Abstract
Introduction: Parkinson’s disease is characterized by non-motor/motor dysfunction midbrain neuronal death and α-synuclein deposits. The accepted hypothesis is that unknown environmental factors induce α-synuclein accumulation in the brain via the enteric nervous system. Material and Methods: Monoclonal antibodies made against recombinant α-synuclein protein or α-synuclein epitope 118–123 were applied to the antigens of 180 frequently consumed food products. The specificity of those antibody-antigen reactions was confirmed by serial dilution and inhibition studies. The Basic Local Alignment Search Tool sequence matching program was used for sequence homologies. Results: While the antibody made against recombinant α-synuclein reacted significantly with 86/180 specific food antigens, the antibody made against α-synuclein epitope 118–123 reacted with only 32/180 tested food antigens. The food proteins with the greatest number of peptides that matched with α-synuclein were yeast, soybean, latex hevein, wheat germ agglutinin, potato, peanut, bean agglutinin, pea lectin, shrimp, bromelain, and lentil lectin. Conclusions: The cross-reactivity and sequence homology between α-synuclein and frequently consumed foods, reinforces the autoimmune aspect of Parkinson’s disease. It is hypothesized that luminal food peptides that share cross-reactive epitopes with human α-synuclein and have molecular similarity with brain antigens are involved in the synucleinopathy. The findings deserve further confirmation by extensive research.
Keywords: Parkinson’s disease, α-synuclein, antibodies, antigen, food, cross-reactivity, sequence homology, synucleinopathy, BLAST, gut-brain axis
1. Introduction
Environmental factors play a major role in human chronic diseases [1]. In addition to infections, chemicals and stress, nutritional behavior is emerging as an important factor, affecting the microbiome\dysbiome balance and their metabolome [2]. Food antigens are involved not only in allergy, but also contribute to autoimmune and neurodegenerative diseases [3,4]. Following ingestion, nutrients are digested into tolerated molecules that are absorbed for the benefit of human health and functions. Facing powerful physical barriers and immune mechanisms, immunogenic food antigens are denied entry, thus avoiding immune stimulation and self-reactions. If a non-naïve antigen circumvents those checkpoints, the mucosal innate and reactive immune systems respond, aiming to neutralize the invader. Indirectly, nutrients impact the human microbiome, thus prokaryotic constituents or their mobilome could penetrate the defense mechanisms and impact human health [5,6] If everything works perfectly, the resulting anti-food antibodies are directed to neutralize the foreign protein by forming a complex that will be eliminated by the cellular immune cells.
However, this complex formation between antibodies and food proteins can result in the activation of a complement cascade and binding to C1q. These anti-nutrient antibodies can cross-react with human self-molecules. This autoimmune cascade can also be evoked when sequence homology or shared immunogenic epitopes exist between those food-originated foreign proteins and the host’s self-determinants [7]. This phenomenon is called molecular mimicry or cross-reactivity, and is often described in various autoimmune conditions [7]. More and more such diseases are associated with various nutritional compounds [8]. Rheumatoid arthritis has been connected to wheat, fish, pork, milk and dairy products, eggs, lectins and agglutinins [7,9,10,11,12]. Multiple sclerosis incidence has been strongly correlated with the consumption of cow’s milk [12,13] and other food products [8]. Celiac disease, dermatitis herpetiformis and celiac ataxia are induced by gluten-containing food products [1,2,3]. Many other autoimmune diseases might benefit from a gluten-free diet [5,14,15,16] or other restrictive dietary regimens [17]. Even an autoimmune neurological disease like polyradiculoneuropathy has been induced by porcine brains, although in this case the exposure of the affected abattoir workers was through aerosolized antigens and not per oral intake [18]. Based on the above studies, it can be concluded that nutrients are associated with autoimmune diseases. Many of the studies were performed on animal models, thus causality and mechanistic pathways are far from being elucidated.
No less interesting is the relationship between neuroinflammatory, neurodegenerative or neuropsychiatric conditions and food consumption. In this regard, bovine milk and dairy products [12], gluten [1,4,19] and red meat [20] have been suggested as high-risk nutrients. Interestingly, milk and dairy products are also among the list of foods said to exacerbate Parkinson’s disease (PD) [21], while some nutritional diets, like the Mediterranean and quasi-vegan diets, might be protective or preventive against it [22,23].
A very logical suggestion was recently suggested by Riccio and Rossano [24]. They concluded that “what determines the organ specificity of the autoimmune-inflammatory process may depend on food antigens resembling proteins of the organ being attacked. This applies to the brain and neuroinflammatory diseases, as to other organs and other diseases.” For example, the late-embryo-abundant group III protein family that is found in plants and seeds such as soybean, wheat, tomato, peanuts and in crustaceans as well, share the epitopes of 11 amino acid residues with α-synuclein (aSN) [25,26,27,28,29,30]. Furthermore, vertebrates, especially fish, fowl and mammals, are the main source of aSN, which after their consumption, can potentially pass the enteric barrier and reach the human brain. In alignment studies, high degrees of sequence homology were found between the aSN of fish, fowl, pig, sheep and cow and the aSN of humans [31,32,33,34,35]. Interestingly, this human-like aSN has been found in the brain and many other animal body tissues and organs, including muscle, bone marrow and ovaries, and in cells such as erythrocytes, platelets, myocytes and neurons [34,36,37,38]. Based on this molecular mimicry between human aSN and aSN from food sources such as mammalian meat, chicken, fish, grains and some plants, we undertook the present study. To our knowledge, there are no current published studies on cross-reactivity and sequence homology between food antigens and aSN. Based on the current enteric origin theory of PD development [39,40,41], the association of high-risk food products with PD [42], and the numerous luminal sources of aSN [43], we hypothesized that frequently ingested foods, if improperly digested in the presence of broken barriers, may enter into the blood. The subsequent production of antibodies against them may contribute to α-synucleinopathy in patients with PD and other neurodegenerative disorders. More so, if cross-reactivity between them coexist with sequence similarity, the two pathways can join together in the process of the autoimmunogenesis in PD. In individuals with elevated levels of aSN antibody in the circulation, these antibodies may react with foods containing aSN, forming immune complexes that manage to cross the blood-brain barriers (BBB) into the brain, where they may induce the formation of Lewy bodies, the hallmarks of PD. All that being said, food is only one of many environmental factors, biological, chemical and pathogenic, that are suspected of being involved somehow in PD, and this mimicry and cross-reactivity between food and anti-aSN antibodies is a new challenging aspect of the disease that deserves further study.
2. Materials and Methods
2.1. Antibodies and Antigens
Monoclonal mouse IgG1 antibody made against recombinant aSN was purchased from R&D Systems, Minneapolis, MN, USA. Monoclonal rabbit IgG antibody made against aSN epitope 118–123, was purchased from Abcam, Cambridge, MA, USA.
2.2. Preparation of Food Antigens
For the preparation of the food antigens, food products in either raw or cooked form were purchased from the supermarket. Food proteins undergo structural epitope transformation when the food is cooked, and dietary preparation was conducted to reflect dietary proteins in either raw or cooked form as represented in typical human diets. A total of 180 different food extracts, representatives of various meats, seafoods, grains, fruits, vegetables, seeds, nuts, beans, spices, gums and more were prepared, in a process similar to the one used in our earlier study [44]. The different foods were ground at 4 °C in either 70% ethanol, or coco buffer containing 0.55 M of NaHCO3, 1 % NaCl pH 8.5. Each food item was kept on the stirrer for 4 h at 25 °C. After each food was stirred, the food processor was decontaminated. The mixture was centrifuged at 2000 g for 15 min, and then the top layer, which contained oil bodies, was discarded. To ensure that all small molecules were removed, the liquid phase from each solvent was dialyzed against a buffer of 0.01 M phosphate buffered saline (PBS) using dialysis bags with a cutoff of 6000 Da for 72 h, with the buffer changed every 24 h. Protein concentration was then measured using a kit provided by Biorad (Hercules, CA, USA). The list of the presently used 180 food’s Current Procedural Terminology (CPT) codes, developed by the American Medical Association, is presented in Supplementary Materials 1.
2.3. Enzyme Linked Immunosorbent Assay (ELISA) for Demonstrating the Reaction of Various Antibodies With Food Antigens
Food antigens at a concentration of one mg/mL were prepared. For coating the ELISA plate, the optimal concentration of each food antigen was determined by examining the concentration of antigens that gave the most reproducible results in quadruplicate. Accordingly, the stock solution was diluted from 1:50–1:200 in 0.1 M carbonate-bicarbonate buffer (pH 9.5). One hundred microliters were added to each well of the polystyrene flat bottom ELISA plate. The plates were then incubated overnight at 4 °C, and then washed 5 times with 200 microliters PBS containing 0.05 % Tween 20 at a pH of 7.4. 200 microliters of 2.5 % bovine serum albumin (BSA) were then added to prevent the nonspecific binding of immunoglobulins, after which the plates were incubated at 4 °C overnight. Plates were subsequently washed as described above, and then unimmunized mouse serum, rabbit serum, and mouse or rabbit monoclonal antibodies made against aSN at optimal dilutions of 1:100–1:300 were added to different groups of wells and were incubated at room temperature for 1 h. They were then washed 5 times with PBS-Tween buffer. We then added one hundred microliters of monoclonal antibody made against aSN, followed by incubation, washing, and the addition of secondary antibody or alkaline phosphatase labeled anti-rabbit or anti-mouse IgG to different wells, after which the plates were incubated again. 100 microliters of para-nitrophenylphosphate (PNPP) in 0.1 mL diethanolamine buffer 1 mg/mL containing 1 mM MgCl2 and sodium azide at a pH of 9.8 were added to start the enzymatic reaction, which was stopped 45 min later with 50 microliters of 1 N NaOH, so that the samples were ready for quantitative analysis by an ELISA reader. The microtiter reader recorded the optic density (OD) at 405 nm to provide quantitative antibody reactivity levels in comparison with control wells coated either with human serum albumin (HSA) as negative control or aSN as positive control. Each analysis was performed in quadruplicate.
2.4. Determination of Specificity of Antigen-Antibody Reaction
To demonstrate the specific binding of aSN antibodies not only to synuclein, but to different food antigens, serial dilutions and inhibition studies in the presence of different food antigens in the liquid phase of the antigen-antibody reaction was performed. Different sets of different ELISA wells were coated with the food antigens. In each set four or five foods were chosen as being representative of the antigens that showed from moderate to strong reactions with aSN antibodies. For the plates using mouse monoclonal antibody, these foods were honey, shrimp, yeast, tofu and quinoa. For the plates using rabbit monoclonal antibody, the foods were wheat germ agglutinin (WGA), lentil lectin, latex hevein, thyme and potato. The aSN antibodies were added serially in dilutions ranging from 1:100 to 1:12,800 to the appropriate set of microtiter strips. After incubation, washing, the addition of a second antibody, and the completion of other ELISA steps, the ODs were recorded at 405 nm.
The inhibition study was done by the addition of various concentrations of a specific antigen to an antibody in liquid phase first, followed by the addition of the antigen-antibody mixture to ELISA microwells coated with the same antigen. For example, to prove that the binding of aSN antibody to yeast, quinoa, tofu, shrimp and honey is specific, the following steps were taken:
1. Eight different ELISA plate wells were coated with optimal concentrations of yeast or another antigen used in this inhibition study.
2. 100 microliters of monoclonal anti-aSN were then added to each of 8 different test tubes.
3. The first tube, tube #1, containing anti-aSN antibody was to serve as the baseline reaction of antibody to each tested antigen, so no antigen (in this case, yeast) was added to this tube. To the other seven tubes containing anti-aSN antibody, we added increasing concentrations of yeast protein. Tube #2 received 2 micrograms, tube #3 received 4 micrograms, and tubes #4–8 received 8, 16, 32, 64, and 128 micrograms of yeast antigen respectively.
4. The eight tubes now contained 100 microliters of aSN-antibody and from 0 to 128 micrograms of yeast protein. The tubes were individually mixed, and then the contents of each tube were added to the appropriate ELISA wells coated with yeast protein described in Step #1.
After incubation, washing, the addition of anti-mouse or anti-rabbit IgG labeled with enzyme, and the completion of additional ELISA steps, the ODs were recorded. The inhibition of anti-aSN antibody binding to specific food antigen-coated ELISA wells by the same antigen in liquid phase was demonstrated graphically to be in proportion to the increased amount of protein used for inhibition. All of these same steps were also used for quinoa, tofu, shrimp and honey, which were the other food antigens used in the inhibition study using mouse monoclonal antibody.
The inhibition of anti-aSN antibody made against the short epitope AA 118–123 of aSN was done in a similar manner using food proteins that had moderate to strong reactions with aSN antibody.
2.5. Amino Acid Sequence Similarity Between Alpha-Synuclein and Food Proteins
We used the NIH/US National Library of Medicine’s Basic Local Alignment Search Tool (BLAST) sequence matching program to study degrees of possible mimicry between the amino acid sequences of aSN and food proteins such as soy bean, yeast, pea lectin, lentil lectin, bean agglutinin (concanavalin-A), wheat germ agglutinin, peanut protein, pineapple bromelain and shrimp. These are foods that had moderate to strong reactions with the aSN antibody. We also ran a sequence match search with proteins for coconut, a food that did not react with this antibody.
2.6. Statistical Analysis
Statistical analysis was performed using STATA 14.2 software. Independent t-tests were performed to evaluate mean differences of optical densities between controls and antigens. A Bonferroni adjustment was conducted to account for type 1 errors with multiple comparisons and alpha was set to <0.001.
3. Results
Based on earlier studies about the cross reactivity of food components and human tissue antigens [7,45,46,47,48,49], the present study explored the reactivity of aSN antibody with 180 different commonly used food products.
3.1. Cross Reactivity Between aSN and Different Food Antigens.
Anti-aSN antibodies had significant reactivity with 86/180 foods, much higher in comparison to the ODs of reagents, controls and the mean ODs of the other 94 foods +3SD (0.50).
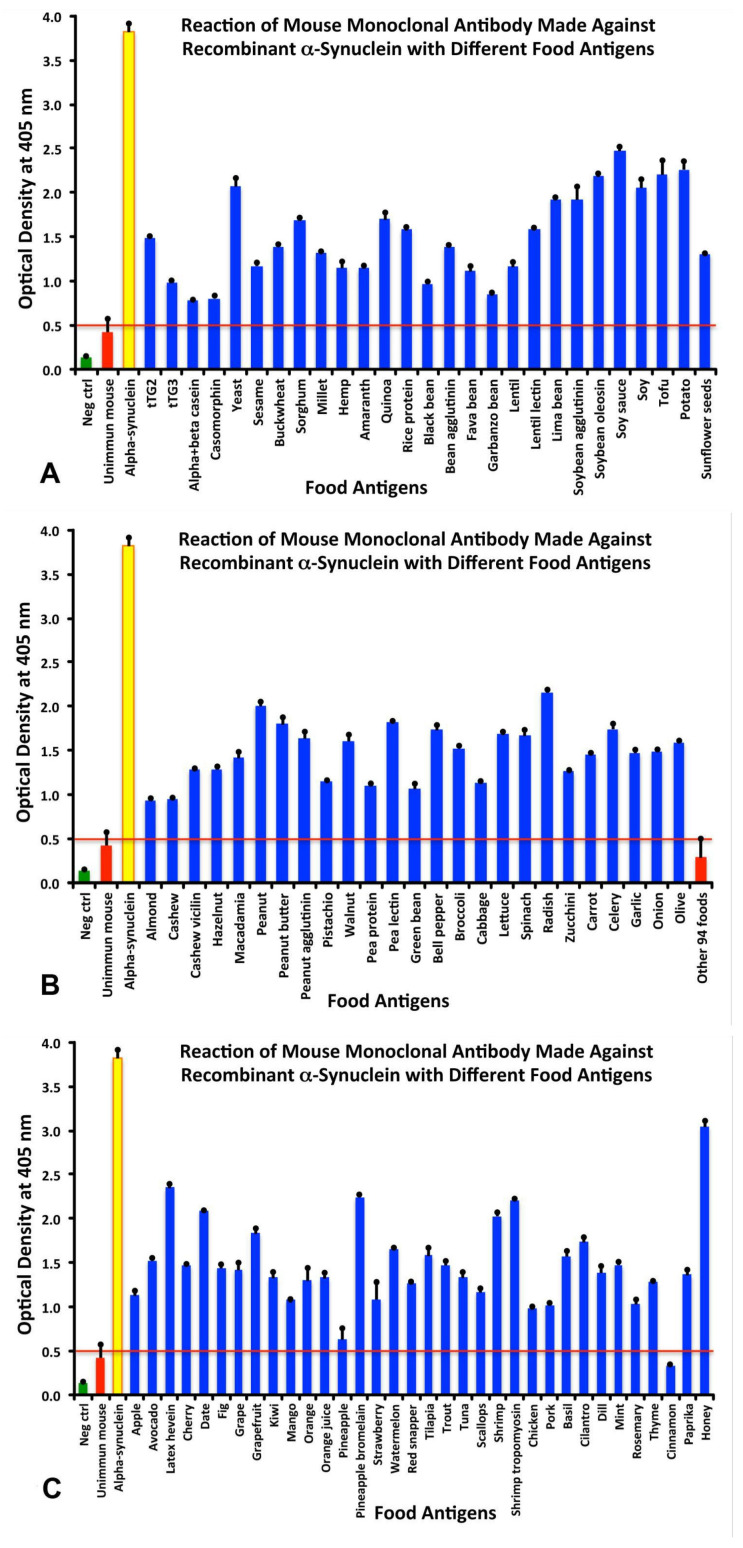
The resulting means +SD of each antigen’s quadruplicate ODs were calculated and are presented in Figure 1A–C. In order to semi-quantify the levels of aSN antibody’s reactivity with the studied food antigens, the following key for the ODs were used: 0–0.50 = insignificant or negative; 0.51–1.0 = low; 1.10–1.80 = moderate; 1.81–2.5 = high; and >2.5 = very high. The highest OD that can be obtained with the laboratory ELISA reader is 4.0.
Figure 1.
(A–C) Reaction of mouse monoclonal antibody made against recombinant aSN with different food antigens. tTG—tissue transglutaminase.
Comparing the reaction of aSN antibody with alpha synuclein (3.82), which was close to the maximum OD of 4.0, the reaction of anti-aSN antibody was strongest with honey (OD 3.0—very high), followed by soy sauce (OD 2.5—high), potato (OD 2.3—high), tofu, radish and soybean oleosin (OD 2.2—high), yeast and soy (OD 2.1—high). Overall, not counting the highest reaction of aSN antibody with honey, 17 foods were graded as high (OD 1.81–2.5), 56 were graded as moderate, 12 were graded as low, and 94 foods had individual ODs that were equal to or less than the mean OD of all these 94 foods plus 3SD, or 0.50 or less, representing the insignificant or negative results (Figure 1A–C). Interestingly, the mean OD plus 3SD that resulted from the reaction between unimmunized mouse serum with all 180 food antigens was also very close to the above cutoff of 0.50 (Figure 1A–C). The difference between the ODs of the aSN-immune-reactive foods versus the non-immune reactive foods was very significant (p < 0.0001).
3.2. Cross Reactivity Between the Epitope 118–123 of aSN with Different Food Antigens
The aSN epitope 118–123 (VDPDNE) is localized at the polar, negatively charged and acidic C-terminal tail of aSN, representing a cleavage site, playing an important role in fibrillar synucleinopathy [50,51,52,53].
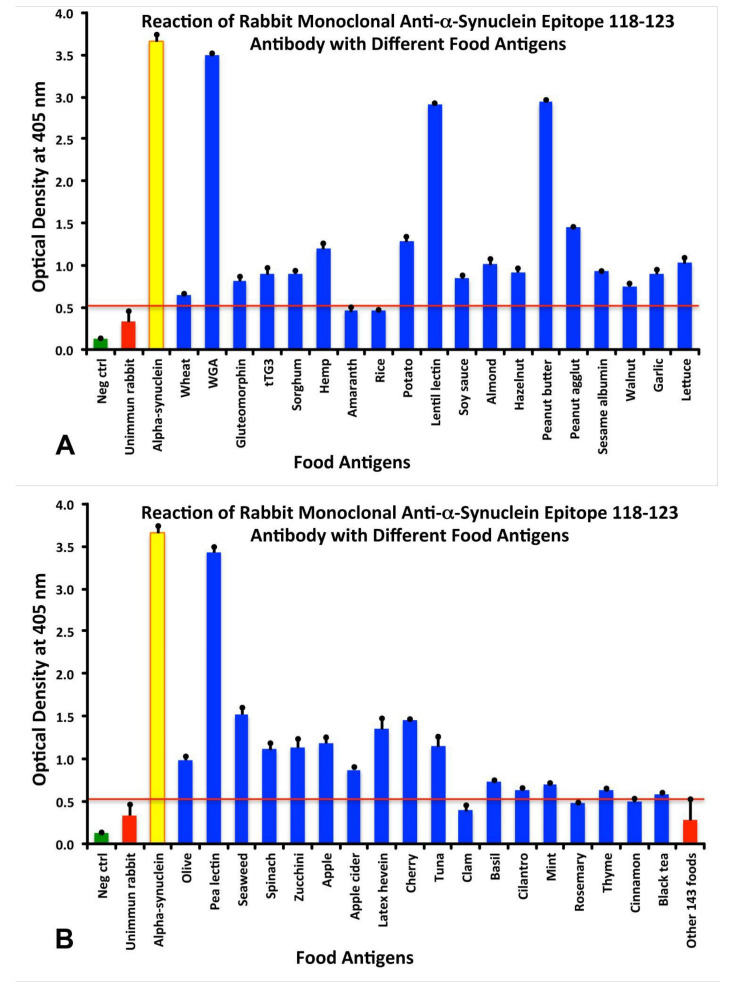
In order to semi-quantify the levels of the reactivity of this epitope’s antibody with the studied food antigens, the following key for the ODs were used: 0–0.51 = insignificant or negative; 0.52–1.0 = low; 1.10–1.80 = moderate; 1.81–2.5 = high; and >2.5 = very high. Whereas the complete sequence aSN protein antibody reacted significantly with 86 food antigens, the monoclonal antibody made against this specific epitope resulted in reactivity with a total of 32 food antigens, ranging from low to high reactivity.
The reaction of anti-aSN antibody was strongest with WGA (OD 3.5—very high), followed by pea lectin (OD 3.4—very high), peanut butter and lentil lectin (OD 2.9—very high). There were no food antigens in the high zone range, 10 food products in the moderate range, and 19 in the low range. All the rest of the tested food antigens were below the cut-off level of 0.52 and were considered negative (Figure 2A,B). The difference between the ODs of the aSN-immune-reactive foods versus the mean OD plus 3SD of the 148 non-immune reactive foods was very significant (p < 0.0001).
Figure 2.
(A,B) Reaction of rabbit monoclonal antibody made against anti-epitope 118–123 with different food antigens. WGA—wheat germ agglutinin, tTG3—tissue transglutaminase 3.
3.3. Analytical Specificity of Anti-aSN Antibody Binding to Selected Food Antigens Performed by Serial Dilutions
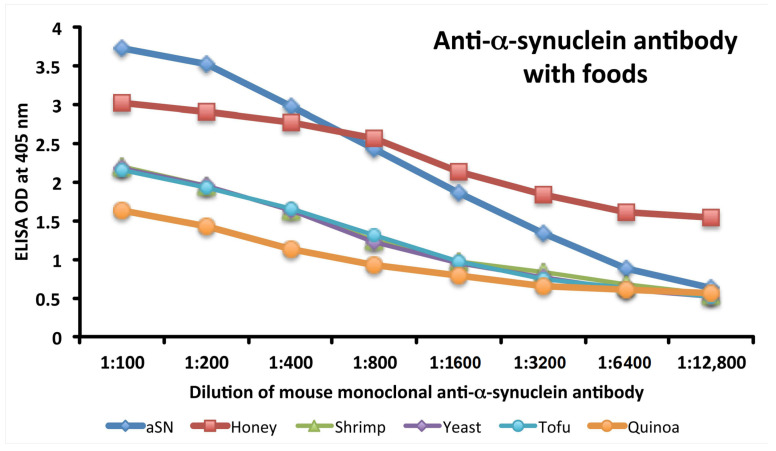
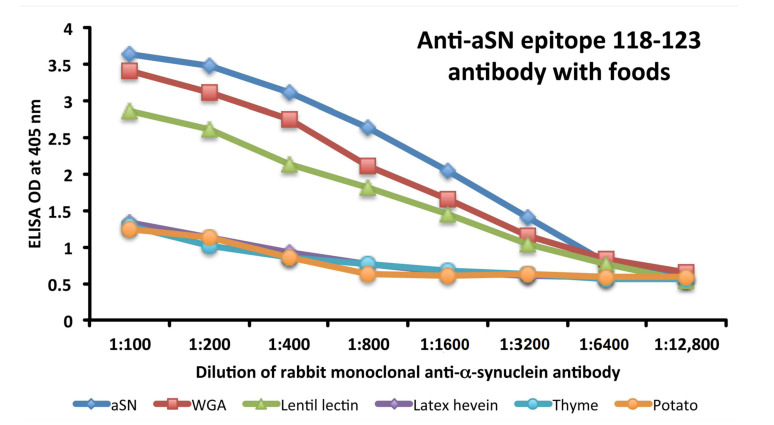
Specificity of anti-aSN antibody binding to various food antigens is demonstrated by serial dilutions for the complete aSN sequence in Figure 3 and for the short aSN epitope 118–123 in Figure 4. The addition of anti-aSN antibody, diluted from 1:100–1:12,800, to a fixed concentration of six different antigens is presented (aSN, yeast, quinoa, tofu, shrimp and honey for the complete aSN sequence, and aSN, wheat germ agglutinin, lentil lectin, latex hevein, thyme and potato for the aSN 118–123 sequence). Plates were incubated and followed by other required ELISA steps. After the addition of substrate and color measurement and calculation of indices, a significant decrease in antibody indices, in proportion to the antibody dilution, was observed. Logically, the highest decline of aSN antibodies was observed against the aSN itself, as a control. The other declines followed precisely their OD values, as shown in Figure 1A–C for the full aSN sequence, and Figure 2A,B for the aSN 118–123 sequence. More specifically, the very high cross reactivity of the aSN full sequence with honey (Figure 1C) had the steepest decline (Figure 3). Yeast, tofu and shrimp with their high OD levels (Figure 1A,C) had a middle decline (Figure 3), while quinoa, with its moderate cross reactivity to aSN (Figure 1A) assumed the lowest decline during the dilution experiment (Figure 3). For the aSN 118–123 sequence, it’s very high cross-reactivity with wheat germ agglutinin (OD 3.5, Figure 2A) had the steepest decline (Figure 4). Lentil lectin with an OD of 2.9 (Figure 2A) assumed a moderate decline, while latex hevein (OD 1.4, Figure 2B), potato (OD 1.3, Figure 2A) and thyme (OD 0.6, Figure 2B) had the lowest declines during the dilution experiment (Figure 4).
Figure 3.
Analytical specificity by serial dilution study for mouse monoclonal anti-aSN. Shown are the reactions of various dilutions of mouse monoclonal anti-aSN antibody with alpha-synuclein (blue diamond), honey (red square), shrimp (green triangle), yeast (purple diamond), tofu (light blue circle) and quinoa (orange circle).
Figure 4.
Analytical specificity by serial dilution study for rabbit monoclonal anti-aSN epitope 118-123. Shown are the reactions of various dilutions of rabbit monoclonal anti-aSN antibody with alpha-synuclein (blue diamond), WGA (red square), lentil lectin (green triangle), latex hevein (purple diamond), thyme (light blue circle) and potato (orange circle). aSN—alpha-synuclein, WGA –wheat germ agglutinin.
3.4. Analytical Specificity of Mouse Monoclonal Anti-aSN Antibody Binding to Selected Food Antigens Performed by Serial Inhibitions
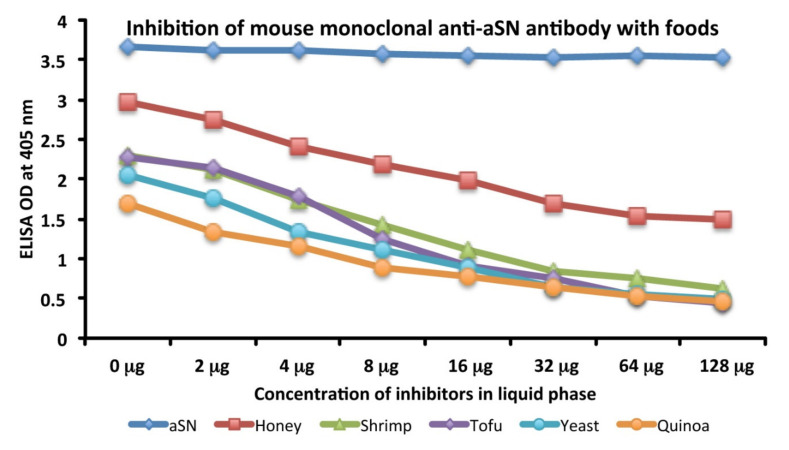
The binding of anti-aSN antibody to aSN as the control in the presence of HSA, or anti-aSN antibody binding to various cross-reactive food antigens was examined using different concentrations of cross-reactive food components, in a liquid phase. Figure 5 shows the inhibition of mouse monoclonal aSN antibody binding in the presence of different concentrations of HSA, or the binding of anti-aSN antibody to yeast, quinoa, tofu, shrimp and honey in the presence of 0–128 micrograms of yeast, quinoa, tofu, shrimp and honey. Compared to HSA, which caused no inhibition in this antibody-antigen reaction, the addition of higher concentrations of specific food antigens to the liquid phase, followed by the addition of aSN antibodies, resulted in a significant decline in the reaction of aSN antibodies to the cross-reactive-food-coated plates. This inhibition was observed even with a lower concentration of nutritional antigens, for example, 2–4 micrograms. Similar to the dilutional assay, the inhibition declines followed precisely their OD values, as shown in Figure 1A–C. More specifically, the very high cross reactivity to honey (Figure 1C) had the steepest decline (Figure 5). Shrimp, tofu and yeast with their high OD levels (Figure 1A,C) had a middle decline (Figure 5), while quinoa, with its more moderate cross reactivity to aSN (Figure 1A) assumed the lowest decline during the dilution experiment (Figure 5). A negative correlation was observed between the concentration of inhibitors in the liquid phase and the corresponding steepness of the antigenic food graphs (Figure 5).
Figure 5.
Analytical specificity by inhibition study for mouse monoclonal anti-aSN antibody binding to various food antigens. Graph shows the inhibition of mouse monoclonal aSN antibody with different concentrations of aSN (blue diamond), honey (red square), shrimp (green triangle), tofu (purple diamond), yeast (light blue circle) and quinoa (orange circle).
3.5. Analytical Specificity of the Rabbit Monoclonal Anti-aSN Epitope 118–123 Antibody Binding to Selected Food Antigens Performed by Serial Inhibitions
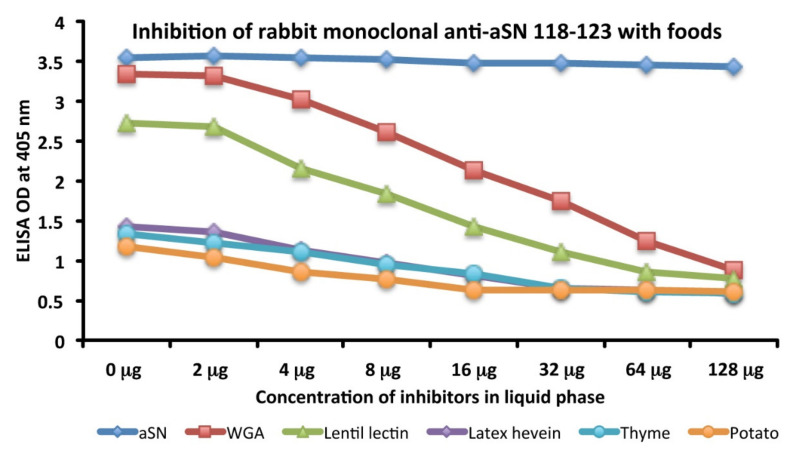
The binding of rabbit monoclonal antibody binding to aSN and various cross-reactive food antigens was examined in the presence of different concentrations of HSA or the same food antigens in the liquid phase of the assay. Inhibition in the binding of rabbit monoclonal anti-aSN Epitope 118–123 antibody to 5 different food antigens and aSN are shown in Figure 6. The graph shows the inhibition of rabbit monoclonal anti-aSN antibody binding to aSN (blue diamond), WGA (red square), lentil lectin (green triangle), latex hevein (purple diamond), thyme (light blue circle) and potato (orange circle) with different concentrations (0–128 microgram) of the same antigen in liquid phase. Compared to HSA, which caused no inhibition in this antibody-antigen inhibition reaction, the addition of increasing concentrations of specific food antigens to the liquid phase, followed by the addition of aSN antibodies, resulted in a significant decline in the reaction of aSN antibodies to the cross-reactive-food-coated plates. This inhibition was observed even with a lower concentration of nutritional antigens, for example, 2–8 micrograms. Similar to the dilutional assay, the inhibition declines followed precisely their OD values, as shown in Figure 2A,B. More specifically, the very high cross reactivity to WGA (OD 3.5, Figure 2A) had the steepest decline (Figure 6). Lentil lectin, with an OD of 2.9, (Figure 2A) assumed a moderate decline, while latex hevein (OD 1.4, Figure 2B), potato (OD 1.3, Figure 2A) and thyme (OD 0.6, Figure 2B) had the lowest decline during the dilution experiment (Figure 6). A negative correlation was observed between the concentration of inhibitors in the liquid phase and the corresponding steepness of the antigenic food graphs (Figure 6).
Figure 6.
Analytical specificity by inhibition study for rabbit monoclonal anti-aSN epitope 118–123. Shown are the reactions of various dilutions of rabbit monoclonal anti-aSN antibody with aSN (blue diamond), WGA (red square), lentil lectin (green triangle), latex hevein (purple diamond), thyme (light blue circle) and potato (orange circle). aSN—Alpha-synuclein; WGA—wheat germ agglutinin.
3.6. Sequence Similarity Between Alpha-Synuclein and Food Antigens
Amino acid sequences for proteins from peanut, yeast, potato, shrimp, pineapple bromelain, bean agglutinin (concanavalin-A), pea lectin, lentil lectin, soybean agglutinin, wheat germ agglutinin, and latex hevein, which had moderate to very strong reactions with aSN antibody (Figure 1A–C), were downloaded from NCBI resources and were compared to the aSN 140 amino acid sequence using the NIH/US National Library of Medicine’s Basic Local Alignment Search Tool (BLAST) sequence matching program.
The food proteins with the greatest number of immunological peptides that matched with aSN were yeast (Saccharomyces cerevisiae), soybean agglutinin, latex hevein, wheat germ agglutinin, peanut, pea lectin, potato, bean agglutinin, and shrimp. Bromelain had two matches, while lentil lectin only had one, but all the other proteins had from four to more than twenty matches with aSN. Even when the cutoff was set for percentage of identity at 50%, the resulting number of matches for many of those proteins was so overwhelming that not all of the instances of similarity could practically be shown in a table (Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7 and Table 8), as was the case with yeast and soybean agglutinin. Honey was the only food for which we were unable to find protein sequences from NCBI, and consequently we were unable to compare its sequence with the aSN one. Finally, in many instances, matches with food proteins occurred in more than one chain or section of the aSN sequence, as are marked with * in Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7 and Table 8.
Table 1.
Potential cross-reactive epitopes between alpha-synuclein proteins and yeast proteins.
| Yeast Protein | Yeast Sequence |
Mapped Start To End | Alpha-Synuclein Sequence |
ID (%) |
|---|---|---|---|---|
| Structure of the S. cerevisiae Srs2 C-terminal domain in complex with PCNA conjugated to SUMO | MDIFSQ-LSRAK | 45–55 | MDVFMKGLSKAK | 58 |
| Proteasome Activator Complex | KATNGVVIATE | 19–29 | KAKEGVVAAAE * | 64 |
| Structure of the ribosomal 80S-eEF2-sordarin complex | FMKKLRAAK---LAAPE | 10–23 | FMKGLSKAKEGVVAAAE * | 53 |
| Crystal Structure of the S. cerevisiae glucokinase, Glk1 | MEKGLAPPKEG | 43–53 | M-KGLSKAKEG | 64 |
| Solution structure of the oxidized form of the Yap1 redox domain | MAKAKCSERGVVINAE | 60–75 | LSKAK--E-GVVAAAE | 56 |
| Cryo-EM Structure of the RIX1-REA1 Pre-60S Particle | KG-SKAK-GVNMAA | 471–482 | KGLSKAKEGV-VAA | 71 |
| Crystal structure of yeast elongation factor 2 in complex with sordarin | SKAGEIVLAA | 813–822 | SKAKEGVVAA * | 70 |
| Solution structure of the SBDbeta domain of yeast Ssa1 | KGRLSKEDIEKMVAEAE | 123–139 | KG-LSKAK-EGVVAAAE | 59 |
| Crystal structure of the RNA recognition motif of yeast eIF3b residues 76–161 | EATGKTK-GFLFV | 48–59 | EAAGKTKEGVLYV * | 69 |
| Structure of the ribosomal 80S-eEF2-sordarin complex from yeast | TKEAAAVAEGKSKQ | 9–22 | TKQGVAEAAGKTKE * | 50 |
| Crystal structure of a Prp8 C-terminal fragment | KQNDEEAAGASTVMKTK | 276–292 | KQGVAEAAG-----KTK * | 53 |
| Crystal Structures of Yeast Transketolase In Complex with Analogs of Thiamin Diphosphate | TPEGVAERAQKT | 650–661 | TKQGVAEAAGKT * | 67 |
| Crystal Structure of Yeast Aspartyl-Trna Synthetase Complexed with Trna Asp | KEGI-EMLRAAGK | 336–347 | KQGVAE---AAGK | 54 |
| A Gated Channel into The Proteasome Core Particle | TKEGVVLGV----EK | 42–52 | TKEGVVHGVATVAEK * | 67 |
| Structure of The Yeast Cytochrome Bc1 Complex Co-Crystallized with An Antibody Fv-Fragment | GSRYATKDGVAH | 22–33 | GSK—TKEGVVH * | 58 |
| Electron cryomicroscopy structure of S. cerevisiae FAS in the Apo state | SKTIKD-LVGGKSTV | 169–182 | SKT-KEGVVHGVATV * | 53 |
| Structure of the Ndi1 protein in complex with the competitive inhibitor, stigmatellin | VHLRTAVAKVEEK | 271–283 | VH---GVATVAEK * | 54 |
| Cryo-EM Structure of the Exocyst Complex | QVNSIGGVVVT | 781–791 | QVTNVGGAVVT | 64 |
| Arx1 pre-60S particle | NVGGALRVPG--AISEK | 44–58 | NVGGAV-VTGVTAVAQK | 53 |
| Crystallographic snapshots of eukaryotic dimethylallyltransferase acting on tRNA | IAGTTG-VGKSQL | 7–18 | IAAATGFVKKDQL * | 62 |
| Crystal structure of yeast aminopeptidase 1 (Ape1) | TAEGYGRIAVA | 104–114 | TVEGAGSIAAA * | 64 |
| Yeast RNA polymerase III initial transcribing complex | DNEDNE-GSEE-----EPE | 579–591 | DNEAYEMPSEEGYQDYEPE * | 53 |
* This match occurred in more than one section of the target sequence. Not all of the instances of identification and similarity are shown due to the overwhelming number of occurrences.
Table 2.
Potential cross-reactive epitopes between alpha-synuclein proteins and soybean agglutinin proteins.
| Soybean Agglutinin Protein | Soybean Agglutinin Sequence | Mapped Start to End | Alpha-Synuclein Sequence |
ID (%) |
|---|---|---|---|---|
| Uncharacterized protein LOC100790514 [Glycine max] | MNGLSK---GVVATA | 178–189 | MKGLSKAKEGVVAAA | 67 |
| Uncharacterized protein LOC100799919 [Glycine max] | GL-KTKEGVVLAVE | 39–51 | GLSKAKEGVVAAAE | 71 |
| Uncharacterized protein LOC100793479 isoform X1 [Glycine max] | FSTGLSKRTSKVAEKKEGTVAGA | 79–101 | FMKGLSK-----A--KEGVVAAA | 52 |
| Coleoptile phototropism protein 1 [Glycine max] | MDYFVKTLSGIKAK-GV | 57–72 | MDVFMKGLS--KAKEGV | 65 |
| Proteinaceous RNase P 2 [Glycine max] | VGEAEFDAGRVKEGVL | 219–234 | VAEA---AGKTKEGVL | 63 |
| Uncharacterized protein At4g13230 [Glycine max] | KTKES-AEHAKDNVVGKTKESAEYV | 121–144 | KTKQGVAEAA-----GKTKEGVLYV | 56 |
| Soyasapogenol B glucuronide galactosyltransferase-like [Glycine max] |
KT-QG--EEEGWLEWLNKKKEGSVLYV | 268–291 | KTKQGVAEAAG------KTKEG-VLYV | 52 |
| Peptidyl-prolyl cis-trans isomerase FKBP62 [Glycine max] | EAAGKKKEEGNVLF | 389–402 | EAAGKTKE--GVLY | 64 |
| 51 kDa seed maturation protein isoform X1 [Glycine max] | VGDAAQKTKE---YV | 164–175 | VAEAAGKTKEGVLYV | 60 |
| Binding partner of ACD11 1 [Glycine max] | KTKEKVLAQDNQGKTEEG | 228–245 | KTKQGV-AEAA-GKTKEG | 56 |
| ATP-dependent DNA helicase Q-like 3 isoform X1 [Glycine max] | SKIK--VV--VATVA | 307–317 | SKTKEGVVHGVATVA | 67 |
| Hypothetical protein GLYMA_15G213400 [Glycine max] | GVVQHGPGGTMATVAE | 1008–1023 | GVV-HG----VATVAE | 63 |
| Proteasome subunit alpha type-5 [Glycine max] | KTKEGVVLAV----EK | 41–51 | KTKEGVVHGVATVAEK | 63 |
| Subtilisin-like protease SBT1.2 [Glycine max] | VTNVGEANSSYVVT-VSA | 692–708 | VTNVGGA----VVTGVTA | 61 |
| AT-hook motif nuclear-localized protein 23 [Glycine max] | VTNVSLRQPASAGAVVT | 112–128 | VTNVG-------GAVVT | 53 |
| NAD-dependent malic enzyme 59 kDa isoform, mitochondrial [Glycine max] | VTAEVGAAVV--CAAVAEK | 543–559 | VT-NVGGAVVTGVTAVAQK | 58 |
| Subtilisin-like protease SBT5.3 [Glycine max] | VTNVGKARSIYKAVVVSPTGVNVTVV | 679–704 | VTNVG------GAVV---TG--VTAV | 50 |
| Pyruvate decarboxylase 2 [Glycine max] | VE---AIQTATG-VKKD | 555–567 | VEGAGSIAAATGFVKKD | 59 |
| 2-oxoglutarate dehydrogenase, mitochondrial [Glycine max] | SAATATGFLKVHQKEQ | 993–1008 | SIAAATGFVK---KDQ | 56 |
| Pyruvate decarboxylase 2 [Glycine max] | VE---AIATATG-PKKDSL | 560–574 | VEGAGSIAAATGFVKKDQL | 58 |
| Autophagy-related protein 18f isoform X2 [Glycine max] | TVSGAA--AAATG--RKNAL | 572–587 | TVEGAGSIAAATGFVKKDQL | 55 |
* This match occurred in more than one section of the target sequence. Not all of the instances of identification and similarity are shown due to the overwhelming number of occurrences.
Table 3.
Potential cross-reactive epitopes between alpha-synuclein proteins and latex hevein proteins.
| Latex Hevein Protein | Latex Hevein Sequence |
Mapped Start to End | Alpha-Synuclein Sequence |
ID (%) |
|---|---|---|---|---|
| Hypothetical protein GH714_008467 | LSKAKGGVVAEEE | 28–40 | LSKAKEGVVAAAE | 77 |
| Hypothetical protein GH714_000288 | KGVLSRKAKEAAVAAAQ | 176–192 | KG-LS-KAKEGVVAAAE * | 71 |
| Hypothetical protein GH714_000288 | KENVVEA | 587–593 | KEGVVAA * | 71 |
| Uncharacterized protein LOC110639732 | LSLKAHDGGVVA | 257–268 | LS-KAKEG-VVA | 67 |
| Lysine-specific histone demethylase 1 homolog 1-like | MKG-----DGVVAAAD | 288–298 | MKGLSKAKEGVVAAAE * | 56 |
| Proteasome subunit alpha type-5 | GL-KTKEGVVLAVE | 39–51 | GLSKAKEGVVAAAE | 71 |
| Hypothetical protein GH714_036586 | LDVFMKGI--AAEG | 223–234 | MDVFMKGLSKAKEG | 64 |
| Hypothetical protein GH714_025383 | DVFMKGI--AAEG | 282–292 | DVFMKGLSKAKEG * | 69 |
| Hypothetical protein GH714_028032 | DVFTKSLSQAK | 237–247 | DVFMKGLSKAK * | 73 |
| ABC transporter B family member 2-like | KEGASEGEVVEAA | 1096–1108 | KEG-----VVAAA | 54 |
| E3 ubiquitin-protein ligase CIP8 | YELPTDD--QDYE | 288–298 | YEMPSEEGYQDYE | 54 |
| The article Zinc finger protein BRUTUS-like At1g18910 | EAYEMPYASE | 583–592 | EAYEMP—SE * | 80 |
| DEAD-box ATP-dependent RNA helicase 56-like isoform X1 | DNDAYE---EE-LLDYEEE | 6–20 | DNEAYEMPSEEGYQDYEPE * | 58 |
| Hypothetical protein GH714_022075 [Hevea brasiliensis] | EAYESSSEESFKD | 180–192 | EAYEMPSEEGYQD | 62 |
| Hypothetical protein GH714_005670 | DNERFDF-NEEPYQQYE | 231–246 | DNEAYEMPSEEGYQDYE | 53 |
| Splicing factor U2af large subunit B-like isoform X4 | YE---EEGYQGNGEDFE | 4–17 | YEMPSEEGYQ----DYE | 53 |
* This match occurred in more than one section of the target sequence. Not all of the instances of identification and similarity are shown due to the overwhelming number of occurrences. Only identifications with the sequences from 1–20 and 121–140 of the complete Latex hevein sequence are shown.
Table 4.
Potential cross-reactive epitopes between alpha-synuclein proteins and wheat germ agglutinin.
| Wheat Germ Agglutinin Protein | Wheat Germ Agglutinin Sequence |
Mapped Start to End | Alpha-Synuclein Sequence |
ID (%) |
|---|---|---|---|---|
| Chain A, Glutaredoxin | LAKAKE-IVASA | 53–63 | LSKAKEGVVAAA | 67 |
| Chain Y, 60s Ribosomal Protein L24 | KAKGKFTAEDV-AAA | 129–142 | KAK-----EGVVAAA | 53 |
| Chain I, 60s Ribosomal Protein L16 | DVGMK-----KKGV | 35–43 | DVFMKGLSKAKEGV | 50 |
| Chain F, 60s Ribosomal Protein L30 | KQKVAAEKIKAAENTK--VIY | 29–47 | KQGV-AE---AAGKTKEGVLY | 52 |
| Chain u, 60s Ribosomal Protein P1 | GGAAAAEEKKE | 80–90 | GVAEAAGKTKE | 55 |
| Chain A, Xylanase Inhibitor Protein I | VHPKNVYYGVAPVAQK | 231–246 | VH------GVATVAEK | 50 |
| Chain H, 60s Ribosomal Protein L6 | EGVTVQ-VAAKVVTV | 14–27 | EGV-VHGVA----TV | 53 |
| Chain M, 40s Ribosomal Protein S12e | VHLV-TVPSAKT | 114–124 | VHGVATV-AEKT | 58 |
| Chain X, Xylanase Inhibitor | GSKPVSKVNV--GV | 109–120 | GSK—TKEGVVHGV * | 50 |
| Chain A, Eukaryotic Translation Initiation Factor 4e-1 | GAVV----SVRQK | 114–122 | GAVVTGVTAVAQK | 54 |
| Chain A, Ribulose bisphosphate carboxylase large chain | EEAGAAVAAESSTG | 51–64 | EGAGS-IAA--ATG | 50 |
| Chain J, 60s Ribosomal Protein L5 | GIQEHIDLGMKYDP | 107–120 | GILE--D--MPVDP | 50 |
* This match occurred in more than one section of the target sequence. Not all of the instances of identification and similarity are shown due to the overwhelming number of occurrences.
Table 5.
Potential cross-reactive epitopes between alpha-synuclein proteins and pea lectin, concanavalin-A (bean agglutinin) and lentil lectin proteins.
| Pea Lectin Protein |
Pea Lectin
Sequence |
Mapped Start to End |
Alpha-Synuclein
Sequence |
ID (%) |
| Chain A, Ferredoxin—nadp reductase, leaf isozyme, chloroplastic | VYMMGL-KGME | 263–272 | VFMKGLSKAKE* | 55 |
| Chain A, Putative aminoaldehyde dehydrogenase | KEDVDVAVAAA | 44–54 | KEGV---VAAA | 64 |
| Chain B, Photosystem II CP47 reaction center protein | EGVAAA | 94–99 | EGVVAA | 83 |
| Chain X, Photosystem II reaction center protein X | KASLKEKVVTGLTAAA | 16–31 | KA--KEGVV----AAA | 56 |
| Chain A, Dihydrolipoamide dehydrogenase | KAEEDGVA | 327–334 | KAKEGVVA | 63 |
| Chain A, Dihydrolipoamide dehydrogenase | FTSGLNLDKIGV | 280–291 | FMKGLSKAKEGV | 50 |
| Chain A, Protein (ferredoxin: nadp + reductase) | VYMCGL-KGME | 263–272 | VFMKGLSKAKE * | 55 |
| Concanavalin-A Protein | Concanavalin-A Sequence |
Mapped
Start to End |
Alpha-Synuclein
Sequence |
ID (%) |
| Chain A, Alpha-mannosidase | KAYEGEV | 518–524 | KAKEGVV | 71 |
| Chain A, Alpha-mannosidase | VENVLDSVV | 48–56 | VTNVGGAVV | 56 |
| Chain A, Urease | GALSIA----FVSKAALDQ | 758–772 | GAGSIAAATGFVKK---DQ * | 53 |
| Chain A, Refined crystal structure of cavavalin from jack bean | EE---QEGVIVKMP | 168–178 | EEGAPQEGILEDMP * | 50 |
| Chain A, Urease | KEEEDA-SEGV | 24–33 | KNEEGAPQEGI | 55 |
| Chain A, Alpha-mannosidase | YE--SSEGDFSDYQ | 609–620 | YEMPSEEG-YQDYE * | 50 |
| Lentil Lectin Protein |
Lentil Lectin
Sequence |
Mapped Start to End |
Alpha-Synuclein
Sequence |
ID (%) |
| Solution structure of Lipid Transfer Protein | AGSITKLNTNNAAA | 56–69 | AGSI-------AAA * | 50 |
* This match occurred in more than one section of the target sequence.
Table 6.
Potential cross-reactive epitopes between alpha-synuclein proteins and potato proteins.
| Potato Protein | Potato Sequence |
Mapped Start to End | Alpha-Synuclein Sequence |
ID (%) |
|---|---|---|---|---|
| The structure of a glutathione synthetase (StGSS1) from Solanum tuberosum in ADP and y-EC bound closed conformation | SSSNEGGVAA | 463–472 | SKAKEGVVAA | 60 |
| Crystal structure of S-adenosylmethionine decarboxylase | KGLRSLSKA | 35–43 | KGL---SKA | 67 |
| Crystal structure of potato Rx-CC domain in complex with RanGAP2-WPP domain | LSKE-E---AAKNAE | 36–46 | LSKAKEGVVAA--AE | 53 |
| Structure determination and refinement at 1.8 A resolution of Disproportionating Enzyme | EGAVSSVARIA | 458–468 | EGVVHGVATVA * | 55 |
| The structure of a glutathione synthetase (StGSS1) from Solanum tuberosum in ADP and y-EC bound closed conformation | GVDMVH--APVA | 54–63 | GV--VHGVATVA | 58 |
| Characterization of Solanum tuberosum Multicystatin and Significance of Core Domains | AATDGG--KK | 60–67 | AAT--GFVKK | 60 |
| >Crystal Structure of Potato Multicystatin | >AATDDAG--KK | >55–63 | >AAT---GFVKK | >55 |
* This match occurred in more than one section of the target sequence.
Table 7.
Potential cross-reactive epitopes between alpha-synuclein proteins and peanut proteins.
| Peanut Protein | Peanut Protein Sequence | Mapped Start to End | Alpha-Synuclein Sequence | ID (%) |
|---|---|---|---|---|
| Chain A, PR 10 protein | GLFRAIEGYVLA | 140–151 | GLSKAKEG-VVA * | 58 |
| Chain A, Stilbene synthase, | AGLKTTGEGLDWGVLF | 357–372 | AG-KTK-E----GVLY | 50 |
| Chain A, PR 10 protein, | VVGGVALPPT-AEKITFETK | 83–101 | VVHGVA---TVAEK----TK * | 55 |
| Chain A, Protein (peanut lectin) | VSGAVVK-VT | 156–164 | VGGAVVTGVT | 70 |
| Chain A, Arachin Arah3 isoform | EQ-----GAIVT | 274–280 | EQVTNVGGAVVT | 50 |
| Chain A, Arachin Arah3 isoform | AVPTGV | 137–142 | AVVTGV | 83 |
| Chain A, Protein (peanut lectin) | AGSIGGGT | 98–105 | AGSIAAAT | 63 |
* This match occurred in more than one section of the target sequence.
Table 8.
Potential cross-reactive epitopes between alpha-synuclein proteins, and shrimp and pineapple bromelain proteins.
| Shrimp Protein | Shrimp Protein Sequence |
Mapped
Start to End |
Alpha-Synuclein Sequence | ID (%) |
| Chain A, Triosephosphate isomerase | FMKTGPLSPNTEVVV | 28–42 | FMK-G-LSKAKEGVV | 60 |
| Chain A, Proliferating cell nuclear antigen | TKEGVKFSAA | 163–172 | TKEGVVHGVA * | 60 |
| Chain A, Triosephosphate isomerase | AIGTGKTA | 170–177 | AVVTGVTA | 63 |
| Chain A, Arginine kinase | QDGILE | 343–348 | QEGILE | 83 |
| Pineapple Bromelain Protein |
Pineapple Bromelain
Sequence |
Mapped
Start to End |
Alpha-Synuclein
Sequence |
ID (%) |
| Three-Dimensional Structure of Pineapple Cystatin | KAKEQVV | 85–91 | KAKEGVV * | 86 |
| Three-Dimensional Structure of Pineapple Cystatin | AEAEAEEEEG | 19–28 | AEAAGKTKEG | 50 |
* This match occurred in more than one section of the target sequence.
Coconut was one of the foods that did not react significantly with the aSN antibody. When we tried to match it with aSN anyway, we found only one promising match (KESGVINEKNIAE), but it only had a 46% match with the aSN sequence (KE-GVVAA---AE).
4. Discussion
Parkinson’s disease is characterized by the abnormal folding of aSN, a protein localized in the substantia nigra in the form of Lewy bodies. Regarding the source of misfolded aSN in the brain, the current hypothesis is that chemicals, infections or other unknown environmental factors penetrate the peripheral nervous system through the gastrointestinal system or through the olfactory bulb in the nasal cavity, subsequently moving on to the adjacent enteric nerve, from there finally reaching the brain, then culminating in widespread aSN pathology at the later stages [43,54,55,56]. The hypothesis is partially supported by the fact that many food sources, including plants and seeds such as soy bean, beans, peanuts, wheat and tomato, as well as crustaceans such as shrimp, contain different proteins that share high degrees of similarity with aSN [25,26,27,28,29,30]. Furthermore, aSN sequences are highly conserved in vertebrates, particularly in mammals. Thus, the consumption of animal food products originated from fish, fowl, and mammals such as cattle, pigs, sheep and goats, especially animal food products containing red blood cells, are the major source of aSN that reach the human gut [33,34,35,36,37].
In the present study, the hypothesis was that if aSN shares molecular similarity with so many foods, then monoclonal antibodies made against aSN should react with some of those food proteins. Looking at it from the other direction, if anti-aSN reacts with different food antigens, then amino acid sequence similarities should exist between those food proteins and aSN.
Following the above-mentioned hypothesis, it can be envisioned that if foods that cross-react with aSN are partially or undigested in the gut due to local eco events, the aSN in those food particles may become misfolded, and its handling by the enteric nerve may help in the transportation of the misfolded aSN into the brain. Based on the increased barriers permeability in PD, the leaky gut and the permeable BBB may also allow aSN aggregates to migrate cephalically [57].
Molecular mimicry between food antigens and self-tissue proteins is one of the possible mechanisms in the initiation of autoimmune diseases [7,8,45,46,47,48,49,58,59]. Intriguingly, based on this mechanism, several aspects of neuroinflammation and autoimmunity have been described in PD [60,61]. Confirmational molecular mimicry between curli, an E. coli protein fibril, and two other protein amyloids, silk and Sup35, produced by certain microbiota, was found to accelerate the aggregation of amyloid protein A and aSN in animal models of amyloidosis and PD [62,63]. Besides molecular mimicry involving microbes, viral infections like herpes simplex-1 and Epstein Barr virus have been detected in the blood samples of PD patients. Interestingly, antibodies targeting the virus’ proteins showed cross-reactivity to human aSN. The C-terminus of LMP1, a late membrane protein of the Epstein Barr virus, presents a strong amino acid sequence similarity to aSN C-tail [50,51,52,53]. However, except for the suggested molecular homology between microbiota or viral proteins and aSN [54,55] and the possibility that high intake of Western diet and red meat might increase the risk of PD [23,64,65], no study, to the best of our knowledge, is available on immunogenic cross-reactivity between numerous specific nutrients and aSN protein. Thus, it was logical to apply monoclonal antibodies made against recombinant aSN or its short epitope to a variety of food antigens commonly consumed by humans.
While antibody made against the short 118–123 epitope of aSN had low to strong reactions with 32 different food antigens, the monoclonal antibody that was prepared against the full-sequence recombinant aSN reacted with 86 out of the 180 tested food antigens. These reactive antigens were prepared from seeds, nuts, beans, fruits, vegetables, seafood, fish, chicken and mammalian meats (Figure 1A–C). The observation that the reaction of anti-aSN antibody was strongest with honey (OD 3.0—very high, Figure 1C) deserve some explanation. Honey is very high in sugars but other constituents are various organic acids, enzymes (invertase, insulase), traces of vitamins and proteins and pollen grains originated from flowers are also found in honey. The anti-aSN-honey cross reactive antibodies, most probably, are directed to the protein part, however, in this assay, sugar moieties might exert an effect. This extensive immunoreactivity between aSN antibodies and so many food antigens may indicate that cross-reactivity through mimicry exists between food-sourced aSN and human aSN.
The reactivity of aSN antibodies with many food antigens was confirmed by the high degree of peptide sequence homologies between aSN and yeast, soybean agglutinin, wheat germ agglutinin, latex hevein, potato, peanut, bean agglutinin (concanavalin-A), pea lectin, shrimp, pineapple bromelain, and lentil lectin (Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7 and Table 8). Using the BLAST matching program, a multiplicity of protein sequences in all those food proteins that showed varying degrees of similarity with aSN, were detected. The cutoff at an ID percentage of 50% was applied, so that only listed matches of 50% or higher were listed. In many instances, repeating sequences were observed in different subunits of the food proteins and the aSN sequences. The highest homology was found between aSN and yeast, followed by soybean agglutinin, WGA, latex hevein, potato, peanut, concanavalin-A, pea lectin, shrimp, bromelain, and lentil lectin. As a contrary proof, coconut protein, which did not react at all with aSN, predictably had no significant homology with aSN. Honey, on the other hand, reacted very strongly with aSN antibody, but we were unable to find a usable protein sequence for honey in the matching program index.
The plethora of these matches between aSN sequences and food antigens may explain why monoclonal antibodies made against aSN proteins reacted with so many food antigens out of the 180 in the present study. It should be noted that the study was limited to the identification of general cross-reactive antibody responses, while the BLAST search was just limited to 12 food antigens. The results may indicate that the aSN antibodies reacted against shared conformational epitopes in the food proteins. The current study design did not specifically include analyses that would capture conformational or non-linear epitopes, but screened any of the food sequences that matched with the aSN sequences, especially the highly recurring ones, hence possibly be conformational epitopes. Conformational epitopes could be major targets of autoantibody production in autoimmune diseases [66,67], resulting in aSN aggregation and associated pathology. Further investigation to identify the specific cross-reactive epitopes will require specific peptide fragmentation inhibition studies, epitope mapping, as well as computational modeling, which were not within the scope of this study.
The present results open a new horizon for food specific antigens that might drive PD. It is a new insight that reflects a connection between the disease and commonly consumed foods that enter the gut lumen, where they are digested, absorbed, sensed and sampled by the pluripotent epithelial and sub-epithelial innate immune cells [6,39]. The reactive mucosal immune B lymphocyte is activated, directly or indirectly, and responds by producing IgG isotype antibodies specifically directed against the nutritional antigens. Those food-specific antibodies, by cross-reacting with so many aSN sequences (Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7 and Table 8), may help not only in the aggregation of aSN but also its delivery to the brain through the enteric nerve, and the broken gut and BBBs.
In support of the dual-hit hypothesis of PD by Braak et al. [54,55,56], Chandra et al. [68] suggested that under the influence of various environmental factors, aSN becomes misfolded in the gut endocrine cells, where, through its communication with the adjacent enteric neurons, the aSN starts its propagation up to the brain [69]. Interestingly, the enteric glial cells influence and are influenced by the enteric epithelial cell, gut microbiome, nutrient content and mucosal immunity. Taken together, those influences might affect tight junction functional integrity and facilitate aSN gut’s permeation [69,70,71,72,73]. Moreover, the gut is very rich in enzymes, both originated from dietary sources, or secreted as the result of gut dysbiosis, which contributes to post-translational modification of proteins (PTMP), including aSN [6,74,75,76,77].
Like many other proteins in the gut, aSN may undergo PTMP by acetylation, phosphorylation, oxidation, ubiquitination, glycation, nitration, deamidation, transamidation and sumoylation. This enzymatic PTMP reaction can result in the alteration of protein structures, affecting their functional and aggregatory capacity [76,77]. Furthermore, luminal aSN aggregation might be enhanced by the interaction of food-sourced aSN and late embryo-abundant (LEA) in the gut with toxic chemicals in the foods, as well as by compounds originated from disturbed gut microbiota, such as bacterial toxins, amyloids, and antibodies in the lumen [43,78,79,80,81,82]. The aggregation of luminal aSN and its entry into the enteric nervous system has been shown to be the next important step in delivering those detrimental molecules into the brain [83].
Simultaneously, aggregated aSN, microbial generated toxins, antigens, amyloids, antibodies, inflammatory cytokines, and undigested food antigens can affect the functional integrity of the tight junctions [3,6,39,43]. The failure of the tight junction integrity results in the entry of those macromolecules into the submucosa, the regional lymph nodes, and into the circulation. An immune response against them results in the production of cytokines and antibodies. The reaction of these antibodies with additional antigens results not only in antibody-antigen binding, but also in the activation of a complement cascade. Together, those two may affect the integrity of the BBB, the regulatory gatekeepers of the brain. Indeed, increased intestinal and BBB permeability has been described in PD and other neuroinflammatory and neurodegenerative disorders [6,84,85,86,87,88,89,90,91,92,93].
Although the presence of some of these factors (antigens, antibodies, immune complexes, aggregated asN, inflammatory cytokines) in the blood may not be directly pathogenic for the brain, it is possible that in the context of BBB permeability, these factors may contribute to the severity of diseases in the brain [87]. Thus, the gut is an ignition source for aggregated aSN and its delivery to the brain, not only by the enteric nervous system, but also by permeable gut and BBB, which altogether, contribute to PD synucleinopathy [43,78,84,88,89].
It is not known if the cross-reactive food specific antibodies against aSN epitopes will induce gain or loss of aSN functions. Most recently, strategies to decrease synucleinopathy by tackling the aSN molecule have been described, including targeting the spread, production, aggregation, and degradation of aSN [94]. One wonders what effects the binding of cross-reactive nutrient-specific antibodies to aSN might have on those functions. For example, specific antibodies could potentially neutralize aSN monomers and/or aggregates extracellularly or even intracellularly, as suggested in the case of intra/nanobodies [95]. Targeted aSN immunotherapy could become a shifting paradigm and disease-modifying therapeutic protocol.
Several consequences of aSN antibodies cross-reacting with a variety of food proteins or specific peptides can be postulated:
The presence of antibodies in the blood that cross-react with aSN may interfere with the accurate measurement of aSN antibodies used in support of PD diagnosis and progression assessment of the disease.
Compromised BBB, which is often observed in PD patients, may result in the entry of food cross-reactive antibodies into the central nervous system, where their binding to aSN can further contribute to Lewy body pathology.
The presence in the blood of food antigens that cross-react to aSN may interfere with treatment modalities in which anti-aSN antibodies are used to target misfolded proteins in PD.
High cross-reactivity of a food-specific antibody with aSN may affect some physiological functions of aSN, thus putting individuals at risk for synucleinopathy.
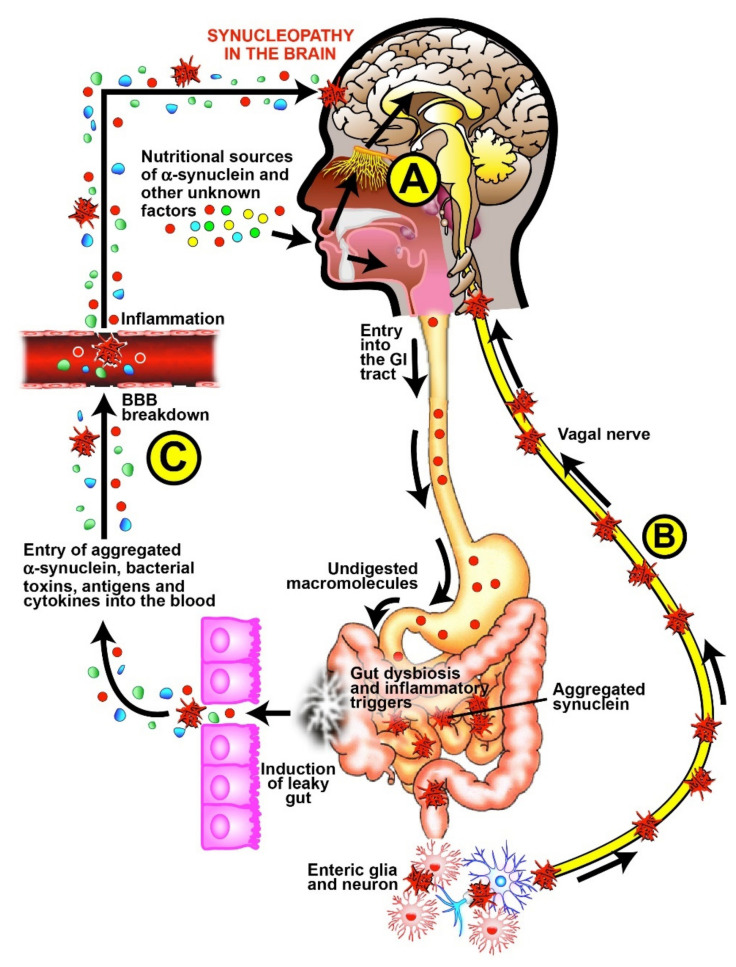
Glutamate neurotransmission and glutamate receptors play a pivotal role in brain physiology and are altered in PD [96,97]. On the other hand, the 33-mer peptide (p57–89) from the a2-gliadin subtype is considered the supramolecule that induces celiac disease [98]. Intriguingly, the component of the NMDA glutamate receptor ion channel—the human GRINA protein has a significant sequence homology with the 33-mer gliadin peptide [99]. The authors suggested that 33-mer gliadin molecule is a natural antagonist interfering with the normal interactions of GRINA, thus impacting the extraintestinal manifestations of celiac disease. This is a pathophysiological example of how molecular similarity between a luminal gluten-originated nutrient might affect PD behavior through the human glutamate receptor GRINA protein. In addition, celiac disease has been associated with “leaky gut” syndrome, an increase in the permeability of the intestinal mucosa [100]. It has also been shown that dietary imbalance impairs BBB properties, potentially favoring transmigration and leading to neuroinflammation [101]. Figure 7 summarizes schematically the triple-hit Hypothesis of alpha-synucleopathy in the gut-brain axes of patients with Parkinson’s disease.
Figure 7.
The triple-hit hypothesis of alpha-synucleopathy in the gut-brain axes of patients with Parkinson’s disease. Nutritional sources of aSN or cross-reactive nutritional peptides enter in the oral cavity. After chewing, some of those transformed substances may penetrate through the olfactory bud in the nasal cavity into the brain (A). The nutritional factors proceed from the oral cavity into the stomach and intestine. If improperly digested or modified, those nutritional substances may induce gut dysbiosis, enhance the production of inflammatory triggers and amyloidogenesis resulting in the aggregation of aSN. The aggregated aSN activates enterocytes/enteroendocrine/subepithelial dendritic cells, in close contact with the enteric glia cells and through the neurons, uptake the aSN aggregates and deliver them to the brain via the vagal nerve (B). The inflammatory mediators trigger leaky gut and enhance the entry of aggregated aSN and other macromolecules into the circulation, igniting further inflammation in the blood. Those molecules together induce breakdowns in the BBB, followed by the entry of the aggregated aSN and other molecules into the brain (C). Thus, the entry of aggregated aSN via the olfactory bulb, the enteric nerve, or broken gut and blood-brain barriers can result in synucleopathy and the formation of Lewy bodies in Parkinson’s disease.
It should be stressed that those possibilities need further investigation. There are some limitations for the present study. It is an in vitro study, but there would definitely be advantages in investigating in vivo, by feeding or injecting the aSN-cross-reactive food epitopes into animal models and observing if they develop PD-like symptoms or pathology. Ingested nutrients are subjected to enzymatic digestion in the stomach and small intestine. The post-digestion antigenicity of the presently studied food products were not explored. As it is, the clinical and pathophysiological impacts of the cross-reactive antibodies were not explored, and thus any conclusions are purely hypothetical, and it is not truly known whether aSN-cross-reactive food epitopes are pathogenic or protective. The strengths of the study are the plethora of food products, which span a wide range of common nutrients. The specificity of the antibodies’ level determination was double checked by dilution, inhibition and epitope similarity. Furthermore, the putative cross-reactivity between aSN and food proteins was supported by ELISA testing and BLAST matching with many lectins, agglutinins and different forms of the foods, such as soybean, soy sauce and tofu, peanut, peanut agglutinin and peanut butter.
Interestingly, in addition to the luminal aSN [34,36,37,38], another frequently ingested nutrient, namely, gluten, was recently suggested as a potential driver of neurodegeneration [100]. The anti-gluten antibodies cross-reactivity [7,46,47,59] and the numerous epitope sequence homologies with human central nervous system peptides [102] direct to the possible pathophysiological pathway of molecular mimicry, operating in neurodegenerative diseases. The food-gut-brain axis might represent an additional avenue, operating in neurodegenerative disease evolvement.
5. Conclusions
Multiple commonly consumed specific food products induce antibodies that cross-react with aSN and its 118–123 sequence. Our findings raise the possibility that antibody’s cross-reactivity supported by specific sequence’s molecular mimicries between nutrients and aSN is operative thus might represent an additional autoimmune feature to PD neurodegeneration. If substantiated in vitro or on PD animal models or in human subjects, this study’s findings might open a new avenue of research in PD pathophysiology. While the biological and pathophysiological cross-reactive and sequence homology effects are far from being unraveled, it is hoped that the current findings will encourage the scientific and clinical communities to explore the food cross-reactive and molecular mimicry phenomena in the PD enigma.
Acknowledgments
The authors would like to thank Joel Bautista for making the figures, editing the manuscript for English corrections, and typing the manuscript for submission.
Abbreviations
Alpha/α synuclein—aSN; Parkinson’s disease—PD; blood-brain barrier—BBB; wheat germ agglutinin—WGA; post-translational modification of proteins-PTMP; optic density-OD.
Supplementary Materials
The list of the presently used 180 food’s Current Procedural Terminology (CPT) codes, developed by the American Medical Association, is presented in Supplementary Materials 1. The following are available online at https://www.mdpi.com/article/10.3390/cells10051111/s1.
Author Contributions
Conceptualization, A.V. and A.L.; Methodology, A.V. and E.V.; Software, A.V.; Validation, A.V., A.L. and E.V.; Formal Analysis, A.V. and E.V.; Investigation, E.V.; Resources, A.V.; Data Curation, A.V. and A.L.; Writing—Original Draft Preparation, A.L. and A.V.; Writing—Review & Editing, A.V. and A.L.; Visualization, A.V. and E.V.; Supervision, A.L.; Project Administration, A.V. All authors have read and agreed to the published version of the manuscript.
Funding
The study was neither funded nor institutionally supported.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available in a publicly accessible repository.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lerner A., Matthias T. In: Mosaic of Autoimmunity, the Novel Factors of Autoimmune Diseases Revisited. 2nd ed. Shoenfield Y., Perricone C., editors. Elsevier; Amsterdam, The Netherlands: 2019. pp. 315–321. Gluten and Autoimmunogenesis. [Google Scholar]
- 2.Lerner A., Matthias T. Autoimmunity in celiac disease: Extra-intestinal manifestations. Autoimm. Rev. 2019;18:241–246. doi: 10.1016/j.autrev.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Lerner A., Matthias T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun Rev. 2015;14:479–489. doi: 10.1016/j.autrev.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Lerner A., Matthias T. Don’t forget the exogenous microbial transglutaminases: It is immunogenic and potentially pathogenic. AIMS Biophysics. 2016;3:546–552. doi: 10.3934/biophy.2016.4.546. [DOI] [Google Scholar]
- 5.Lerner A., Shoenfeld Y., Matthias T. A Review: Gluten ingestion side effects and withdrawal advantages in non-celiac autoimmune diseases. Nutritional Rev. 2017;75:1046–1058. doi: 10.1093/nutrit/nux054. [DOI] [PubMed] [Google Scholar]
- 6.Lerner A., Matthias T. Gut—the Trojan horse in remote organs’ autoimmunity. J. Clin. Cell Immunol. 2016;7:401. [Google Scholar]
- 7.Vojdani A., Gushgari L.R., Vojdani E. Interaction between food antigens and the immune system: Association with autoimmune disorders. Autoimmun Rev. 2020;19:102459. doi: 10.1016/j.autrev.2020.102459. [DOI] [PubMed] [Google Scholar]
- 8.Gershteyn I.M., Ferreira L.M.R. Immunodietica: A data-driven approach to investigate interactions between diet and autoimmune disorders. J. Transl Autoimmun. 2019;1:100003. doi: 10.1016/j.jtauto.2019.100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hvatum M., Kanerud L., Hällgren R., Brandtzaeg P. The gut-joint axis: Cross reactive food antibodies in rheumatoid arthritis. Gut. 2006;55:1240–1247. doi: 10.1136/gut.2005.076901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lerner A., Matthias T. Rheumatoid arthritis-celiac disease relationship: Joints get that gut feeling. Autoimm Rev. 2015;14:1038–1047. doi: 10.1016/j.autrev.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Lerner A., Matthias T. The gut feeling of the joints: Celiac disease and rheumatoid arthritis are related. Internat J. Celiac Dis. 2019;7:21–25. [Google Scholar]
- 12.Borba V.V., Lerner A., Matthias T., Shoenfeld Y. Bovine milk proteins as a trigger for autoimmune diseases: Myth or reality? Internat J. of Celiac Dis. 2020;8:4–15. [Google Scholar]
- 13.Esposito S., Bonavita S., Sparaco M., Gallo A., Tedeschi G. The role of diet in multiple sclerosis: A review. Rev. Nutr Neurosci. 2018;21:377–390. doi: 10.1080/1028415X.2017.1303016. [DOI] [PubMed] [Google Scholar]
- 14.Lerner A., Ramesh A., Matthias T. Are non-celiac autoimmune diseases responsive to gluten-free diet? Internat J. Celiac Dis. 2017;5:164–167. doi: 10.12691/ijcd-5-4-6. [DOI] [Google Scholar]
- 15.Lerner A., Matthias T. Going gluten free in non-celiac autoimmune diseases: The missing ingredient. Expert Rev. Clin. Immunol. 2018;14:873–875. doi: 10.1080/1744666X.2018.1524757. [DOI] [PubMed] [Google Scholar]
- 16.Lerner A., Berthelot L., Jeremias P., Abbad L., Matthias T., Monteiro R.C. Gut-kidney axis: Gluten, transglutaminase, celiac disease and IgA nephropathy. J. Clin. Cell Immunol. 2017;8:499–503. doi: 10.4172/2155-9899.1000499. [DOI] [Google Scholar]
- 17.Choi I.Y., Lee C., Longo V.D. Nutrition and fasting mimicking diets in the prevention and treatment of autoimmune diseases and immunosenescence. Mol. Cell Endocrinol. 2017;455:4–12. doi: 10.1016/j.mce.2017.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holzbauer S.M., DeVries A.S., Sejvar J.J., Lees C.H., Adjemian J., McQuiston J.H., Medus C., Lexau C.A., Harris J.R., Sergio E Recuenco S.E., et al. Epidemiologic investigation of immune-mediated polyradiculoneuropathy among abattoir workers exposed to porcine brain. PLoS ONE. 2010;5:e9782. doi: 10.1371/journal.pone.0009782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohan M., Okeoma C.M., Sestak K. Dietary Gluten and Neurodegeneration: A Case for Preclinical Studies. Int. J. Mol. Sci. 2020;21:5407. doi: 10.3390/ijms21155407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grant W.B. Using Multicountry Ecological and Observational Studies to Determine Dietary Risk Factors for Alzheimer’s Disease. J. Am. Coll Nutr. 2016;35:476–489. doi: 10.1080/07315724.2016.1161566. [DOI] [PubMed] [Google Scholar]
- 21.Seidl S.E., Santiago J.A., Bilyk H., Potashkin J.A. The emerging role of nutrition in Parkinson’s disease. Front. Aging Neurosci. 2014;6 doi: 10.3389/fnagi.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maraki M.I., Yannakoulia M., Stamelou M., Stefanis L., Xiromerisiou G., Kosmidis M.H., Dardiotis E., Hadjigeorgiou G.M., Sakka P., Costas A Anastasiou C.A., et al. Mediterranean diet adherence is related to reduced probability of prodromal Parkinson’s disease. Mov. Disord. 2019;34:48–57. doi: 10.1002/mds.27489. [DOI] [PubMed] [Google Scholar]
- 23.McCarty F.M., Lerner A. Low risk of Parkinson’s disease in quasi-vegan cultures may reflect GCN2-mediated upregulation of parkin. Adv. Nutr. 2020;12:355–362. doi: 10.1093/advances/nmaa112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riccio P., Rossano R. Undigested food and gut microbiota may cooperate in the pathogenesis of neuroinflammatory diseases: A matter of barriers and a proposal on the origin of organ specificity. Nutrients. 2019;11:2714. doi: 10.3390/nu11112714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Artur M.A.S., Zhao T., Ligterink W., Schranz E., Hilhorst H.W.M. Dissecting the genomic diversification of late embryogenesis abundant (LEA) protein gene families in plants. Genome Biol. Evol. 2019;11:459–471. doi: 10.1093/gbe/evy248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y., Yang M., Cheng H., Sun N., Simu Liu S., Li S., Wang Y., Zheng Y., Uversky V.N. The effect of phosphorylation on the salt-tolerance-related functions of the soybean protein PM18, a member of the group-3 LEA protein family. Biochim. Biophys. Acta Proteins Proteom. 2017;1865:1291–1303. doi: 10.1016/j.bbapap.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 27.Liu H., Xing M., Yang W., XMu X., Wang X., Lu F., Wang Y., Zhang L. Genome-wide Identification of and functional tnsights tnto the late embryogenesis abundant (LEA) gene family in bread wheat (Triticum aestivum) Sci. Rep. 2019;9:13375. doi: 10.1038/s41598-019-49759-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma A., Kumar D., Kumar S., Rampuria S., Reddy A.R., Kirti P.B. Ectopic expression of an atypical hydrophobic group 5 LEA protein from wild peanut Arachis diogoi confers abiotic stress tolerance in tobacco. PLoS ONE. 2016;11:e0150609. doi: 10.1371/journal.pone.0150609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao J., Li X. Identification and phylogenetic analysis of late embryogenesis abundant proteins family in tomato (Solanum lycopersicum) Planta. 2015;241:757–772. doi: 10.1007/s00425-014-2215-y. [DOI] [PubMed] [Google Scholar]
- 30.Wang W., Meng B., Chen W., Ge X., Liu S., Yu J. A proteomic study on post diapaused embryonic development of brine shrimp (Artemia francis-cana) Proteomics. 2007;7:3580–3591. doi: 10.1002/pmic.200700259. [DOI] [PubMed] [Google Scholar]
- 31.George J.M. The Synucleins. Genome Biol. 2002;3 doi: 10.1186/gb-2001-3-1-reviews3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaccaro R., Toni M., Casini A., Vivacqua G., Yu S., D’este L., Cioni C. Localization of α- synuclein in teleost central nervous system: Immunohistochemical and Western blot evidence by 3D5 monoclonal antibody in the common carp, Cyprinus carpio. J. Comp. Neurol. 2015;523:1095–1124. doi: 10.1002/cne.23722. [DOI] [PubMed] [Google Scholar]
- 33.Tiunova A.A., Anokhin K.V., Saha A.R., Schmidt O., Hanger D.P., Anderton B.H., Davies A.M., Ninkina N.N., Buchman V.L. Chicken synucleins: Cloning and expression in the developing embryo. Mech Dev. 2000;99:195–198. doi: 10.1016/S0925-4773(00)00484-6. [DOI] [PubMed] [Google Scholar]
- 34.Killinger B.A., Labrie V. Vertebrate food products as a potential source of prion-like α-synuclein. NPJ Parkinsons Dis. 2017;3:33. doi: 10.1038/s41531-017-0035-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan J., Zhao Y. Evolutionary aspects of the synuclein super-family and sub-families based on large-scale phylogenetic and group-discrimination analysis. Biochem. Biophys. Res. Commun. 2013;441:308–317. doi: 10.1016/j.bbrc.2013.09.132. [DOI] [PubMed] [Google Scholar]
- 36.Barbour R., Kling K., Anderson J.P., Banducci K., Cole T., Diep L., Fox M., Goldstein J.M., Soriano F., Seubert P., et al. Red blood cells are the major source of alpha-synuclein in blood. Neurodegener. Dis. 2008;5:55–59. doi: 10.1159/000112832. [DOI] [PubMed] [Google Scholar]
- 37.Tian C., Liu G., Gao L., Soltys D., Pan C., Stewart T., Shi M., Xie Z., Liu N., Feng T., et al. Erythrocytic α-synuclein as a potential biomarker for Parkinson’s disease. Transl Neurodegener. 2019;8:15. doi: 10.1186/s40035-019-0155-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez-Freire M., Semba R.D., Ubaida-Mohien C., Fabbri E., Scalzo P., Højlund K., Dufresne C., Lyashkov A., Ferrucci L. The human skeletal muscle proteome project: A reappraisal of the current literature. J. Cachexia Sarcopenia Muscle. 2017;8:5–18. doi: 10.1002/jcsm.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lerner A., Neidhöfer S., Matthias T. The gut microbiome feelings of the brain: Perspective for Non-Microbiologists. Microorganisms. 2017;5:66. doi: 10.3390/microorganisms5040066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vacchi E., Kaelin-Lang A., Melli G. Tau and alpha synuclein synergistic effect in neurodegenerative diseases: When the periphery is the core. Int. J. Mol. Sci. 2020;21:5030. doi: 10.3390/ijms21145030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kujawska M., Jodynis-Liebert J. What is the evidence that Parkinson’s disease is a prion disorder, which originates in the gut? Int. J. Mol. Sci. 2018;19:3573. doi: 10.3390/ijms19113573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boulos C., Yaghi N., Hayeck R.E., Heraoui G.N., Fakhoury-Sayegh N. Nutritional risk factors, microbiota and Parkinson’s disease: What Is the current evidence? Nutrients. 2019;11:1896. doi: 10.3390/nu11081896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lerner A. The intestinal luminal sources of a-synuclein: A gastroenterologist perspective. Nutr. Rev. 2021 doi: 10.1093/nutrit/nuab024. [DOI] [PubMed] [Google Scholar]
- 44.Kharrazian D., Herbert M., Vojdani A. Detection of islet cell immune reactivity with low glycemic index foods: Is this a concern for type 1 diabetes? J. Diabetes Res. 2017;2017:4124967. doi: 10.1155/2017/4124967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vojdani A., Turnpaugh C.C. Antibodies against Group A Streptococcus, dopamine receptors, and ganglioside GM1 cross-react with a variety of food antigens, potentially interfering with biomarkers for PANS and PANDAS. Biomark. Neuropsychiatry. 2020;3:100023. doi: 10.1016/j.bionps.2020.100023. [DOI] [Google Scholar]
- 46.Vojdani A., O’Bryan T., Green J.A., McCandless J., Woeller K.N., Vojdani E., Nourian A.A., Cooper E.L. Immune response to dietary proteins, gliadin and cerebellar peptide in children with autism. Nutr. Neurosci. 2004;7:151–161. doi: 10.1080/10284150400004155. [DOI] [PubMed] [Google Scholar]
- 47.Alaedini A., Okamoto H., Briani C., Wollenberg K., Shill H.A., Bushara K.O., Sander H.W., Green P.H.R., Hallett M., Latov M. Immune cross-reactivity in celiac disease: Anti-gliadin antibodies bind to neuronal synapsin I. J. Immunol. 2007;178:6590–6595. doi: 10.4049/jimmunol.178.10.6590. [DOI] [PubMed] [Google Scholar]
- 48.Vojdani A., Tarash I. Cross-reaction between gliadin and different food and tissue antigens. Food Nutr. Sci. 2013;4:20–32. doi: 10.4236/fns.2013.41005. [DOI] [Google Scholar]
- 49.Vojdani A., Vojdani E. Immunoreactivity of anti-AbP-42 specific antibody with toxic chemical and food antigens. J. Alzheimers Dis. Parkinsonism. 2018;8:441. doi: 10.4172/2161-0460.1000441. [DOI] [Google Scholar]
- 50.McGlinchey R.P., Lacy S.M., Huffer K.E., Tayebi N., Sidransky E., Lee J.C. C-terminal α-synuclein truncations are linked to cysteine cathepsin activity in Parkinson’s disease. J. Biol. Chem. 2019;294:9973–9984. doi: 10.1074/jbc.RA119.008930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ulusoy A., Febbraro F., Jensen P.H., Kirik D., Romero-Ramos M. Co-expression of C-terminal truncated α-synuclein enhances full-length α-synuclein-induced pathology. Eur. J. Neurosci. 2010;32:409–422. doi: 10.1111/j.1460-9568.2010.07284.x. [DOI] [PubMed] [Google Scholar]
- 52.van der Wateren I.M., Knowles T.P.J., Buell A.K., Dobson C.M., Galvagnion C. C-terminal truncation of α-synuclein promotes amyloid fibril amplification at physiological pH. Chem. Sci. 2018;9:5506–5516. doi: 10.1039/C8SC01109E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levitan K., Chereau D., Cohen S.I.A., Knowles T.P.J., Dobson C.M., Fink A.L., Anderson J.P., Goldstein J.M., Millhauser G.L. Conserved C-terminal charge exerts a profound influence on the aggregation rate of α-synuclein. J. Mol. Biol. 2011;411:329–333. doi: 10.1016/j.jmb.2011.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Braak H., Rub U., Gai P., Del Tredici K. Idiopathic Parkinson’s disease: Possible routes by which invulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Transm. 2003;110:517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- 55.Hawkes C.H., Del Tredici K., Braak H. Parkinson’s disease: A dual-hit hypothesis. Neuropathol. Appl. Neurobiol. 2007;33:599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Del Tredici K., Braak H. Review: Sporadic Parkinson’s disease: Development and distribution of α-synuclein pathology. Neuropathol. Appl. Neurobiol. 2016;42:33–50. doi: 10.1111/nan.12298. [DOI] [PubMed] [Google Scholar]
- 57.Schwiertz A., Spiegel J., Dillmann U., Grundmann D., Bürmann J., Faßbender K., Schäfer K.H., Unger M.M. Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson’s disease. Parkinsonism Relat. Disord. 2018;50:104–107. doi: 10.1016/j.parkreldis.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 58.Vojdani A. Lectins, agglutinins, and their role in autoimmune reactivities. Altern. Ther. Health Med. 2015;21:46–51. [PubMed] [Google Scholar]
- 59.Vojdani A. Reaction of food-specific antibodies with different tissue antigens. Int. J. Food Sci Tech. 2020;55:1800–1815. doi: 10.1111/ijfs.14467. [DOI] [Google Scholar]
- 60.De Virgilio A., Greco A., Fabbrini G., Inghilleri M., Rizzo M.I., Gallo A., Conte M., Rosato C., Ciniglio Appiani M., de Vincentiis M. Parkinson’s disease: Autoimmunity and neuroinflammation. Autoimmun. Rev. 2016;15:1005–1011. doi: 10.1016/j.autrev.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 61.Garretti F., Agalliu D., Lindestam Arlehamn C.S., Sette A., Sulzer D. Autoimmunity in Parkinson’s disease: The role of α-synuclein-specific T cells. Front. Immunol. 2019;10:303. doi: 10.3389/fimmu.2019.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lundmark K., Westermark G.T., Olsén A., Westermark P. Protein fibrils in nature can enhance amyloid protein A amyloidosis in mice: Cross-seeding as a disease mechanism. Proc. Natl. Acad. Sci. USA. 2005;102:6098–6102. doi: 10.1073/pnas.0501814102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miraglia F., Colla E. Microbiome, Parkinson’s Disease and molecular mimicry. Cells. 2019;8:222. doi: 10.3390/cells8030222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okubo H., Miyake Y., Sasaki S., Murakami K., Tanaka K., Fukushima W., Kiyohara C., Tsuboi Y., Yamada T., Oeda T., et al. Dietary patterns and risk of Parkinson’s disease: A case-control study in Japan. Eur. J. Neurol. 2012;19:681–688. doi: 10.1111/j.1468-1331.2011.03600.x. [DOI] [PubMed] [Google Scholar]
- 65.Coimbra C.G., Junqueira V.B.C. High doses of riboflavin and the elimination of dietary red meat promote the recovery of some motor functions in Parkinson’s disease patients. Braz. J. Med. Biol. Res. 2003;36:1409–1417. doi: 10.1590/S0100-879X2003001000019. [DOI] [PubMed] [Google Scholar]
- 66.Benvenga S., Guarneri F. Molecular mimicry and autoimmune thyroid disease. Rev. Endocr. Metab. Disord. 2016;17:485–498. doi: 10.1007/s11154-016-9363-2. [DOI] [PubMed] [Google Scholar]
- 67.Lyons-Weiler J. Pathogenic priming likely contributes to serious and critical illness and mortality in COVID-19 via autoimmunity. J. Transl. Autoimm. 2020;3:100051. doi: 10.1016/j.jtauto.2020.100051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chandra R., Hiniker A., Kuo Y.M., Nussbaum R.L., Liddle R.A. α-Synuclein in gut endocrine cells and its implications for Parkinson’s disease. JCI Insight. 2017;2:e92295. doi: 10.1172/jci.insight.92295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seguella L., Capuano R., Sarnelli G., Esposito G. Play in advance against neurodegeneration: Exploring enteric glial cells in gut-brain axis during neurodegenerative diseases. Expert Rev. Clin. Pharmacol. 2019;12:555–564. doi: 10.1080/17512433.2019.1612744. [DOI] [PubMed] [Google Scholar]
- 70.Vergnolle N., Cirillo C. Neurons and glia in the enteric nervous system and epithelial barrier function. Physiology (Bethesda) 2018;33:269–280. doi: 10.1152/physiol.00009.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cirillo C., Sarnelli G., Esposito G., Turco F., Steardo L., Cuomo R. S100B protein in the gut: The evidence for enteroglial-sustained intestinal inflammation. World J. Gastroenterol. 2011;17:1261–1266. doi: 10.3748/wjg.v17.i10.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fitzgerald E., Murphy S., Martinson H.A. Alpha-synuclein pathology and the role of microbiota in Parkinson’s disease. Front. Neurosci. 2019;13:369. doi: 10.3389/fnins.2019.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khalil M., Zhang Z., Engel M.A. Neuro-immune networks in gastrointestinal disorders. Visc. Med. 2019;35:52–60. doi: 10.1159/000496838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barrett P.J., Greenamyre J.T. Post-translational Modification of α-synuclein in Parkinson’s disease. Brain Res. 2015;1628:247–253. doi: 10.1016/j.brainres.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 75.Oueslati A., Fournier M., Lashuel H.A. Role of post-translational modifications in modulating the structure, function and toxicity of alpha-synuclein: Implications for Parkinson’s disease pathogenesis and therapies. Prog. Brain Res. 2010;183:115–145. doi: 10.1016/S0079-6123(10)83007-9. [DOI] [PubMed] [Google Scholar]
- 76.González N., Arcos-López T., König A., Quintanar L., Menacho Márquez M., Outeiro T.F., Fernández C.O. Effects of alpha-synuclein post-translational modifications on metal binding. J. Neurochem. 2019;150:507–521. doi: 10.1111/jnc.14721. [DOI] [PubMed] [Google Scholar]
- 77.Lerner A., Aminov R., Matthias T. Dysbiosis may trigger autoimmune diseases via inappropriate posttranslational modification of host proteins. Front. Microbiol. 2016;7:84. doi: 10.3389/fmicb.2016.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anselmi L., Bove C., Coleman H., Le K., Subramanian M.P., Venkiteswaran K., Subramanian T., Travagli R.A. Ingestion of subthreshold doses of environmental toxins induces ascending Parkinsonism in the rats. NPJ Parkinson’s Dis. 2018;4:30. doi: 10.1038/s41531-018-0066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sampson T.R., Challis C., Jain N., Moiseyenko A., Ladinsky M.S., Shastri G.G., Thron T., Needham B.D., Horvath I., Debelius J.W., et al. A gut bacterial amyloid promotes α-synuclein aggregation and motor impairment in mice. Elife. 2020;9:e53111. doi: 10.7554/eLife.53111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Batista C.R.A., Gomes G.F., Candelario-Jalil E., Fiebich B.L., de Oliveira A.C.P. Lipopolysaccharide-induced neuroinflammation as a bridge to understand neurodegeneration. Int. J. Mol. Sci. 2019;20:2293. doi: 10.3390/ijms20092293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim C., Lv G., Lee J.S., Jung B.C., Masuda-Suzukake M., Hong C.-S., Elvira Valera E., Lee H.-J., Paik S.R., Hasegawa M., et al. Exposure to bacterial endotoxin generates a distinct strain of α-synuclein fibril. Sci. Rep. 2016;6:30891. doi: 10.1038/srep30891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barbut D., Stolzenberg E., Zasloff M. Gastrointestinal immunity and alpha-synuclein. J. Parkinson’s Dis. 2019;9:S313–S322. doi: 10.3233/JPD-191702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim S., Kwon S.H., Kam T.-I., Panicker N., Karuppgounder S.S., Lee S., Lee J.H., Kim W.R., Kook M., Foss C.A., et al. Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson’s disease. Neuron. 2019;103:627–641. doi: 10.1016/j.neuron.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Forsyth C.B., Shannon K.M., Kordower J.H., Voigt R.M., Shaikh M., Jaglin J.A., Estes J.D., Dodiya H.B., Keshavarzian A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS ONE. 2011;6:e28032. doi: 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mulak A., Koszewicz M., Panek-Jeziorna M., Koziorowska-Gawron E., Budrewicz S. Fecal calprotectin as a marker of the gut immune system activation is elevated in Parkinson’s disease. Front. Neurosci. 2019;13:992. doi: 10.3389/fnins.2019.00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Challis C., Hori A., Sampson T.R., Yoo B.B., Challis R.C., Hamilton A.M., Mazmanian K.M., Volpicelli-Daley L.A., Gradinaru V. Gut-seeded α-synuclein fibrils promote gut dysfunction and brain pathology specifically in aged mice. Nat. Neurosci. 2020;23:327–336. doi: 10.1038/s41593-020-0589-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van IJzendoorn S.C.D., Derkinderen P. The intestinal barrier in Parkinson’s disease: Current state of knowledge. J. Parkinson’s Dis. 2019;9:S323–S329. doi: 10.3233/JPD-191707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kelly L.P., Carvey P.M., Keshavarzian A., Shannon K.M., Shaikh M., Bakay R.A.E., Kordower J.H. Progression of intestinal permeability changes and alpha-synuclein expression in a mouse model of Parkinson’s disease. Mov. Disord. 2014;29:999–1009. doi: 10.1002/mds.25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maes M., Sirivichayakul S., Kanchanatawan B., Vojdani A. Upregulation of the intestinal paracellular pathway with breakdown of tight and adherens junctions in deficit schizophrenia. Mol. Neurobiol. 2019;56:7056–7073. doi: 10.1007/s12035-019-1578-2. [DOI] [PubMed] [Google Scholar]
- 90.Maes M., Sirivichayakul S., Kanchanatawan B., Vojdani A. Breakdown of the paracellular tight and adherens junctions in the gut and blood brain barrier and damage to the vascular barrier in patients with deficit schizophrenia. Neurotox. Res. 2019;36:306–322. doi: 10.1007/s12640-019-00054-6. [DOI] [PubMed] [Google Scholar]
- 91.Nagele R.G., Clifford P.M., Siu G., Levin E.C., Acharya N.K., Han M., Kosciuk M.C., Venkataraman V., Zavareh S., Zarrabi S., et al. Brain reactive autoantibodies prevalent in human sera increase intraneuronal amyloid-β (1–42) deposition. J. Alzheimers Dis. 2011;25:605–622. doi: 10.3233/JAD-2011-110098. [DOI] [PubMed] [Google Scholar]
- 92.Houser M.C., Tansey M.G. The gut-brain axis: Is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinson’s Dis. 2017;3:3. doi: 10.1038/s41531-016-0002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Klingelhoefer L., Reichmann H. Pathogenesis of Parkinson disease—the gut-brain axis and environmental factors. Nat. Rev. Neurol. 2015;11:625–636. doi: 10.1038/nrneurol.2015.197. [DOI] [PubMed] [Google Scholar]
- 94.Fields C.R., Bengoa-Vergniory N., Wade-Martins R. Targeting alpha-synuclein as a therapy for Parkinson’s disease. Front. Mol. Neurosci. 2019;12:299. doi: 10.3389/fnmol.2019.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tran H.T., Chung C.H.-Y., Iba M., Zhang B., Trojanowski J.Q., Luk K.C., Lee V.M.Y. α-Synuclein immunotherapy blocks uptake and template propagation of misfolded α-synuclein and neurodegeneration. Cell Rep. 2014;7:2054–2065. doi: 10.1016/j.celrep.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang Z., Zhang S., Fu P., Zhang Z., Lin K., Ko J.K.-S., Yung K.K.-L. Roles of Glutamate Receptors in Parkinson’s Disease. Int. J. Mol. Sci. 2019;20:4391. doi: 10.3390/ijms20184391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jiménez-González V., Ogalla-García E., García-Quintanilla M., García-Quintanilla A. Deciphering GRINA/Lifeguard1: Nuclear Location, Ca2+ Homeostasis and Vesicle Transport. Int. J. Mol. Sci. 2019;20:4005. doi: 10.3390/ijms20164005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baron M.V., Troncone R., Auricchio S. Gliadin peptides as triggers of the proliferative and stress/innate immune response of the celiac small intestinal mucosa. Int. J. Mol. Sci. 2014;15:20518–20537. doi: 10.3390/ijms151120518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Garcia-Quintanilla A., Miranzo-Navarro D. Extraintestinal manifestations of celiac disease: 33-mer gliadin binding to glutamate receptor GRINA as a new explanation. Bioessays. 2016;38:427–439. doi: 10.1002/bies.201500143. [DOI] [PubMed] [Google Scholar]
- 100.Obrenovich M.E.M. Leaky gut, leaky brain? Microorganisms. 2018;6:107. doi: 10.3390/microorganisms6040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Costa de Aquino C., Leitão R.A., Oliveira Alves L.A., Coelho-Santos V., Guerrant R.L., Ribeiro C.F., Malva J.O., Silva A.P., Oriá R.B. Effect of hypoproteic and high-fat diets on hippocampal blood-brain barrier permeability and oxidative stress. Front. Nutr. 2018;5:131. doi: 10.3389/fnut.2018.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lerner A., Benzvi C. “Let Food Be Thy Medicine”: Gluten and Potential Role in Neurodegeneration. Cells. 2021;10:756. doi: 10.3390/cells10040756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available in a publicly accessible repository.