Abstract
Hepatocellular carcinoma (HCC) is a common malignancy, and the hepatitis B virus (HBV) is its major pathogenic factor. Over the past decades, it has been confirmed that HBV infection could promote disease progression through a variety of mechanisms, ultimately leading to the malignant transformation of liver cells. Many factors have been identified in the pathogenesis of HBV-associated HCC (HBV-HCC), including HBV gene integration, genomic instability caused by mutation, and activation of cancer-promoting signaling pathways. As research in the progression of HBV-HCC progresses, the role of many new mechanisms, such as epigenetics, exosomes, autophagy, metabolic regulation, and immune suppression, is also being continuously explored. The occurrence of HBV-HCC is a complex process caused by interactions across multiple genes and multiple steps, where the synergistic effects of various cancer-promoting mechanisms accelerate the process of disease evolution from inflammation to tumorigenesis. In this review, we aim to provide a brief overview of the mechanisms involved in the occurrence and development of HBV-HCC, which may contribute to a better understanding of the role of HBV in the occurrence and development of HCC.
Keywords: hepatocellular carcinoma, hepatitis B virus, carcinogenic mechanisms, therapy
Introduction
Liver cancer is predicted to be the sixth most commonly diagnosed cancer and the fourth leading cause of cancer-related deaths worldwide in 2018. Primary liver cancers include hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma, and combined carcinoma, with HCC accounting for 75–85% of cases.1 The main risk factors for HCC include chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV), aflatoxin-contaminated foodstuffs, heavy alcohol intake, and type 2 diabetes. According to the latest statistics, approximately 50–80% of HCC cases worldwide are caused by HBV infection.2 Various pathogenic factors have also been shown to cooperate with HBV to improve the incidence of HCC.3,4
Hepatotropic HBV is a DNA virus with a partly double-stranded relaxed circular DNA (rcDNA). According to the different degrees of the whole HBV genome sequence, HBV can be divided into 10 genotypes (A-J).5 The HBV gene mainly encodes four kinds of antigens, namely HBsAg, HBcAg, HBeAg, and HBxAg, the expression of which has been proven to be related to the occurrence of HCC.
HBV infection can promote hepatocellular carcinogenesis through direct or indirect mechanisms. On the one hand, HBV can increase the instability of the host cell genome and cause epigenetic remodeling of host DNA, resulting in chromosomal remodeling and the abnormal expression of oncogenes and tumor suppressor genes by integrating into or inducing mutations in host genes. It can also cause the malignant transformation of liver cells by activating various cancer-related signaling pathways, regulating cell metabolism, and other mechanisms. On the other hand, the liver microenvironment changes through HBV infection-induced chronic inflammation, the interaction between the virus and the innate immune cells, and adaptive immune cells, which help the virus evade immune surveillance and promote disease evolution from inflammation to the formation of a tumor. Further research on the mechanisms of HCC induced by HBV infection can provide reliable new methods and new ideas for the prevention, diagnosis, and treatment of HBV-HCC. In this review, the main mechanisms associated with HBV-HCC are described based on the latest research progress in this field.
HBV Structure
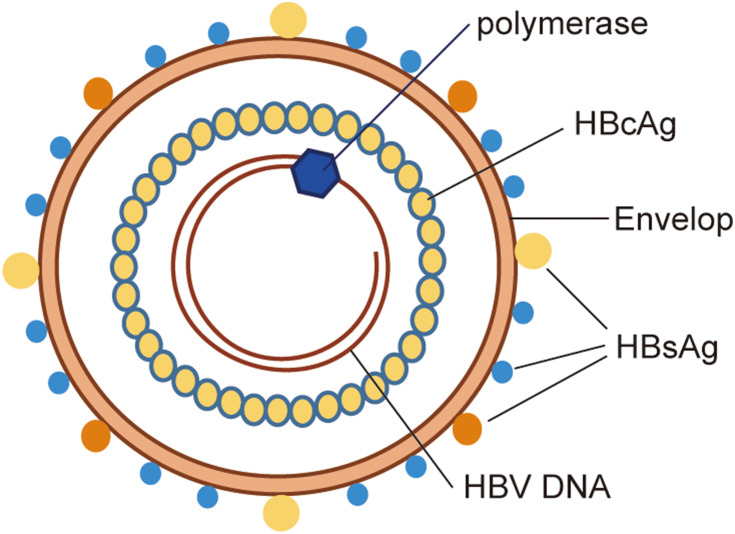
The intact HBV consists of a double envelope and a core granule (Figure 1). The envelope is composed of a lipid bilayer and different proteins. The lipid bilayer contains the S antigen, as well as the pre-S1 and pre-S2 antigens, which together constitute the large, medium, and small protein forms on the envelope, collectively known as the hepatitis B surface antigen (HBsAg). The surface of the core particle is the true viral capsid, which is composed of the HBV core antigen (HBcAg). The core particle contains circular and partially double-stranded DNA and polymerase, and is therefore involved in the replication of the HBV genome. In addition, there is also a nucleocapsid-related soluble E antigen in the serum called HBeAg.
Figure 1.
Schematic representation of HBV structure. The intact HBV consists of a double envelope and a core granule. The lipid bilayer contains the large, medium and small protein forms on the envelope, collectively known as HBsAg. The surface of the core particle is the true viral capsid, which is composed of the HBcAg. The core particle contains circular and partially double-stranded DNA and polymerase.
Abbreviations: HBsAg, hepatitis B surface antigen; HBcAg, hepatitis B core antigen; HBV, hepatitis B virus.
The HBV genome contains four open reading frames (ORFs), namely ORF-P, -S, -C, and -X. The four ORFs overlap each other, and their regulatory sequences also overlap among them. ORF-P encodes DNA polymerase, reverse transcriptase, and RNase H. It also encodes the terminal proteins that have primase activity. ORF-S consists of the pre-S1, pre-S2, and S genes, which encode the HBsAg proteins, namely the pre-S1 protein, pre-S2 protein and S protein, respectively. ORF-C is composed of the pre-C and C genes, which encode HBcAg and HBeAg. The E antigen can be secreted into the serum and participate in the immune regulation of the host. ORF-X encodes the minimum X protein, which has a trans-regulatory function and can activate the enhancers and promoters of homologous or heterologous genes. It is also closely related to the ability of HBV to infect cells and the occurrence of HCC.
HBV and Gene Mutation
Mutations in both the HBV gene and somatic genes can promote the malignant transformation of liver cells. Various factors can lead to relevant mutations in the HBV gene, including long-term infection, the special structure of the HBV genome, and the wide, long-term, and non-standard application of NAs (nucleoside/nucleotide analogs), as well as host immune stress, which are conducive to virus survival. Genomic mutations can change the biological behavior and pathogenicity of the virus and are closely related to the malignant transformation of liver disease. Mutations in the HBV genome do not occur randomly throughout the viral DNA, but are often concentrated in specific regions. For example, double mutations in G1896A of the pre-C region and T1762/A1764 in the basal core promoter (BCP) region have been identified as risk predictors of HCC.6 Point mutations, deletions, and insertions in the pre-S1 and pre-S2 regions are common in patients with chronic hepatitis B (CHB) and HCC. The mutated pre-S region is more likely to retain the virus in cells, thus promoting the malignant transformation of cells, and is a strong risk factor for the occurrence of HCC.7 In patients with cirrhosis and HCC, mutations at specific sites in the S region lead to premature stop codons. The expression of truncated S protein can often be detected, which results in the activation of the endoplasmic reticulum stress pathway, induction of DNA oxidative damage, and genomic instability, promoting the formation of HCC. In addition, mutations in the HBV can help it evade immune surveillance. It has been demonstrated that the long-term accumulation of epitope mutations in HBc and HBe under immune pressure from cytotoxic T lymphocytes (CTLs) can reduce the virus-specific CTL response, helping viruses evade immune surveillance and disease progression.8–10
A large number of somatic mutations occur in the host genome after long-term stimulation of chronic inflammation caused by HBV infection. Some relevant mutations suitable for cell survival will then be screened out and play a significant role in a tumor-promoting effect. Proteogenomic analysis identified five significant mutant genes in tumor tissues of 159 patients with HBV-associated HCC, including TP53 (58%), CTNNB1 (19%), Axin1 (18%), Keap1 (7%), and RB1 (6%).11 Among them, TP53, Axin1, and RB1 are common tumor suppressor genes, while CTNNB1 is an oncogene. KEAP1 is an important regulatory factor of cell resistance to oxidative stress, and a disorder of the KEAP1-NRF2 pathway is closely related to the induction of drug resistance in malignant tumors.12 In addition, it has also been confirmed that many genes, including TERT, ARID1A, ARID2, and NFE2L2, are mutated in HBV-associated HCC.13,14 These genes have been shown to be closely related to the occurrence of a variety of malignant tumors, including HCC,15–17 which suggests the potential effect of virus infection on somatic cell mutation and the eventual formation of tumors.
HBV DNA Integration
The integration of viral DNA into the host genome is an important molecular mechanism of HCC occurrence. Although it is not necessary for viral replication, the integration of HBV DNA into the host chromosome has been demonstrated in approximately 85% to 90% of tumor cells from patients with HCC.18 The integration of HBV DNA into the host genome can result in the rearrangement, partial loss, or acquisition of viral sequences. Although the host gene loci for HBV integration are random,19 the integration of HBV DNA into certain genomic loci can enable the integrated cells to acquire the ability of clonal growth, producing more mutations, and promoting malignant transformation. Integration of HBV DNA into host genes is often incomplete, which leads to the destruction of both host and HBV genes, and even the rearrangement of gene sequences, leading to the abnormal expression of oncogenes and tumor suppressor genes. Studies have shown that integration of HBV DNA into host cells can result in significantly high copy number variation (CNV) of chromosomes near the integration sites, causing hepatic cell genome instability and inducing mutations, chromosomal deletions, and gene rearrangements.20,21 Therefore, exploring the priority integration regions in the HBV genome and priority integration sites in the human genome is an important molecular basis for understanding the occurrence of HCC.
With the development of sequencing techniques, the detection of HBV DNA integration is no longer limited to traditional PCR-based detection methods.22 HBV integration in patients with HCC was analyzed based on HBV probes and high-throughput sequencing, and showed that integration breakpoints can be distributed in all gene regions of the virus. HBV DNA sequences integrated into the host genome include the X, C, enhancer, and S genes, among which the X and C genes are the most common integration genes in the genomes of patients with HCC.23 X gene integration can directly activate oncogenes (such as Myc, Ras, Src, and CyclinD1) and inhibit the expression of tumor suppressor genes (such as P53 and Rb), thus participating in the occurrence of HCC. C-terminal truncated X protein (Ct-HBx) expression can promote hepatocyte proliferation and reprogram cell metabolism by inhibiting thioredoxin-interacting protein (TXNIP).24 Furthermore, Ct-HBx regulates the transcription of Caveolin-1 and stabilizes LRP6 to maintain the activation of β-catenin, promote the progression of HBV-associated HCC,25 and enhance the invasion and metastasis of HCC cells.26,27 In addition, integrated viral DNA can lead to the persistent expression of mutated and truncated HBx, HBsAg, and HBcAg proteins. A high expression of these proteins is associated with endoplasmic reticulum and mitochondrial stress responses and may increase the risk of HCC.28,29
By analyzing the tumor and adjacent non-tumor tissues from patients with HCC, HBV DNA fragments were found to be readily integrated into fragile sites (intergene regions, repeat regions, CpG islands, and telomeres) and functional regions (including genes related to cell survival, metabolism, and cell cycle regulation) of the host genome. The carcinogenicity of HBV integration depends to some extent on the function of the host genes targeted by HBV in HCC.30 Repeated HBV integration can be detected in many tumor-associated genes. Integration hotspots such as TERT, MLL4, CCNA2, CCNE1, and FN1 were previously detected through whole genome sequencing (WGS). Using high-throughput viral integration detection (HIVID) technology, it has been found that HBV integration also exists in many new genes such as NRG3, UNC5D, CTNND2, PTPRD, and AHRR, which are closely related to tumor progression.31,32 The targeted genes for HBV integration have also been shown to be significantly enriched in cancer-related pathways, such as MAPK, extracellular matrix-receptor interactions, and Hedgehog signaling, and the integration frequency of HBV is expected to increase along with the progression from hepatitis B to HCC.33 The most common HBV integration site occurs in the telomerase reverse transcriptase gene (TERT). The HBx gene promoter, C gene promoter, and enhancer have been found to integrate into the TERT or nearby regulatory regions.34 Repeated integration of the promoter augments the expression of TERT.35 Reactivated telomerase can trigger the clonal proliferation of cells, leading to a malignant transformation and the eventual development of HCC.36 Recent studies have shown that the binding of E74-like ETS transcription factor 4 (ELF4) to chimeric HBV EnhI in the TERT promoter region is the molecular mechanism of HBV integration-mediated telomerase activation.37
It is worth noting that HBV DNA integration has been found in approximately 70% of HCC patients with occult HBV infection (OBI) in recent years, mainly involving the virus X and preS/S genomic regions.38,39 HBV DNA gene integration is associated with changes in tumor suppressor genes, mutations in the p53 gene, and genomic instability in HCC patients with OBI.40,41 This is also an important reason why patients with OBI tend to develop HCC.
HBV and microRNA
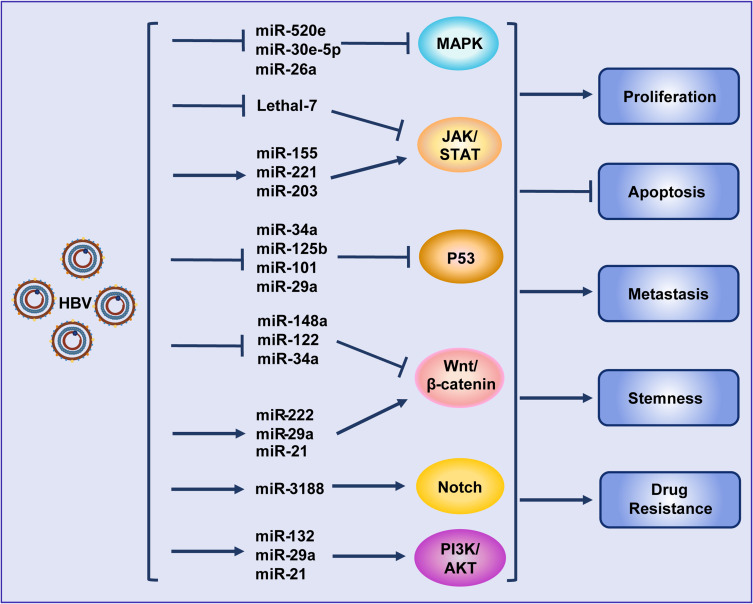
In recent years, a large number of studies have shown that miRNAs play an important role in HBV replication as well as in the occurrence and development of HBV-associated HCC. Various miRNAs can promote HBV replication via different mechanisms.42–45 Our previous studies revealed that HBV could target RIG-I and RIG-G and inhibit STAT1 expression by inducing miR-146a expression, impairing the clearance of the virus via the innate immune system.46,47 By analyzing the global miRNA expression profiles in HBV-associated HCC, it was found that the expression of many miRNAs, such as miR-150 and miR-342-3p, was altered.48,49 Analysis of a large number of clinical samples showed that miRNAs such as miR-375, miR-25, and let-7f could be used as HBV-specific miRNAs, showing potential clinical value for the prediction and diagnosis of HBV+ HCC.50,51 MiRNAs have also been demonstrated to be closely related to chemotherapy resistance in HBV-associated HCC.52 Moreover, HBV can further target different oncogenes53 or intervene in multiple signaling pathways, such as Wnt, MAPK, STAT, P53, Akt and Notch, by regulating the expression of different miRNAs to promote HCC48,54,55 (Figure 2). The structural proteins of HBV can also regulate the expression of some miRNAs in a transcription factor-dependent manner, thus regulating the progress of HCC. For example, HBx promotes the expression of miR-1269b in an NF-κB-dependent manner, promoting the proliferation and migration of HCC cells.56 HBx also interacts with c-Myc to inhibit miRNA-192-3p expression and promote HBV replication.57
Figure 2.
Hepatitis B virus promotes hepatocellular carcinoma by intervening various signal pathways through different microRNAs. Lines ending with arrows or bars indicate promotion or inhibitory effects, respectively.
Abbreviation: HBV, hepatitis B virus.
In addition to miRNAs expressed by host cells, in recent years, HBV has also been found to encode miRNAs to promote the disease process. In HBV-associated HCC, HBV-miR-2 can act as an oncogene, inhibit cell apoptosis, promote cell growth, and enhance the migration and invasion ability of HCC cells. Moreover, HBV-miR-2 can be detected in both the tissues and sera of patients with HBV+ HCC.58 HBV-miR-3 controls viral replication in host cells59 and promotes HCC growth by regulating the expression of the host gene protein phosphatase 1A (PPM1A).60 HBV-miR-3 can also affect disease progression caused by persistent HBV infection by inhibiting SOCS5, activating the STAT1-IFN signaling pathway, and inducing the M1 polarization of macrophages.59
Conversely, in addition to the above promoting effects, some miRNAs have also been found to exert inhibitory effects on HBV-associated HCC processes. HBX-LINE1 integrated in HBV+ HCC can downregulate miRNA-122 expression,61 while ADAR1 can protect against HBV infection by upregulating miRNA-122 in liver cells,62 suggesting that miRNA-122 may inhibit the progression of HBV infection to HCC. MiRNA-520a and miR-302c-3p can inhibit HBV replication and reduce the survival rate of HBV+ HCC cells.63,64 Restoration of miR-193b expression increased the sensitivity of HBV+ HCC to sorafenib.65 These phenomena indicate that the role of miRNAs in the progression of HBV-associated HCC is complex but not simply promotive or suppressive, influencing tumor progression by affecting viral replication, or directly promoting or inhibiting the development of tumors.
HBV and Epigenetic Modification
Recent studies on epigenetic modifications have revealed its key role in cancer development.66 HBV can cause epigenetic remodeling by changing the methylation state of DNA and the post-translational modification of histones, which is an important pathogenesis mechanism of HBV-associated HCC. Bioinformatics analysis showed that the methylation levels of tumor tissues from patients with HBV-associated HCC were significantly higher than those from non-infected HCC patients, where 68% of the methylated genes were associated with cancer development,67 suggesting that HBV infection may affect gene methylation in tumor tissues. The expression of HBV structural proteins (mainly HBx) can cause abnormal epigenetic remodeling, activating oncogenes and silencing tumor suppressor genes.66,68 In HBV+ HCC, HBx downregulates the expression of ASPP1 and ASPP2 genes through DNA methylation modification, and dampens their interaction with the p53 protein, which inhibits HCC apoptosis, promotes HCC growth, and is closely related to the occurrence of early liver cancer.69 HBx can also promote the binding of methyltransferases DNMT1 and DNMT3A to the gene promoters of SFRP1 and SFRP5, leading to the silencing of SFRP1 and SFRP5, promoting tumor progression. Inhibition of DNMT1 can inactivate Wnt signaling and reduce the expression levels of the Wnt target genes C-Myc and CyclinD1, impeding the growth and invasion of HBV-associated HCC in vitro and in vivo. HBx protein can also stabilize WDR5 (a core subunit of histone H3 lysine 4 methyltransferase) by inhibiting its ubiquitination, promoting the methylation modification of target gene promoters and promoting the formation and growth of tumors in HCC tumor-bearing mice. Moreover, WDR5 can also promote HBV transcription through H3K4Me3 methylation modification of HBV cccDNA.70 In addition to methylation modification, the expression of histone deacetylase HDAC1 was significantly increased in the tumor tissues of patients with HBV+ HCC. Furthermore, it was found that HBx upregulates metastasis-associated protein 1 (MTA1) and HDAC1 at the transcriptional level, which stabilizes HIF-1α and promotes HCC invasion and metastasis in HBx-transgenic mice.71 These phenomena indicate that epigenetic remodeling of HBV is very important for the pathogenesis of HCC.
In addition, epigenetic mechanisms are involved in the regulation of HBV replication. The presence of HBV cccDNA is an important reason for the difficulty in curing HBV infection. The acetylation of histones binding to cccDNA in HCC can promote the transcription of cccDNA at the epigenetic level, thereby promoting HBV replication. By interacting with lncRNA-DLEU2, HBx can regulate viral cccDNA transcription, viral replication, and host cancer-related gene expression at the epigenetic level.72
HBV Involved in Activation of Tumor-Promoting Signaling Pathways
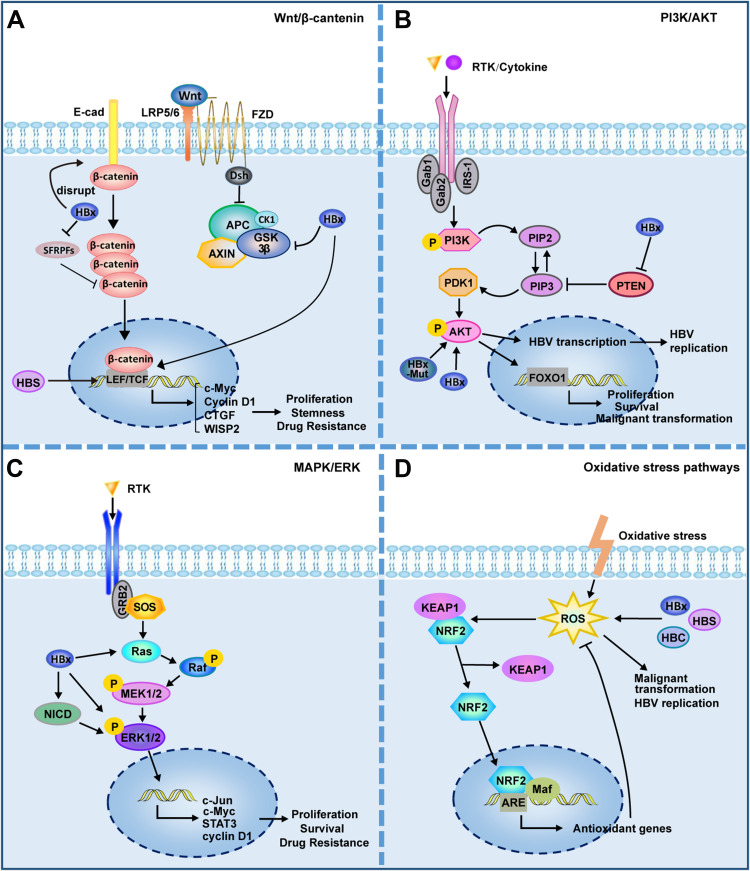
The carcinogenicity of HBV is closely related to the activation of multiple cancer-related signaling pathways in their host cells (Figure 3). The above described HBV mutations and integrations lead to the abnormal expression of multiple molecules in host cells, most of which are located in key locations of oncogenic signaling pathways. HBV structural proteins are also involved in disease progression by regulating multiple signaling pathways through mechanisms such as regulation of miRNAs or epigenetics.
Figure 3.
HBV is involved in activation of cancer-related signaling pathways. (A) Wnt/β-catenin signalling pathway. HBV can activate Wnt/β-catenin signaling by activating TCF or inhibiting GSK3β; HBx can silence the SFRPs to activate Wnt signaling; HBx promotes the disintegration of the E-cadherin complex with β-catenin; HBsAg can up–regulate the expression of LEF-1. (B) PI3K/AKT signalling pathway. HBx can inhibit PTEN, a suppressor of PIP3; HBx and HBx mutant can activate Akt directly. (C) MAPK/ERK signalling pathway. HBx can activate Ras and ERK directly; HBx can activate ERK by activating Notch signal. (D) Oxidative stress pathways. The structural proteins of HBV, HBx, HBS and HBC, can induce ROS production. ROS has been shown to play a direct promoting role in viral replication and malignant transformation. ROS can activate KEAP1/NRF2/ARE pathway, which plays an important role in resistance to ROS. Lines ending with arrows or bars indicate activating or inhibitory effects respectively.
Abbreviations: LEF, lymphoid enhancer-binding factor; LRP, low-density lipoprotein receptor-related protein; TCF, T cell factor; FZD, frizzled; E-cad, E-cadherin; SFRPs, secreted frizzled-related proteins; RTK, receptor protein tyrosine kinase; IRS-1, Insulin receptor substrate 1; HBx, hepatitis B X protein; HBx-Mut, HBx mutant; NICD, Notch intracellular domain; ROS, reactive oxygen species; HBC, hepatitis B core antigen; HBS, hepatitis B surface antigen; HBV, hepatitis B virus.
Wnt/β-Catenin Pathway
An abnormal activation of the Wnt/β-catenin signaling pathway has been observed in approximately 66% of patients with HCC.14 Various molecules, including the protein components of HBV, HCV, and HIF can activate the Wnt/β-catenin pathway in HCC cells.73,74 The activation of this pathway is closely related to the occurrence, development, stemness, and drug resistance of HCC.75,76 For example, HBx interacts directly with MyH9 to activate the Wnt/β-catenin/c-Jun signaling pathway, promoting the formation of stemness, metastasis, proliferation, and sorafenib resistance in HCC.77 HBx competitively binds to APC and replaces the degradable complex of β-catenin, which causes the accumulation of β-catenin in the nucleus and activates Wnt signaling, promoting malignant cell transformation.78 As mentioned above, HBV can maintain the activation of β-catenin through a variety of mechanisms, and is closely related to the expression of downstream cyclin D1 and c-Myc. In addition, activated β-catenin can also interact with other transcription factors, such as TCF and HIF-1α, to regulate the expression of target genes and promote disease progression.79
PI3K/AKT Pathway
The PI3K/Akt pathway is involved in the control of cell growth and proliferation and is abnormally activated in different tumors.80 As a tumor suppressor, PTEN can negatively regulate the PI3K/Akt pathway, while HBx can regulate liver cell proliferation and aggravate HBV-related carcinogenesis by inhibiting PTEN and activating Akt.81–83 HBx and Akt also regulate cell proliferation and malignant transformation through a synergistic effect.84 The HBx mutant K130M/V131I also promotes HCC formation by activating Akt signaling in a transgenic mouse model.85 Akt signaling is also involved in the regulation of HBV transcription and replication.63
MAPK Pathway
MAPK signaling plays an important role in HBV replication, immune escape, and the promotion of tumor cell survival and drug resistance. In HBV-infected cells, p38 MAPK plays a crucial role in HBsAg/HBeAg secretion, viral replication, and the formation of HBV cccDNA.86,87 In the presence of HBV, HBx induced the expression of pro-oncogenic MAPK14 and promoted tumorigenesis, while the inhibition of MAPK14 can largely reverse the HBV-induced resistance of HCC cells to sorafenib.88 HBx relieves the inhibition of ERK by activating Notch signaling, which promotes the growth of HCC.89 HBcAg induces the upregulation of B7-H1 through the activation of the Akt, ERK, and P38 signaling pathways, which inhibits the clearance of HBV DNA and promotes the apoptosis of dendritic cells,90 thereby benefiting HBV to evade immune surveillance.
Oxidative Stress Pathways
Oxidative stress plays an important role in tumor occurrence and development. Reactive oxygen species (ROS) levels are increased in the liver and blood of patients infected with HBV, which are related to the severity of the disease and the replication status of HBV.91–93 The structural proteins HBV, HBx, HBS, and HBC can also induce ROS production.94 For example, HBx can destroy mitochondrial membrane potential and induce ROS production by interacting with the mitochondrial outer membrane or affecting the respiratory complex.95,96 ROS have been shown to play a direct promoting role in HBV-induced liver fibrosis and liver cancer.97,98 The KEAP1/NRF2/ARE pathway plays an important role in anti-oxidation and resistance to ROS-induced damage.99 In HBV-infected tumor cells, activation of NRF2 can reduce the level of intracellular HBV pGRNA and inhibit viral replication, proving that intervening the expression of NRF2 might be an effective means of antiviral therapy.
HBV and Autophagy
In chronic infection, HBV can activate autophagy through different pathways,100 promoting the replication of HBV and the formation and release of virus particles, thus aggravating the disease process.101–103 For example, HBs can activate autophagy by triggering endoplasmic reticulum stress.104 HBx can enhance autophagy and promote viral replication by binding to PI3KC3 or by activating miR-192-3p-XIAP signaling.105 Recent studies have found that although the complete autophagy process can promote HBV replication, late autophagic degradation will degrade a certain amount of virus and HBsAg, suggesting that autophagy plays a dual role in HBV infection.106,107
In HCC, the level of autophagy is significantly reduced, and a large number of studies have shown that autophagy can inhibit tumorigenesis.108,109 Compared with paracancerous tissues, the expression levels of autophagy-related genes BECN1 and ATG5 were decreased in the tumor tissues of patients with HBV-associated HCC, accompanied by a reduced level of autophagy.110 Activation of autophagy can also selectively degrade cyclin D1 and block the cell cycle in the G1 phase, then inhibit DNA synthesis, cell proliferation, and HCC formation.111 In addition to affecting tumor cells, autophagy can also play an anti-tumor role by activating the immune response. HBx-modified tumor cells can act as a vaccine, which can activate T cell-mediated anti-tumor immune responses targeting HBx-positive HCC cells by inducing autophagy. These results suggest that autophagy can negatively modulate the tumorigenesis of HBV-associated HCC.112 Although a large number of studies have confirmed that the level and role of autophagy changes at different stages of HBV infection, how the mutual regulation of HBV and autophagy affects the disease progression from chronic hepatitis to tumor remains to be further explored.
HBV and Metabolism
In recent years, metabolic reprogramming has been considered an important mechanism in the development of malignant tumors. The liver is the metabolic site of carbohydrates, fat, and protein. Therefore, the occurrence and development of HCC is closely related to metabolic reprogramming. As a key pathogenic factor of HCC, HBV replication and viral proteins play a crucial role in the metabolic reprogramming of tumor cells and stromal cells. The newest multidimensional gene proteomics study systematically revealed the important role of abnormal liver-specific metabolic pathways in HBV-associated HCC. Multi-omics analysis of paired tumor and adjacent liver tissues from patients with HBV-associated HCC revealed that more than 80% of liver-specific genes were downregulated at the protein level. The expression of most proteins in liver-specific metabolic pathways is also significantly reduced in tumors. However, the key enzymes in cholesterol metabolism, ammonia/glutamine metabolism, and those in the glycolysis pathway were upregulated in tumors, accompanied by a significant activation of lipid biosynthesis-related enzymes.11 These results indicate that liver-specific metabolic pathways are reprogrammed in HBV-associated HCC and suggest that metabolic changes induced by HBV infection may be involved in the occurrence and development of HCC. HBx protein can promote the survival and stemness of tumor cells by upregulating glycolysis and fatty acid oxidation, resulting in the growth of tumors in mice.113,114 Ct-HBx can reshape glucose metabolism to promote the progression of HCC.24 The liver is an important glycogen storage and metabolic organ. The HBx-HDAC1 complex was found to inhibit the expression of glycogen synthase 2 (GYS2) and regulate glycogen metabolism by interacting with acetylated P53, thus promoting the growth of HCC.115 HBp protein can trigger glycolysis through the FoxO3/miRNA-30b-5p/MINPP1 signaling axis, enhancing the proliferation and migration of tumor cells.116
In addition to its influence on tumor cells, metabolic reprogramming induced by HBV may also play a role in promoting tumorigenesis by affecting the extracellular microenvironment. For example, HBV can help the virus evade innate immune recognition by activating glycolysis, promoting HK activity and lactic acid production, and blocking RLR signaling by inhibiting RIG-I/MAVS interactions.117 In livers with chronic HBV infection, amino acid depletion affects protein and nucleotide synthesis in T cells, which in turn affects the activation and function of these cells.118 This may be an important mechanism that promotes the progression of HBV-associated HCC. Currently, studies on specific HBV-associated mechanisms of metabolic reprogramming that are involved in the occurrence and development of HCC are still limited. Whether every structural protein of HBV can directly or indirectly regulate the key molecules in the metabolic pathways and participate in metabolic reprogramming, and whether the key enzymes in metabolic pathways play a tumor-promoting role with their non-metabolic enzyme activity in HBV-associated HCC, remain to be further explored.
HBV and Intestinal Microbiota
The intestinal flora plays an important role in human metabolism and physiological functions. Because of the existence of the “liver-gut axis,” intestinal flora and their metabolites can also influence the progression of liver disease. Chronic viral infection or cirrhosis remains a major cause of liver cancer. The disturbance of intestinal flora was higher in patients with CHB.119 Changes in intestinal flora composition and function in patients with early CHB suggest the potential role of intestinal flora in the progression of CHB.120 Changes in intestinal microbiota composition and diversity have also been detected in patients with HBV-associated HCC, and these microbiota have been shown to be involved in the regulation of different body functions.120,121 As an important pathogen-associated molecular pattern, LPS plays an important role in the progression of liver disease caused by the liver-gut axis. LPS promotes the secretion of inflammatory factors by activating TLR4/NF-κB, regulates the responses of hepatocytes, Kupffer cells, and hepatic stellate cells, and then promotes the occurrence of HCC.122 Regulating the composition of the intestinal flora has been proposed as an adjunct therapy to reduce bacterial translocation and prevent HCC progression. However, the pathogenesis of HBV-associated chronic liver disease and HCC caused by changes in the intestinal microbiota is still not fully understood.
HBV and Immune Tolerance
With the development of research, an increasing number of researchers have realized that the occurrence and development of tumors is not only due to the proliferation and malignant transformation of the cells themselves, but that the immunosuppressive microenvironment also plays a decisive role. A variety of stromal cells, including immune cells, form a complex regulatory network with tumor cells, which jointly promote the occurrence and development of malignant tumors.
HBV and Immunosuppressive Microenvironment
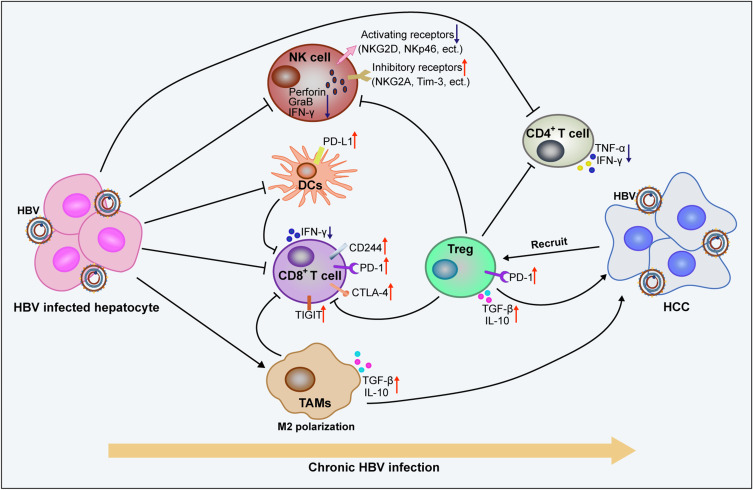
The inhibitory effect of HBV on the innate and adaptive immune cells leads to obstacles in virus recognition and clearance, which aggravates HBV-induced chronic inflammation and promotes the malignant transformation of liver cells (Figure 4). In patients with CHB, HBsAg can inhibit the expression and activation of STAT3 in NK cells, thus significantly inhibiting the activation and function of NK cells.123 Impaired NK cells show a defective clearance effect on HBV, which further accelerates the progression of hepatitis to HCC.124,125 HBeAg can further inhibit LPS-induced activation of the NLRP3 inflammasome and production of IL-1β by inhibiting NF-κB phosphorylation and ROS production, which contribute to the formation of HBV-induced immune tolerance.126 HBV can also inhibit the secretion of antiviral cytokines by macrophages and promote the polarization of cells from M1 to M2, which contributes to the establishment and maintenance of viral infection.127 HBcAg can induce the upregulation of B7-H1 and promote the apoptosis of DCs, which helps the immune escape of HBV.90 In the course of chronic HBV infection, the expression of PD-1, CTLA-4, CD244, and other inhibitory receptors on virus-specific CD8+ T cells increases, which mediates T cell depletion.128,129 It was also detected that the mitochondrial superoxide content in patients with CHB was significantly higher than that in healthy and cured infection groups, which correlated with CD8+ T cell depletion.130 All these negative HBV-induced immunomodulatory effects contribute to the progression of CHB to HCC. Interestingly, a high expression of TIGIT was found to maintain CD8+ T cell resistance to HBV in HBsAg transgenic mice. However, HBsAg vaccination could induce the activation of antigen-specific CTLs in the liver of TIGIT-blocked mice. Notably, the immune responses induced in the process of clearing the virus could lead to liver inflammation, which eventually develops into HCC.131 These findings suggest that the immune response plays an important role in the progression of hepatitis to HCC.
Figure 4.
Chronic HBV infection promotes the formation of immunosuppressive microenvironments and promotes the malignant transformation of liver cells. Long lines ending with arrows or bars indicate activating or inhibitory effects respectively. Short arrows pointing up or down indicate up-regulated or down-regulated.
Abbreviations: TAMs, tumor-associated macrophages; NK cell, natural killer cell; DCs, dendritic cells; Treg, regulatory T cell; HBV, hepatitis B virus.
The immunosuppressive microenvironment also plays an important role in promoting tumor progression. In patients with HBV-associated HCC, the increased peripheral blood neutrophil/lymphocyte ratio (NLR) and increased number of Foxp3+ Treg cells were positively correlated with disease progression.132,133 TGF-β-miR-34a-CCL22 signaling can induce Treg cell infiltration and promote HBV+ HCC metastasis.134 Moreover, the imbalance between Th17 and Treg cells is a risk factor for the progression of HCC in HBV-infected patients.135 Compared with patients with chronic hepatitis B or cirrhosis, the expression of PD-1 and TIGIT in peripheral blood CD4+ and CD8+ T cells is significantly upregulated in patients with HBV-associated HCC and is closely associated with accelerated disease progression and poor prognosis.136 The tumor microenvironment of HBV-associated HCC also showed more severe immunosuppression and exhaustion than that of non-virus-associated HCC,137 which contributes to the poor prognosis associated with HBV-associated HCC. The specific mechanisms by which HBV regulates the exhaustion of immune cells and promote the formation of an immunosuppressive tumor microenvironment remain to be further explored.
HBV and Extracellular Vesicles
Extracellular vesicles (EVs), as carriers and transporters, can directly transfer proteins, lipids, and nucleic acids between various cells in the microenvironment and play an important role in regulating the progression of malignant tumors. The level of miRNAs with negative immunologic modulation roles in EVs secreted by HBV-infected liver cells was increased. These EVs can be taken up by innate immune cells, inhibiting cellular function, and helping the virus resist the host immune response.138,139
As an important member of EVs, exosomes play an important regulatory role in HBV-related diseases.138 In addition to playing a regulatory role by delivering miRNAs, exosomes secreted by HBV-infected cells contain a variety of HBV RNA, DNA, and protein components. HBx can regulate the biosynthesis of exosomes in the host by increasing the packaging of HBx mRNA and protein, and delivering them to uninfected liver cells, thereby creating a microenvironment conducive to viral replication.140 In HBV transgenic mice, exosomes secreted by HBV-infected cells can also interfere with the clearance of HBV-replicating cells by inhibiting the immune response. HBV-positive exosomes derived from the serum of patients with CHB can be taken up by NK cells, thereby mediating the functional damage of NK cells and inhibiting the antiviral function of NK cells.141 These results indicate that exosomes can regulate viral infection, survival, and replication through intercellular communication. In tumor tissues, tumor-derived exosomes can transport IFITM2 to DCs, thereby inhibiting the secretion of IFN-α and blocking the antiviral effect of IFN-α.142 Exosomes derived from HBV+ HCC can help tumor cells resist chemotherapy.143
Prevention and Treatment
HBV is an important pathogenic factor for the occurrence and development of HCC; therefore, the prevention and treatment of viral infection is very important to reduce the incidence and mortality of HBV-associated HCC. Although HBV vaccination can effectively control the spread of the virus, the incidence of hepatitis B has not yet been significantly reduced due to the low vaccination rate among adults. At present, antiviral drugs for CHB include interferon and nucleoside/nucleotide analogs. Although antiviral therapy can significantly inhibit chronic HBV replication, it does not completely clear HBV with the current therapeutic methods.144
It has been demonstrated in chronic HBV-infected mice and human HBV+ HCC that 3p-HBx-siRNA inhibits viral replication, activates NK and T cells, promotes DC maturation, reduces the proportion of MDSCs, promotes the production of RIG-I-mediated type I interferon and proinflammatory cytokines, and activates the antiviral effect of the innate and adaptive immune systems.145 Targeting STAT3 can inhibit viral replication and inhibit proliferation and angiogenesis of HBV-associated HCC, showing a good anti-tumor effect in vivo.123 Exosomes have also been shown to mediate the transport of IFN-α from non-infected cells to virus-infected hepatocytes, playing an antiviral role.146,147 This suggests that exosomes can act as effective vectors for antiviral therapy. Exosomes can also play an antiviral role as immune adjuvants.148
In addition, research on the prevention and treatment of chronic hepatitis B with therapeutic vaccines has also made great progress in recent years. Vaccines based on HBx can restore the immune response of HBV-specific CD4+ T cells and CD8+ T cells, showing a good therapeutic effect on chronic HBV infection.149 The combined use of Poly I:C as an HBV vaccine adjuvant can overcome the systemic tolerance caused by chronic HBV infection, improve the function of DCs, reverse the exhaustion of HBV-specific CD8+ T cells, and induce the formation of long-term immune memory, which can efficiently prevent the recurrence of HBV infections.150 More recently, Wang et al designed a dual-targeting HBV pre-S1 nanoparticle vaccine based on ferritin nanoparticles, which can simultaneously target resident SIGNR1+ macrophages and SIGNR1+ DCs in mouse lymph nodes, and promote the activation of B cells and Tfh cells, inducing the production of antibodies. This nanoparticle vaccine induces a high-level and persistent anti-preS1 response, which results in an efficient viral clearance and a partial serological conversion in chronic HBV-infected mice, exerting a significant anti-HBV therapeutic effect.151
Conclusions
In conclusion, multiple mechanisms are involved in the regulation of the disease process from HBV infection to tumorigenesis. The occurrence and development of HBV-associated HCC is a result of the accumulation of multiple factors and the interactions of various mechanisms. With the development of research, new pathogenic mechanisms have been proposed, but there are still many problems that need to be solved. The prevention and treatment of HBV infection is important for the prevention and treatment of HCC. Anti-HBV therapy is still the main treatment for chronic hepatitis B; and although existing antiviral drugs can inhibit HBV replication, they are not effective in clearing HBV, and the incidence of HBV-associated HCC is still high due to repeated viral infection. Raising awareness regarding the importance of hepatitis B vaccination is the most cost-effective means of preventing HBV infection and transmission. New therapies, such as gene therapy and immunoregulatory therapy, are also being studied, but most of these studies are still in the laboratory stage. In terms of stability, drug resistance, and actual efficacy, further evaluation of these therapies is required. Also, further research on the mechanisms of the occurrence and development of HBV-associated HCC is of great significance for us to gain a more comprehensive understanding of the disease process and to find more effective therapeutic targets and strategies to treat HCC.
Acknowledgments
We are grateful to the Editage (www.editage.cn) for English language editing.
Funding Statement
This work was supported by the Natural Science Foundation of China (No. 81972694 and 81972686) and the National Major Science & Technology Project for Control and Prevention of Major Infectious Diseases in China (No.2018ZX10301401).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15(Suppl 4):5–13. doi: 10.1634/theoncologist.2010-S4-05 [DOI] [PubMed] [Google Scholar]
- 3.Donato F, Tagger A, Gelatti U, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002;155(4):323–331. doi: 10.1093/aje/155.4.323 [DOI] [PubMed] [Google Scholar]
- 4.Wild CP, Montesano R. A model of interaction: aflatoxins and hepatitis viruses in liver cancer aetiology and prevention. Cancer Lett. 2009;286(1):22–28. doi: 10.1016/j.canlet.2009.02.053 [DOI] [PubMed] [Google Scholar]
- 5.Liu CJ, Kao JH. Global perspective on the natural history of chronic hepatitis B: role of hepatitis B virus genotypes A to J. Semin Liver Dis. 2013;33(2):97–102. doi: 10.1055/s-0033-1345716 [DOI] [PubMed] [Google Scholar]
- 6.Wei F, Zheng Q, Li M, Wu M. The association between hepatitis B mutants and hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore). 2017;96(19):e6835. doi: 10.1097/MD.0000000000006835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pollicino T, Cacciola I, Saffioti F, Raimondo G. Hepatitis B virus PreS/S gene variants: pathobiology and clinical implications. J Hepatol. 2014;61(2):408–417. doi: 10.1016/j.jhep.2014.04.041 [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Wu Y, Deng M, et al. CD8(+) T-cell response-associated evolution of hepatitis B virus core protein and disease progress. J Virol. 2018;92(17). doi: 10.1128/JVI.02120-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu JF, Ni YH, Chen HL, Hsu HY, Chang MH. The impact of hepatitis B virus precore/core gene carboxyl terminal mutations on viral biosynthesis and the host immune response. J Infect Dis. 2014;209(9):1374–1381. doi: 10.1093/infdis/jit638 [DOI] [PubMed] [Google Scholar]
- 10.Sun L, Zhang Y, Zhao B, et al. A new unconventional HLA-A2-restricted epitope from HBV core protein elicits antiviral cytotoxic T lymphocytes. Protein Cell. 2014;5(4):317–327. doi: 10.1007/s13238-014-0041-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao Q, Zhu H, Dong L, et al. Integrated proteogenomic characterization of HBV-related hepatocellular carcinoma. Cell. 2019;179(2):561–577 e522. doi: 10.1016/j.cell.2019.08.052 [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Tao S, Liao L, et al. TRIM25 promotes the cell survival and growth of hepatocellular carcinoma through targeting Keap1-Nrf2 pathway. Nat Commun. 2020;11(1):348. doi: 10.1038/s41467-019-14190-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trung NT, Hoan NX, Trung PQ, et al. Clinical significance of combined circulating TERT promoter mutations and miR-122 expression for screening HBV-related hepatocellular carcinoma. Sci Rep. 2020;10(1):8181. doi: 10.1038/s41598-020-65213-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Totoki Y, Tatsuno K, Covington KR, et al. Trans-ancestry mutational landscape of hepatocellular carcinoma genomes. Nat Genet. 2014;46(12):1267–1273. doi: 10.1038/ng.3126 [DOI] [PubMed] [Google Scholar]
- 15.Hu C, Li W, Tian F, et al. Arid1a regulates response to anti-angiogenic therapy in advanced hepatocellular carcinoma. J Hepatol. 2018;68(3):465–475. doi: 10.1016/j.jhep.2017.10.028 [DOI] [PubMed] [Google Scholar]
- 16.Sun X, Ou Z, Chen R, et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63(1):173–184. doi: 10.1002/hep.28251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan X, Larsson C, Xu D. Mechanisms underlying the activation of TERT transcription and telomerase activity in human cancer: old actors and new players. Oncogene. 2019;38(34):6172–6183. doi: 10.1038/s41388-019-0872-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Y. Hepatitis B virus-associated hepatocellular carcinoma. Adv Exp Med Biol. 2017;1018:11–21. [DOI] [PubMed] [Google Scholar]
- 19.Brechot C, Gozuacik D, Murakami Y, Paterlini-Brechot P. Molecular bases for the development of hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC). Semin Cancer Biol. 2000;10(3):211–231. doi: 10.1006/scbi.2000.0321 [DOI] [PubMed] [Google Scholar]
- 20.Sung WK, Zheng H, Li S, et al. Genome-wide survey of recurrent HBV integration in hepatocellular carcinoma. Nat Genet. 2012;44(7):765–769. doi: 10.1038/ng.2295 [DOI] [PubMed] [Google Scholar]
- 21.Fujimoto A, Totoki Y, Abe T, et al. Whole-genome sequencing of liver cancers identifies etiological influences on mutation patterns and recurrent mutations in chromatin regulators. Nat Genet. 2012;44(7):760–764. doi: 10.1038/ng.2291 [DOI] [PubMed] [Google Scholar]
- 22.Matsubara K, Tokino T. Integration of hepatitis B virus DNA and its implications for hepatocarcinogenesis. Mol Biol Med. 1990;7(3):243–260. [PubMed] [Google Scholar]
- 23.Lee WY, Bachtiar M, Choo CCS, Lee CG. Comprehensive review of Hepatitis B virus-associated hepatocellular carcinoma research through text mining and big data analytics. Biol Rev Camb Philos Soc. 2019;94(2):353–367. doi: 10.1111/brv.12457 [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Yan Q, Gong L, et al. C-terminal truncated HBx initiates hepatocarcinogenesis by downregulating TXNIP and reprogramming glucose metabolism. Oncogene. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mao X, Tey SK, Ko FCF, et al. C-terminal truncated HBx protein activates caveolin-1/LRP6/beta-catenin/FRMD5 axis in promoting hepatocarcinogenesis. Cancer Lett. 2019;444:60–69. doi: 10.1016/j.canlet.2018.12.003 [DOI] [PubMed] [Google Scholar]
- 26.Li W, Li M, Liao D, et al. Carboxyl-terminal truncated HBx contributes to invasion and metastasis via deregulating metastasis suppressors in hepatocellular carcinoma. Oncotarget. 2016;7(34):55110–55127. doi: 10.18632/oncotarget.10399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sze KM, Chu GK, Lee JM, Ng IO. C-terminal truncated hepatitis B virus x protein is associated with metastasis and enhances invasiveness by C-Jun/matrix metalloproteinase protein 10 activation in hepatocellular carcinoma. Hepatology. 2013;57(1):131–139. doi: 10.1002/hep.25979 [DOI] [PubMed] [Google Scholar]
- 28.Choi YM, Lee SY, Kim BJ. Naturally occurring Hepatitis B virus mutations leading to endoplasmic reticulum stress and their contribution to the progression of hepatocellular carcinoma. Int J Mol Sci. 2019;20(3). doi: 10.3390/ijms20030597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montalbano R, Honrath B, Wissniowski TT, et al. Exogenous hepatitis B virus envelope proteins induce endoplasmic reticulum stress: involvement of cannabinoid axis in liver cancer cells. Oncotarget. 2016;7(15):20312–20323. doi: 10.18632/oncotarget.7950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Zhang J, Yang Z, et al. The function of targeted host genes determines the oncogenicity of HBV integration in hepatocellular carcinoma. J Hepatol. 2014;60(5):975–984. doi: 10.1016/j.jhep.2013.12.014 [DOI] [PubMed] [Google Scholar]
- 31.Zhao LH, Liu X, Yan HX, et al. Genomic and oncogenic preference of HBV integration in hepatocellular carcinoma. Nat Commun. 2016;7:12992. doi: 10.1038/ncomms12992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li W, Zeng X, Lee NP, et al. HIVID: an efficient method to detect HBV integration using low coverage sequencing. Genomics. 2013;102(4):338–344. doi: 10.1016/j.ygeno.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 33.Yang L, Ye S, Zhao X, et al. Molecular characterization of HBV DNA integration in patients with hepatitis and hepatocellular carcinoma. J Cancer. 2018;9(18):3225–3235. doi: 10.7150/jca.26052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Souza S, Lau KC, Coffin CS, Patel TR. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J Gastroenterol. 2020;26(38):5759–5783. doi: 10.3748/wjg.v26.i38.5759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toh ST, Jin Y, Liu L, et al. Deep sequencing of the hepatitis B virus in hepatocellular carcinoma patients reveals enriched integration events, structural alterations and sequence variations. Carcinogenesis. 2013;34(4):787–798. doi: 10.1093/carcin/bgs406 [DOI] [PubMed] [Google Scholar]
- 36.Nault JC, Ningarhari M, Rebouissou S, Zucman-Rossi J. The role of telomeres and telomerase in cirrhosis and liver cancer. Nat Rev Gastroenterol Hepatol. 2019;16(9):544–558. doi: 10.1038/s41575-019-0165-3 [DOI] [PubMed] [Google Scholar]
- 37.Sze KM, Ho DW, Chiu YT, et al. Hepatitis B virus-telomerase reverse transcriptase promoter integration harnesses host ELF4, resulting in telomerase reverse transcriptase gene transcription in hepatocellular carcinoma. Hepatology. 2020;73:23–40. doi: 10.1002/hep.31231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saitta C, Tripodi G, Barbera A, et al. Hepatitis B virus (HBV) DNA integration in patients with occult HBV infection and hepatocellular carcinoma. Liver Int. 2015;35(10):2311–2317. doi: 10.1111/liv.12807 [DOI] [PubMed] [Google Scholar]
- 39.Wong DK, Cheng SCY, Mak LL, et al. Among patients with undetectable Hepatitis B surface antigen and hepatocellular carcinoma, a high proportion has integration of HBV DNA into hepatocyte DNA and no cirrhosis. Clin Gastroenterol Hepatol. 2020;18(2):449–456. doi: 10.1016/j.cgh.2019.06.029 [DOI] [PubMed] [Google Scholar]
- 40.Matsuzaki Y, Chiba T, Hadama T, et al. HBV genome integration and genetic instability in HBsAg-negative and anti-HCV-positive hepatocellular carcinoma in Japan. Cancer Lett. 1997;119(1):53–61. doi: 10.1016/S0304-3835(97)00249-8 [DOI] [PubMed] [Google Scholar]
- 41.Mak LY, Wong DK, Pollicino T, Raimondo G, Hollinger FB, Yuen MF. Occult hepatitis B infection and hepatocellular carcinoma: epidemiology, virology, hepatocarcinogenesis and clinical significance. J Hepatol. 2020;73(4):952–964. doi: 10.1016/j.jhep.2020.05.042 [DOI] [PubMed] [Google Scholar]
- 42.Chen L, Ming X, Li W, et al. The microRNA-155 mediates hepatitis B virus replication by reinforcing SOCS1 signalling-induced autophagy. Cell Biochem Funct. 2020;38(4):436–442. doi: 10.1002/cbf.3488 [DOI] [PubMed] [Google Scholar]
- 43.Dai X, Zhang W, Zhang H, et al. Modulation of HBV replication by microRNA-15b through targeting hepatocyte nuclear factor 1alpha. Nucleic Acids Res. 2014;42(10):6578–6590. doi: 10.1093/nar/gku260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin J, Tang S, Xia L, et al. MicroRNA-501 promotes HBV replication by targeting HBXIP. Biochem Biophys Res Commun. 2013;430(4):1228–1233. doi: 10.1016/j.bbrc.2012.12.071 [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Li Y. miR-146 promotes HBV replication and expression by targeting ZEB2. Biomed Pharmacother. 2018;99:576–582. doi: 10.1016/j.biopha.2018.01.097 [DOI] [PubMed] [Google Scholar]
- 46.Hou Z, Zhang J, Han Q, et al. Hepatitis B virus inhibits intrinsic RIG-I and RIG-G immune signaling via inducing miR146a. Sci Rep. 2016;6:26150. doi: 10.1038/srep26150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hou ZH, Han QJ, Zhang C, Tian ZG, Zhang J. miR146a impairs the IFN-induced anti-HBV immune response by downregulating STAT1 in hepatocytes. Liver Int. 2014;34(1):58–68. doi: 10.1111/liv.12244 [DOI] [PubMed] [Google Scholar]
- 48.Wang G, Dong F, Xu Z, et al. MicroRNA profile in HBV-induced infection and hepatocellular carcinoma. BMC Cancer. 2017;17(1):805. doi: 10.1186/s12885-017-3816-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morishita A, Fujita K, Iwama H, et al. Role of microRNA-210-3p in hepatitis B virus-related hepatocellular carcinoma. Am J Physiol Gastrointest Liver Physiol. 2020;318(3):G401–G409. doi: 10.1152/ajpgi.00269.2019 [DOI] [PubMed] [Google Scholar]
- 50.Zhu HT, Liu RB, Liang YY, et al. Serum microRNA profiles as diagnostic biomarkers for HBV-positive hepatocellular carcinoma. Liver Int. 2017;37(6):888–896. doi: 10.1111/liv.13356 [DOI] [PubMed] [Google Scholar]
- 51.Chen S, Chen H, Gao S, et al. Differential expression of plasma microRNA-125b in hepatitis B virus-related liver diseases and diagnostic potential for hepatitis B virus-induced hepatocellular carcinoma. Hepatol Res. 2017;47(4):312–320. doi: 10.1111/hepr.12739 [DOI] [PubMed] [Google Scholar]
- 52.Li R, Xu T, Wang H, et al. Dysregulation of the miR-325-3p/DPAGT1 axis supports HBV-positive HCC chemoresistance. Biochem Biophys Res Commun. 2019;519(2):358–365. doi: 10.1016/j.bbrc.2019.08.116 [DOI] [PubMed] [Google Scholar]
- 53.Sadri Nahand J, Bokharaei-Salim F, Salmaninejad A, et al. microRNAs: key players in virus-associated hepatocellular carcinoma. J Cell Physiol. 2019;234(8):12188–12225. doi: 10.1002/jcp.27956 [DOI] [PubMed] [Google Scholar]
- 54.Tian JH, Liu WD, Zhang ZY, et al. Influence of miR-520e-mediated MAPK signaling pathway on HBV replication and regulation of hepatocellular carcinoma cells via targeting EphA2. J Viral Hepat. 2018. [DOI] [PubMed] [Google Scholar]
- 55.Lou W, Liu J, Ding B, et al. Identification of potential miRNA-mRNA regulatory network contributing to pathogenesis of HBV-related HCC. J Transl Med. 2019;17(1):7. doi: 10.1186/s12967-018-1761-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kong XX, Lv YR, Shao LP, et al. HBx-induced MiR-1269b in NF-kappaB dependent manner upregulates cell division cycle 40 homolog (CDC40) to promote proliferation and migration in hepatoma cells. J Transl Med. 2016;14(1):189. doi: 10.1186/s12967-016-0949-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J, Chen J, Liu Y, et al. Hepatitis B virus induces autophagy to promote its replication by the axis of miR-192-3p-XIAP through NF kappa B signaling. Hepatology. 2019;69(3):974–992. doi: 10.1002/hep.30248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yao L, Zhou Y, Sui Z, et al. HBV-encoded miR-2 functions as an oncogene by downregulating TRIM35 but upregulating RAN in liver cancer cells. EBioMedicine. 2019;48:117–129. doi: 10.1016/j.ebiom.2019.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Zhao X, Sun L, Mu T, et al. An HBV-encoded miRNA activates innate immunity to restrict HBV replication. J Mol Cell Biol. 2020;12(4):263–276. doi: 10.1093/jmcb/mjz104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chavalit T, Nimsamer P, Sirivassanametha K, et al. Hepatitis B virus-encoded microRNA (HBV-miR-3) regulates host gene PPM1A related to hepatocellular carcinoma. Microrna. 2020;9(3):232–239. doi: 10.2174/2211536608666191104105334 [DOI] [PubMed] [Google Scholar]
- 61.Liang HW, Wang N, Wang Y, et al. Hepatitis B virus-human chimeric transcript HBx-LINE1 promotes hepatic injury via sequestering cellular microRNA-122. J Hepatol. 2016;64(2):278–291. doi: 10.1016/j.jhep.2015.09.013 [DOI] [PubMed] [Google Scholar]
- 62.Sartorius B, Makarova J, Sartorius B, et al. The regulatory role of microRNA in Hepatitis-B Virus-Associated Hepatocellular Carcinoma (HBV-HCC) pathogenesis. Cells. 2019;8(12):1504. doi: 10.3390/cells8121504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun W, Zhang J, Chen J. MicroRNA-520a suppresses HBV replication in HepG2.2.15 cells by inactivating AKT. J Int Med Res. 2018;46(11):4693–4704. doi: 10.1177/0300060518792780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hamada-Tsutsumi S, Naito Y, Sato S, et al. The antiviral effects of human microRNA miR-302c-3p against hepatitis B virus infection. Aliment Pharmacol Ther. 2019;49(8):1060–1070. doi: 10.1111/apt.15197 [DOI] [PubMed] [Google Scholar]
- 65.Mao K, Zhang J, He C, et al. Restoration of miR-193b sensitizes Hepatitis B virus-associated hepatocellular carcinoma to sorafenib. Cancer Lett. 2014;352(2):245–252. doi: 10.1016/j.canlet.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 66.Flavahan WA, Gaskell E, Bernstein BE. Epigenetic plasticity and the hallmarks of cancer. Science. 2017;357:6348. doi: 10.1126/science.aal2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song MA, Kwee SA, Tiirikainen M, et al. Comparison of genome-scale DNA methylation profiles in hepatocellular carcinoma by viral status. Epigenetics. 2016;11(6):464–474. doi: 10.1080/15592294.2016.1151586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park IY, Sohn BH, Yu E, et al. Aberrant epigenetic modifications in hepatocarcinogenesis induced by hepatitis B virus X protein. Gastroenterology. 2007;132(4):1476–1494. doi: 10.1053/j.gastro.2007.01.034 [DOI] [PubMed] [Google Scholar]
- 69.Zhao J, Wu G, Bu F, et al. Epigenetic silence of ankyrin-repeat-containing, SH3-domain-containing, and proline-rich-region- containing protein 1 (ASPP1) and ASPP2 genes promotes tumor growth in hepatitis B virus-positive hepatocellular carcinoma. Hepatology. 2010;51(1):142–153. doi: 10.1002/hep.23247 [DOI] [PubMed] [Google Scholar]
- 70.Gao W, Jia Z, Tian Y, et al. HBx protein contributes to liver carcinogenesis by H3K4me3 modification through stabilizing WD repeat domain 5 protein. Hepatology. 2020;71(5):1678–1695. doi: 10.1002/hep.30947 [DOI] [PubMed] [Google Scholar]
- 71.Yoo YG, Na TY, Seo HW, et al. Hepatitis B virus X protein induces the expression of MTA1 and HDAC1, which enhances hypoxia signaling in hepatocellular carcinoma cells. Oncogene. 2008;27(24):3405–3413. doi: 10.1038/sj.onc.1211000 [DOI] [PubMed] [Google Scholar]
- 72.Salerno D, Chiodo L, Alfano V, et al. Hepatitis B protein HBx binds the DLEU2 lncRNA to sustain cccDNA and host cancer-related gene transcription. Gut. 2020;69(11):2016–2024. doi: 10.1136/gutjnl-2019-319637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aicher S, Kakkanas A, Cohen L, et al. Differential regulation of the Wnt/beta-catenin pathway by hepatitis C virus recombinants expressing core from various genotypes. Sci Rep. 2018;8(1):11185. doi: 10.1038/s41598-018-29078-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Daud M, Rana MA, Husnain T, Ijaz B. Modulation of Wnt signaling pathway by hepatitis B virus. Arch Virol. 2017;162(10):2937–2947. doi: 10.1007/s00705-017-3462-6 [DOI] [PubMed] [Google Scholar]
- 75.Liu Y, Ye X, Zhang JB, et al. PROX1 promotes hepatocellular carcinoma proliferation and sorafenib resistance by enhancing beta-catenin expression and nuclear translocation. Oncogene. 2015;34(44):5524–5535. doi: 10.1038/onc.2015.7 [DOI] [PubMed] [Google Scholar]
- 76.Huang M, Chen C, Geng J, et al. Targeting KDM1A attenuates Wnt/beta-catenin signaling pathway to eliminate sorafenib-resistant stem-like cells in hepatocellular carcinoma. Cancer Lett. 2017;398:12–21. doi: 10.1016/j.canlet.2017.03.038 [DOI] [PubMed] [Google Scholar]
- 77.Lin X, Li AM, Li YH, et al. Silencing MYH9 blocks HBx-induced GSK3beta ubiquitination and degradation to inhibit tumor stemness in hepatocellular carcinoma. Signal Transduct Target Ther. 2020;5(1):13. doi: 10.1038/s41392-020-0111-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hsieh A, Kim HS, Lim SO, Yu DY, Jung G. Hepatitis B viral X protein interacts with tumor suppressor adenomatous polyposis coli to activate Wnt/beta-catenin signaling. Cancer Lett. 2011;300(2):162–172. doi: 10.1016/j.canlet.2010.09.018 [DOI] [PubMed] [Google Scholar]
- 79.Monga SP. Beta-catenin signaling and roles in liver homeostasis, injury, and tumorigenesis. Gastroenterology. 2015;148(7):1294–1310. doi: 10.1053/j.gastro.2015.02.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8(8):627–644. doi: 10.1038/nrd2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chung TW, Lee YC, Ko JH, Kim CH. Hepatitis B virus X protein modulates the expression of PTEN by inhibiting the function of p53, a transcriptional activator in liver cells. Cancer Res. 2003;63(13):3453–3458. [PubMed] [Google Scholar]
- 82.Ha HL, Yu DY. HBx-induced reactive oxygen species activates hepatocellular carcinogenesis via dysregulation of PTEN/Akt pathway. World J Gastroenterol. 2010;16(39):4932–4937. doi: 10.3748/wjg.v16.i39.4932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim GW, Imam H, Khan M, et al. HBV-induced increased N6 methyladenosine modification of PTEN RNA affects innate immunity and contributes to HCC. Hepatology. 2020;73:533–547. doi: 10.1002/hep.31313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khattar E, Mukherji A, Kumar V. Akt augments the oncogenic potential of the HBx protein of hepatitis B virus by phosphorylation. FEBS J. 2012;279(7):1220–1230. doi: 10.1111/j.1742-4658.2012.08514.x [DOI] [PubMed] [Google Scholar]
- 85.Chiu AP, Tschida BR, Sham TT, et al. HBx-K130M/V131I promotes liver cancer in transgenic mice via AKT/FOXO1 signaling pathway and arachidonic acid metabolism. Mol Cancer Res. 2019;17(7):1582–1593. doi: 10.1158/1541-7786.MCR-18-1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chang WW, Su IJ, Chang WT, Huang W, Lei HY, Lei H-Y. Suppression of p38 mitogen-activated protein kinase inhibits hepatitis B virus replication in human hepatoma cell: the antiviral role of nitric oxide. J Viral Hepat. 2008;15(7):490–497. doi: 10.1111/j.1365-2893.2007.00968.x [DOI] [PubMed] [Google Scholar]
- 87.Kim SY, Kim H, Kim SW, et al. An effective antiviral approach targeting Hepatitis B virus with NJK14047, a novel and selective biphenyl amide p38 mitogen-activated protein kinase inhibitor. Antimicrob Agents Chemother. 2017;61(8). doi: 10.1128/AAC.00214-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Witt-Kehati D, Fridkin A, Alaluf MB, Zemel R, Shlomai A. Inhibition of pMAPK14 overcomes resistance to sorafenib in hepatoma cells with Hepatitis B virus. Transl Oncol. 2018;11(2):511–517. doi: 10.1016/j.tranon.2018.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liao B, Zhou H, Liang H, Li C. Regulation of ERK and AKT pathways by hepatitis B virus X protein via the Notch1 pathway in hepatocellular carcinoma. Int J Oncol. 2017;51(5):1449–1459. doi: 10.3892/ijo.2017.4126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li M, Zhou ZH, Sun XH, et al. Hepatitis B core antigen upregulates B7-H1 on dendritic cells by activating the AKT/ERK/P38 pathway: a possible mechanism of hepatitis B virus persistence. Lab Invest. 2016;96(11):1156–1164. doi: 10.1038/labinvest.2016.96 [DOI] [PubMed] [Google Scholar]
- 91.Valgimigli M, Valgimigli L, Trere D, et al. Oxidative stress EPR measurement in human liver by radical-probe technique. Correlation with etiology, histology and cell proliferation. Free Radic Res. 2002;36(9):939–948. doi: 10.1080/107156021000006653 [DOI] [PubMed] [Google Scholar]
- 92.Fujita N, Sugimoto R, Ma N, et al. Comparison of hepatic oxidative DNA damage in patients with chronic hepatitis B and C. J Viral Hepat. 2008;15(7):498–507. doi: 10.1111/j.1365-2893.2008.00972.x [DOI] [PubMed] [Google Scholar]
- 93.Bolukbas C, Bolukbas FF, Horoz M, Aslan M, Celik H, Erel O. Increased oxidative stress associated with the severity of the liver disease in various forms of hepatitis B virus infection. BMC Infect Dis. 2005;5:95. doi: 10.1186/1471-2334-5-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ivanov AV, Valuev-Elliston VT, Tyurina DA, et al. Oxidative stress, a trigger of hepatitis C and B virus-induced liver carcinogenesis. Oncotarget. 2017;8(3):3895–3932. doi: 10.18632/oncotarget.13904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee HR, Cho YY, Lee GY, You DG, Yoo YD, Kim YJ. A direct role for hepatitis B virus X protein in inducing mitochondrial membrane permeabilization. J Viral Hepat. 2018;25(4):412–420. doi: 10.1111/jvh.12831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee YI, Hwang JM, Im JH, et al. Human hepatitis B virus-X protein alters mitochondrial function and physiology in human liver cells. J Biol Chem. 2004;279(15):15460–15471. doi: 10.1074/jbc.M309280200 [DOI] [PubMed] [Google Scholar]
- 97.Xie H, Xie D, Zhang J, et al. ROS/NF-kappaB signaling pathway-mediated transcriptional activation of TRIM37 promotes HBV-associated hepatic fibrosis. Mol Ther Nucleic Acids. 2020;22:114–123. doi: 10.1016/j.omtn.2020.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yuan K, Lei Y, Chen HN, et al. HBV-induced ROS accumulation promotes hepatocarcinogenesis through Snail-mediated epigenetic silencing of SOCS3. Cell Death Differ. 2016;23(4):616–627. doi: 10.1038/cdd.2015.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046 [DOI] [PubMed] [Google Scholar]
- 100.Zhang HT, Chen GG, Hu BG, et al. Hepatitis B virus x protein induces autophagy via activating death-associated protein kinase. J Viral Hepat. 2014;21(9):642–649. doi: 10.1111/jvh.12191 [DOI] [PubMed] [Google Scholar]
- 101.Wang X, Lin Y, Liu S, et al. O-GlcNAcylation modulates HBV replication through regulating cellular autophagy at multiple levels. FASEB J. 2020;34(11):14473–14489. doi: 10.1096/fj.202001168RR [DOI] [PubMed] [Google Scholar]
- 102.He Q, Song X, Huang Y, et al. Dexamethasone stimulates Hepatitis B Virus (HBV) replication through autophagy. Med Sci Monit. 2018;24:4617–4624. doi: 10.12659/MSM.906250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xie M, Yang Z, Liu Y, Zheng M. The role of HBV-induced autophagy in HBV replication and HBV related-HCC. Life Sci. 2018;205:107–112. doi: 10.1016/j.lfs.2018.04.051 [DOI] [PubMed] [Google Scholar]
- 104.Li J, Liu Y, Wang Z, et al. Subversion of cellular autophagy machinery by hepatitis B virus for viral envelopment. J Virol. 2011;85(13):6319–6333. doi: 10.1128/JVI.02627-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sir D, Tian Y, Chen WL, Ann DK, Yen TS, Ou JH. The early autophagic pathway is activated by hepatitis B virus and required for viral DNA replication. Proc Natl Acad Sci U S A. 2010;107(9):4383–4388. doi: 10.1073/pnas.0911373107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin Y, Wu C, Wang X, et al. Hepatitis B virus is degraded by autophagosome-lysosome fusion mediated by Rab7 and related components. Protein Cell. 2019;10(1):60–66. doi: 10.1007/s13238-018-0555-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lin Y, Wu C, Wang X, et al. Synaptosomal-associated protein 29 is required for the autophagic degradation of hepatitis B virus. FASEB J. 2019;33(5):6023–6034. doi: 10.1096/fj.201801995RR [DOI] [PubMed] [Google Scholar]
- 108.Rautou PE, Mansouri A, Lebrec D, Durand F, Valla D, Moreau R. Autophagy in liver diseases. J Hepatol. 2010;53(6):1123–1134. doi: 10.1016/j.jhep.2010.07.006 [DOI] [PubMed] [Google Scholar]
- 109.Zhang H, Zhang Y, Zhu X, et al. DEAD box protein 5 inhibits liver tumorigenesis by stimulating autophagy via interaction with p62/SQSTM1. Hepatology. 2019;69(3):1046–1063. doi: 10.1002/hep.30300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lan SH, Wu SY, Zuchini R, et al. Autophagy suppresses tumorigenesis of hepatitis B virus-associated hepatocellular carcinoma through degradation of microRNA-224. Hepatology. 2014;59(2):505–517. doi: 10.1002/hep.26659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu SY, Lan SH, Liu HS. Degradative autophagy selectively regulates CCND1 (cyclin D1) and MIR224, two oncogenic factors involved in hepatocellular carcinoma tumorigenesis. Autophagy. 2019;15(4):729–730. doi: 10.1080/15548627.2019.1569918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yan Y, Liu N, Lu L, et al. Autophagy enhances antitumor immune responses induced by irradiated hepatocellular carcinoma cells engineered to express hepatitis B virus X protein. Oncol Rep. 2013;30(2):993–999. doi: 10.3892/or.2013.2531 [DOI] [PubMed] [Google Scholar]
- 113.Chen YY, Wang WH, Che L, et al. BNIP3L-dependent mitophagy promotes HBx-induced cancer stemness of hepatocellular carcinoma cells via glycolysis metabolism reprogramming. Cancers (Basel). 2020;12(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang MD, Wu H, Huang S, et al. HBx regulates fatty acid oxidation to promote hepatocellular carcinoma survival during metabolic stress. Oncotarget. 2016;7(6):6711–6726. doi: 10.18632/oncotarget.6817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen SL, Zhang CZ, Liu LL, et al. A GYS2/p53 negative feedback loop restricts tumor growth in HBV-related hepatocellular carcinoma. Cancer Res. 2019;79(3):534–545. doi: 10.1158/0008-5472.CAN-18-2357 [DOI] [PubMed] [Google Scholar]
- 116.Chen W, Jiang J, Gong L, et al. Hepatitis B virus P protein initiates glycolytic bypass in HBV-related hepatocellular carcinoma via a FOXO3/miRNA-30b-5p/MINPP1 axis. J Exp Clin Cancer Res. 2021;40(1):1. doi: 10.1186/s13046-020-01803-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhou L, He R, Fang P, et al. Hepatitis B virus rigs the cellular metabolome to avoid innate immune recognition. Nat Commun. 2021;12(1):98. doi: 10.1038/s41467-020-20316-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fisicaro P, Boni C, Barili V, Laccabue D, Ferrari C. Strategies to overcome HBV-specific T cell exhaustion: checkpoint inhibitors and metabolic re-programming. Curr Opin Virol. 2018;30:1–8. doi: 10.1016/j.coviro.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 119.Kapil S, Duseja A, Sharma BK, et al. Small intestinal bacterial overgrowth and toll-like receptor signaling in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2016;31(1):213–221. doi: 10.1111/jgh.13058 [DOI] [PubMed] [Google Scholar]
- 120.Zeng Y, Chen S, Fu Y, et al. Gut microbiota dysbiosis in patients with hepatitis B virus-induced chronic liver disease covering chronic hepatitis, liver cirrhosis and hepatocellular carcinoma. J Viral Hepat. 2020;27(2):143–155. doi: 10.1111/jvh.13216 [DOI] [PubMed] [Google Scholar]
- 121.Liu Q, Li F, Zhuang Y, et al. Alteration in gut microbiota associated with hepatitis B and non-hepatitis virus related hepatocellular carcinoma. Gut Pathog. 2019;11:1. doi: 10.1186/s13099-018-0281-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Toffanin S, Cornella H, Harrington A, Llovet JM. HCC is promoted by bacterial translocation and TLR-4 signaling: a new paradigm for chemoprevention and management. Hepatology. 2012;56(5):1998–2000. doi: 10.1002/hep.26080 [DOI] [PubMed] [Google Scholar]
- 123.Zheng B, Yang Y, Han Q, Yin C, Pan Z, Zhang J. STAT3 directly regulates NKp46 transcription in NK cells of HBeAg-negative CHB patients. J Leukoc Biol. 2019;106(4):987–996. doi: 10.1002/JLB.2A1118-421R [DOI] [PubMed] [Google Scholar]
- 124.Sun C, Sun H, Zhang C, Tian Z. NK cell receptor imbalance and NK cell dysfunction in HBV infection and hepatocellular carcinoma. Cell Mol Immunol. 2015;12(3):292–302. doi: 10.1038/cmi.2014.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu D, Han Q, Hou Z, Zhang C, Zhang J. miR-146a negatively regulates NK cell functions via STAT1 signaling. Cell Mol Immunol. 2017;14(8):712–720. doi: 10.1038/cmi.2015.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yu X, Lan P, Hou X, et al. HBV inhibits LPS-induced NLRP3 inflammasome activation and IL-1beta production via suppressing the NF-kappaB pathway and ROS production. J Hepatol. 2017;66(4):693–702. doi: 10.1016/j.jhep.2016.12.018 [DOI] [PubMed] [Google Scholar]
- 127.Faure-Dupuy S, Delphin M, Aillot L, et al. Hepatitis B virus-induced modulation of liver macrophage function promotes hepatocyte infection. J Hepatol. 2019;71(6):1086–1098. doi: 10.1016/j.jhep.2019.06.032 [DOI] [PubMed] [Google Scholar]
- 128.Raziorrouh B, Schraut W, Gerlach T, et al. The immunoregulatory role of CD244 in chronic hepatitis B infection and its inhibitory potential on virus-specific CD8+ T-cell function. Hepatology. 2010;52(6):1934–1947. doi: 10.1002/hep.23936 [DOI] [PubMed] [Google Scholar]
- 129.Tjwa ET, van Oord GW, Hegmans JP, Janssen HL, Woltman AM. Viral load reduction improves activation and function of natural killer cells in patients with chronic hepatitis B. J Hepatol. 2011;54(2):209–218. doi: 10.1016/j.jhep.2010.07.009 [DOI] [PubMed] [Google Scholar]
- 130.Fisicaro P, Barili V, Montanini B, et al. Targeting mitochondrial dysfunction can restore antiviral activity of exhausted HBV-specific CD8 T cells in chronic hepatitis B. Nat Med. 2017;23(3):327–336. doi: 10.1038/nm.4275 [DOI] [PubMed] [Google Scholar]
- 131.Zong L, Peng H, Sun C, et al. Breakdown of adaptive immunotolerance induces hepatocellular carcinoma in HBsAg-tg mice. Nat Commun. 2019;10(1):221. doi: 10.1038/s41467-018-08096-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu X, He L, Han J, et al. Association of neutrophil-lymphocyte ratio and T lymphocytes with the pathogenesis and progression of HBV-associated primary liver cancer. PLoS One. 2017;12(2):e0170605. doi: 10.1371/journal.pone.0170605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tajiri K, Baba H, Kawai K, et al. Neutrophil-to-lymphocyte ratio predicts recurrence after radiofrequency ablation in hepatitis B virus infection. J Gastroenterol Hepatol. 2016;31(7):1291–1299. doi: 10.1111/jgh.13287 [DOI] [PubMed] [Google Scholar]
- 134.Yang P, Li QJ, Feng Y, et al. TGF-beta-miR-34a-CCL22 signaling-induced Treg cell recruitment promotes venous metastases of HBV-positive hepatocellular carcinoma. Cancer Cell. 2012;22(3):291–303. doi: 10.1016/j.ccr.2012.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li K, Liu H, Guo T. Th17/Treg imbalance is an indicator of liver cirrhosis process and a risk factor for HCC occurrence in HBV patients. Clin Res Hepatol Gastroenterol. 2017;41(4):399–407. doi: 10.1016/j.clinre.2016.12.004 [DOI] [PubMed] [Google Scholar]
- 136.Liu X, Li M, Wang X, et al. PD-1(+) TIGIT(+) CD8(+) T cells are associated with pathogenesis and progression of patients with hepatitis B virus-related hepatocellular carcinoma. Cancer Immunol Immunother. 2019;68(12):2041–2054. doi: 10.1007/s00262-019-02426-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lim CJ, Lee YH, Pan L, et al. Multidimensional analyses reveal distinct immune microenvironment in hepatitis B virus-related hepatocellular carcinoma. Gut. 2019;68(5):916–927. doi: 10.1136/gutjnl-2018-316510 [DOI] [PubMed] [Google Scholar]
- 138.Li S, Li S, Wu S, Chen L. Exosomes modulate the viral replication and host immune responses in HBV infection. Biomed Res Int. 2019;2019:2103943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kakizaki M, Yamamoto Y, Yabuta S, Kurosaki N, Kagawa T, Kotani A. The immunological function of extracellular vesicles in hepatitis B virus-infected hepatocytes. PLoS One. 2018;13(12):e0205886. doi: 10.1371/journal.pone.0205886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kapoor NR, Chadha R, Kumar S, Choedon T, Reddy VS, Kumar V. The HBx gene of hepatitis B virus can influence hepatic microenvironment via exosomes by transferring its mRNA and protein. Virus Res. 2017;240:166–174. doi: 10.1016/j.virusres.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 141.Yang Y, Han Q, Hou Z, Zhang C, Tian Z, Zhang J. Exosomes mediate hepatitis B virus (HBV) transmission and NK-cell dysfunction. Cell Mol Immunol. 2017;14(5):465–475. doi: 10.1038/cmi.2016.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shi Y, Du L, Lv D, et al. Exosomal interferon-induced transmembrane protein 2 transmitted to dendritic cells inhibits interferon alpha pathway activation and blocks anti-Hepatitis B virus efficacy of exogenous interferon alpha. Hepatology. 2019;69(6):2396–2413. doi: 10.1002/hep.30548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu DX, Li PP, Guo JP, et al. Exosomes derived from HBV-associated liver cancer promote chemoresistance by upregulating chaperone-mediated autophagy. Oncol Lett. 2019;17(1):323–331. doi: 10.3892/ol.2018.9584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Petersen J, Thompson AJ, Levrero M. Aiming for cure in HBV and HDV infection. J Hepatol. 2016;65(4):835–848. doi: 10.1016/j.jhep.2016.05.043 [DOI] [PubMed] [Google Scholar]
- 145.Han Q, Hou Z, Yin C, Zhang C, Zhang J. 5ʹ-triphosphate siRNA targeting HBx elicits a potent anti-HBV immune response in pAAV-HBV transfected mice. Antiviral Res. 2019;161:36–45. doi: 10.1016/j.antiviral.2018.11.006 [DOI] [PubMed] [Google Scholar]
- 146.Yao Z, Qiao Y, Li X, et al. Exosomes exploit the virus entry machinery and pathway to transmit alpha interferon-induced antiviral activity. J Virol. 2018;92(24). doi: 10.1128/JVI.01578-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li J, Liu K, Liu Y, et al. Exosomes mediate the cell-to-cell transmission of IFN-alpha-induced antiviral activity. Nat Immunol. 2013;14(8):793–803. doi: 10.1038/ni.2647 [DOI] [PubMed] [Google Scholar]
- 148.Jesus S, Soares E, Cruz MT, Borges O. Exosomes as adjuvants for the recombinant hepatitis B antigen: first report. Eur J Pharm Biopharm. 2018;133:1–11. doi: 10.1016/j.ejpb.2018.09.029 [DOI] [PubMed] [Google Scholar]
- 149.Horng JH, Lin WH, Wu CR, et al. HBV X protein-based therapeutic vaccine accelerates viral antigen clearance by mobilizing monocyte infiltration into the liver in HBV carrier mice. J Biomed Sci. 2020;27(1):70. doi: 10.1186/s12929-020-00662-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhao HJ, Han QJ, Wang G, et al. Poly I:C-based rHBVvac therapeutic vaccine eliminates HBV via generation of HBV-specific CD8(+) effector memory T cells. Gut. 2019;68(11):2032–2043. doi: 10.1136/gutjnl-2017-315588 [DOI] [PubMed] [Google Scholar]
- 151.Wang W, Zhou X, Bian Y, et al. Dual-targeting nanoparticle vaccine elicits a therapeutic antibody response against chronic hepatitis B. Nat Nanotechnol. 2020;15(5):406–416. doi: 10.1038/s41565-020-0648-y [DOI] [PMC free article] [PubMed] [Google Scholar]