Abstract
The study aims to evaluate the infection prevalence, virulence gene distribution and antimicrobial resistance of Aeromonas hydrophila associated in diseased outbreaks of cultured freshwater fish in Northern Vietnam. The confirmed A. hydrophila were screened for the presence of the five pitutative-virulence genes including aerolysin (aerA), hemolysin (hlyA), cytotonic enterotoxin (act), heat-labile cytotonic enterotoxin (alt), and heat-stable enterotoxin (ast), and examined the susceptibility to 16 antibiotics. A total of 236 A. hydrophila isolates were recovered and confirmed from 506 diseased fish by phenotypic tests, PCR assays, and gyrB, rpoB sequenced analyses, corresponding to the infection prevalence at 46.4%. A total of 88.9% of A. hydrophila isolates harbored at least one of the tested virulence genes. The genes aerA and act were most frequently found (80.5% and 80.1%, respectively) while the ast gene was absent in all isolates. The resistance to oxacillin, amoxicillin and vancomycin exhibited the highest frequencies (>70%), followed by erythromycin, oxytetracycline, florfenicol, and sulfamethoxazole/trimethoprim (9.3–47.2%). The multiple antibiotic resistance (MAR) index ranged between 0.13–0.88 with 74.7% of the isolates having MAR values higher than 0.2. The results present a warning for aquaculture farmers and managers in preventing the spread of A. hydrophila and minimizing antibiotic resistance of this pathogen in fish farming systems.
Keywords: Aeromonas hydrophila, infection prevalence, virulence genes, antimicrobial resistances, freshwater fish, Vietnam
1. Introduction
Freshwater fish farming represents a substantial proportion in the aquaculture industry, particularly in developing countries providing food and livelihood and contribution to the regional economy [1]. A great diversity of freshwater aquatic organisms has been cultured, of which, tilapia; carp; and catfish are the major species in global aquaculture production [1,2]. Recently, the farming systems of these species have been shifted from simple traditional to intensive culture methods [1,3]. In the intensive practices, animals are confined in densely stocked and often high organic load conditions which trigger the development and spread of pathogenic bacteria [4,5]. The problems and economic losses related to bacterial diseases have, therefore, become increasingly frequent.
Aeromonas spp. are commonly found in freshwater, estuarine, and saltwater environments [6,7,8]. Members of this genus have been the focus of attention because of their potential to act as pathogens on a wide range of hosts not only in fish, amphibians, and reptiles but also in mammals, including humans [8,9]. Among Aeromonas species that cause fish diseases, A. hydrophila has been identified as one of the most dangerous pathogens causing mortality outbreaks in a diversity of cultured fish species [6,10] and has been responsible for huge economic losses in various countries [11,12].
The infection of farmed and wild fish by A. hydrophila is characterized as hemorrhagic septicemia with signs of ulceration, hemorrhaging, and fin erosion [6]. The disease displays chronic traits that persist for weeks, the mortality rate gradually increases and high cumulative mortality can occur [13]. More critically, due to the capacity of human infection, the A. hydrophila diseased fish proposed a potential risk to human health through foodborne [14]. The capacity of A. hydrophila to cause diseases relates to a variety of virulence factors which are complex and multifactorial [8,15]. Among them, hemolysin (hlyA), aerolysin (aerA), cytotoxic heat-labile enterotoxin (act), cytotonic heat-labile enterotoxin (alt), and cytotonic heat-stable enterotoxin (ast) are well-known virulence factors and likely related to the hemorrhagic septicemia symptoms on the infected fish and animals [16,17].
To prevent and treat bacterial infections in aquaculture, antibiotics are commonly used via medicated feed or direct addition to the culture water [18]. These administration methods often result in heavy use of antibiotics, broader aquatic areas exposed and a wide range of bacteria impacted to the drugs in comparison to the use of antibiotics in terrestrial animal production [19]. The potential consequences of antibiotic overuse or misuse include the development of antibiotic-resistant bacteria, transferring of resistance traits to the bacterial community, and reducing the efficacy of antibiotic treatment for human and animal diseases [20,21].
Tilapia, channel catfish and carp (common carp and grass carp) are main culture species in Northern Vietnam. Their fast growth rate, ease to culture, and ability to fetch very good market prices have contributed towards the rapid expansion of the culture area, and they have become favorable culture species in this region. Although intensive farming systems of those freshwater fish have rapidly developed, increased in economic importance, and contributed to the achievement of the top 3 seafood exporting countries [22], the information on infection by A. hydrophila in freshwater fish is limited, particularly in Northern Vietnam. The aim of this study was, therefore, to identify the prevalence of infection, the presence of the genes encoding relevant virulence factors and antimicrobial resistance of A. hydrophila isolated from freshwater fish in this region.
2. Results
2.1. Clinical Signs and Gross Lesions
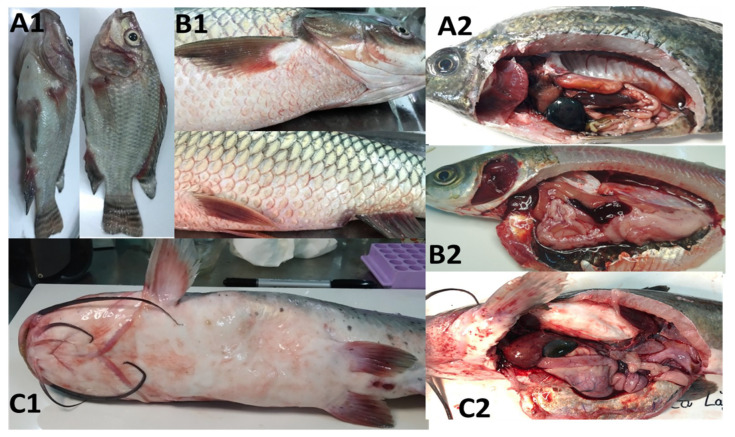
The diseased freshwater fish in this study exhibited obvious critical symptoms, including abnormal swimming behavior at the water surface, reduction in feed intake, pale or darker skin with or without ulcer formation, hemorrhage around their mouth, operculum, fin bases, fin erosion and swollen belly with hemorrhagic protrusion from the anal opening was also observed (Figure 1). In most of the inspected fish samples, gross lesions were mainly hemorrhagic and enlarged liver, gallbladder and spleen, empty or partially empty stomach and intestine, and enlarged and darkened posterior kidney. The impacted ponds/cages initially showed sporadic mortality and later mass mortality of infected fish.
Figure 1.
Clinical symptoms and gross lesions of diseased fish infected with A. hydrophila. The clinical signs of heavy hemorrhage around the fish’s mouth, operculum, and fin bases, and fin erosion of tilapia (A1), grass carp (B1) and channel catfish (C1); and gross lesions of enlarged gall bladder and liver, enlarged and darkened spleen, hemorrhage and empty stomach and intestines in infected tilapia (A2), grass carp (B2), channel catfish (C2).
2.2. Aeromonas Hydrophila Identification
A total of 458 bacterial isolates from diseased fish grew well on the Rimler–Shotts (RS) medium with small smooth and yellow colonies consisting of Gram-negative, rod-shaped, and motile bacilli were recovered and tentatively identified as Aeromonas species. The results of the phenotypic tests revealed that 338/458 isolates were identical to those of the reference A. hydrophila strain ATCC 7966 (Supplementary Table S1), accounted for 73.1% of the total isolates recovered from diseased fish.
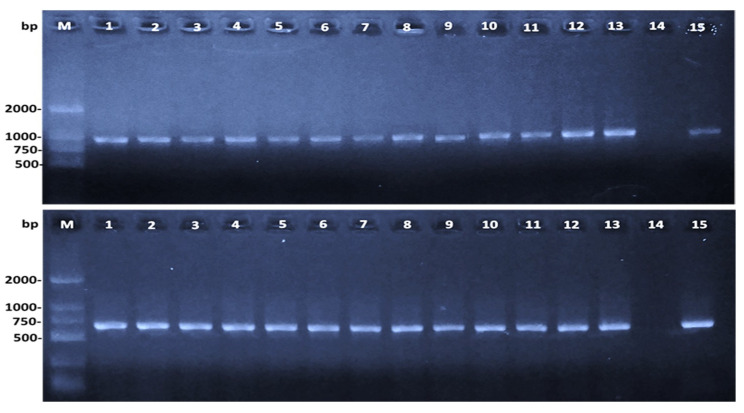
PCR assays successfully amplified both the 16S rRNA gene of Aeromonas genus (953 bp) and species-specific A. hydrophila gene (625 bp) of 255/338 isolates, including 102, 95 and 58 isolates from tilapia, carp, and channel catfish, respectively (Figure 2).
Figure 2.
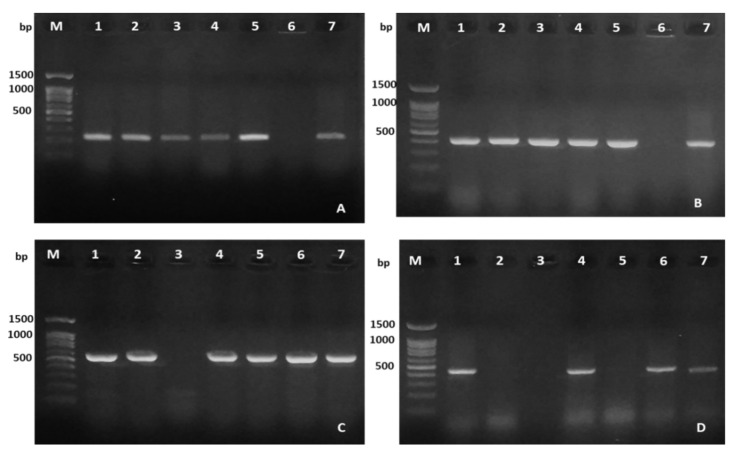
Amplified products of representative A. hydrophila isolates from tilapia (n = 5), carp (n = 4) and channel catfish (n = 4) of Aeromonas genus (953 bp)—upper ranges and A. hydrophila species (625 bp)—lower ranges: M: DNA ladder; lane 1–13 representative isolates from diseased fish; lane 14-negative control; lane 15-positive control (A. hydrophila ATCC 7966).
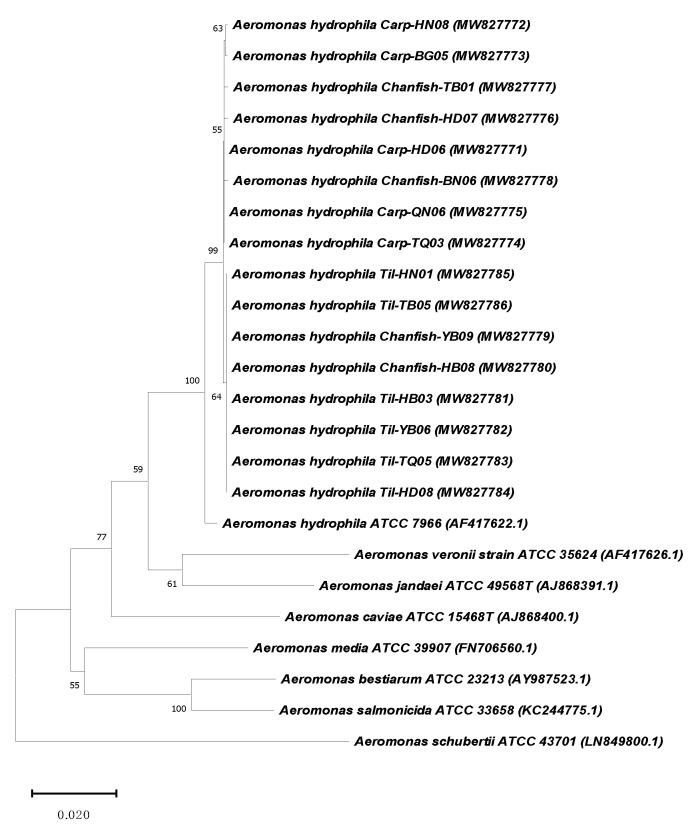
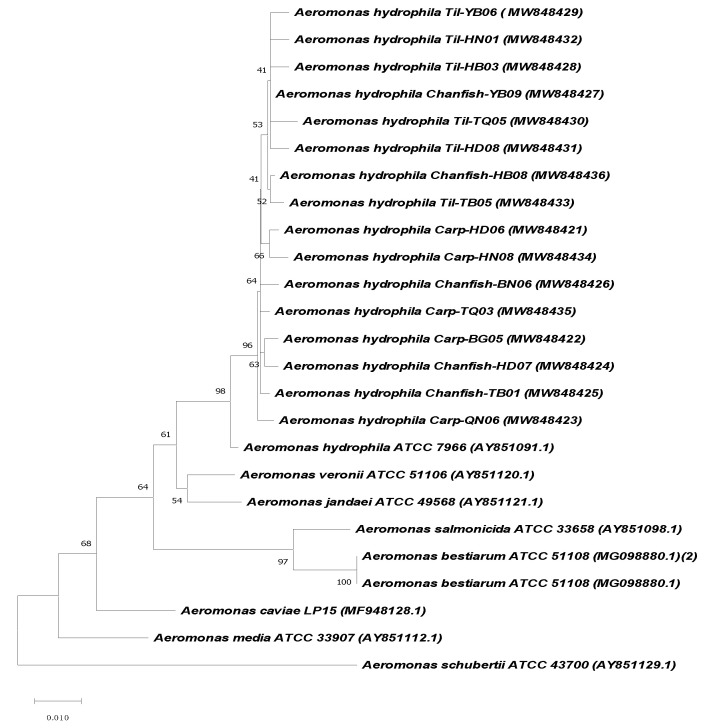
The BLASTing analyses of gyrB and rpoB sequences revealed that 236/255 isolates were most precise identification as A. hydrophila due to their sequences shared the highest identities to those of other A. hydrophila in Genbank database and to those of the reference A. hydrophila strain ATCC 7966 (ranged 99.1–99.2%, and 98.4–99.1% for gyrB and rpoB sequences, respectively) (Table S2). Another 12 isolates with the sequence similarity below 97% (both or neither gyrB and/or rpoB) to the reference strain were subjected as misidentification. Due to a large number of isolates, the sequences of gyrB and rpoB genes from 16 representative isolates (including the highest and lowest sequence identities to those of A. hydrophila ATCC 7966) were submitted to Genbank under accession number MW827771-MW827786 (for gyrB genes), MW848421- MW848436 (for rpoB genes) and used for phylogenetic analysis. The derived neighbor-joining trees using gyrB and rpoB sequences in this study with other Aeromonas species in Genbank database revealed that the isolates of the present study are most closely related to A. hydrophila strain ATCC 7966 (Figure 3 and Figure 4). Based on the number of A. hydrophila isolates that were conclusively identified (236 isolates), the detection frequency of this pathogen was at 46.4%, on average, and no significant difference was shown in the frequencies of this bacteria detected on diseased tilapia, carp and channel catfish (p > 0.05) (Table 1).
Figure 3.
Phenotypic analysis based on gyrB sequences of the representative isolates recovered from the three fish hosts with those of other of Aeromonas species retrieved from Genbank using the neighbor-joining method. Bootstraps of 2000 replicates were performed.
Figure 4.
Phenotypic analysis based on rpoB sequences of the representative isolates recovered from the three fish hosts with those of other of Aeromonas species retrieved from Genbank using the neighbor-joining method. Bootstraps of 2000 replicates were performed.
Table 1.
Prevalence of A. hydrophila isolated from diseased tilapia, carp and channel catfish.
| Fish Species | Total no. of Samples | Number of Isolates | ||||
|---|---|---|---|---|---|---|
| Recovered on Rimler–Shotts Medium | Confirmed by Phenotypic Tests | Confirmed by PCR | Confirmed bySequencing | Detection Frequencies (%) |
||
| Tilapia | 198 | 187 | 136 | 102 | 93 | 47.0 |
| Carp | 187 | 156 | 118 | 95 | 89 | 47.6 |
| Channel catfish | 121 | 115 | 84 | 58 | 54 | 44.6 |
| Total/mean | 506 | 458 | 338 | 255 | 236 | 46.4 |
2.3. Virulence Genes Characteristics
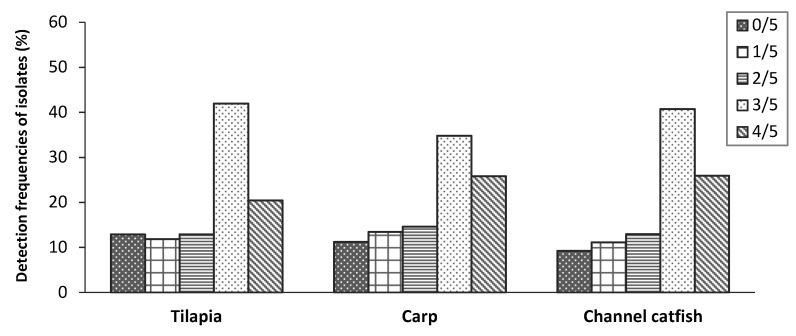
The presence of the five putative virulence genes in A. hydrophila isolated from diseased fish samples is shown in Table 2 and Figure 5 and Figure 6. Of all isolates, no selected virulence gene was detected in 11.1% of the isolates while the other 88.9% isolates harbored at least one virulence gene. The Fisher’s exact test showed no significant difference in the detection frequency of each virulence gene on A. hydrophila isolates among the three fish species (p > 0.05). The highest frequencies of the isolates (39.2%) were found carrying 3/5 virulence genes and the proportion of isolates harboring 1/5, 2/5 and 4/5 virulence genes accounted for 12.1%, 13.5% and 24.1%, respectively. On average, aerolysin (aerA, 80.5%) and cytotoxic enterotoxin (act, 80.1%) were the most frequently detected virulence genes in A. hydrophila isolates from the three freshwater fish species. Hemolysin A (hlyA) was detected at a lower frequency of 59.7%, followed by the occurrence rates of heat-labile cytotonic enterotoxin (alt) gene 44.9%. In contrast, the heat-stable enterotoxin (ast) gene was not detected in any of the 236 isolates.
Table 2.
Detection frequencies of the five virulence genes in A. hydrophila isolated from tilapia, carp and channel catfish.
| Virulence Genes | Detection Frequencies % (N) | |||
|---|---|---|---|---|
| Tilapia (n = 93) |
Carp (n = 89) |
Channel Catfish (n = 54) |
Mean (n = 236) |
|
| hlyA | 58.1 (54) | 63.0 (56) | 57.5 (31) | 59.7 (141) |
| aerA | 83.9 (78) | 79.8 (71) | 76.0 (41) | 80.5 (190) |
| act | 83.9 (78) | 76.5 (68) | 79.7 (43) | 80.1 (189) |
| alt | 42.0 (39) | 47.2 (42) | 46.3 (25) | 44.9 (106) |
| ast | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Figure 5.
The detection frequencies of the A. hydrophila isolates from cultured tilapia, carp, and channel catfish carrying 0–4 virulence genes out of the 5 tested genes in the study.
Figure 6.
PCR amplification of virulence genes carried in representative A. hydrophila isolates. M: DNA ladder; lane 1–7 representative amplified products of act gene (232 bp) (A); aerA gene (431 bp) (B); hlyA gene (597 bp) (C); and alt gene (442 bp) (D).
2.4. Antibiotic Susceptibility Patterns
Examination of antimicrobial susceptibility to 16 antimicrobials was performed on all 236 confirmed A. hydrophila isolates in this study. The results demonstrated that all isolates from tilapia, carp and channel catfish exhibited a serious and varying degree of resistance to all the tested antimicrobial agents.
Oxacillin (Ox), amoxicillin (Ax) and vancomycin (Va) showed the highest resistance frequencies (>70%) in A. hydrophila isolates from the three fish species. The resistance rate reached up to 100% for oxacillin and amoxicillin in the isolates from carp and channel catfish. The resistance ratio to neomycin (Ne) was approximately 80% of the isolates from tilapia, significantly higher than those of the isolates from carp (21.3%) and channel catfish (29.6%) (p < 0.05, Table 3 and Table 4).
Table 3.
Antimicrobial susceptibility profiles of A. hydrophila isolates from tilapia, carp and channel catfish.
| Antimicrobial Agents | Antimicrobial Susceptibility of A. hydrophila (N = 236) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tilapia (n = 93) | Carp (n = 89) | Channel Catfish (n = 54) | |||||||
| S (%) | I (%) | R (%) | S (%) | I (%) | R (%) | S (%) | I (%) | R (%) | |
| Penicillins (PNs) | |||||||||
| Oxacillin (Ox) | 0 (0) | 4.4 (4) | 95.7 (89) | 0 (0) | 0 (0) | 100 (89) | 0 (0) | 9.3 (5) | 90.8 (49) |
| Amoxicillin (Ax) | 15.1 (14) | 7.6 (7) | 77.5 (72) | 3.4 (3) | 0(0) | 96.7 (86) | 0 (0) | 0 (0) | 100 (54) |
| Β-Lactam/β-Lactamase inhibitor combination (BL/BLI) | |||||||||
| Amoxicillin-Clavulanic acid (AC) | 76.4 (71) | 15.1 (14) | 8.7 (8) | 73.1 (65) | 18 (16) | 9.0 (8) | 68.6 (37) | 16.7 (9) | 14.9 (8) |
| Cephems (CPs) | |||||||||
| Ceftriaxone (Ct) | 69.9 (65) | 4.4 (4) | 25.9 (24) | 95.6 (85) | 4.5 (4) | 0 (0) | 90.8 (49) | 9.3 (5) | 0(0) |
| Cefuroxime (Cu) | 81.8 (76) | 5.4 (5) | 13.0 (12) | 96.7 (86) | 0 (0) | 3.4 (3) | 100 (54) | 0(0) | 0(0) |
| Cefotaxime (Cx) | 77.5 (72) | 0 (0) | 22.6 (21) | 96.7 (86) | 0 (0) | 3.4 (3) | 100 (54) | 0(0) | 0(0) |
| Macrolides (MCs) | |||||||||
| Erythromycin (Er) | 24.8 (23) | 32.3 (30) | 43.1 (40) | 38.3 (34) | 14.7 (13) | 47.2 (42) | 57.5 (31) | 33.4 (18) | 9.3 (5) |
| Quinolones (QLs) | |||||||||
| Nalidixic (Na) | 54.9 (51) | 0 (0) | 45.2 (42) | 46.1 (41) | 3.4 (3) | 50.6 (45) | 53.8 (29) | 9.3 (5) | 37.1 (20) |
| Sulfonamides (SULs) (Folate pathway inhibitors) | |||||||||
| Sulfamethoxzole- Trimethoprim (SM/TM) |
36.6 (34) | 18.3 (17) | 45.2 (42) | 75.3 (67) | 0 (0) | 24.8 (22) | 68.6 (37) | 9.3 (5) | 22.3 (12) |
| Aminoglycosides (AMGs) | |||||||||
| Neomycin (Ne) | 4.4 (4) | 16.2 (15) | 79.6 (74) | 42.7 (38) | 36.0 (32) | 21.4 (19) | 38.9 (21) | 31.5 (17) | 29.7 (16) |
| Glycopeptide (GLs) | |||||||||
| Vancomycin (Va) | 3.3 (3) | 15.1 (14) | 81.8 (76) | 6.8 (6) | 20.3 (18) | 73.1 (65) | 0 (0) | 16.7 (9) | 83.4 (45) |
| Flouroquinolones (FQNs) | |||||||||
| Ofloxacin (Of) | 49.5 (46) | 8.7 (8) | 42 (39) | 79.8 (71) | 3.4 (3) | 16.9 (15) | 61.2 (33) | 38.9 (21) | 0 (0) |
| Norfloxacin (No) | 63.5 (59) | 24.8 (23) | 11.9 (11) | 83.2 (74) | 3.4 (3) | 13.5 (12) | 77.8 (42) | 22.3 (12) | 0 (0) |
| Tetracyclines (TCs) | |||||||||
| Doxycycline (Dx) | 88.2 (82) | 9.7 (9) | 2.2 (2) | 75.3 (67) | 18 (16) | 6.8 (6) | 100 (54) | 0 (0) | 0(0) |
| Oxytetracycline (OTC) | 59.2 (55) | 4.4 (4) | 36.6 (34) | 55.1 (49) | 0 (0) | 45 (40) | 70.4 (38) | 0 (0) | 29.7 (16) |
| Amphenicols (AMPs) | |||||||||
| Florfenicol (Fl) | 72.1 (67) | 3.3 (3) | 24.8 (23) | 76.5 (68) | 0 (0) | 23.6 (21) | 83.4 (45) | 0 (0) | 16.7 (9) |
| Overall | |||||||||
| Average (%) | 48.5 | 10.6 | 40.9 | 59.0 | 7.6 | 33.4 | 60.6 | 12.3 | 27.1 |
R = resistant; I = intermediate; S = susceptibility; n = number of isolates from each source; N: total number of isolates.
Table 4.
Antimicrobial-resistant phenotypes of the isolates confirmed from tilapia, carp and channel catfish.
| No of Drugs | Resistance Phenotypes | The Ratio of Isolates—% (N) | ||
|---|---|---|---|---|
| Tilapia n = 93 |
Carp n = 89 |
Channel Catfish n = 54 |
||
| 2 | Ox + Ax | 2.2 (2) | 3.4 (3) | 16.7 (9) |
| 3 | Ox + Ne + Va | 7.6 (7) | 0 (0) | 0 (0) |
| Ox + Ax + Ne | 3.3 (3) | 0 (0) | 0 (0) | |
| Ox + Ax + Na | 0 (0) | 2.3 (2) | 0 (0) | |
| Ox + Ax + Va | 0 (0) | 21.4 (19) | 20.4 (11) | |
| 4 | Ox + Ax + Na + Va | 4.4 (4) | 3.4 (3) | 13 (7) |
| Ox + Ax + Of + Ne | 3.3 (3) | 3.4 (3) | 0 (0) | |
| Ox + Ax + Ne + Va | 2.2 (2) | 0 (0) | 0 (0) | |
| Ox + Ax + Va + Fl | 0 (0) | 4.5 (4) | 0 (0) | |
| 5 | Ox + Ax + Na + Ne + Va | 3.3 (3) | 0 (0) | 11.2 (6) |
| Ox + Ax + Er + Ne + Va | 5.4 (5) | 5.7 (5) | 0 (0) | |
| Ox + Ax + SM/TM + Fl + OTC | 0 (0) | 3.4 (3) | 0 (0) | |
| Ox + Ax + Na + Va + OTC | 0 (0) | 3.4 (3) | 7.5 (4) | |
| Ox + Ax + SM/TM + Va + OTC | 0 (0) | 1.2 (1) | 0 (0) | |
| Ox + Ax + Er + Na + OCT | 0 (0) | 18 (16) | 0 (0) | |
| 6 | Ox + Ct + Cx + Va + Fl + OTC | 5.4 (5) | 0 (0) | 0 (0) |
| Ox + Ax + Er + SM/TM + Ne + Va | 3.3 (3) | 0 (0) | 0 (0) | |
| Ox + Ax + SM/TM + Ne + Va + OTC | 3.3 (3) | 3.4 (3) | 0 (0) | |
| Ox + Ax + Er + Na + Ne + OTC | 2.2 (2) | 0 (0) | 0 (0) | |
| Ox + Ax + Cu + SM/TM + Ne + Va | 3.3 (3) | 0 (0) | 0 (0) | |
| Ox + Ax + Ac + Er + Ne + Va | 4.4 (4) | 0 (0) | 9.3 (5) | |
| Ox + Ax + Er + Of + Ne + Va | 1.1 (1) | 0 (0) | 0 (0) | |
| Ox + Ax + SM/TM + Va + Fl + OTC | 0 (0) | 0 (0) | 7.5 (4) | |
| Ax + SM/TM + Ne + Va + Fl + OTC | 0 (0) | 0 (0) | 9.3 (5) | |
| 7 | Ox + Ax + Ct + Er + Ne + Va + OTC | 3.3 (3) | 0 (0) | 0 (0) |
| Ox + Ax + SM/TM + Ne + Va + Fl + OTC | 2.2 (2) | 0 (0) | 0 (0) | |
| Ox + Ax + Na + SM/TM + Ne + Va + Fl | 3.3 (3) | 0 (0) | 0 (0) | |
| Ox + Ax + Na + Of + Ne + Va + OTC | 4.4 (4) | 0 (0) | 0 (0) | |
| Ox + Ax + Ac + Na + Of + No + Va | 0 (0) | 3.4 (3) | 0 (0) | |
| Ox + Ax + Ac + Na + SM/TM + Va + OTC | 0 (0) | 0 (0) | 5.6 (3) | |
| 8 | Ox + Ct + Cx + Cu + Of + Ne + Va + OTC | 2.2 (2) | 0 (0) | 0 (0) |
| Ox + Ct + Cx + Na + Of + SM/TM + Ne + OTC | 3.3 (3) | 0 (0) | 0 (0) | |
| Ox + Ax + Er + Na + Of + SM/TM + Ne + Va | 5.4 (5) | 0 (0) | 0 (0) | |
| Ox + Ax + Er + Na + Of + No + SM/TM + Va | 5.4 (5) | 4.5 (4) | 0 (0) | |
| Ox + Ax + Ac + Er + Na + Of + Ne + Va | 1.1 (1) | 3.4 (3) | 0 (0) | |
| Ox + Ax + Er + No + SM/TM + Va + Fl + OTC | 0 (0) | 3.4 (3) | 0 (0) | |
| Ox + Ax + Er + Na + SM/TM + Va + Fl + OTC | 0 (0) | 2.3 (2) | 0 (0) | |
| 9 | Ct + Cx + Na + Of + No + SM/TM + Ne + Fl + OTC | 4.4 (4) | 0 (0) | 0 (0) |
| Ox + Ax + Er + Na + Of + SM/TM + Va + Fl + OTC | 3.3 (3) | 0 (0) | 0 (0) | |
| Ox + Ax + Er + Na + SM/TM + Va + Dx + Fl + OTC | 0 (0) | 4.5 (4) | 0 (0) | |
| 10 | Ox + Ax + Er + Na + Of + SM/TM + Ne + Va + Fl + OTC | 1.1 (1) | 0 (0) | 0 (0) |
| Ox + Ax + Cu + Cx + Er + Na + Ne + Va + Fl + OTC | 0 (0) | 3.4 (3) | 0 (0) | |
| 12 | Ox + Ax + Ct + Cu + Cx + Er + Na + Of + SM/TM + Ne + Va + Fl | 2.2 (2) | 0 (0) | 0 (0) |
| Ox + Ax + Ac + Ct + Cu + Cx + Er + Of + SM/TM + Ne + Va + Fl | 3.3 (3) | 0 (0) | 0 (0) | |
| Ox + Ax + Ac + Er + Na + Of + No + SM/TM + Ne + Va + Fl + OTC | 0 (0) | 2.3 (2) | 0 (0) | |
| 14 | Ox + Ax + Ct + Cu + Cx + Er + Na + Of + No + SM/TM + Ne + Va + Dx + OTC | 2.2 (2) | 0 (0) | 0 (0) |
All of the A. hydrophila isolates from channel catfish were susceptible to the six antimicrobial agents including cefotaxime (Ct), ceftriaxone (Cx), cefuroxime (Cu), ofloxacin (Of), norfloxacin (No), and doxycycline (Dx). A similar resistance outcome to cefotaxime was also detected in the isolates from carp and low resistance frequencies (<15%) were observed to the other five drugs. In contrast, the isolates from tilapia exhibited variable degrees of resistance to those antimicrobial agents at below 15% to cefuroxime, norfloxacin and doxycycline; and from 22.6 to 41.9% for cefotaxime, ceftriaxone and ofloxacin. All isolates expressed resistance frequencies below 15% to the combination of amoxicillin and clavulanic acid and slightly higher resistance frequencies (16.7–24.7%) were observed to florfenicol.
Relatively higher resistance ratios were found in A. hydrophila isolates from tilapia and carp to erythromycin (Er), nalidixic acid (Na) and oxytetracycline (OTC) (36.6–50.6%) compared to those of isolates from channel catfish (9.3–37.0%). The resistance rate of tilapia isolates to the combination of sulfamethoxazole and trimethoprim (SM/TM, 45.2%) was significantly higher than that of isolates from carp (22.2%) and channel catfish (24.7%), (p < 0.05).
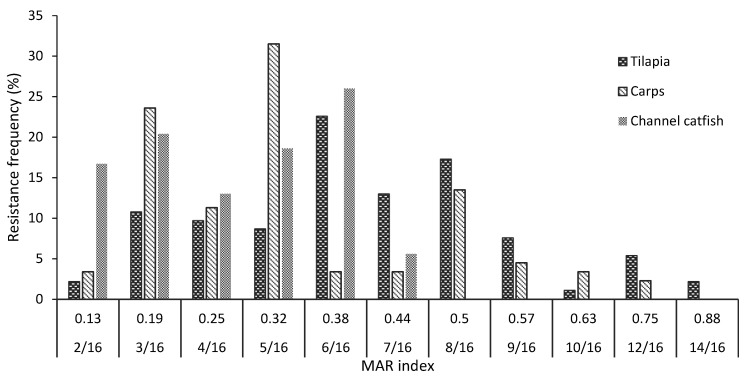
The overall multiple antibiotic resistance (MAR) index ranged from 0.13 to 0.88, corresponding to 100% A. hydrophila isolates resistant to at least two antimicrobial agents. The highest MAR values of tilapia, carp, and channel catfish isolates were 0.88, 0.75, and 0.44, respectively (Figure 7). In total, 74.7% of the A. hydrophila isolates were observed with MAR ≥ 0.2 (87.6% of isolates from tilapia, followed by those of carp and channel catfish, at 73.3 and 63.2%, respectively). Almost 64% of the tilapia isolates were resistant to 6/16 to 8/16 antimicrobial agents, while 16.3% of the isolates showed resistance to more than 8 drugs (MAR > 0.5). On carp, more than 66% of the isolates resisted 3/16 to 5/16 antibiotics while more than 94% of the isolates from channel catfish resisted 2/16 to 6/16 of the antimicrobial agents.
Figure 7.
The distributions of multiple antibiotic resistances (MAR) of A. hydrophila isolates from cultured tilapia, carp, and channel catfish. None of the isolates was resistant to only 0 or 1 agent. The values 2/16 to 14/16 mean that the isolates showed resistance to 2 to 14 out of 16 antimicrobial agents with the corresponding MAR values.
3. Materials and Methods
3.1. Sample Locations and Clinical Examination of Diseased Fish
In 2019–2020, a total of 506 diseased fish (moribund or recently died) of three cultured species including tilapia (198 samples), carp (mainly common carp and grass carp; 187 samples), and channel catfish (121 samples) were collected from 54 farms of the major culture areas located in sixteen provinces in Northern Vietnam (Figure 8). The fish samples were placed in sterile sealed plastic bags, transferred to the laboratory of the Department of Aquatic Environment and Fish Pathology—Faculty of Fisheries, Vietnam National University of Agriculture (VNUA) in a cold box (below 4 °C), and immediately analyzed. Clinical signs and gross features of all diseased fish were observed and recorded.
Figure 8.
A map showing the provincial sites (indicated by red color) from which diseased fish were collected in the study.
3.2. Bacterial Culture and Isolation
For detection of A. hydrophila in fish, the skin surface was first sterilized by swabbing with 70% ethyl alcohol before making dissection incisions. Samples of liver, kidney and spleen were streaked on Rimler–Shotts (RS) agar plates (Himedia, India), followed by incubation at 28 °C for 18–24 h. The presumptive colonies (yellow) were selected and re-isolated three times, followed by the determination of colony and bacterial morphology. Subsequently, isolates were stored in brain heart infusion (BHI) broth supplemented with 15% glycerol and kept at −80 °C for further examination.
3.3. Bacterial Identification
Conventional identification of Aeromonas species based on phenotypic methods is challenging due to the heterogeneous nature of the species [23]. Identification of A. hydrophila in the present study was, therefore, conducted using a combination of phenotypic tests and molecular methods. For phenotypic identification, the following tests were conducted including shape and color of colonies on Rimler–Shotts media, cell morphology, motility, catalase, cytochrome oxidase (OX), Voges–Proskauer (VP), hemolysis of sheep RBCs, sensitivity to 0/129 (150 µg), indole production (IND), urease production (URE), citrate utilization (CIT), glucose fermentation, H2S production, arginine dihydrolase (ADH), lysine decarboxylase (LDC), ornithine decarboxylase (ODC), melibiose fermentation (MEL), amygdalin fermentation (AMY), arabinose fermentation (ARA), inositol fermentation (INO), mannitol fermentation (MAN), tryptophane deaminase (TDA), gelatin hydrolysis (GEL). The isolates were selected if all test results were identical to those of the reference A. hydrophila strain ATCC 7966 (Supplementary Table S1).
For molecular identification, the genomic DNA of bacterial isolates was extracted using the InstaGene matrix (Bio-Rad laboratories, Hercules, CA, USA) according to the manufacturer’s protocol. All the suspected isolates which were screened by the phenotypic tests were subjected to PCR examination using primer sets to detect specific Aeromonas genus (953 bp) and Aeromonas hydrophila species (AeroH; 625 bp) as described in previous studies [24,25] (Table 5). PCR reaction mixtures and conditions were modified from previous study [25]. In brief, PCR reaction mixtures (total 20 µL) included 10 µL of Gotaq Green Master Mix (Promega, Madison, WI, USA), 1.5 µL of specific primer (each of forward and reverse), 3 µL DNA template and 4 µL of DNA-free distilled water. The mixtures were then placed in a thermal cycler for amplification under the following conditions: an initial denaturation of 4 min at 94 °C; 35 cycles consisting of denaturation at 95 °C for 30 s, annealing at 58 °C for 30 s, and extension step at 72 °C for 60 s; and a final extension for 7 min at 72 °C. The amplified products were then analyzed by electrophoresis on a 1% agarose gel containing Redsafe nucleic acid staining solution (Intron, Korea). The images were captured digitally using a Gel image system (Hercules, CA, USA).
Table 5.
Primers used for A. hydrophila identification in this study.
| Gene | Primers | DNA Sequence (5′→3′) | Product Size (bp) | References |
|---|---|---|---|---|
| 16S rRNA | Aero16S-F | CTACTTTTGCCGGCGAGCGG TGATTCCCGAAGGCACTCCC |
953 | [24] |
| Aero16S-R | ||||
| AeroH | AeroH-F | GAAAGGTTGATGCCTAATACGTA | 625 | [25] |
| AeroH-R | CGTGCTGGCAACAAAGGACAG | |||
| gyrB | gyrB 3F | TCCGGCGGTCTGCACGGCGT | 1110 | [30] |
| gyrB 14R | TTGTCCGGGTTGTACTCGTC | |||
| rpoB | PasrpoB-L | GCAGTGAAAGARTTCTTTGGTTC | 560 | [31] |
| RpoB-R | GTTGCATGTTNGNACCCAT |
Due to the complexity of Aeromonas species identification, the specific genes sequence analyses were conducted after PCR examination [26]. The DNAs of the PCR-positive isolates were used for sequencing the housekeeping genes gyrB (~1100 pb) and rpoB (560 bp) with the corresponding primer sets presented in Table 5. The sequences of gyrB and rpoB genes of the suspected A. hydrophila isolates were evaluated the identity to other Aeromonas species and A. hydrophila ATCC 7966 from the Genbank database. The representative sequences of gyrB and rpoB genes and those of other Aeromonas species were aligned using ClustalW [27], and phylogenetic trees were then constructed using the neighbor-joining method [28] in Mega X software [29].
3.4. Virulence Genes Detection
The presence of the genes encoding enterotoxins (act, alt, ast) and hemolysins (hlyA and aerA) in the genome of A. hydrophila isolates was detected by PCR using specific primers described in previous studies [32,33,34] (Table S3). The protocols of DNA extraction and PCR reaction mixture were similar to those used in bacterial identification on the corresponding primers. The thermal conditions consisted of an initial denaturation of 94 °C for 3 min; followed by 35 cycles of amplification, each consisting of 94 °C denaturation for 30 s, annealing for 50 s at 1 °C below the lowest Tm of a given primer pair reported by the previous authors (Table S3); and 72 °C extension for 10 min. The PCR products were then subjected to electrophoresis, visualized, and photographed in a similar manner to the protocol used for bacterial identification.
3.5. Antimicrobial Susceptibility Testing
The susceptibility of A. hydrophila isolates to antibiotics was examined using the disc diffusion technique according to the guidelines of the Clinical Laboratory Standards Institute (CLSI) [35]. Sixteen antibiotics of 11 antibiotic classes/subclasses were tested including two penicillins: oxacillin (Ox, 1 µg) and amoxicillin (Ax, 10 µg); one β-Lactam/β-Lactamase inhibitor combination (BL/BLIs): amoxicillin-clavulanic acid (Ac, 20/10 µg); three cephalosporins: cefotaxime (Ct, 30 µg), cefuroxime (Cu, 30 µg), and ceftriaxone (Cx, 30 µg); one macrolide: erythromycin (Er, 15 µg); one quinolone: nalidixic acid (Na, 30 µg); one sulfonamide: sulfamethoxazole/trimethoprim (SM/TM, 23.75/1.25 µg); one aminoglycosides: neomycin (Ne, 30 µg); one glycopeptide: vancomycin (Va, 30 µg); two fluoroquinolones: ofloxacin (Of, 5 µg) and norfloxacin (No, 10 µg); two tetracyclines: doxycycline (Dx, 30 µg) and oxytetracycline (OTC, 30 µg); and one amphenicol: florfenicol (Fl, 30 µg). The selected antibiotics are the commonly used drugs or highly detected frequencies in aquatic environments surrounding the aquaculture areas in Vietnam [36,37,38,39].
Aeromonas hydrophila isolates were grown in Mueller–Hinton (MH) broth and were adjusted to reach a McFarland turbidity of 0.5. The suspension was then spread onto Mueller–Hinton agar by a sterilized cotton swab. Antibiotic discs were placed onto the inoculated plates and incubated at 28 °C. The zones of inhibition were recorded and classified as susceptible, intermediate, and resistant according to zone diameter interpretation standards described in the CLSI M100-S25 [35]. Since as the CLSI breakpoint for A. hydrophila includes only a few antimicrobial agents, CLSI breakpoints for Enterobacteria were instead used. The multiple antibiotic resistance (MAR) index of the isolates was calculated as described by Krumperman [40], in which MAR = a/b, where ‘a’ represents the number of antibiotics to which the isolate was resistant and ‘b’ represents the total number of antibiotics to which the isolates were exposed for susceptibility testing.
3.6. Data Analysis
The prevalence of infection, detection frequencies of virulence genes and antibiotic resistance ratios of A. hydrophila isolated from the three fish species (tilapia, carp, and channel catfish) were compared with Fisher’s exact test using SPSS software (version 20). For all the analyses, statistical significance was determined if a two-tailed p-value was no more than 0.05.
4. Discussion
Freshwater fish farming has accounted for almost 50% of the world’s aquaculture production [1]. Of this, tilapia, carp and catfish represent the primary cultured species globally and in Northern Vietnam [41]. The farming systems of these species have recently been shifted toward intensive modes characterized by a high organic matter load and high stocking densities, which are the favorable conditions for bacterial disease outbreaks. In the present study, 46.4% of diseased fish with the typical hemorrhagic septicemia symptoms were infected with A. hydrophila, which reveals the predominance of this pathogen in freshwater fish farming in Northern Vietnam and constitutes a huge economic loss due to the high accumulative mortality of fish in impacted ponds/cages. The current study showed no significant difference in detection frequencies of A. hydrophila among diseased tilapia, carp, and channel catfish. The development of cage culture with multiple species in open systems (rivers, lakes) and/or inadequate water management in earth ponds probably caused similar infection frequencies between fish, creating favorable conditions to spread this pathogen among fish farms.
Aeromonas hydrophila has emerged as an important foodborne pathogen worldwide [42,43]. The consumption of contaminated water and aquatic food products are considered the main sources of human Aeromonas infection [44]. A. hydrophila can cause gastrointestinal as well as extra-intestinal infections in humans [45,46]. There is evidence demonstrated the relationship between the presence of virulence-associated factors and the virulence increase in fish and other hosts [47]. In the present study, 88.9% of the A. hydrophila isolates from diseased fish carried the tested virulence genes and 39.2% of isolates carried 3/5 virulence genes. The result indicates that most of the A. hydrophila isolates from diseased tilapia, carp and channel catfish had a high potential pathogenicity on fish and potentially on fish consumers. Their infection is likely responsible for hemorrhagic septicemia symptoms in the fish hosts. However, although all the fish infected with A. hydrophia in this study exhibited similar clinical symptoms and gross lesions, 11.1% of the isolates had none of the tested virulence genes. The results suggest for these isolates the possible involvement of other virulence genes and pathogenic factors not investigated in this study, such as genes encoding proteases and lipase, in inducing hemorrhagic septicemia symptoms in diseased fish [34,48], or the concurrence of infection with other pathogens [49].
The heterogeneity in the frequencies of the virulence gene among A. hydrophila isolates in the present and previous studies demonstrates the variability of virulence gene profiles of the isolates. The present study showed that aerolysin aerA and cytotoxic enterotoxin act genes (harbored by 80.5%, and 80.1% of isolates, respectively) were most prevalent. Gene aerA codes a pore-forming toxin that binds to receptors on the target cell membrane while act has enterotoxic, hemolytic, and cytotoxic activities and involved in tissue damage and fluid secretion in intestinal epithelial cells of infected fish [17,50]. The high prevalence of aerA and act genes was also reported by previous studies [51,52]. Hemolysin hlyA is another important virulence factor, not only in lysing red blood cells but in cytotoxic activity against a broad range of species and cell types [53,54]. In the current work, the hlyA gene was detected in 57.5 to 63.0% of isolates which is higher than reported by El-Bahar et al. at 9.09% of A. hydrophila isolates from diseased tilapia in Egypt [51]. Meanwhile, Hayati et al. recorded a very high distribution of hlyA gene (95%) in this pathogen from farmed and wild tilapia, climbing perch and catfish sampled in Malaysia [55]. Heat labile enterotoxin (alt) contributes to promoting fluid accumulation in the small intestine of animals [56]. Our study showed 42.5% of the isolates carrying the alt gene which was lower than those reported by Rather et al. [57] on A. hydrophila isolated from various fish species. Heat stable cytotonic enterotoxin ast causes CHO cells to elongate and evokes intestinal fluid accumulation [56]. However, in the present study, the ast gene was absent in all A. hydrophila isolates, in contrast to 70% of A. hydrophila isolates from tilapia and channel catfish in Egypt carrying the ast gene [58]. The variabilities in detection frequencies of virulence genes among A. hydrophila isolates might be due to the geographical distribution of strains and the possibility of horizontal gene transfer [21,59].
Assessments of antimicrobial susceptibility are important to monitor the severity of antimicrobial resistance and to select proper drugs for disease treatments in fish farming, minimizing the risk to human health. In intensive farming systems, there is a widespread and often unregulated use of antimicrobial agents to control infectious diseases [4], resulting in the emergence of reservoirs of antimicrobial-resistant bacteria in fish and other aquatic animals, as well as in the aquatic environment [60,61]. From these reservoirs, resistance genes may be disseminated by horizontal gene transfer and reach human pathogens, or drug-resistant pathogens from the aquatic environment may reach humans directly [62]. The severity of antimicrobial resistance in A. hydrophila has been reported in various cultured species [63]. In the present study, 100% A. hydrophila isolates from tilapia, carp and channel catfish displayed variable degrees of antimicrobial resistance to the 16 agents of 11 antibiotic subclasses and most of the isolates were resistant to multiple antimicrobial agents. This is the first study carried out on a wide range of important cultured freshwater fish in the Northern Vietnam where the intensive farming systems have been expanded rapidly in recent years. The result reveals that antibiotic resistance of A. hydrophila in freshwater fish farming in Vietnam is close to the situation occurring in many other countries and adds to the global concerns.
Despite the divergences in the susceptibility patterns of the A. hydrophila isolates to the tested antibiotics, the tilapia isolates exhibited an overall higher degree of resistance to those drugs. The highest resistance rates of A. hydrophila isolates from freshwater fish species were observed for penicillins (oxacillin and amoxicillin) group (>90%) and vancomycin (>73%). The present result is consistent with previous reports and has been explained due to the intrinsic resistance of Aeromonas spp. to beta-lactams and glycopeptide antibiotics [64,65]. Although most of the isolates were resistant to penicillins, very low resistance frequencies of A. hydrophila isolates were shown for the combination of amoxicillin and clavulanic acid (AC) because of the capacity of clavulanic acid for beta-lactamase inhibition, preventing bacteria from destroying amoxicillin [46]. Significantly lower resistance frequencies (p < 0.001) of A. hydrophila isolates from carp and channel catfish (21.3–29.6%) to neomycin were detected in comparison to that of isolates from tilapia (80%). Regarding the Cephem subclass, most isolates from carp and channel catfish were susceptible to the second and the third cephalosporins (Ct, Cu and Cx), but a reduced susceptibility has been shown on tilapia isolates to these agents. Although Aeromonas spp. are generally susceptible to later-generation cephalosporins [21,66], the increased resistance to the second- and third-generation cephalosporins has been observed in clinical and seafood isolates [15]. High susceptibility of the isolates from carp and channel catfish and lower susceptibility of the tilapia isolates were also observed for fluoroquinolones (Of and No) and sulfonamides (SM/TM). The dissimilarity in resistance patterns of A. hydrophila isolates among fish species might imply historical differences in the use of antimicrobial agents in different fish culture system in terms of frequencies, quantity, utilization methods and drug species [67]. Similar phenomena of the high variability in antibiotic resistance frequencies on Aeromonas spp. from a diversity of fish species in different geographical regions were also previously reported [65,68].
The multiple antibiotic resistance (MAR) index has been used to indicate the degrees of antibiotic utilization. A MAR index value higher than 0.2 reflects the bacterial isolates from high-risk sources of antibiotic contamination where antibiotics are often used [40]. The present study showed an average of 74.7% A. hydrophila isolates from the three fish species having a MAR value higher than 0.2, with the highest frequency (87.6%) of the isolates from tilapia, followed by that of the isolates from carp (73.3%) and channel catfish (63.3%). The result demonstrates the overall high prevalence of multiple antibiotic resistances in A. hydrophila isolates from freshwater fish in Vietnam. The higher frequencies of antibiotic resistance in tilapia isolates in comparison to those from carp and channel catfish might imply the more frequent utilization of antimicrobial agents in tilapia farming.
5. Conclusions
The infection prevalence of Aeromonas hydrophila has been determined at 46.4% associated with freshwater fish disease in Northern Vietnam. Most A. hydrophila isolates harbored at least one of the five virulence genes (aerA, hlyA, alt, act, and ast) that posed a high potential pathogenicity to fish mortality. The majority of A. hydrophila isolates in the present study showed multiple antibiotic resistances when exposed to 16 antibiotics. These results might be a warning to farmers and aquaculture managers regarding to mitigating the spread of A. hydrophila in culture systems, enhance the awareness of the appropriate uses of antibiotics in fish farms. In addition, other environmentally friendly treatments alternatives such as herbal therapy, phages, and vaccination should be encouraged to apply to reduce the antibiotic resistance, improve food quality, and minimize negative impacts to human and environment.
Acknowledgments
The authors are grateful to Terutoyo Yoshida (Fish Pathology Division, University of Miyazaki) for his support during conducting experiments. We thank Steve Webb, Henry Kaspar and Norman Ragg (Cawthron Institute, New Zealand) for their valuable comments on the manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antibiotics10050532/s1, Table S1 Biochemical characterization of A. hydrophila in the present study; Table S2: The identities of gyrB and rpoB sequences from A. hydrophila isolates in present study and those of A. hydrophila ATCC ATCC 7966; Table S3: Primers used for the detection of virulence genes in A. hydrophila.
Author Contributions
Conceptualization, T.D.H. and D.T.N.; Methodology, T.D.H., D.T.N. and L.T.D.; Validation, T.D.H., D.T.N., N.T.H.G. and K.V.V.; Formal Analysis, D.T.N., N.T.H.G. and D.V.L.; Investigation, T.D.H.; Resources, K.V.V., D.T.N., N.T.H.G. and L.T.D.; Data Curation, D.T.N., D.V.L., and L.T.D.; Writing—Original Draft Preparation, D.T.N. and T.D.H.; Funding Acquisition, T.D.H. All authors reviewed and contributed to the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Grant-in-Aid for Scientific Research from Ministry of Agriculture and rural development, Vietnam, grant number ĐTKHCN.WB.11/20.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Faculty of fisheries, Vietnam National University of Agriculture (protocol code FFVNUA.15620-KHCN and date of approval: 10 February 2019).
Data Availability Statement
No new data available.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Davis D., Nguyen T., Li M., Gatlin D.M., O’Keefe T. Advances in aquaculture nutrition: Catfish, tilapia and carp nutrition. In: Burnell G., Allan G., editors. New Technologies in Aquaculture. Woodhead Publishing; Cambridge, UK: 2009. pp. 440–458. [DOI] [Google Scholar]
- 2.Eissa A., Moustafa M., El-Husseiny I., Saeid S., Saleh O., Borhan T. Identification of some skeletal deformities in freshwater teleosts raised in Egyptian aquaculture. Chemosphere. 2009;77:419–425. doi: 10.1016/j.chemosphere.2009.06.050. [DOI] [PubMed] [Google Scholar]
- 3.Mzula A., Wambura P.N., Mdegela R.H., Shirima G.M. Present status of aquaculture and the challenge of bacterial diseases in freshwater farmed fish in Tanzania; A call for sustainable strategies. Aquac. Fish. 2020 doi: 10.1016/j.aaf.2020.05.003. [DOI] [Google Scholar]
- 4.Heuer O.E., Kruse H., Grave K., Collignon P., Karunasagar I., Angulo F.J. Human health consequences of use of antimicrobial agents in aquaculture. Clin. Infect. Dis. 2009;49:1248–1253. doi: 10.1086/605667. [DOI] [PubMed] [Google Scholar]
- 5.Tavares-Dias M., Martins M.L. An overall estimation of losses caused by diseases in the Brazilian fish farms. J. Parasit. Dis. 2017;41:913–918. doi: 10.1007/s12639-017-0938-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dias M.K., Sampaio L.S., Proietti-Junior A.A., Yoshioka E.T., Rodrigues D.P., Rodriguez A.F., Ribeiro R.A., Faria F.S., Ozório R.O., Tavares-Dias M. Lethal dose and clinical signs of Aeromonas hydrophila in Arapaima gigas (Arapaimidae), the giant fish from Amazon. Vet. Microbiol. 2016;188:12–15. doi: 10.1016/j.vetmic.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Papadopoulou C., Economou E., Zakas G., Salamoura C., Dontorou C., Apostolou J. Microbiological and pathogenic contaminants of seafood in Greece. J. Food Qual. 2007;30:28–42. doi: 10.1111/j.1745-4557.2007.00104.x. [DOI] [Google Scholar]
- 8.Janda J.M., Abbott S.L. The genus Aeromonas: Taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 2010;23:35–73. doi: 10.1128/CMR.00039-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plumb J.A., Hanson L.A. Health Maintenance and Principal Microbial Diseases of Cultured Fishes. John Wiley & Sons; Hoboken, NJ, USA: 2010. pp. 1–483. [DOI] [Google Scholar]
- 10.Abdel-Latif H.M., Khafaga A.F. Natural co-infection of cultured Nile tilapia Oreochromis niloticus with Aeromonas hydrophila and Gyrodactylus cichlidarum experiencing high mortality during summer. Aquac. Res. 2020;51:1880–1892. doi: 10.1111/are.14538. [DOI] [Google Scholar]
- 11.Abd-El-Malek A.M. Incidence and virulence characteristics of Aeromonas spp. in fish. Vet. World. 2017;10:34–37. doi: 10.14202/vetworld.2017.34-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar R., Pande V., Singh L., Sharma L., Saxena N., Thakuria D., Singh A.K., Sahoo P.K. Pathological findings of experimental Aeromonas hydrophila infection in golden mahseer (Tor putitora) Fish Aquac. J. 2016;7:160. [Google Scholar]
- 13.Zhang D., Moreira G.S., Shoemaker C., Newton J.C., Xu D.-H. Detection and quantification of virulent Aeromonas hydrophila in channel catfish tissues following waterborne challenge. FEMS Microbiol. Lett. 2016;363:1–5. doi: 10.1093/femsle/fnw080. [DOI] [PubMed] [Google Scholar]
- 14.Palu A.P., Gomes L.M., Miguel M.A.L., Balassiano I.T., Queiroz M.L.P., Freitas-Almeida A.C., de Oliveira S.S. Antimicrobial resistance in food and clinical Aeromonas isolates. Food Microbiol. 2006;23:504–509. doi: 10.1016/j.fm.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Lee H.J., Hoel S., Lunestad B.T., Lerfall J., Jakobsen A.N. Aeromonas spp. isolated from ready-to-eat seafood on the Norwegian market: Prevalence, putative virulence factors and antimicrobial resistance. J. Appl. Microbiol. 2020;130:1380–1393. doi: 10.1111/jam.14865. [DOI] [PubMed] [Google Scholar]
- 16.Albert M.J., Ansaruzzaman M., Talukder K.A., Chopra A.K., Kuhn I., Rahman M., Mollby R. Prevalence of enterotoxin genes in Aeromonas spp. isolated from children with diarrhea, healthy controls, and the environment. J. Clin. Microbiol. 2000;38:3785–3790. doi: 10.1128/jcm.38.10.3785-3790.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sha J., Kozlova E.V., Chopra A.K. Role of various enterotoxins in Aeromonas hydrophila-induced gastroenteritis: Generation of enterotoxin gene-deficient mutants and evaluation of their enterotoxic activity. Infect. Immun. 2002;70:1924–1935. doi: 10.1128/IAI.70.4.1924-1935.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rico A., Phu T.M., Satapornvanit K., Min J., Shahabuddin A., Henriksson P.J., Murray F.J., Little D.C., Dalsgaard A., Van den Brink P.J. Use of veterinary medicines, feed additives and probiotics in four major internationally traded aquaculture species farmed in Asia. Aquaculture. 2013;412:231–243. doi: 10.1016/j.aquaculture.2013.07.028. [DOI] [Google Scholar]
- 19.Le T.X., Munekage Y. Residues of selected antibiotics in water and mud from shrimp ponds in mangrove areas in Viet Nam. Mar. Pollut. Bull. 2004;49:922–929. doi: 10.1016/j.marpolbul.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Rhodes G., Huys G., Swings J., Mcgann P., Hiney M., Smith P., Pickup R.W. Distribution of oxytetracycline resistance plasmids between aeromonads in hospital and aquaculture environments: Implication of Tn1721 in dissemination of the tetracycline resistance determinant Tet A. Appl. Environ. Microbiol. 2000;66:3883–3890. doi: 10.1128/AEM.66.9.3883-3890.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yano Y., Hamano K., Tsutsui I., Aue-umneoy D., Ban M., Satomi M. Occurrence, molecular characterization, and antimicrobial susceptibility of Aeromonas spp. in marine species of shrimps cultured at inland low salinity ponds. Food Microbiol. 2015;47:21–27. doi: 10.1016/j.fm.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Rabobank, World Seafood Trade Map 2019. [(accessed on 2 May 2019)]; Available online: https://research.rabobank.com/far/en/sectors/animal-protein/world-seafood-trade-map.html.
- 23.Hoai T.D., Trang T.T., Van Tuyen N., Giang N.T.H., Van Van K. Aeromonas veronii caused disease and mortality in channel catfish in Vietnam. Aquaculture. 2019;513:734425. doi: 10.1016/j.aquaculture.2019.734425. [DOI] [Google Scholar]
- 24.Lee C., Cho J.C., Lee S.H., Lee D.G., Kim S.J. Distribution of Aeromonas spp. as identified by 16S rDNA restriction fragment length polymorphism analysis in a trout farm. Appl. Microbiol. 2002;93:976–985. doi: 10.1046/j.1365-2672.2002.01775.x. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen M.E., Hoi L., Schmidt A.S., Qian D., Shimada T., Shen J.Y., Larsen J.L. Is Aeromonas hydrophila the dominant motile Aeromonas species that causes disease outbreaks in aquaculture production in the Zhejiang Province of China? Dis. Aquat. Organ. 2001;46:23–29. doi: 10.3354/dao046023. [DOI] [PubMed] [Google Scholar]
- 26.Samayanpaulraj V., Sivaramapillai M., Palani S.N., Govindaraj K., Velu V., Ramesh U. Identification and characterization of virulent Aeromonas hydrophila Ah17 from infected Channa striata in river Cauvery and in vitro evaluation of shrimp chitosan. Food Sci. Nutr. 2020;8:1272–1283. doi: 10.1002/fsn3.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson J.D., Higgins D.G., Gibson T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saitou N., Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 29.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yanez M., Catalán V., Apráiz D., Figueras M., Martinez-Murcia A. Phylogenetic analysis of members of the genus Aeromonas based on gyrB gene sequences. Int. J. Syst. Evol. Microbiol. 2003;53:875–883. doi: 10.1099/ijs.0.02443-0. [DOI] [PubMed] [Google Scholar]
- 31.Korczak B., Christensen H., Emler S., Frey J., Kuhnert P. Phylogeny of the family Pasteurellaceae based on rpoB sequences. Int. J. Syst. Evol. Microbiol. 2004;54:1393–1399. doi: 10.1099/ijs.0.03043-0. [DOI] [PubMed] [Google Scholar]
- 32.Heuzenroeder M.W., Wong C.Y., Flower R.L. Distribution of two hemolytic toxin genes in clinical and environmental isolates of Aeromonas spp.: Correlation with virulence in a suckling mouse model. FEMS Microbiol. Lett. 1999;174:131–136. doi: 10.1111/j.1574-6968.1999.tb13559.x. [DOI] [PubMed] [Google Scholar]
- 33.Kingombe C.I.B., Huys G., Tonolla M., Albert M.J., Swings J., Peduzzi R., Jemmi T. PCR detection, characterization, and distribution of virulence genes in Aeromonas spp. Appl. Environ. Microbiol. 1999;65:5293–5302. doi: 10.1128/AEM.65.12.5293-5302.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nawaz M., Khan S.A., Khan A.A., Sung K., Tran Q., Kerdahi K., Steele R. Detection and characterization of virulence genes and integrons in Aeromonas veronii isolated from catfish. Food Microbiol. 2010;27:327–331. doi: 10.1016/j.fm.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Patel J.B., Cockerill F.R., Bradfoord P.A. CLSI M100-S25 performance standards for antimicrobial susceptibility testing; twenty-fifth informational supplement. Clin. Lab. Stand. Inst. 2015;35:1–240. [Google Scholar]
- 36.Hedberg N., Stenson I., Pettersson M.N., Warshan D., Nguyen-Kim H., Tedengren M., Kautsky N. Antibiotic use in Vietnamese fish and lobster sea cage farms; implications for coral reefs and human health. Aquaculture. 2018;495:366–375. doi: 10.1016/j.aquaculture.2018.06.005. [DOI] [Google Scholar]
- 37.Hoa P.T.P., Managaki S., Nakada N., Takada H., Shimizu A., Anh D.H., Viet P.H., Suzuki S. Antibiotic contamination and occurrence of antibiotic-resistant bacteria in aquatic environments of northern Vietnam. Sci. Total Environ. 2011;409:2894–2901. doi: 10.1016/j.scitotenv.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 38.Binh V.N., Dang N., Anh N.T.K., Thai P.K. Antibiotics in the aquatic environment of Vietnam: Sources, concentrations, risk and control strategy. Chemosphere. 2018;197:438–450. doi: 10.1016/j.chemosphere.2018.01.061. [DOI] [PubMed] [Google Scholar]
- 39.Do T.C.M.V., Nguyen D.Q., Nguyen T.D., Le P.H. Development and validation of a LC-MS/MS method for determination of multi-class antibiotic residues in aquaculture and river waters, and photocatalytic degradation of antibiotics by TiO2 nanomaterials. Catalysts. 2020;10:356. doi: 10.3390/catal10030356. [DOI] [Google Scholar]
- 40.Krumperman P.H. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl. Environ. Microbiol. 1983;46:165–170. doi: 10.1128/AEM.46.1.165-170.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Huong N., Cuong T.H., Thu T.T.N., Lebailly P. Efficiency of Different Integrated Agriculture Aquaculture Systems in the Red River Delta of Vietnam. Fish. Aquac. J. 2017;8:493. doi: 10.4172/2150-3508.1000230. [DOI] [Google Scholar]
- 42.Igbinosa I.H., Igumbor E.U., Aghdasi F., Tom M., Okoh A.I. Emerging Aeromonas species infections and their significance in public health. Sci. World J. 2012;2012:1–13. doi: 10.1100/2012/625023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pal M. Is Aeromonas hydrophila a potential pathogen of food safety concern. J. Food Microbiol. 2018;2:1–2. [Google Scholar]
- 44.Hoel S., Vadstein O., Jakobsen A.N. The significance of mesophilic Aeromonas spp. in minimally processed ready-to-eat seafood. Microorganisms. 2019;7:1–25. doi: 10.3390/microorganisms7030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parker J.L., Shaw J.G. Aeromonas spp. clinical microbiology and disease. J. Infect. 2011;62:109–118. doi: 10.1016/j.jinf.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Vila J., Marco F., Soler L., Chacon M., Figueras M.J. In vitro antimicrobial susceptibility of clinical isolates of Aeromonas caviae, Aeromonas hydrophila and Aeromonas veronii biotype sobria. J. Antimicrob. Chemothe. 2002;49:701–702. doi: 10.1093/jac/49.4.701. [DOI] [PubMed] [Google Scholar]
- 47.Li J., Ni X.D., Liu Y.J., Lu C.P. Detection of three virulence genes alt, ahp and aerA in Aeromonas hydrophila and their relationship with actual virulence to zebrafish. J. Appl. Microbiol. 2011;110:823–830. doi: 10.1111/j.1365-2672.2011.04944.x. [DOI] [PubMed] [Google Scholar]
- 48.Jiravanichpaisal P., Roos S., Edsman L., Liu H., Söderhäll K. A highly virulent pathogen, Aeromonas hydrophila, from the freshwater crayfish Pacifastacus leniusculus. J. Invertebr. Pathol. 2009;101:56–66. doi: 10.1016/j.jip.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Nicholson P., Mon-on N., Jaemwimol P., Tattiyapong P., Surachetpong W. Coinfection of tilapia lake virus and Aeromonas hydrophila synergistically increased mortality and worsened the disease severity in tilapia (Oreochromis spp.) Aquaculture. 2020;520:734746. doi: 10.1016/j.aquaculture.2019.734746. [DOI] [Google Scholar]
- 50.Xu X.J., Ferguson M.R., Popov V.L., Houston C.W., Peterson J.W., Chopra A.K. Role of a cytotoxic enterotoxin in Aeromonas-mediated infections: Development of transposon and isogenic mutants. Infect. Immun. 1998;66:3501–3509. doi: 10.1128/IAI.66.8.3501-3509.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.El-Bahar H.M., Ali N.G., Aboyadak I.M., Khalil S.A.E.S., Ibrahim M.S. Virulence genes contributing to Aeromonas hydrophila pathogenicity in Oreochromis niloticus. Int. Microbiol. 2019;4:479–490. doi: 10.1007/s10123-019-00075-3. [DOI] [PubMed] [Google Scholar]
- 52.Oliveira S.T., Veneroni-Gouveia G., Costa M.M. Molecular characterization of virulence factors in Aeromonas hydrophila obtained from fish. Pesquisa Veterinária Brasileira. 2012;32:701–706. doi: 10.1590/S0100-736X2012000800004. [DOI] [Google Scholar]
- 53.Ristow L.C., Welch R.A. Hemolysin of uropathogenic Escherichia coli: A cloak or a dagger? Biochimica et Biophysica Acta (BBA)-Biomembranes. 2016;1858:538–545. doi: 10.1016/j.bbamem.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 54.Tomás J.M. The main Aeromonas pathogenic factors. ISRN Microbiol. 2012;2012:1–22. doi: 10.5402/2012/256261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hayati H.R., Hassan M., Ong B., Abdelhadi Y., Hidayahanum H.N., Sharifah R., Faten A.N., Kuttichantran S., Alsaid M. Virulence genes detection of Aeromonas hydrophila originated from diseased freshwater fishes. Adv. Environ. Biol. 2015;9:22–26. [Google Scholar]
- 56.Chopra A.K., Houston C.W. Enterotoxins in Aeromonas-associated gastroenteritis. Microbes Infect. 1999;1:1129–1137. doi: 10.1016/S1286-4579(99)00202-6. [DOI] [PubMed] [Google Scholar]
- 57.Rather M.A., Willayat M.M., Wani S.A., Hussain S.A., Shah S.A. Enterotoxin gene profile and molecular epidemiology of Aeromonas species from fish and diverse water sources. J. Appl. Microbiol. 2019;127:921–931. doi: 10.1111/jam.14351. [DOI] [PubMed] [Google Scholar]
- 58.Abd El Tawab A.A., Maarouf A.A., El Hofy F.I., El Mougy E.E. Detection of some virulence genes in A. hydrophila and A. caviae isolated from fresh water fishes at Qalubia Governorate. Benha Vet. Med. J. 2017;33:489–503. doi: 10.21608/bvmj.2017.30598. [DOI] [Google Scholar]
- 59.Hoel S., Vadstein O., Jakobsen A.N. Species distribution and prevalence of putative virulence factors in mesophilic Aeromonas spp. isolated from fresh retail sushi. Front. Microbiol. 2017;8:931. doi: 10.3389/fmicb.2017.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aoki T. Present and future problems concerning the development of resistance in aquaculture. In: Michel C., Alderman D., editors. Chemotherapy in Aquaculture: From Theory to Reality. Office International des EÉpizooties; Paris, France: 1992. pp. 254–262. [Google Scholar]
- 61.Akinbowale O.L., Peng H., Barton M. Antimicrobial resistance in bacteria isolated from aquaculture sources in Australia. J. Appl. Microbiol. 2006;100:1103–1113. doi: 10.1111/j.1365-2672.2006.02812.x. [DOI] [PubMed] [Google Scholar]
- 62.WHO . Joint FAO/OIE/WHO Expert Workshop on Non-Human Antimicrobial Usage and Antimicrobial Resistance: Scientific Assessment: Geneva, 1–5 December 2003 (No. WHO/CDS/CPE/ZFK/2004.7) World Health Organization; Geneva, Switzerland: 2004. [Google Scholar]
- 63.Stratev D., Stoev S., Vashin I., Daskalov H. Some varieties of pathological changes in experimental infection of carps (Cyprinus carpio) with Aeromonas hydrophila. J. Aquac. Eng. Fish. Res. 2015;1:191–202. doi: 10.3153/JAEFR15019. [DOI] [Google Scholar]
- 64.Fosse T., Giraud-Morin C., Madinier I. Phenotypes of beta-lactam resistance in the genus Aeromonas. Pathologie-Biologie. 2003;51:290. doi: 10.1016/S0369-8114(03)00027-0. [DOI] [PubMed] [Google Scholar]
- 65.Borella L., Salogni C., Vitale N., Scali F., Moretti V.M., Pasquali P., Alborali G.L. Motile aeromonads from farmed and wild freshwater fish in northern Italy: An evaluation of antimicrobial activity and multidrug resistance during 2013 and 2016. Acta Vet. Scand. 2020;62:6. doi: 10.1186/s13028-020-0504-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dahanayake P.S., Hossain S., Wickramanayake M.V.K.S., Heo G.J. Prevalence of virulence and antimicrobial resistance genes in Aeromonas species isolated from marketed cockles (Tegillarca granosa) in Korea. Lett. Appl. Microbiol. 2020;71:94–101. doi: 10.1111/lam.13261. [DOI] [PubMed] [Google Scholar]
- 67.Yuan K., Wang X., Chen X., Zhao Z., Fang L., Chen B., Jiang J., Luan T., Chen B. Occurrence of antibiotic resistance genes in extracellular and intracellular DNA from sediments collected from two types of aquaculture farms. Chemosphere. 2019;234:520–527. doi: 10.1016/j.chemosphere.2019.06.085. [DOI] [PubMed] [Google Scholar]
- 68.Ottaviani D., Santarelli S., Bacchiocchi S., Masini L., Ghittino C., Bacchiocchi I. Occurrence and characterization of Aeromonas spp. in mussels from the Adriatic Sea. Food Microbiol. 2006;23:418–422. doi: 10.1016/j.fm.2005.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data available.