Abstract
Spices have long been used to improve food flavor, due to their appealing fragrance and sensory attributes. Nowadays, spices-based bioactives, particularly phenolic compounds, have gained attention due to their wide range of significant effects in biological systems. The present study was conducted to characterize the 12 widely used spices (allspice, black cardamom, black cumin, black pepper, cardamom, cinnamon, clove, cumin, fennel, nutmeg, star-anise, and turmeric) for their phenolics with the liquid chromatography-electrospray ionization quadrupole time-of-flight mass spectrometry (LC-ESI-QTOF-MS2), polyphenols estimation, and their antioxidant potential. Total phenolics, total flavonoids, and total tannin content and their antioxidant activities were estimated in all spices. Clove and allspice had the highest value of total polyphenol content (215.14 and 40.49 mg gallic acid equivalent (GAE) per g of sample), while clove and turmeric had the highest total flavonoids (5.59 mg quercetin equivalent (QE) per g of sample) and total tannin contents (23.58 mg catechin equivalent (CE) per g of sample), respectively. On the other hand, black cumin and black pepper had the highest phosphomolybdate activity (15.61 and 15.43 mg ascorbic acid equivalent (AAE) per g of sample), while clove was almost identified with highest free radical scavenging capacity. A positive correlation was observed among phenolic compounds and antioxidant activities. In this quest, a total of 79 phenolic compounds were tentatively characterized by using LC-ESI-QTOF-MS2 including 26 phenolic acids, 33 flavonoids, 16 other polyphenols, and 4 lignans. The high-performance liquid chromatography coupled with photodiode array detector (HPLC-PDA) quantification of phenolic compounds exhibited higher phenolic acids. These results provided us some valuable information that spices have powerful antioxidant potential that can be further used in human food and animal feed as a supplement for different health promoting applications.
Keywords: spices, polyphenols, antioxidant activities, characterization, identification, quantification, HPLC-PDA, LC-MS/MS
1. Introduction
Herbs and spices have been used worldwide as a food ingredient since ancient times to improve food flavor and storage stability, due to their sensory and preservative characteristics. Herbs and spices have also been used in eastern medicine (naturopathy) for centuries to heal aches, wounds, joint inflammation, sprains, and bone fractions in the body. The global production of herbs and spices has been increased due to their wide use in the food industry, pharmaceutical industry, cosmetic industry, feed industry as a natural feed additive, and the research and development sector. Herbs and spices are known to have various health promoting aspects including digestive stimulant, antioxidant, antimicrobial, antiviral, anti-inflammatory, antidiabetic, anti-obesity, antipyretic, anti-hypertensive, anti-depression, anti-carcinogenic, anti-tumor, anti-HIV, anti-Parkinson disease, cardio- and neuroprotective properties [1,2,3,4]. This health-promoting potential is attributed to the bioactive compounds, including polyphenols present in herbs and spices. Polyphenols include flavonoids, stilbenes, phenolic acids, and lignans, which have attracted nutritionists and food specialist’s attention, due to their potential health outcomes. These bioactives contribute to the different biological activities. Nowadays, phenolic compounds have attracted great attention due to their multiple biological activities like free radical scavenging capacity, metal chelation, inhibition of cellular proliferation, modulation of signal transduction pathways, and enzymatic activity [5].
Herbs and spices have been studied in various countries for their antioxidant potential and human health. Herbs and spices contain natural antioxidants that help to reduce the oxidative stress caused by the high concentration of free radicals in biological systems. Thus, herbs and spices could be used to ameliorate or prevent health issues resulting from chronic oxidative stress and metabolic syndromes or disorders [6]. The distribution of phenolic compounds in different herbs and spices have been previously explored, however a comprehensive profiling of these phenolics is still lacking, due to their complex structure and nature. The chemical characterization of these phenolic compounds is usually carried out after extraction and filtration of samples using liquid chromatography- mass spectrometry (LC-MS). The mass spectrometry is a reliable analytical technique that is widely used to tentatively elucidate the unknown compounds from the complex samples of different plant materials including herbs and spices.
To achieve this study’s objective, the phenolic contents in allspice, black cardamom, black cumin, black pepper, cardamom, cinnamon, clove, cumin, fennel, nutmeg, star-anise, and turmeric were determined through total phenolic contents (TPC), total flavonoid contents (TFC), and total tannin contents (TTC), while the antioxidant potential of all spices was determined through 2,2′-diphenyl-1-picrylhy-drazyl (DPPH), ferric reducing antioxidant power (FRAP), 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) assay (ABTS), reducing power assay (RPA), ferrous ion chelating assay (FICA), hydroxyl radical scavenging assay (•OH-RSA) and phosphomolybdate assay (PMA). Further, liquid chromatography-electrospray ionization quadrupole time-of-flight mass spectrometry (LC-ESI-QTOF-MS2) was used for the characterization and identification of phenolic compounds, while high-performance liquid chromatography coupled with photodiode array detector (HPLC-PDA) was used for the quantification of targeted phenolics from different spices. This study supports the use of spices as a potential source of polyphenols in different sectors including food, nutraceutical, pharmaceutical, cosmetic as well as in feed, due to their strong antioxidant potential.
2. Materials and Methods
2.1. Chemicals and Reagents
Analytical grade chemicals were used for extraction and characterization of spices. Most of the chemicals used for extraction and characterization were purchased from Sigma-Aldrich (Castle Hill, NSW, Australia). Folin–Ciocalteu’s phenol reagent, gallic acid, L-ascorbic acid, vanillin, hexahydrate aluminum chloride, sodium phosphate, iron(III) chloride hexahydrate (Fe[III]Cl3·6H2O), sodium phosphate dibasic hepta-hydrate, sodium phosphate monobasic monohydrate, trichloroacetic acid, hydrated sodium acetate, hydrochloric acid, ethylenediaminetetraacetic acid (EDTA), ferrozine, iron (II) chloride, iron (III) chloride, 3-hydrobenzoic acid, ammonium molybdate, quercetin, catechin, iron (II) sulphate heptahydrate, DPPH, 2,4,6tripyridyl-s-triazine (TPTZ), potassium ferrocyanide (III), and ABTS were purchased from the Sigma Aldrich (Castle Hill, NSW, Australia) for the estimation of polyphenols and antioxidant potential. Sodium carbonate anhydrous and hydrogen peroxide (30%) were purchased from Chem-Supply Pty Ltd. (Adelaide, SA, Australia) and 98% sulfuric acid was purchased from RCI Labscan (Rongmuang, Thailand). HPLC and LC-MS grade reagents include methanol, ethanol, acetonitrile, formic acid, iron (III) chloride anhydrous, and glacial acetic acid were purchased from Thermo Fisher Scientific Inc. (Scoresby, VIC, Australia). To perform various in vitro bioactivities and antioxidant assays, 96 well-plates were purchased from Thermo Fisher Scientific (Scoresby, VIC, Australia). Additionally, HPLC vials (1 mL) were purchased from Agilent technologies (Melbourne, VIC, Australia).
2.2. Preparation and Extraction of Phenolic Compounds
Spices (whole + powder) were purchased from a local market and ground into fine powder with a grinder. The methods of Vallverdú-Queralt, et al. [7] and Feng, et al. [8] were used for the extraction of phenolic compounds from spices with some modifications. Briefly, the extracts were prepared by using the 70% ethanol in Milli-Q water with 0.1% formic acid. After adding the 30 mL solvent in 2 g sample in triplicate, the samples were placed in orbital shaker (ZWYR-240 incubator shaker, Labwit, Ashwood, VIC, Australia) for 16 h for possible extraction of phenolic compounds at 20 °C and 150 rpm. Then, all samples were centrifuged (ROTINA380R, Hettich Refrigerated Centrifuge, Tuttlingen, Baden-Württemberg, Germany) at 4000 rpm for 15 min. The supernatant was collected and stored at −20 °C for further antioxidant potential. The extracts were filtered by using a 0.45 μL syringe filter for LC-MS analysis in HPLC vials.
2.3. Antioxidant Assays
The antioxidant assays were carried out by following the protocols of Subbiah, et al. [9], Suleria, et al. [10] and Zhu, et al. [11] with some modifications, all tests were performed in triplicate. The 96-well plates were used to determine the potential of each spice extract. The standard curves were constructed against the different concentrations of standards by using the Multiskan microplate photometer to record the absorbance.
2.3.1. Determination of Total Polyphenols
The TPC of all spices were determined by using the method of Vallverdú-Queralt, Regueiro, Martínez-Huélamo, Alvarenga, Leal and Lamuela-Raventos [7] with some modifications. To start, 25 μL (25% Folin–Ciocalteu reagent v/v) with 200 μL water (Milli-Q) were added to 25 μL of sample extracts in 96-well plates. Then, the plate was incubated for 5 min at 25 °C. Finally, 25 μL (10% v/v sodium carbonate) were added in reaction mixture and placed in the dark for 60 min at 25 °C and absorbance was recorded at 765 nm. The TPC was quantified by constructing the standard curve against gallic acid ranging from 0 to 200 μg/mL in ethanol. The results were documented as milligram gallic acid equivalents (GAE) per gram dry weight of samples.
2.3.2. Determination of Flavonoid Contents
The TFC were determined by using the AlCl3 colorimetric method of Muhammad, et al. [12] with modifications. In this, 80 μL of the sample extract were mixed with 80 μL 2% aluminum chloride solution and 120 μL sodium acetate aqueous solution (50 g/L) in 96-well plates. The reaction mixture was placed in the dark at 25 °C for 2.5 h and absorbance was recorded at 440 nm. Measurement of all samples were made in triplicate and TFC were quantified by constructing a standard curve against 0–50 μg/mL quercetin in methanol. The results were expressed as milligram quercetin equivalents (QE) per gram dry weight of the samples (r2 = 0.999).
2.3.3. Determinations of Total Tannins
The TTC in spices were calculated by using the method of Feng, Dunshea and Suleria [8] with some modifications by using the 96-well plate method. To do this, 25 µL of sample solution was mixed with 150 µL of 4% vanillin solution in a 96-well plate. A total of 25 µL of 32% sulfuric acid was added to the mixture and allowed to incubate at 25 °C for 15 min and absorbance was recorded at 500 nm. Measurements for all samples was made in triplicate and quantification was done by constructing a standard curve with 0–1000 µg/mL catechin solution in methanol. The results were expressed as mg catechin equivalents (CE) per gram dry weight of the samples.
2.3.4. DPPH Assay
The DPPH free radical scavenging potential of all samples was estimated by using the method of Sokamte, et al. [13] with modifications. To do this, 25 µL sample extract and 275 µL 0.1 M solution of DPPH in methanol were mixed in 96-well plate method. The reaction mixture was placed in the dark for 30 min at room temperature and absorbance was recorded at 517 nm. Anti-radical capacity of all the samples was estimated by constructing the standard curve against 0–50 µg/mL ascorbic acid in water. The results were expressed as milligram ascorbic acid equivalents per gram dry weight of the samples (mg AAE/g).
2.3.5. FRAP Assay
The ferric reducing activity of all the samples was estimated by using the method of Chen, et al. [14] with some modifications. The FRAP reagent was prepared by mixing 300 mM sodium acetate buffer, 10 mM TPTZ and 20 mM ferric chloride in the ratio of 10:1:1 (v/v/v). To add this, 20 µL sample extract were mixed with 280 µL FRAP reagent in 96-well plate. The reaction mixture was placed at 37 °C for 10 min and the absorbance was measured at 593 nm. The quantification of all the results was made by constructing the standard curve again 0–50 µg/mL ascorbic acid in water. The results were expressed as mg AAE/g.
2.3.6. ABTS Radical Scavenging Assay
The ABTS radical scavenging activity of all the samples was measured by using the method of Severo, et al. [15] with some modifications. To do this, 7 mM ABTS solution was mixed with 140 mM potassium persulfate solution. The reaction mixture was allowed to incubate in the dark for 16 h to generate an ABTS+ solution. The ABTS+ solution was diluted with ethanol to make its absorbance to 0.70 ± 0.02 at 734 nm. After this, 10 µL of sample extract was mixed with 290 µL of ABTS+ solution in 96-well plate and allowed to incubate at 25 °C for 6 min and the absorbance was recorded at 734 nm. The quantification was completed by constructing the standard curve against 0–150 µg/mL of ascorbic acid in water. The results were expressed as mg AAE/g.
2.3.7. Reducing Power Assay (RPA)
The reducing power activity was determined by modifying the method of Ferreira, et al. [16]. A total of 10 μL extract, 25 μL of 0.2 M phosphate buffer (pH 6.6), and 25 μL of K3[Fe(CN)6] were added, sequentially followed by incubation at 25 °C for 20 min. Then, 25 μL of 10% TCA solution was added to stop the reaction followed by the addition of 85 μL of water and 8.5 μL of FeCl3 and incubated for further 15 min at 25 °C. Next, the absorbance was measured at a wavelength of 750 nm. Ascorbic acid from 0 to 300 μg/mL was used to obtain a standard curve and data was presented in mg AAE/g.
2.3.8. Hydroxyl Radical Scavenging Activity (•OH-RSA)
The Fenton-type reaction method of Smirnoff and Cumbes [17] was used to determine •OH-RSA with some modifications. A 50 μL extract was mixed with 50 μL of 6 mM FeSO4·7H2O and 50 μL of 6 mM H2O2 (30%), followed by incubation at 25 °C for 10 min. After incubation, 50 μL of 6 mM 3-hydrooxybenzoic acid were added and absorbance was measured at a wavelength of 510 nm. Ascorbic acid from 0 to 300 μg/mL was used to obtain a standard curve and data was presented in mg AAE/g.
2.3.9. Ferrous Ion Chelating Activity (FICA)
The Fe2+ chelating activity of the samples were measured, according to Dinis, et al. [18] with modifications. A total of 15 μL extract was mixed with 85 μL of water, 50 μL of 2 mM ferrous chloride (with additional 1:15 dilution in water) and 50 μL of 5 mM ferrozine (with additional 1:6 dilution in water), followed by incubation at 25 °C for 10 min. Then the absorbance was measured at a wavelength of 562 nm. EDTA from concentrations of 0 to 50 μg/mL was used to obtain a standard curve and data was presented as mg EDTA/g.
2.3.10. Phosphomolybdate Assay (PMA)
The PMA of all the spices was measured by using the method of Nićiforović, et al. [19] with modifications. For the PMA, 40 μL of each spice extract was added to 260 μL of phosphomolybdate reagent (0.6 M H2SO4, 0.028 M sodium phosphate and 0.004 M ammonium molybdate). The mixture was incubated at 95 °C for 90 min, cooled at room temperature and absorbance was measured at 695 nm. A standard curve was generated using concentrations of 0–200 μg/mL ascorbic acid and the results were expressed as mg AAE/g.
2.4. LC-ESI-QTOF-MS2 Characterization of Phenolic Compounds
The identification and characterization of polyphenols from sample extracts was completed by using the method of Hong, et al. [20] and Zhong, et al. [21] with modifications. To do this, the phenolic compound characterization was performed on an Agilent 1200 HPLC with an Agilent 6520 Accurate Mass Q-TOF LC-MS2 (Agilent Technologies, Santa Clara, CA, USA). The separation was conducted using a Synergi Hydro-RP 80 Å, reverse phase column (250 mm × 4.6 mm, 4 μm particle size) with protected C18 ODS (4.0 × 2.0 mm) guard column (Phenomenex, Lane Cove, NSW, Australia). In brief, the mobile phase consisted of water/formic acid (99.9:0.1, v/v; eluent A) and acetonitrile/water/formic acid (95:5:0.1, eluent B). The gradient profile was as follows: 0–10% B (0–5 min), 10–25% B (5–25 min), 25–35% B (25–35 min), 35–40% B (35–45 min), 40–55% B (45–75 min), 55–80% B (75–80 min), 80–90% B (80–82 min), 90–100% B (82–85 min), isocratic 0% B (85–90 min). A 6 µL aliquot of each spice extract was injected and the flow rate was set at 0.8 mL/min. Peaks were identified in both positive and negative ion modes with the capillary and nozzle voltage set to 3.5 kV and 500 V, respectively. Additionally, following conditions were maintained; (i) nitrogen gas temperature at 325 °C, (ii) sheath gas flow rate of 5 L/min at 325 °C, (iii) nitrogen gas nebulization at 30 psi. A complete mass scan ranging from m/z 50 to 900 was used, MS/MS analyses was carried out in automatic mode with collision energy (10, 15, and 30 eV) for fragmentation. Peak identification was performed in both positive and negative modes, while the instrument control, data acquisition, and processing were performed using LC-ESI-QTOF-MS2 MassHunter workstation software (Qualitative Analysis, 152 version B.06.01, Agilent Technologies, Santa Clara, CA, USA).
2.5. HPLC-PDA Analysis
The quantification of polyphenols from spices were executed by following the method of Tang, et al. [22] and Gu, et al. [23] with some modifications. Water Alliance (2690) HPLC equipped with photo array detector (PDA) was used for this purpose. The same column was used as for LC/MS. In brief, the mobile phase of water/acetic acid (98:2, v/v; eluent A) and acetonitrile/water/acetic acid (50:49.5:0.5, eluent B). Sample and column temperature were unchecked (room temperature). The gradient profile was as follows: 10% B (0 min), 25% B (20 min), 35% B (30 min), 40% B (40 min), 55% B (70 min), 80% B (75 min), 100% B (77 min), 100% B (79 min), 10% B (82–85 min). A 20 µL aliquot of each spice extract was injected and the flow rate was set at 0.8 mL/min. Standard calibration curves were used for identification and quantification of the 20 compounds in triplicate.
2.6. Statistical Analysis
The data of the phenolic contents and the antioxidant assays were represented as the means ± standard deviation and one-way analysis of variance (ANOVA) was used to test for differences in mean values between different samples, followed by Tukey’s honestly significant differences (HSD) multiple rank test at p < 0.05. ANOVA was performed by Minitab Program for Windows version 18.0 (Minitab, LLC, State College, PA, USA). For correlation between polyphenols and antioxidant activities, XLSTAT-2019.1.3 (Addinsoft Inc. New York, NY, USA) was used.
3. Results and Discussion
The screening, characterization, and verification of polyphenols from most widely used spices were tentatively achieved with the help of LC-ESI-QTOF-MS2. A significant correlation between phenolic compounds and their antioxidant potential was achieved.
3.1. Polyphenols Estimation of Spices
The estimation of polyphenols in different spices was achieved through TPC, TFC, and TTC. Nowadays, spices gaining more interest due to their potent polyphenols including phenolic acids, flavonoids, and tannins with significant antioxidant potential. In Table 1, the polyphenols estimation and antioxidant potential of different spice extracts are summarized.
Table 1.
Polyphenol contents and antioxidant activity of twelve spices.
| Spices | TPC (mg GAE/g) |
TFC (mg QE/g) |
TTC (mg CE/g) |
DPPH (mg AAE/g) |
FRAP (mg AAE/g) |
ABTS (mg AAE/g) |
RPA (mg AAE/g) |
FICA mg EDTA/g |
OH-RSA (mg AAE/g) |
PMA (mg AAE/g) |
|---|---|---|---|---|---|---|---|---|---|---|
| Allspice | 40.49 ± 1.92 b | 0.52 ± 0.08 f | 14.96 ± 0.14 b | 14.35 ± 0.15 a | 3.64 ± 0.33 b | 37.05 ± 2.32 b | 28.44 ± 3.57 b | 1.86 ± 0.12 c | 52.17 ± 3.46 a | 8.08 ± 0.52 d |
| Black Cardamom | 5.54 ± 0.19 g | 0.15 ± 0.02 g | 3.86 ± 0.08 cd | 4.03 ± 0.17 cd | 1.81 ± 1.60 c | 6.41 ± 0.39 e | 8.61 ± 3.16 de | 2.44 ± 0.08 b | 43.25 ± 0.42 b | 5.72 ± 0.16 e |
| Black Cumin | 4.02 ± 0.23 g | 1.73 ± 0.25 e | 3.08 ± 0.26 cd | 3.47 ± 0.04 d | 1.50 ± 0.98 c | 3.94 ± 0.32 f | 4.22 ± 0.13 f | 1.47 ± 0.16 c | 17.46 ± 0.76 e | 15.61 ± 1.05 a |
| Black Pepper | 8.06 ± 0.08 f | 0.57 ± 0.16 e | 3.04 ± 0.31 cd | 3.61 ± 0.14 d | 0.10 ± 0.09 d | 7.61 ± 0.36 e | 10.05 ± 0.88 d | 2.70 ± 0.16 b | 19.66 ± 0.31 e | 15.43 ± 0.39 a |
| Cardamom | 3.30 ± 0.32 gh | 0.13 ± 0.04 g | 1.73 ± 0.05 d | 2.91 ± 0.06 de | 0.15 ± 0.02 d | 8.50 ± 0.35 e | 8.00 ± 1.25 de | 2.26 ± 0.07 b | 46.65 ± 1.10 b | 6.48 ± 0.16 e |
| Cinnamon | 34.53 ± 2.29 c | 0.52 ± 0.23 f | 12.77 ± 2.25 b | 10.99 ± 0.14 b | 1.06 ± 0.49 c | 28.75 ± 0.86 c | 27.22 ± 3.25 b | 1.32 ± 0.05 c | 17.25 ± 1.36 e | 12.06 ± 0.83 b |
| Clove | 215.14 ± 7.33 a | 5.59 ± 0.33 a | 4.96 ± 0.13 c | 14.16 ± 0.04 a | 6.52 ± 0.13 a | 111.94 ± 1.16 a | 37.20 ± 3.85 a | 3.37 ± 0.08 a | 31.87 ± 2.85 c | 8.45 ± 2.76 d |
| Cumin | 10.76 ± 0.15 e | 3.62 ± 0.12 c | 2.04 ± 0.12 d | 5.62 ± 0.83 cd | 0.21 ± 0.08 d | 9.34 ± 0.20 e | 16.98 ± 1.34 c | 1.13 ± 0.10 c | 9.62 ± 0.46 f | 9.66 ± 0.48 cd |
| Fennel | 8.31 ± 0.03 f | 0.19 ± 1.8 g | 1.68 ± 0.09 d | 5.15 ± 0.47 cd | 0.53 ± 0.49 d | 7.11 ± 0.36 e | 9.52 ± 3.42 d | 1.17 ± 0.18 c | 47.25 ± 0.95 b | 8.16 ± 0.39 d |
| Nutmeg | 14.85 ± 0.41 e | 2.22 ± 0.21 d | 4.33 ± 0.15 c | 6.92 ± 0.36 c | 0.81 ± 0.30 d | 14.15 ± 0.52 d | 7.35 ± 2.67 e | 1.05 ± 0.02 cd | 23.89 ± 0.17 d | 10.82 ± 1.49 c |
| Star Anise | 23.87 ± 0.58 d | 0.95 ± 0.20 f | 13.84 ± 0.51 b | 8.78 ± 0.16 bc | 1.16 ± 0.31 c | 27.89 ± 1.04 c | 18.78 ± 4.73 c | 0.63 ± 0.15 d | 12.89 ± 0.92 ef | 9.66 ± 0.27 cd |
| Turmeric | 23.81 ± 0.70 d | 4.30 ± 0.26 b | 23.58 ± 0.87 a | 7.46 ± 0.09 c | 1.15 ± 0.34 c | 27.80 ± 1.19 c | 9.48 ± 1.19 d | 2.16 ± 0.08 b | 47.18 ± 0.35 b | 11.06 ± 0.73 bc |
Values are mean ± standard deviation per gram powder weight; n = 3 samples per sample. Values within the same column with different superscript letters (a–h) are significantly different from each other (p < 0.05). TPC (total phenolic contents); TFC (total flavonoid contents); TTC (total tannin contents); DPPH (2,2′-diphenyl-1-picrylhydrazyl assay); FRAP (ferric reducing antioxidant power assay); ABTS (2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid assay); PMA (phosphomolybdate assay); GAE (gallic acid equivalents); QE (quercetin equivalents); CE (catechin equivalents); AAE (ascorbic acid equivalents); RPA (reducing power assay); FICA (ferrous ion chelating activity); •OH-RSA (hydroxyl-radical scavenging activity); EDTA (ethylenediaminetetraacetic acid).
Highest phenolic contents were found in clove, allspice, and cinnamon which contained 215.14, 40.49, and 34.53 mg GAE/g, respectively, while the least value of TPC was reported in cardamom (3.30 mg GAE/g). Previously, Shan, et al. [24] reported the same trend with highest TPC value of clove (14.38 g GAE/100 g), while overall our values of TPC were higher in spices which they listed in their study. The higher value of TPC for cinnamon and cumin (34.53 mg GAE/g, 10.78 mg GAE/g) as compared to the previous reported value (5.82 mg GAE/g, 4.98 mg GAE/g) [7] indicates that 70% ethanol with 0.1% formic acid enabled better extraction in comparison to 50% ethanol with 0.1% formic acid. This might have been also due to different species or cultivars used for extraction and quantification between the current and latter study. In another study, Słowianek and Leszczyńska [25] reported the highest TPC values for clove and cinnamon as compared to other spices. A wide range of TPC values provided in this study reflects the diversity of phenolic compounds in spices and their capacity to reduce the Folin-Ciocalteu Reagent. The variations in TPC can be attributed to extraction conditions (solvent type, concentrations, solvent to sample ratio and time and temperature combinations for extraction) [26], cultivar differences, and the geographical location of the spices where they were grown.
On the other hand, highest value of TFC was found in clove (5.59 mg QE/g) and turmeric (4.30 mg QE/g) while the lowest values were found in cardamom (0.13 mg QE/g) and fennel (0.19 QE/g), respectively. In previous studies, TFC value for turmeric was reported in the range of 0.84–4.34 mg QE/g [27,28]. Overall, spices contain flavonoids in very much lower quantities than other phenolic compounds. Previously, the lowest flavonoid concentrations were reported in cardamom, fennel, and star anise [29]. Total flavonoids in cinnamon had been reported in the range of 2.03–3.3 g QE/100 g DW [30]. In contrast, TTC value of turmeric (23.58 mg QE/g) was significantly higher as compared to other listed spices, while fennel had the lowest TTC value (1.68 mg QE/g).
Phenolic compounds have gained attention due to their potent antioxidant potential and being an index for nutritional assessment of food components. Thus, the characterization of spices with advanced analytical techniques like LC-ESI-QTOF-MS2 can deliver more authentic, reliable, and valuable information for their applications in the pharmaceutical, cosmetics, food, and feed industries.
3.2. Antioxidant Potential of Spices
Polyphenols are the vital antioxidant components in plants that have different potential biological activities. They are considered as multifunctional compounds and acts as reducing agents, radical oxygen scavengers, hydrogen atom donators, and metal chelators in the biological systems. The antioxidant potential of spices was further investigated based on different mechanisms, including the radical scavenging ability and the sample’s reducing power. For this purpose, DPPH, ABTS and FRAP, RPA, •OH-RSA, FICA, and PMA assays were conducted, and the results reported in Table 1.
The DPPH, •OH-RSA, FICA, and ABTS assays mainly have been used for measuring the free radical scavenging activity of the bioactive compounds mainly related to polyphenols [31], while the FRAP assay evaluates the ability of samples to donate electrons to reduce a Fe+3-TPTZ complex to a blue Fe+2-TPTZ complex.
DPPH has the ability to donate hydrogen ion or scavenge free radicals in the biological system. When DPPH solution is mixed with a spice extract, it denotes hydrogen atom and ultimately reduce the violet color. DPPH, FRAP, and ABTS values (14.16 mg AAE/g, 6.52 mg AAE/g, and 111.94 mg AAE/g) of clove were significantly higher as compared to other listed spices, while cardamom, black pepper, and black cumin had the least values of DPPH, FRAP, and ABTS (2.91 mg AAE/g, 0.10 mg AAE/g and 3.94 mg AAE/g), respectively. Previously, Wojdyło, et al. [32], Vallverdú-Queralt, Regueiro, Martínez-Huélamo, Alvarenga, Leal and Lamuela-Raventos [7], Patra, et al. [33], Shan, Cai, Sun and Corke [24], Dvorackova, Snoblova, Chromcova and Hrdlicka [26], Przygodzka, Zielińska, Ciesarová, Kukurová and Zieliński [29], and Muhammad, et al. [34] researched different spices for their phenolic compounds and antioxidant potential. The higher value of DPPH, FRAP, and ABTS for clove could be attributed towards the higher contents of total phenolics and flavonoids, which are potential antioxidants agents. Cinnamon and clove were reported with the highest antioxidant activity in many studies compared to other spices [20,21,24,25,31].
Reactive oxygen species (ROS) cause damage to cellular biomolecules, such as carbohydrates, proteins, lipids, and nucleic acids. Antioxidants in spices play a crucial role for the inactivation of ROS by inhibiting their generation in the biological system [35]. Different assays were conducted to estimate the radical scavenging and reducing power of spices. RPA (37.20 mg AAE/g) and FICA (3.37 mg EDTA/g) values of clove were significantly higher compared to other spices. The chelating ability mainly depends on the type of functional group used for iron chelation. •OH-RSA assay was also conducted to estimate the scavenging ability of spices. Highest value of •OH-RSA was found in allspice (52.17 mg AAE/g), while the least value was found in cumin (9.62 mg AAE/g). On the other hand, in the PMA assay the highest value was recorded for black cumin and black pepper (15. 61 mg AAE/g and 15.43 mg AAE/g), while cardamom and black cardamom had the least values (6.48 mg AAE/g and 5.72 mg AAE/g), respectively. Previously, Yadav and Bhatnagar [36] also found the higher value of ferrous ion chelating activity for clove compared to other studied spices. Overall, the higher antioxidant potential of clove could be attributed towards higher concentration of total polyphenols and flavonoids compared to other selected spices. Previously, different studies were conducted for the estimation of free radical scavenging abilities of spices [13,24,37,38,39,40]. In the complex biological systems, different mechanisms contribute to the oxidative reactions through which different ROS may be produced which may affect the cellular biomolecules. The results from conducted assays showed that spice extracts have different scavenging capacities depending upon their ability to scavenge reactive species in the biological systems.
Interestingly, antioxidant activities vary in spices depending upon the method used for extraction. The spice extracts are a complex mixture of bioactive compounds. The phenolic acids and flavonoid content in each spice mainly depend on cultivar, geographic locations, and climate conditions. There is a list of methods to determine the antioxidant potential and every technique has its benefits and limitations. Conclusively, our results depict that each spice’s antioxidant activity has its own tendency depending upon the phenolic profile or method used to quantify it. To estimate targeted antioxidant potential of spices, a wide range of in vitro assays can be applied, while the identification and confirmations of these antioxidant compounds can be attained by using the advanced analytical techniques including LC-ESI-QTOF-MS2.
Phenolic compounds are a diverse group of compounds gaining popularity in the field of research for their health-beneficial potential. The potential antioxidant mechanisms of polyphenols can make them a target to extend lipid rich foods′ shelf life. Moreover, spices contain a wide range of antimicrobial phenolic constituents, which have food preserving properties during storage. Additionally, phytochemicals have complex natures, thus, there is no single method that reflects the same antioxidant potential of polyphenols due to multiple mechanisms and reactions in the biological system. Therefore, LC-MS characterization is one of the advanced research tools that execute the polyphenols profiling and help to understand the total antioxidant potential of spices. The polyphenol constituents in spices and their antioxidant capacity demonstrated that further research should be conducted to identify the actual contribution of phenolic compounds while eliminating or minimizing the contribution of non-phenolic compounds towards antioxidant potential.
3.3. LC-ESI-QTOF-MS2 Characterization of the Phenolic Compounds
Herbs and Spices have a significant interest due to their bioactive constituents especially phenolic compounds that may exert potential effects on human health. LC-ESI-QTOF-MS2 is an advanced analytical technique used to characterize and identify the bioactive compounds from different fruits, vegetables, and medicinal plants, including herbs and spices. An untargeted qualitative analysis of 12 spices was achieved through liquid chromatography in negative ([M–H]−) and positive ([M+H]+) modes of ionization coupled with QTOF-MS2 (Supplementary data—Figures S1 and S2).
The tentative identification of phenolic compounds was executed through Personal Compound Database Library (PCDL) via Agilent MassHunter Qualitative Analysis B.06.00 Software (Agilent Technologies, Santa Clara, CA, USA). The compounds with PCDL library score more than 80 and mass error less than 5 ppm were stipulated for further MS2 characterization, identification, and verification based on mass to charge ratio (m/z). In the present scrutiny of the spices, LC-ESI-MS2 permitted the tentative characterization and identification of 79 phenolic compounds including 26 phenolic acids, 33 flavonoids, 4 lignans, and 16 other polyphenols (Table 2).
Table 2.
Characterization of phenolic compounds from twelve spices through LC-ESI-QTOF-MS2.
| No. | Proposed Compounds |
Molecular Formula |
RT (min) |
Ionization ESI (+/−) |
Molecular Weight | Theoretical (m/z) | Observed (m/z) |
Error (ppm) | MS2 Product Ions |
Spices |
|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic acids | ||||||||||
| Hydroxybenzoic acids | ||||||||||
| 1 | Gallic acid 4-O-glucoside | C13H16O10 | 6.117 | [M-H]− | 332.0743 | 331.0670 | 331.0672 | 0.6 | 169, 125 | Cl |
| 2 | Gallic acid | C7H6O5 | 11.187 | [M-H]− | 170.0215 | 169.0142 | 169.0140 | −1.2 | 125 | * Ci, SA |
| 3 | 4-Hydroxybenzoic acid 4-O-glucoside | C13H16O8 | 11.212 | ** [M-H]− | 300.0845 | 299.0772 | 299.0784 | 4 | 255, 137 | * SA, Cu, Ci |
| 4 | Protocatechuic acid 4-O-glucoside | C13H16O9 | 12.462 | [M-H]− | 316.0794 | 315.0721 | 315.0724 | 1.0 | 153 | * Ci, Cu, Tu, SA |
| 5 | 2,3-Dihydroxybenzoic acid | C7H6O4 | 15.201 | [M-H]− | 154.0266 | 153.0193 | 153.0197 | 2.6 | 109 | * Ci, Nu, AS, Fe, BCu, Bca |
| 6 | 2-Hydroxybenzoic acid | C7H6O3 | 18.754 | ** [M-H]− | 138.0317 | 137.0244 | 137.0247 | 2.2 | 93 | * Ci, Tu, Cu, BCu, Fe, SA, AS, BP, BCa, Nu, |
| 7 | Ellagic acid | C14H6O8 | 30.403 | [M-H]− | 302.0063 | 300.9990 | 300.9993 | 1.0 | 284, 229, 201 | * Cl, Fe, AS, BCu |
| 8 | Paeoniflorin | C23H28O11 | 37.249 | [M-H]− | 480.1632 | 479.1559 | 479.1551 | −1.7 | 449, 357, 327 | Cl |
| Hydroxycinnamic acids | ||||||||||
| 9 | Cinnamic acid | C9H8O2 | 9.107 | [M-H]− | 148.0524 | 147.0451 | 147.0455 | 2.7 | 103 | SA |
| 10 | 3-Caffeoylquinic acid | C16H18O9 | 14.784 | ** [M-H]− | 354.0951 | 353.0878 | 353.0867 | −3.1 | 253, 190, 144 | * Cu, BCa, BCu, AS, Fe |
| 11 | Caffeic acid | C9H8O4 | 15.526 | [M-H]− | 180.0423 | 179.0350 | 179.0350 | 0.0 | 143, 133 | * Cu, BCa |
| 12 | Caffeoyl glucose | C15H18O9 | 15.526 | [M-H]− | 342.0951 | 341.0878 | 341.0866 | −3.5 | 179, 161 | * Cu, SA |
| 13 | 1-Sinapoyl−2-feruloylgentiobiose | C33H40O18 | 17.508 | [M-H]− | 724.2215 | 723.2142 | 723.2137 | −0.7 | 529, 499 | Cu |
| 14 | p-Coumaric acid 4-O-glucoside | C15H18O8 | 18.297 | [M-H]− | 326.1002 | 325.0929 | 325.0935 | 1.8 | 163 | * Cu, BCa |
| 15 | Ferulic acid 4-O-glucuronide | C16H18O10 | 20.092 | [M-H]− | 370.0900 | 369.0827 | 369.0809 | −4.9 | 193 | Cu |
| 16 | 3-p-Coumaroylquinic acid | C16H18O8 | 23.499 | [M-H]− | 338.1002 | 337.0929 | 337.0939 | 3.0 | 265, 173, 162 | Cl |
| 17 | p-Coumaroyl tartaric acid | C13H12O8 | 24.022 | ** [M-H]− | 296.0532 | 295.0459 | 295.0458 | −0.3 | 115 | * BCa, AS, BCu, Fe |
| 18 | Ferulic acid | C10H10O4 | 24.277 | ** [M-H]− | 194.0579 | 193.0506 | 193.0506 | 0.0 | 178, 149, 134 | Cu *, BCa, Ci, Fe, AS, BCu |
| 19 | m-Coumaric acid | C9H8O3 | 34.468 | ** [M-H]− | 164.0473 | 163.0400 | 163.0397 | −1.8 | 119 | * Ci, Nu, Ca, BCa, Tu, Cu |
| 20 | Cinnamoyl glucose | C15H18O7 | 39.905 | ** [M-H]− | 310.1053 | 309.0980 | 309.0991 | 3.6 | 147, 131, 103 | * Fe, BCu, AS, Ci, SA |
| 21 | 1,5-Dicaffeoylquinic acid | C25H24O12 | 41.696 | [M-H]− | 516.1268 | 515.1195 | 515.1210 | 2.9 | 353, 335, 191, 179 | Cu |
| 22 | 3-Feruloylquinic acid | C17H20O9 | 73.409 | [M-H]− | 368.1107 | 367.1034 | 367.1051 | 4.6 | 298, 288, 192, 191 | Tu |
| Hydroxyphenyl acetic acids | ||||||||||
| 23 | 2-Hydroxy-2-phenylacetic acid | C8H8O3 | 12.269 | ** [M-H]− | 152.0473 | 151.0400 | 151.0397 | −2.0 | 136, 92 | * Ci, Fe, AS, BCu, BCa |
| 24 | 3,4-Dihydroxyphenylacetic acid | C8H8O4 | 12.767 | ** [M-H]− | 168.0423 | 167.0350 | 167.0343 | −4.2 | 149, 123 | * Ci, Ca, BCa |
| Hydroxyphenylpropanoic acids | ||||||||||
| 25 | Dihydroferulic acid 4-O-glucuronide | C16H20O10 | 11.157 | [M-H]− | 372.1056 | 371.0983 | 371.0967 | −4.3 | 195 | * Cu, BCu, AS, Fe |
| 26 | 3-Hydroxy-3-(3-hydroxyphenyl)propionic acid | C9H10O4 | 15.891 | [M-H]− | 182.0579 | 181.0506 | 181.0509 | 1.7 | 163, 135, 119 | Cu |
| Flavonoids | ||||||||||
| Flavanols | ||||||||||
| 27 | (+)-Catechin 3-O-gallate | C22H18O10 | 5.723 | [M-H]− | 442.0900 | 441.0827 | 441.0848 | 4.8 | 289, 169, 125 | Cu |
| 28 | (+)-Gallocatechin | C15H14O7 | 17.148 | [M-H]− | 306.0740 | 305.0667 | 305.0674 | 2.3 | 261, 219 | Ca |
| 29 | (+)-Catechin | C15H14O6 | 22.355 | ** [M-H]− | 290.0790 | 289.0717 | 289.0718 | 0.3 | 245, 205, 179 | * Ci, Nu, SA |
| 30 | 4′-O-Methyl-(-)-epigallocatechin 7-O-glucuronide | C22H24O13 | 23.154 | [M-H]− | 496.1217 | 495.1144 | 495.1138 | −1.2 | 451, 313 | Cl |
| 31 | Procyanidin trimer C1 | C45H38O18 | 24.969 | ** [M-H]− | 866.2058 | 865.1985 | 865.1972 | −1.5 | 739, 713, 695 | * Ci, Nu |
| 32 | Procyanidin dimer B1 | C30H26O12 | 28.514 | [M-H]− | 578.1424 | 577.1351 | 577.1350 | −0.2 | 451 | * Ci, Nu |
| Flavones | ||||||||||
| 33 | Apigenin 6,8-di-C-glucoside | C27H30O15 | 21.450 | ** [M-H]− | 594.1585 | 593.1512 | 593.1502 | −1.7 | 503, 473 | Ci, SA, BP, Cu |
| 34 | 6-Hydroxyluteolin 7-O-rhamnoside | C21H20O11 | 24.912 | ** [M-H]− | 448.1006 | 447.0933 | 447.0931 | −0.4 | 301 | * Ci, Cl, Fe, AS, BP, BCu |
| 35 | Rhoifolin | C27H30O14 | 26.866 | [M-H]− | 578.1636 | 577.1563 | 577.1567 | 0.7 | 413, 269 | BP |
| 36 | Apigenin 6-C-glucoside | C21H20O10 | 27.726 | ** [M-H]− | 432.1056 | 431.0983 | 431.0976 | −1.6 | 413, 341, 311 | * Ci, Cu, SA |
| 37 | Apigenin 7-O-(6′′-malonyl-apiosyl-glucoside) | C29H30O17 | 36.541 | [M-H]− | 650.1483 | 649.1410 | 649.1440 | 4.6 | 605 | Cu |
| 38 | Apigenin 7-O-glucuronide | C21H18O11 | 66.907 | [M-H]− | 446.0849 | 445.0776 | 445.0761 | −3.4 | 269, 175 | Tu |
| Flavanones | ||||||||||
| 39 | Hesperetin 3′-O-glucuronide | C22H22O12 | 29.866 | [M-H]− | 478.1111 | 477.1038 | 477.1022 | −3.4 | 301, 175, 113, 85 | * Ca, Cl, SA |
| Flavonols | ||||||||||
| 40 | 3′-O-Methyl-(-)-epicatechin 7-O-glucuronide | C22H24 O12 | 21.751 | [M-H]− | 480.1268 | 479.1195 | 479.1179 | −3.3 | 149, 121 | Clove |
| 41 | Patuletin 3-O-glucosyl-(1->6)-[apiosyl(1->2)]-glucoside | C33H40O22 | 22.623 | [M-H]− | 788.2011 | 787.1938 | 787.1932 | −0.8 | 625, 463, 301, 271 | * Fe, AS, BCu |
| 42 | Kaempferol 3-O-glucosyl-rhamnosyl-galactoside | C33H40O20 | 24.301 | [M-H]− | 756.2113 | 755.2040 | 755.2031 | −1.2 | 285 | Ci |
| 43 | Myricetin 3-O-rutinoside | C27H30O17 | 25.374 | [M-H]− | 626.1483 | 625.1410 | 625.1427 | 2.7 | 301 | * Cu, Ca |
| 44 | Kaempferol 3,7-O-diglucoside | C27H30O16 | 26.515 | ** [M-H]− | 610.1534 | 609.1461 | 609.1466 | 0.8 | 447, 285 | * Ci, SA |
| 45 | Myricetin 3-O-rhamnoside | C21H20O12 | 29.644 | [M-H]− | 464.0955 | 463.0882 | 463.0892 | 2.2 | 317 | * Ci, SA |
| 46 | Quercetin 3-O-arabinoside | C20H18O11 | 31.369 | [M-H]− | 434.0849 | 433.0776 | 433.0783 | 1.6 | 301 | SA |
| 47 | Isorhamnetin | C16H12O7 | 51.072 | ** [M-H]− | 316.0583 | 315.0510 | 315.0516 | 1.9 | 300, 271 | * SA, Ci, Cl |
| Dihydroflavonols | ||||||||||
| 48 | Dihydroquercetin 3-O-rhamnoside | C21H22O11 | 29.998 | [M-H]− | 450.1162 | 449.1089 | 449.1080 | −2.0 | 303 | Cu |
| Isoflavonoids | ||||||||||
| 49 | 2′-Hydroxyformononetin | C16H12O5 | 10.792 | [M+H]+ | 284.0685 | 285.0758 | 285.0766 | 2.8 | 270, 229 | * Fe, AS, BCu |
| 50 | 3′-Hydroxygenistein | C15H10O6 | 23.177 | ** [M+H]+ | 286.0477 | 287.0550 | 287.0563 | 4.5 | 269, 259 | * Fe, Cu, AS, BCu, SA, Cl |
| 51 | 5,6,7,3′,4′-Pentahydroxyisoflavone | C15H10O7 | 33.232 | ** [M+H]+ | 302.0427 | 303.0500 | 303.0498 | −0.7 | 285, 257 | * Ci, SA, BCu, AS, Fe |
| 52 | 3′-Hydroxydaidzein | C15H10O5 | 33.061 | [M-H]− | 270.0528 | 269.0455 | 269.0459 | 1.5 | 241, 225, 213, 181 | * Cu, Nu |
| 53 | 4′-Methoxy-2′,3,7-trihydroxyisoflavanone | C16H14O6 | 36.725 | [M-H]− | 302.0790 | 301.0717 | 301.0711 | −2.0 | 283 | Cl |
| 54 | 3′,4′,7-Trihydroxyisoflavanone | C15H12O5 | 47.982 | [M-H]− | 272.0685 | 271.0612 | 271.0611 | −0.4 | 177, 151, 119, 107 | Cl |
| 55 | 3′-Hydroxymelanettin | C16H12O6 | 50.696 | [M-H]− | 300.0634 | 299.0561 | 299.0553 | −2.7 | 284 | Cu, Cl |
| 56 | Violanone | C17H16O6 | 58.320 | [M-H]− | 316.0947 | 315.0874 | 315.0866 | −2.5 | 300, 285, 135 | Cl |
| 57 | 2-Dehydro-O-desmethylangolensin | C15H12O4 | 65.406 | [M-H]− | 256.0736 | 255.0663 | 255.0672 | 3.5 | 135, 119 | Cl |
| 58 | 6′′-O-Malonyldaidzin | C24H22O12 | 74.313 | [M+H]+ | 502.1111 | 503.1184 | 503.1190 | 1.2 | 255 | Nu |
| 59 | Dihydrobiochanin A | C16H14O5 | 83.912 | [M+H]+ | 286.0841 | 287.0914 | 287.0904 | −3.5 | 269, 203, 175 | Nu |
| Other polyphenols | ||||||||||
| Hydroxycoumarins | ||||||||||
| 60 | Coumarin | C9H6O2 | 4.079 | [M+H]+ | 146.0368 | 147.0441 | 147.0438 | −2.0 | 103, 91 | Ci |
| 61 | Scopoletin | C10H8O4 | 8.681 | [M-H]− | 192.0423 | 191.0350 | 191.0350 | 0.0 | 176 | * Fe, AS, Cl, BP, BCu, Ca |
| 62 | Esculin | C15H16O9 | 16.338 | [M-H]− | 340.0794 | 339.0721 | 339.0713 | −2.4 | 177 | Cu |
| 63 | Esculetin | C9H6O4 | 21.656 | [M-H]− | 178.0266 | 177.0193 | 177.0197 | 2.3 | 149, 133, 89 | Cu |
| Hydroxybenzoketones | ||||||||||
| 64 | 2-Hydroxy-4-methoxyacetophenone 5-sulfate | C9H10O7S | 12.758 | [M-H]− | 262.0147 | 261.0074 | 261.0063 | −4.2 | 181, 97 | * Cu |
| 65 | 2,3-Dihydroxy-1-guaiacylpropanone | C10H12O5 | 14.893 | ** [M-H]− | 212.0685 | 211.0612 | 211.0607 | −2.4 | 167, 123, 105, 93 | * Cu, Ci |
| Curcuminoids | ||||||||||
| 66 | Curcumin | C21H20O6 | 41.696 | ** [M-H]− | 368.1260 | 367.1187 | 367.1178 | −2.5 | 217 | * Ci, Tu |
| 67 | Demethoxycurcumin | C20H18O5 | 42.828 | [M-H]− | 338.1154 | 337.1081 | 337.1080 | −0.3 | 217 | * Ci |
| Tyrosols | ||||||||||
| 68 | 3,4-DHPEA-AC | C10H12O4 | 10.480 | ** [M-H]− | 196.0736 | 195.0663 | 195.0663 | 0.0 | 135 | Ci |
| 69 | Hydroxytyrosol | C8H10O3 | 13.986 | [M-H]− | 154.0630 | 153.0557 | 153.0553 | −2.6 | 123 | BP |
| 70 | Demethyloleuropein | C24H30O13 | 16.589 | [M-H]− | 526.1686 | 525.1613 | 525.1631 | 3.4 | 495 | Cu |
| 71 | 3,4-DHPEA-EDA | C17H20O6 | 18.780 | [M-H]− | 320.1260 | 319.1187 | 319.1181 | −1.9 | 275, 195 | Ci |
| 72 | Hydroxytyrosol 4-O-glucoside | C14H20O8 | 18.913 | ** [M-H]− | 316.1158 | 315.1085 | 315.1073 | −3.8 | 153, 123 | Ci, BP |
| Phenolic terpenes | ||||||||||
| 73 | Rosmanol | C20H26O5 | 26.775 | [M+H]+ | 346.1780 | 347.1853 | 347.1847 | −1.7 | 301, 241, 231 | * Fe, AS, BCu |
| 74 | Carnosic acid | C20H28O4 | 86.739 | [M-H]− | 332.1988 | 331.1915 | 331.1911 | −1.2 | 287, 269 | * Ca, BCa |
| Other polyphenols | ||||||||||
| 75 | Salvianolic acid C | C26H20O10 | 41.017 | [M-H]− | 492.1056 | 491.0983 | 491.0971 | −2.4 | 311, 267, 249 | Cl |
| Lignans | ||||||||||
| 76 | Sesamin | C20H18O6 | 5.578 | ** [M-H]− | 354.1103 | 353.1030 | 353.102 | −2.8 | 338, 163 | * Ca, Nu, BP |
| 77 | Enterolactone | C18H18O4 | 24.192 | [M+H]+ | 298.1205 | 299.1278 | 299.1267 | −3.7 | 281, 187, 165 | * Ci, BCu, AS, Fe |
| 78 | Conidendrin | C20H20O6 | 32.552 | [M+H]+ | 356.1260 | 357.1333 | 357.1324 | −2.5 | 339, 221, 206 | Ci |
| 79 | Secoisolariciresinol-sesquilignan | C30H38O10 | 34.727 | [M-H]− | 558.2465 | 557.2392 | 557.2386 | −1.1 | 539, 521, 509, 361 | Ci |
* = compound detected more than one spice, data presented only with asterisk, ** = Compound found in both positive [M+H]+ and negative modes [M-H]−. RT stands for “retention time”. Spices were presented with abbreviations. Cinnamon (Ci), Clove (Cl), Allspice (AS), Nutmeg (Nu), Fennel (Fe), Star Anise (SA), Black Pepper (BP), Black Cardamom (BCa), Black Cumin (BCu), Cardamom (Ca), Turmeric (Tu), Cumin (Cu).
3.3.1. Phenolic Acids
Phenolic acids are the phenolic compounds, which were present in considerable amount in different spices. Phenolic acids are found ubiquitously in herbs and spices and widely reported for their potential prospective including antioxidant, anti-microbial, anti-inflammatory, antimutagenic, and anticancer, etc. [41]. In our study, a total of 26 phenolic acids were tentatively identified including 08 hydroxybenzoic acids, 14 hydroxycinnamic acids, 2 hydroxyphenylacetic acids, and 2 hydroxyphenylpropanoic acids (Table 2). Most of the phenolic acids exhibited the neutral loss of CO2 (44 Da) and hexosyl moiety (162 Da).
Hydroxybenzoic Acids
The compound 1 (m/z 331.0672) and compound 4 (m/z 315.0724) were identified as gallic acid 4-O-glucoside and protocatechuic acid 4-O-glucoside, which exhibited the product ions at m/z 169 and m/z 125, and m/z 153 through the loss of hexosyl moiety and CO2, while the compounds 2 (m/z 169.0140), 5 (m/z 153.0197), and 6 (m/z 137.0247) were identified as gallic acid, 4-hydroxybenzoic acid 4-O-glucoside, and 2-hydroxybenzoic acid which showed the product ions at m/z 125, m/z 109, and m/z 93 via the loss of CO2 (44 Da), respectively [10]. Previously, Yisimayili, et al. [42] had also reported the compound 1 in their studies. The compound 7 (m/z 300.9993) was identified as ellagic acid, which possess a wide range of biological activities like anti-radical, antimicrobial, anti-inflammatory, and anti-carcinogenic, and exhibited the product ions at m/z 284, m/z 229, and m/z 209 [43]. The compound 8 (m/z 479.1551) was identified as paeoniflorin in clove with product ions at m/z 449, m/z 357, and m/z 327 in the negative mode with the neutral loss of formaldehyde (CH2O-30 Da) from precursor ion and benzoic acid (C7H6O2-122 Da) from precursor and product ions, respectively [44].
Hydroxycinnamic Acids
Moreover, hydroxycinnamic acids collectively detected more in numbers than other phenolic acids in this quest. According to our study, a total of 14 hydroxycinnamic acids were identified with significant antioxidant potential. The compound 9 (m/z 147.0455) and compound 19 (m/z 163.0397) were detected as cinnamic acid and m-coumaric acid with the product ions at m/z 103 and m/z 119 with the neutral loss of CO2 (44 Da). Previously, coumaric acid was also reported in cinnamon and cumin (spices), along with rosemary, thyme, oregano, and bay (herbs) [7]. The compound 11 (m/z 179.0350—caffeic acid) produced product ions at m/z 143 (neutral loss—2H2O- 36 Da) and m/z 133 (neutral loss—HCOOH—46 Da) while compound 12 (Caffeoyl glucose) produced caffeic acid ion at m/z 179 with the loss of glucoside (162 Da). The compound 15 (Ferulic acid 4-O-glucuronide-C16H18O10) produced product ion at m/z 193 with the neutral loss of glucuronide moiety (176 Da). Ferulic acid (compound 18) was identified in six different spices including cinnamon, cumin, allspice, etc. Moreover, ferulic acid produced product acid, which possess a wide range of biological activities like anti-radical, antimicrobial, anti-inflammatory, and anti-carcinogenic, and exhibited the product ions at m/z 284, m/z 229, and m/z 209 [43]. The compound 8 (m/z 479.1551) was identified as paeoniflorin in clove with product ions at m/z 449, m/z 357, and m/z 327 in the negative mode with the neutral loss of formaldehyde (CH2O—30 Da) from precursor ion and benzoic acid (C7H6O2—122 Da) from precursor and product ions, respectively [44].
Hydroxycinnamic Acids
Moreover, hydroxycinnamic acids collectively detected more in numbers than other phenolic acids in this quest. According to our study, a total of 14 hydroxycinnamic acids were identified with significant antioxidant potential. The compound 9 (m/z 147.0455) and compound 19 (m/z 163.0397) were detected as cinnamic acid and m-coumaric acid with the product ions at m/z 103 and m/z 119 with the neutral loss of CO2 (44 Da). Previously, coumaric acid was also reported in cinnamon and cumin (spices), along with rosemary, thyme, oregano, and bay (herbs) [7].
The compound 11 (m/z 179.0350—caffeic acid) produced product ions at m/z 143 (neutral loss—2H2O—36 Da) and m/z 133 (neutral loss—HCOOH—46 Da), while compound 12 (Caffeoyl glucose) produced caffeic acid ion at m/z 179 with the loss of glucoside (162 Da). The compound 15 (Ferulic acid 4-O-glucuronide-C16H18O10) produced product ion at m/z 193 with the neutral loss of glucuronide moiety (176 Da). Ferulic acid (compound 18) was identified in six different spices including cinnamon, cumin, allspice, etc. Moreover, ferulic acid produced product ions at m/z 178, m/z 149, and m/z 134 with the neutral loss of CH3 (15 Da), CO2 (44 Da), and CH3 with CO2 (59 Da), respectively, from the precursor ion in negative mode. The compounds 21 (1,5-Dicaffeoylquinic acid) and compound 22 (3-Feruloylquinic acid) were identified in cumin and turmeric both in negative modes. The compound 21 produced fragment ions at m/z 353, m/z 335, m/z 191, and m/z 179 with the neutral loss of C9H6O3 (162 Da), C9H8O4 (180 Da), C18H12O6 (324 Da), and C16H16O8 (336 Da) from parent ion (m/z 515.1210), respectively [45,46].
Other Phenolic Acids
The compound 23 (m/z 151.0397) was characterized as 2-hydroxy-2-phenylacetic acid, which produced product ions at m/z 136 and m/z 92 with one unit of H2O (18 Da) and CO2 (44 Da). Compound 25 (dihydroferulic acid 4-O-glucuronide-C16H20O10) was identified in negative mode which produced a fragment ion at m/z 195 by losing the glucuronide (176 Da), which was confirmed through MS2 [10].
3.3.2. Flavonoids
Flavonoids are the most abundant class of secondary plant metabolites, used for pharmaceutical, medicinal, and cosmetic applications due to their antioxidative, anti-inflammatory, anti-carcinogenic, and anti-mutagenic properties [47]. In our study, 33 flavonoids in total were identified including 8 flavonols, 6 flavanols, 6 flavones, 1 flavanone, 1 dihydroflavonol, and 11 Isoflavonoids (Table 2).
Flavanols
The compound 28 (gallocatechin) were tentatively identified at m/z 305.0674 in negative mode. In MS2 experiment, the product ions m/z 261 and m/z 219 were formed with the loss of CO2 (44 Da) and C3O2 and H2O (86 Da). The compound 29 (catechin) was identified at m/z 289.0742 [M-H]− and product ions formed at m/z 245, m/z 205, and m/z 179 by the loss of CO2, flavonoid A ring and flavonoid B ring in MS2 from the precursor ion, respectively [48].
Flavones and Flavanones
Apigenin 6-8-di-C glucoside (compound 33) was identified in negative mode at m/z 593.1502, which produced fragment ions at m/z 503 and m/z 473 [49]. Rhoifolin (compound 35) was found in negative mode at m/z 577.1567 with the product ions m/z 413 and m/z 269 with the loss of rhamnose moiety and H2O (164 Da) along with hexosyl moiety plus rhamnose moiety (308 Da) from the parent ion [50]. Compound 37 (apigenin 7-O-(6′′-malonyl-apiosyl-glucoside) was characterized at m/z 649.1440 and produced fragment ion at m/z 605 with the loss of CO2 (44 Da) and only present in cumin. A precursor ion of compound 38 (m/z 445.0761) was tentatively identified as apigenin 7-O-glucuronide, which produced the product ions at m/z 269 and m/z 175 through the removal of glucuronide and glucuronide ‘C’ ring, respectively [51].
Flavonols and Dihydroflavonols
The compound 44 at m/z 609.1466 was characterized as kaempferol 3,7-O-diglucoside and produced fragments ions at m/z 447 [M-H-162] and m/z 285 [M-H-324], which were confirmed through MS2 [52]. The compound 45 (m/z 463.0892) and the compound 48 (m/z 449.1080) produced the daughter ions at m/z 317 and m/z 303 with the removal of rhamnoside moiety (146 Da) from the parent ion. The Compound 46 (m/z 433.0783) was identified as quercetin 3-O-arabinoside which produced fragment ion at m/z 301, due to loss of C5H8O4 (132 Da) [53].
Isoflavonoids
3′-Hydroxygenistein (compound 50; C15H10O6) was tentatively characterized at m/z 287.0563 [M+H]+, which produced fragment ions at m/z 269 and m/z 259 and confirmed through MS/MS via the removal of H2O (18 Da) and CO (28 Da) from the parent ion. The compound 54 were identified in negative mode at m/z 271.0611 and tentatively characterized as 3′,4′,7-trihydroxyisoflavanone. In MS2, fragment ions were present at m/z 177, m/z 151 and m/z 119 through the loss of C6H6O [M-H-94], C8H8O [M-H-120], and C7H7O4 [M-H-152] [54]. Violanone (compound 56) was produced fragment ions at m/z 300, m/z 285, and m/z 135 with the removal of CH3 (15 Da), 2CH3 (30 Da), and C10H12O3 (180 Da), respectively from the precursor ion (m/z 315.0866) [55].
Procyanidins (Tannins)
Procyanidin trimmer C1 (compound 31; [M-H]−) and procyanidin dimmer B1 (compound 32) were identified in cinnamon and nutmeg at m/z (865.1972) and m/z 577.1350, which produced fragments ions at m/z 739, m/z 713, and m/z 695; and m/z 451, respectively. The fragment ion at m/z 739 was attributed due to heterocyclic ring fission [M-H-126], while fragment ion at m/z 451 due to cleavage between C4-C5 and O-C2 of one pyran ring, which leads to the loss of ring “A” [56]. The procyanidins are the second most abundant phenolic compounds with antioxidant, anti-inflammatory, anti-cancer, and anti-cardiovascular activities [57].
3.3.3. Other Polyphenols
A total of 15 other polyphenols were identified from the different spices, which were further divided into three hydroxycoumarins, two hydroxybenzoketones, two curcuminoids, five tyrosols, two phenolic terpenes, and one other polyphenols (Table 2). Coumarin (compounds 60) was found in [M+H]+ mode at m/z 147.0438. In the MS2, coumarin showed the product ion peaks at m/z 103 with the loss of CO2 and m/z 91with the loss of 2CO, respectively, while in the MS2 spectra of m/z 191.0350, peak at m/z 176 with the loss of CH3 was characterized as scopoletin in negative mode (compound 61) [58]. Curcuminoids are the main bioactive compounds of turmeric present in many plant-based foods stuff. The compounds 66 (m/z 367.1178) and 67 (m/z 337.1080) were identified as curcumin and demthoxycurcumin. Both compounds were produced fragment ions at m/z 217 with the removal of (50 Da). Curcumin is well documented due to potent pharmacological activities including antioxidant, anti-inflammatory, anti-microbial, anti-mutagenic anti-angiogenic, and antidiabetic [59,60]. Additionally, curcumin is useful to treat irritable bowel syndrome due to its ability to modulate the gut microbiota [61]. Hydroxytyrosol (compound 69) was observed in negative mode at m/z 153.0553, which was further identified through MS2 due to loss of CH2OH [62]. Carnosic acid (compound 74) was characterized at m/z 331.1911 [M-H]− and produced daughter ions at m/z 287 and m/z 269 with the removal of CO2 (44 Da) from the parent ion and further removal of H2O (18 Da) from daughter ion. Carnosic acid was previously reported in cinnamon, thyme, oregano, and rosemary [7].
3.3.4. Lignans
Spices bioactive compounds, such as lignans, characterized through LC-MS have remarkable antioxidant and anti-carcinogenic properties. Consumption of plants lignans have been reported to protect or reduce the spread of ovarian cancer, breast cancer, and prostate cancer in humans. A total of four lignans were identified in selected spices. The compound 77 in [M+H]+ at m/z 299.1267 was identified as enterolactone which showed fragments at m/z 281, m/z 187, and m/z 165 with the neutral loss of H2O (18 Da), C6H8O2 (112 Da) and C9H8O2 (148 Da), respectively [63].
The LC-ESI-QTOF-MS2 was used as an excellent tool for the characterization and identification of phenolic compounds from twelve spices. Its application to spices allowed us to identify 79 phenolic compounds with their fragment ions. According to our best knowledge, no single study had been conducted in which all these compounds were identified before. The identification of these phenolic constituents in spices with significant antioxidant potential, can lead further in-vivo research to understand their health benefits.
3.4. Heatmap and Hierarchical Analysis of Quantified Polyphenols in Spices
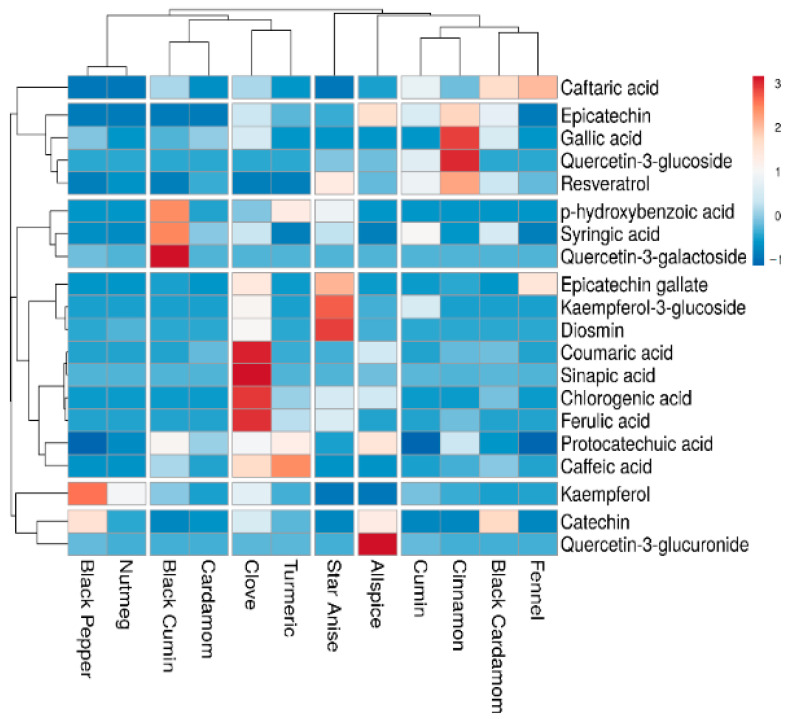
A heatmap was constructed for further analyzing the HPLC-PDA data of twenty phenolic compounds from selected spices (Figure 1). Six clusters were generated in row and column wise and highlighted with hierarchical clustering.
Figure 1.
Heatmap showing “distribution and concentration” of quantified phenolic compounds (mg/g) in all spices. Red color boxes indicating the higher concentration while blue color boxes showing lower or zero concentration.
The difference in clustering indicates the variation of concentration of phenolic compounds in spices. The variation in color profile indicates the abundance of different phenolic acids and flavonoids. Overall, clove was identified with more compounds from which mainly phenolic acids were in abundance. The red color boxes in clove (sinapic acid, caffeic acid, chlorogenic acid, coumaric acid, and kaempferol) depicts the high concentration of these compounds as compared to other compounds [24]. On the other hand, epicatechin gallate, diosmin, resveratrol, and kaempferol-3-glucoside were identified comparatively with higher concentration in star-anise. Moreover, syringic acid is the only phenolic acid which was abundantly found in cumin and less or absent from other spices. In cinnamon, gallic acid and quercetin-3-glucoside were present at high concentrations, while ferulic acid, epicatechin, protocatechuic acid, coumaric acid, syringic acid, and resveratrol were less abundant. Previously, epicatechin, p-coumaric acid, syringic acid, ferulic acid, and quercetin were quantified in cinnamon and cumin [7]. Higher concentration of quercetin-3-galactoside and protocatechuic acid were observed in black cumin along with p-hydroxybenzoic acid, caftaric acid, kaempferol, diosmin, and epicatechin gallate. These results were in accordance to Feng, Dunshea and Suleria [8], who quantified diosmin, kaempferol, p-hydroxybenzoic acid, and protocatechuic acid in their study. Caftaric acid and epicatechin were detected only in fennel with reasonable amounts. Very minute quantities pf phenolic compounds were present in nutmeg and cardamom. Quercetin-3-galactoside, syringic acid, p-hydroxybenzoic acid were found in abundance along with protocatechuic acid, caffeic acid, caftaric acid, and kaempferol in black cumin, while kaempferol and catechin were found with significant amount in black cardamom compared to other phenolic acids. Previously, caffeic acid, catechin derivatives, kaempferol, and other phenolic acids (chlorogenic and ferulic acid) were quantified in cinnamon, cumin, and star-anise [24].
Different studies had been conducted to quantify phenolic compounds from herbs and spices, still there is a gap due to their complex structures and influence of environmental conditions on secondary metabolite concentrations among different cultivars.
3.5. Correlation of Polyphenols and Antioxidant Activities
Since polyphenols found in herbs and spices have vital antioxidant properties, we investigated TPC, TFC, and TTC in 12 spices. The value of TPC was found in the range of 4.02–215.14 mg GAE/g, while the average was calculated as 32.72 mg GAE/g (Table 1). The highest TPC value was observed for clove and lowest in black cumin. The activities of DPPH, FRAP, ABTS, RPA, •OH-RSA, FICA, and PMA were used to measure the antioxidant potential of spice extracts. A regression analysis was performed to evaluate the correlation among the results of conducted assays (Table 3).
Table 3.
Pearson’s correlation between antioxidant capacity by different antioxidant assays.
| Variables | TPC | TFC | TTC | DPPH | FRAP | ABTS | RPA | •OH-RSA | FICA | PMA | Phenolic Acids |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TFC | 0.60 * | ||||||||||
| TTC | 0.05 | 0.26 | |||||||||
| DPPH | 0.70 * | 0.30 | 0.51 | ||||||||
| FRAP | 0.90 ** | 0.43 | 0.15 | 0.76 ** | |||||||
| ABTS | 0.98 ** | 0.59 * | 0.22 | 0.79 ** | 0.90 ** | ||||||
| RPA | 0.78 ** | 0.28 | 0.28 | 0.91 ** | 0.75 ** | 0.83 ** | |||||
| •OH-RSA | 0.04 | −0.14 | 0.20 | 0.10 | 0.23 | 0.08 | −0.05 | ||||
| FICA | 0.56 * | 0.36 | −0.09 | 0.12 | 0.54 * | 0.51 | 0.25 | 0.40 | |||
| PMA | −0.17 | 0.18 | 0.05 | −0.18 | −0.25 | −0.19 | −0.21 | −0.59 * | −0.12 | ||
| Phenolic Acids | 0.65 * | 0.46 | 0.02 | 0.27 | 0.73 ** | 0.61 * | 0.34 | 0.13 | 0.54 * | −0.21 | |
| Flavonoids | 0.47 | 0.01 | 0.20 | 0.62 * | 0.46 | 0.51 | 0.71 ** | −0.42 | 0.09 | 0.12 | 0.16 |
* Significant correlation at p ≤ 0.05; ** Significant correlation at p ≤ 0.01.
Highly significant positive correlation was observed between total phenolic contents and antioxidant activities of DPPH, FRAP and ABTS, RPA, FICA, while TFC only correlated with ABTS. Phenolic acids positively correlated with TPC, FRAP, ABTS, and FICA, while flavonoids positively correlated with DPPH and RPA, respectively. A positive correlation was previously reported between antioxidant activities and total polyphenols of herbs and spices [64]. Interestingly, a negative correlation was found between TPC, TFC, and TTC with PMA, •OH-RSA, and FICA, respectively. DPPH, FRAP, ABTS, RPA, •OH-RSA, and FICA also negatively correlated with PMA. Phenolic acids and flavonoids negatively correlated with PMA and •OH-RSA, respectively. It has been established that total phenolics are responsible for antioxidant potential of plant foods [20,31,62]. Several factors influence the correlation including, the range of tested samples, concentrations quantified, and different antioxidant assays applied. In our study, DPPH, FRAP, and ABTS were highly correlated to each other. Same trend was reported by Kim, Yang, Lee and Kang [28] where they found that TPC had high correlation with antioxidant activities as compared to TFC.
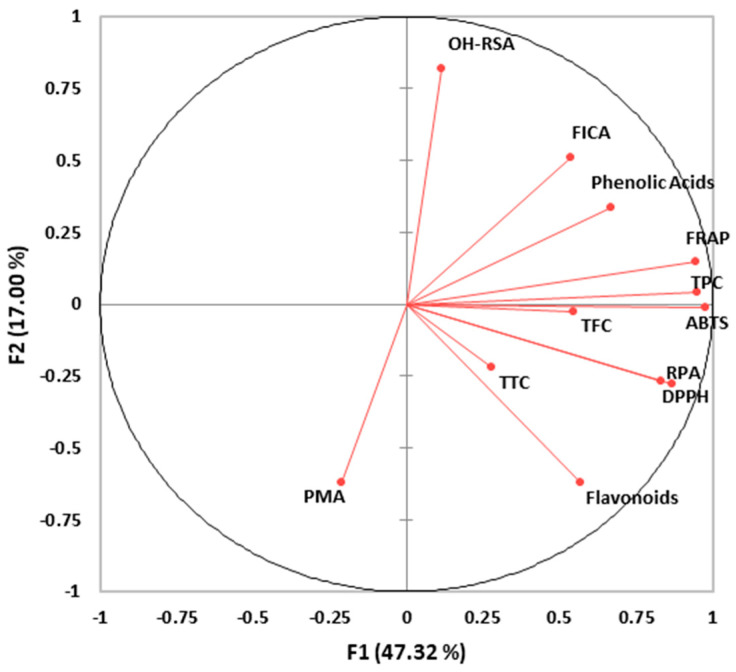
Principal component analysis (Figure 2) clearly indicated that phenolic acids and flavonoids were more correlated with TPC, ABTS, FRAP, TFC, DPPH, RPA, FICA, and TTC with higher scores, while PMA showed negative correlation with •OH-RSA. Moreover, flavonoids and phenolic acids negatively correlated with •OH-RSA and PMA, respectively, which indicated the diversity of bioactive compounds in spices.
Figure 2.
Principal Component Analysis (PCA) of the phenolic contents (TPC, TFC, TTC, phenolic acids, and flavonoids) and their antioxidant capacities (DPPH, FRAP, ABTS, RPA, •OH-RSA, FICA, and PMA) of twelve spices.
It is suggested after analyzing the results that antioxidant activities may also be attributed towards non-phenolic constituents in spice extracts. Although, simple phenols do not contribute much to antioxidant activities, but they react with Folin-Ciocalteu reagent. Results from current study indicate that different phenolic compounds show different antioxidant activities depending upon their structure, synergistic action, concentration, and antagonistic behavior with other compounds present in each spice extract.
Our results exhibited the higher concentration of phenolic constituents in spices with significant antioxidant activity. Moreover, further quantitative analysis of major phenolic compounds in selected spices through LC-ESI-QqQ-MS/MS could provide better understanding of the relationship between phenolics, structure and their antioxidant potential.
4. Conclusions
It is concluded that spices have a significant amount of phenolic contents with considerable in-vitro antioxidant capacity. The results of DPPH, FRAP, ABTS, RPA, FICA, •OH-RSA, and PMA showed that spices have high free radical scavenging activity and reducing power. Furthermore, total phenolic content, total flavonoid content, and the twelve spices′ antioxidant capacity were different from each other. From the advanced LC-ESI-QTOF-MS2 analytical technique applied for identification and characterization of the polyphenols in spices; a total of 79 polyphenols were tentatively identified in our study. These results make the picture clearer that spices have powerful antioxidant potential that can be further used in human food and animal feed industries as a supplement for different health prospective. The frequent use of these spices could make a substantial contribution to the performance and wellbeing of animals and human health. Due to extended anti-radical capacities of spices, they validate their use in pharmaceutical, nutraceutical, food, and feed industries. Awareness among people should be created about their bioavailability for nutritional and medicinal values. The in-vivo bioavailability, bioaccessibility, and toxicological studies should be conducted in order to commercialize these secondary metabolites. Further research about the polyphenol’s composition and their antioxidant capacities from spices is required to better understand their mechanisms to implement them in functional and nutraceutical foods.
Acknowledgments
We would like to thank Nicholas Williamson, Shuai Nie and Swati Varshney from the Mass Spectrometry and Proteomics Facility, Bio 21 Molecular Science and Biotechnology Institute, the University of Melbourne, Australia. We are also thankful to the University of Melbourne, Australia, and Higher Education Commission of Pakistan for their scholarship support. Moreover, we are also thankful to Yasmeen Bashmil, Amrit BK, Ahsan Aziz, Hafza Fasiha Zahid, Zhenzhao Li, Yun Xiong, Yit Tao Loo, and master’s students for their moral support.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antiox10050721/s1, Figure S1. Base Peak Chromatograms of 12 spices in negative (Red) and positive (Blue) mode. Figure S2. Extracted ion chromatogram and their mass spectrum.
Author Contributions
Conceptualization, A.A., H.A.R.S., and F.R.D.; methodology, A.A., H.W., and H.A.R.S.; software, A.A. and H.A.R.S.; validation, A.A. and H.A.R.S.; visualization A.A. and H.A.R.S.; formal analysis, A.A. and H.W.; investigation, A.A.; H.A.R.S., and F.R.D.; resources, H.A.R.S. and F.R.D.; data curation, A.A. and H.A.R.S.; writing—original draft preparation, A.A.; writing—review and editing, A.A., J.J.C., H.A.R.S., E.N.P., and F.R.D.; supervision, H.A.R.S., J.J.C., E.N.P., and F.R.D.; project administration, H.A.R.S., and F.R.D.; funding acquisition, H.A.R.S., and F.R.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Melbourne under the “McKenzie Fellowship Scheme” (Grant No. UoM-18/21), the “Faculty Research Initiative Funds” and “Richard WS Nicholas Agricultural Science Scholarship” funded by the Faculty of Veterinary and Agricultural Sciences, the University of Melbourne, Australia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tapsell L.C., Hemphill I., Cobiac L., Patch C.S., Sullivan D.R., Fenech M., Roodenrys S., Keogh J.B., Clifton P.M., Williams P.G., et al. Health benefits of herbs and spices: The past, the present, the future. Med. J. Aust. 2006;185:S1–S24. doi: 10.5694/j.1326-5377.2006.tb00548.x. [DOI] [PubMed] [Google Scholar]
- 2.Singh N., Rao A.S., Nandal A., Kumar S., Yadav S.S., Ganaie S.A., Narasimhan B. Phytochemical and pharmacological review of cinnamomum verum j. Presl-a versatile spice used in food and nutrition. Food Chem. 2020;338:127773. doi: 10.1016/j.foodchem.2020.127773. [DOI] [PubMed] [Google Scholar]
- 3.Gupta M. Pharmacological properties and traditional therapeutic uses of important indian spices: A review. Int. J. Food Prop. 2010;13:1092–1116. doi: 10.1080/10942910902963271. [DOI] [Google Scholar]
- 4.Leja K.B., Czaczyk K. The industrial potential of herbs and spices? A mini review. Acta Sci. Pol. Technol. Aliment. 2016;15:353–365. doi: 10.17306/J.AFS.2016.4.34. [DOI] [PubMed] [Google Scholar]
- 5.Del Rio D., Rodriguez-Mateos A., Spencer J.P.E., Tognolini M., Borges G., Crozier A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013;18:1818–1892. doi: 10.1089/ars.2012.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yashin A., Yashin Y., Xia X., Nemzer B. Antioxidant activity of spices and their impact on human health: A review. Antioxidants. 2017;6:70. doi: 10.3390/antiox6030070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vallverdú-Queralt A., Regueiro J., Martínez-Huélamo M., Alvarenga J.F.R., Leal L.N., Lamuela-Raventos R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014;154:299–307. doi: 10.1016/j.foodchem.2013.12.106. [DOI] [PubMed] [Google Scholar]
- 8.Feng Y., Dunshea F.R., Suleria H.A.R. Lc-esi-qtof/ms characterization of bioactive compounds from black spices and their potential antioxidant activities. J. Food Sci. Technol. 2020;57:4671–4687. doi: 10.1007/s13197-020-04504-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subbiah V., Zhong B., Nawaz M.A., Barrow C.J., Dunshea F.R., Suleria H.A. Screening of phenolic compounds in australian grown berries by lc-esi-qtof-ms/ms and determination of their antioxidant potential. Antioxidants. 2021;10:26. doi: 10.3390/antiox10010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suleria H.A., Barrow C.J., Dunshea F.R.J.F. Screening and characterization of phenolic compounds and their antioxidant capacity in different fruit peels. Foods. 2020;9:1206. doi: 10.3390/foods9091206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu C., Chou O., Lee F.Y., Wang Z., Barrow C.J., Dunshea F.R., Suleria H.A.R. Characterization of phenolics in rejected kiwifruit and their antioxidant potential. Processes. 2021;9:781. doi: 10.3390/pr9050781. [DOI] [Google Scholar]
- 12.Muhammad D.R.A., Praseptiangga D., Van de Walle D., Dewettinck K. Interaction between natural antioxidants derived from cinnamon and cocoa in binary and complex mixtures. Food Chem. 2017;231:356–364. doi: 10.1016/j.foodchem.2017.03.128. [DOI] [PubMed] [Google Scholar]
- 13.Sokamte T.A., Mbougueng P.D., Tatsadjieu N.L., Sachindra N.M. Phenolic compounds characterization and antioxidant activities of selected spices from cameroon. S. Afr. J. Bot. 2019;121:7–15. doi: 10.1016/j.sajb.2018.10.016. [DOI] [Google Scholar]
- 14.Chen X.-X., Feng H.-L., Ding Y.-M., Chai W.-M., Xiang Z.-H., Shi Y., Chen Q.-X. Structure characterization of proanthocyanidins from caryota ochlandra hance and their bioactivities. Food Chem. 2014;155:1–8. doi: 10.1016/j.foodchem.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Severo J., Tiecher A., Chaves F.C., Silva J.A., Rombaldi C.V. Gene transcript accumulation associated with physiological and chemical changes during developmental stages of strawberry cv. Camarosa. Food Chem. 2011;126:995–1000. doi: 10.1016/j.foodchem.2010.11.107. [DOI] [Google Scholar]
- 16.Ferreira I.C.F.R., Baptista P., Vilas-Boas M., Barros L. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast portugal: Individual cap and stipe activity. Food Chem. 2007;100:1511–1516. doi: 10.1016/j.foodchem.2005.11.043. [DOI] [Google Scholar]
- 17.Smirnoff N., Cumbes Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry. 1989;28:1057–1060. doi: 10.1016/0031-9422(89)80182-7. [DOI] [Google Scholar]
- 18.Dinis T.C.P., Madeira V.M.C., Almeida L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 19.Nićiforović N., Mihailović V., Masković P., Solujić S., Stojković A., Muratspahić D.P. Antioxidant activity of selected plant species; potential new sources of natural antioxidants. Food Chem. Toxicol. 2010;48:3125–3130. doi: 10.1016/j.fct.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Hong Y., Wang Z., Barrow C.J., Dunshea F.R., Suleria H.A.R. High-throughput screening and characterization of phenolic compounds in stone fruits waste by lc-esi-qtof-ms/ms and their potential antioxidant activities. Antioxidants. 2021;10:234. doi: 10.3390/antiox10020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong B., Robinson N.A., Warner R.D., Barrow C.J., Dunshea F.R., Suleria H.A. Lc-esi-qtof-ms/ms characterization of seaweed phenolics and their antioxidant potential. Marine drugs. 2020;18:331. doi: 10.3390/md18060331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang J., Dunshea F.R., Suleria H.A.R. Lc-esi-qtof/ms characterization of phenolic compounds from medicinal plants (hops and juniper berries) and their antioxidant activity. Foods. 2020;9:7. doi: 10.3390/foods9010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu C., Howell K., Dunshea F.R., Suleria H.A.R. Lc-esi-qtof/ms characterisation of phenolic acids and flavonoids in polyphenol-rich fruits and vegetables and their potential antioxidant activities. Antioxidants. 2019;8:405. doi: 10.3390/antiox8090405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shan B., Cai Y.Z., Sun M., Corke H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005;53:7749–7759. doi: 10.1021/jf051513y. [DOI] [PubMed] [Google Scholar]
- 25.Słowianek M., Leszczyńska J. Antioxidant properties of selected culinary spices. Herba Pol. 2016;62:29–41. doi: 10.1515/hepo-2016-0003. [DOI] [Google Scholar]
- 26.Dvorackova E., Snoblova M., Chromcova L., Hrdlicka P. Effects of extraction methods on the phenolic compounds contents and antioxidant capacities of cinnamon extracts. Food Sci. Biotechnol. 2015;24:1201–1207. doi: 10.1007/s10068-015-0154-4. [DOI] [Google Scholar]
- 27.Tacouri D.D., Ramful-Baboolall D., Puchooa D. In vitro bioactivity and phytochemical screening of selected spices used in mauritian foods. Asian Pac. J. Trop. Dis. 2013;3:253–261. doi: 10.1016/S2222-1808(13)60066-3. [DOI] [Google Scholar]
- 28.Kim I.-S., Yang M.-R., Lee O.-H., Kang S.-N. Antioxidant activities of hot water extracts from various spices. Int. J. Mol. Sci. 2011;12:4120–4131. doi: 10.3390/ijms12064120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Przygodzka M., Zielińska D., Ciesarová Z., Kukurová K., Zieliński H. Comparison of methods for evaluation of the antioxidant capacity and phenolic compounds in common spices. Lwt Food Sci. Technol. 2014;58:321–326. doi: 10.1016/j.lwt.2013.09.019. [DOI] [Google Scholar]
- 30.Yang C.-H., Li R.-X., Chuang L.-Y. Antioxidant activity of various parts of cinnamomum cassia extracted with different extraction methods. Molecules. 2012;17:7294–7304. doi: 10.3390/molecules17067294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang D., Dunshea F.R., Suleria H.A.R. Lc-esi-qtof/ms characterization of australian herb and spices (garlic, ginger, and onion) and potential antioxidant activity. J. Food Process. Preserv. 2020;44:e14497. doi: 10.1111/jfpp.14497. [DOI] [Google Scholar]
- 32.Wojdyło A., Oszmiański J., Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105:940–949. doi: 10.1016/j.foodchem.2007.04.038. [DOI] [Google Scholar]
- 33.Patra K., Jana S., Mandal D.P., Bhattacharjee S. Evaluation of the antioxidant activity of extracts and active principles of commonly consumed indian spices. J. Environ. Pathol. Toxicol. Oncol. 2016;35:299–315. doi: 10.1615/JEnvironPatholToxicolOncol.2016016387. [DOI] [PubMed] [Google Scholar]
- 34.Muhammad J.I., Masood S.B., Iqra S., Hafiz A.R.S. Physicochemical and antioxidant properties of pizza dough-base enriched with black cumin (nigella sativa) extracts. Curr. Nutr. Food Sci. 2019;15:508–516. [Google Scholar]
- 35.Adjimani J.P., Asare P. Antioxidant and free radical scavenging activity of iron chelators. Toxicol. Rep. 2015;2:721–728. doi: 10.1016/j.toxrep.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yadav A.S., Bhatnagar D. Free radical scavenging activity, metal chelation and antioxidant power of some of the indian spices. Biofactors. 2007;31:219–227. doi: 10.1002/biof.5520310309. [DOI] [PubMed] [Google Scholar]
- 37.Hinneburg I., Dorman H.J.D., Hiltunen R. Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem. 2006;97:122–129. doi: 10.1016/j.foodchem.2005.03.028. [DOI] [Google Scholar]
- 38.Sasikumar J.M., Erba O., Egigu M.C. In vitro antioxidant activity and polyphenolic content of commonly used spices from ethiopia. Heliyon. 2020;6:e05027. doi: 10.1016/j.heliyon.2020.e05027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ene-Obong H., Onuoha N., Aburime L., Mbah O. Chemical composition and antioxidant activities of some indigenous spices consumed in nigeria. Food Chem. 2018;238:58–64. doi: 10.1016/j.foodchem.2016.12.072. [DOI] [PubMed] [Google Scholar]
- 40.Viuda-Martos M., Ruiz Navajas Y., Sánchez Zapata E., Fernández-López J., Pérez-Álvarez J.A. Antioxidant activity of essential oils of five spice plants widely used in a mediterranean diet. Flavour Fragr. J. 2010;25:13–19. doi: 10.1002/ffj.1951. [DOI] [Google Scholar]
- 41.Kumar N., Goel N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019;24:e00370. doi: 10.1016/j.btre.2019.e00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yisimayili Z., Abdulla R., Tian Q., Wang Y., Chen M., Sun Z., Li Z., Liu F., Aisa H.A., Huang C. A comprehensive study of pomegranate flowers polyphenols and metabolites in rat biological samples by high-performance liquid chromatography quadrupole time-of-flight mass spectrometry. J. Chromatogr. A. 2019;1604:460472. doi: 10.1016/j.chroma.2019.460472. [DOI] [PubMed] [Google Scholar]
- 43.Senes C.E.R., Rodrigues C.A., Nicácio A.E., Boeing J.S., Maldaner L., Visentainer J.V. Determination of phenolic acids and flavonoids from myrciaria cauliflora edible part employing vortex-assisted matrix solid-phase dispersion (va-mspd) and uhplc-ms/ms. J. Food Compos. Anal. 2021;95:103667. doi: 10.1016/j.jfca.2020.103667. [DOI] [Google Scholar]
- 44.Yang S., Zhang X., Dong Y., Sun G., Jiang A., Li Y. Cleavage rules of mass spectrometry fragments and rapid identification of chemical components of radix paeoniae alba using uhplc-q-tof-ms. Phytochem. Anal. 2021 doi: 10.1002/pca.3029. [DOI] [PubMed] [Google Scholar]
- 45.Lin H., Zhu H., Tan J., Wang H., Wang Z., Li P., Zhao C., Liu J. Comparative analysis of chemical constituents of moringa oleifera leaves from china and india by ultra-performance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry. Molecules. 2019;24:942. doi: 10.3390/molecules24050942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parejo I., Jauregui O., Sánchez-Rabaneda F., Viladomat F., Bastida J., Codina C. Separation and characterization of phenolic compounds in fennel (foeniculum vulgare) using liquid chromatography-negative electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2004;52:3679–3687. doi: 10.1021/jf030813h. [DOI] [PubMed] [Google Scholar]
- 47.Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: An overview. J. Nutr. Sci. 2016;5:e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gülçin İ., Gören A.C., Taslimi P., Alwasel S.H., Kılıc O., Bursal E. Anticholinergic, antidiabetic and antioxidant activities of anatolian pennyroyal (mentha pulegium)-analysis of its polyphenol contents by lc-ms/ms. Biocatal. Agric. Biotechnol. 2020;23:101441. doi: 10.1016/j.bcab.2019.101441. [DOI] [Google Scholar]
- 49.Makita C., Chimuka L., Steenkamp P., Cukrowska E., Madala E. Comparative analyses of flavonoid content in moringa oleifera and moringa ovalifolia with the aid of uhplc-qtof-ms fingerprinting. S. Afr. J. Bot. 2016;105:116–122. doi: 10.1016/j.sajb.2015.12.007. [DOI] [Google Scholar]
- 50.Ledesma-Escobar C.A., Priego-Capote F., Robles-Olvera V.J., De Castro M.D.L. Changes in the composition of the polar fraction of persian lime (citrus latifolia) during fruit growth by lc–qtof ms/ms analysis. Food Chem. 2017;234:262–268. doi: 10.1016/j.foodchem.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 51.Hussain F., Jahan N., Rahman K.-U., Sultana B., Jamil S. Identification of hypotensive biofunctional compounds of Coriandrum sativum and evaluation of their angiotensin-converting enzyme (ace) inhibition potential. Oxidative Med. Cell. Longev. 2018;2018:4643736. doi: 10.1155/2018/4643736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guijarro-Díez M., Nozal L., Marina M.L., Crego A.L. Metabolomic fingerprinting of saffron by lc/ms: Novel authenticity markers. Anal. Bioanal. Chem. 2015;407:7197–7213. doi: 10.1007/s00216-015-8882-0. [DOI] [PubMed] [Google Scholar]
- 53.Kumar S., Singh A., Kumar B. Identification and characterization of phenolics and terpenoids from ethanolic extracts of phyllanthus species by hplc-esi-qtof-ms/ms. J. Pharm. Anal. 2017;7:214–222. doi: 10.1016/j.jpha.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Z., Barrow C.J., Dunshea F.R., Suleria H.A.R. A comparative investigation on phenolic composition, characterization and antioxidant potentials of five different australian grown pear varieties. Antioxidants. 2021;10:151. doi: 10.3390/antiox10020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu R., Ye M., Guo H., Bi K., Guo D.A. Liquid chromatography/electrospray ionization mass spectrometry for the characterization of twenty-three flavonoids in the extract of dalbergia odorifera. Rapid Commun. Mass Spectrom. 2005;19:1557–1565. doi: 10.1002/rcm.1936. [DOI] [PubMed] [Google Scholar]
- 56.Baskaran R., Pullencheri D., Somasundaram R. Characterization of free, esterified and bound phenolics in custard apple (Annona squamosa L.) fruit pulp by uplc-esi-ms/ms. Food Res. Int. 2016;82:121–127. doi: 10.1016/j.foodres.2016.02.001. [DOI] [Google Scholar]
- 57.Hou K., Wang Z. Application of nanotechnology to enhance adsorption and bioavailability of procyanidins: A review. Food Rev. Int. 2021:1–15. doi: 10.1080/87559129.2021.1888970. [DOI] [Google Scholar]
- 58.Sharifi-Rad J., Song S., Ali A., Subbiah V., Taheri Y., Suleria H.A.R. Lc-esi-qtof-ms/ms characterization of phenolic compounds from pyracantha coccinea m. Roem. And their antioxidant capacity. Cellu. Molec. Biol. 2021;67:201–211. doi: 10.14715/cmb/2021.67.1.29. [DOI] [PubMed] [Google Scholar]
- 59.Razzaq P.A., Iftikhar M., Faiz A., Aman F., Ijaz A., Iqbal S., Khalid A., Sarwar S. A comprehensive review on antidiabetic properties of turmeric. Life Sci. J. 2020;17:26–39. [Google Scholar]
- 60.Hewlings S.J., Kalman D.S. Curcumin: A review of its effects on human health. Foods. 2017;6:92. doi: 10.3390/foods6100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jakubczyk K., Drużga A., Katarzyna J., Skonieczna-Żydecka K. Antioxidant potential of curcumin-a meta-analysis of randomized clinical trials. Antioxidants. 2020;9:1092. doi: 10.3390/antiox9111092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hamden K., Allouche N., Damak M., Elfeki A. Hypoglycemic and antioxidant effects of phenolic extracts and purified hydroxytyrosol from olive mill waste in vitro and in rats. Chem. Biol. Interact. 2009;180:421–432. doi: 10.1016/j.cbi.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 63.Geng C.-A., Chen H., Chen X.-L., Zhang X.-M., Lei L.-G., Chen J.-J. Rapid characterization of chemical constituents in saniculiphyllum guangxiense by ultra fast liquid chromatography with diode array detection and electrospray ionization tandem mass spectrometry. Int. J. Mass Spectrom. 2014;361:9–22. doi: 10.1016/j.ijms.2014.01.021. [DOI] [Google Scholar]
- 64.Lu M., Yuan B., Zeng M., Chen J. Antioxidant capacity and major phenolic compounds of spices commonly consumed in china. Food Res. Int. 2011;44:530–536. doi: 10.1016/j.foodres.2010.10.055. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in Supplementary Material.