Res. Ed.) 1982;284:152–154. doi: 10.1136/bmj.284.6310.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel P.S., Shah M.H., Jha F.P., Raval G.N., Rawal R.M., Patel M.M., Patel J.B., Patel D.D. Alterations in plasma lipid profile patterns in head and neck cancer and oral precancerous conditions. Indian J. Cancer. 2004;41:25–31. [PubMed] [Google Scholar]
- 13.Yao J.J., He X.J., Lawrence W.R., Zhang W.J., Kou J., Zhang F., Zhou G.Q., Wang S.Y., Sun Y. Prognostic Value of Circulating Lipoprotein in Patients with Locoregionally Advanced Nasopharyngeal Carcinoma. Cell. Physiol. Biochem. 2018;48:285–292. doi: 10.1159/000491728. [DOI] [PubMed] [Google Scholar]
- 14.Li G., Da M., Zhang W., Wu H., Ye J., Chen J., Ma L., Gu N., Wu Y., Song X. Alteration of serum lipid profile and its prognostic value in head and neck squamous cell carcinoma. J. Oral Pathol. Med. 2016;45:167–172. doi: 10.1111/jop.12344. [DOI] [PubMed] [Google Scholar]
- 15.Kyrgiou M., Kalliala I., Markozannes G., Gunter M.J., Paraskevaidis E., Gabra H., Martin-Hirsch P., Tsilidis K.K. Adiposity and cancer at major anatomical sites: Umbrella review of the literature. BMJ. 2017;356:j477. doi: 10.1136/bmj.j477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mika A., Sledzinski T. Alterations of specific lipid groups in serum of obese humans: A review. Obes. Rev. 2017;18:247–272. doi: 10.1111/obr.12475. [DOI] [PubMed] [Google Scholar]
- 17.Hu Q., Peng J., Chen X., Li H., Song M., Cheng B., Wu T. Obesity and genes related to lipid metabolism predict poor survival in oral squamous cell carcinoma. Oral Oncol. 2019;89:14–22. doi: 10.1016/j.oraloncology.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Varady K.A., Tussing L., Bhutani S., Braunschweig C.L. Degree of weight loss required to improve adipokine concentrations and decrease fat cell size in severely obese women. Metabolism. 2009;58:1096–1101. doi: 10.1016/j.metabol.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 20.Piepoli M.F., Hoes A.W., Agewall S., Albus C., Brotons C., Catapano A.L., Cooney M.T., Corra U., Cosyns B., Deaton C., et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur. Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicholls S.J., Tang W.H., Scoffone H., Brennan D.M., Hartiala J., Allayee H., Hazen S.L. Lipoprotein(a) levels and long-term cardiovascular risk in the contemporary era of statin therapy. J. Lipid Res. 2010;51:3055–3061. doi: 10.1194/jlr.M008961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boldrup L., Coates P.J., Laurell G., Nylander K. Differences in p63 expression in SCCHN tumours of different sub-sites within the oral cavity. Oral Oncol. 2011;47:861–865. doi: 10.1016/j.oraloncology.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Boldrup L., Coates P.J., Wahlgren M., Laurell G., Nylander K. Subsite-based alterations in miR-21, miR-125b, and miR-203 in squamous cell carcinoma of the oral cavity and correlation to important target proteins. J. Carcinog. 2012;11:18. doi: 10.4103/1477-3163.104007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailwad S.A., Singh N., Jani D.R., Patil P., Singh M., Deep G., Singh S. Alterations in serum lipid profile patterns in oral cancer: Correlation with histological grading and tobacco abuse. Oral Health Dent. Manag. 2014;13:573–579. [PubMed] [Google Scholar]
- 25.Ganjali S., Ricciuti B., Pirro M., Butler A.E., Atkin S.L., Banach M., Sahebkar A. High-Density Lipoprotein Components and Functionality in Cancer: State-of-the-Art. Trends Endocrinol. Metab. 2019;30:12–24. doi: 10.1016/j.tem.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Allampallam K., Dutt D., Nair C., Shetty V., Mundle S., Lisak L., Andrews C., Ahmed B., Mazzone L., Zorat F., et al. The clinical and biologic significance of abnormal lipid profiles in patients with myelodysplastic syndromes. J. Hematother. Stem Cell Res. 2000;9:247–255. doi: 10.1089/152581600319469. [DOI] [PubMed] [Google Scholar]
- 27.Guan X., Liu Z., Zhao Z., Zhang X., Tao S., Yuan B., Zhang J., Wang D., Liu Q., Ding Y. Emerging roles of low-density lipoprotein in the development and treatment of breast cancer. Lipids Health Dis. 2019;18:137. doi: 10.1186/s12944-019-1075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kreger B.E., Anderson K.M., Schatzkin A., Splansky G.L. Serum cholesterol level, body mass index, and the risk of colon cancer. The Framingham Study. Cancer. 1992;70:1038–1043. doi: 10.1002/1097-0142(19920901)70:5<1038::AID-CNCR2820700505>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 29.Li T., Qian Y., Li H., Deng J. Combination of serum lipids and cancer antigens as a novel marker for colon cancer diagnosis. Lipids Health Dis. 2018;17:261. doi: 10.1186/s12944-018-0911-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao B., Yu M., Sang C., Bi B., Chen J. Dyslipidemia and non-small cell lung cancer risk in Chinese population: A case-control study. Lipids Health Dis. 2018;17:278. doi: 10.1186/s12944-018-0925-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou T., Zhan J., Fang W., Zhao Y., Yang Y., Hou X., Zhang Z., He X., Zhang Y., Huang Y., et al. Serum low-density lipoprotein and low-density lipoprotein expression level at diagnosis are favorable prognostic factors in patients with small-cell lung cancer (SCLC) BMC Cancer. 2017;17:269. doi: 10.1186/s12885-017-3239-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen P., Han L., Wang C., Jia Y., Song Q., Wang J., Guan S., Tan B., Liu B., Jia W., et al. Preoperative serum lipids as prognostic predictors in esophageal squamous cell carcinoma patients with esophagectomy. Oncotarget. 2017;8:41605–41619. doi: 10.18632/oncotarget.15651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li A.J., Elmore R.G., Chen I.Y., Karlan B.Y. Serum low-density lipoprotein levels correlate with survival in advanced stage epithelial ovarian cancers. Gynecol. Oncol. 2010;116:78–81. doi: 10.1016/j.ygyno.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hollander D., Kampman E., van Herpen C.M. Pretreatment body mass index and head and neck cancer outcome: A review of the literature. Crit. Rev. Oncol. Hematol. 2015;96:328–338. doi: 10.1016/j.critrevonc.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Karnell L.H., Sperry S.M., Anderson C.M., Pagedar N.A. Influence of body composition on survival in patients with head and neck cancer. Head Neck. 2016;38(Suppl. 1):E261–E267. doi: 10.1002/hed.23983. [DOI] [PubMed] [Google Scholar]
- 36.Iyengar N.M., Kochhar A., Morris P.G., Morris L.G., Zhou X.K., Ghossein R.A., Pino A., Fury M.G., Pfister D.G., Patel S.G., et al. Impact of obesity on the survival of patients with early-stage squamous cell carcinoma of the oral tongue. Cancer. 2014;120:983–991. doi: 10.1002/cncr.28532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author.