Abstract
Background -
To determine the frequency of malformations that would be identified in the limited surface examination of a newborn by the delivering nurse midwife in a resource-limited setting.
Methods -
The limited surface examination will identify visible external anomalies, but not abnormalities inside the mouth, most heart defects, undescended testes, inguinal hernias, hip dysplasia, peripheral vascular anomalies and some internal anomalies. The findings in a malformations surveillance program, involving 289,365 births in Boston, have been used to establish the prevalence rate of malformations that would be identified and not identified. In African countries, the number of anomalies to be identified should also be reduced by excluding polydactyly, postaxial, type B, a common minor finding, from the list of potential malformations.
Results -
2.05% (n=5,941) of the 289,365 births surveyed had one or more malformations. The abnormalities that would have been missed, using surface exam alone, accounted for 0.5% of all of malformations identified and reduced the overall prevalence rate of malformations to 1.5%. In addition, excluding all infants with isolated postaxial polydactyly, type B reduced the expected prevalence rate of malformations to 1.3% in unexposed newborn infants.
Conclusion -
A limited surface examination can detect the majority of malformations among newborn infants.
Keywords: malformations, inclusion and exclusion criteria
INTRODUCTION
When infants have been exposed at conception to a potentially teratogenic medication, the first question asked is whether the exposed infants have an increased frequency of congenital malformations.
There is a spectrum of structural abnormalities or malformations that can be identified, some called “major malformations” and others, “minor malformations.” About 2% of newborns have a major malformation, defined as a structural abnormality with surgical, medical or cosmetic importance (Correa, 2007; Feldkamp, 2017; Toufaily, 2018). A minor anomaly has been defined, arbitrarily, as occurring in less than 4% of infants (Marden, 1964) and as not having surgical, medical or cosmetic importance. The prevalence rates of malformations differ by the birth status of the exposed fetus, being highest in miscarriages (Shepard, 1989), next in stillborn infants (Holmes, 2018a) and lowest in liveborn infants (Correa, 2007). The number of abnormalities identified increases as the infant gets older (Correa, 2007; Thomas, 2018). In addition, other teratogenic effects include growth restriction or small for gestational age infants (Hernandez-Diaz, 2017), microcephaly (Johansson, 2016) and deficits in cognitive function (Cohen, 2019).
The findings identified when examining newborn infants also include many common physical features that are not malformations: minor anomalies (preauricular skin tag), birthmarks (hemangiomas), positional deformities (plagiocephaly), characteristics of prematurity (patent ductus arteriosus), transient findings (muscular ventricular septal defect), findings in newborn screening (hearing loss) and genetic disorders (Down Syndrome). In the analysis of the pediatricians’ examination findings in 1,000 consecutive newborn infants in Boston, the physical findings excluded as not being malformations were 18 times (n=320) more common than the abnormalities considered malformations (n=18) [Holmes, 2011].
The methods that have been used to identify the presence of malformations have included: 1) an examination by a study physician, following a protocol to improve consistency (Holmes, 2001); 2) reading the examination findings recorded in all newborn infants’ medical records by a physician to identify those with malformations (Holmes, 2012; Toufaily, 2018; Holmes, 2018c); 3) a tabulation of the ICD-9 or ICD-10 codes in the infants’ discharge diagnoses that indicate the presence of specific abnormalities in the infants being surveyed (Cooper, 2006); 4) local health care staff providing written descriptions of visible structural anomalies visible on physical examination and photographs of these abnormalities (Li, 2003). These methods are highly sensitive for determining the presence of congenital abnormalities, but are resource-intensive and may not be feasible in low-resource locations.
We present here the effect of using the limited surface examination by the nurse midwife, who has delivered the infant, to determine the presence of malformations in a newborn infant. The limited surface examination described in this report has been used successfully in the Tsepamo Study in Botswana, in which the potential teratogenicity of the HIV medications was evaluated (Zash, 2019). To determine the frequency of the malformations that would be missed by the nurse midwife because of the limited nature of the surface examination, we have used the findings in an active malformations surveillance program in Boston. By selecting the types of malformations identifiable through a limited surface exam (as confirmed in the Tsepamo Study experience) with the full range of malformations in the Boston surveillance, we established the baseline prevalence rate for malformations when using this more limited method of examination.
METHODS
The surface examination of a newborn infant requires inspection from the top of the head down to the toes. The number of malformations expected in a complete surface examination has been determined by analyzing the structural abnormalities identified by examining pediatricians and pathologists in 289,365 births in the Active Malformations Surveillance Program in Boston (Holmes, 2012; Toufaily, 2018; Holmes, 2018c). This Malformations Surveillance Program began in 1972 at the Boston Lying-In Hospital, a hospital that merged with three other hospitals to form the Brigham and Women’s Hospital, and continued until 2012. The surveillance was carried out in a daily review, by a Research Assistant (Monday through Saturday plus holidays), of the pediatricians’ examination findings, all consultants’ notes and laboratory results for liveborn infants. In addition, pathology reports of the autopsies on any deceased liveborn and stillborn infants and elective terminations for fetal anomalies were reviewed by the Research Assistants. The time window for identifying an abnormality was between birth and five days of age; in the later years of this surveillance program most infants were discharged on the second day of life. The day an abnormality was considered identified was the day when the signs or symptoms were first seen or heard and recorded by the examining physician (Holmes, 2012; Toufaily, 2018; Holmes, 2018c).
Each malformation identified was assigned an ICD-9 code, including the British Pediatric Association extension (Crawshaw, 1995), by the research staff. New codes were assigned for specific abnormalities not included in the ICD-9 codes.
In the Active Malformations Surveillance Program, infants with single, or isolated, malformations and multiple malformations were identified and tabulated. For the analysis described here, only the findings in the infants of mothers who had always planned to deliver at this hospital were used. It excludes women who had planned to deliver at another hospital, but transferred their care after the detection, prenatally, of an apparent abnormality in the fetus. The mother’s transfer status was based on information provided in a study-based postpartum interview of the mother by a Research Assistant or from a review of the mother’s medical record (Holmes, 2018b).
In African countries, special consideration is needed as to whether to include or exclude the presence of postaxial polydactyly, type B (Figure 1) as a malformation. In the Active Malformations Surveillance Program in Boston, 0.9% of the African-American infants had this minor finding (Holmes, 2018b). By contrast, the frequency was 0.03% among white infants. This type of polydactyly was isolated in 95% of the affected infants and had no medical significance. This isolated type of polydactyly has never been caused by a human teratogen (Holmes, 2002). Because it is so common in African infants, isolated postaxial polydactyly has been subtracted from the total number of malformations identified to establish a more significant baseline malformation rate.
1.
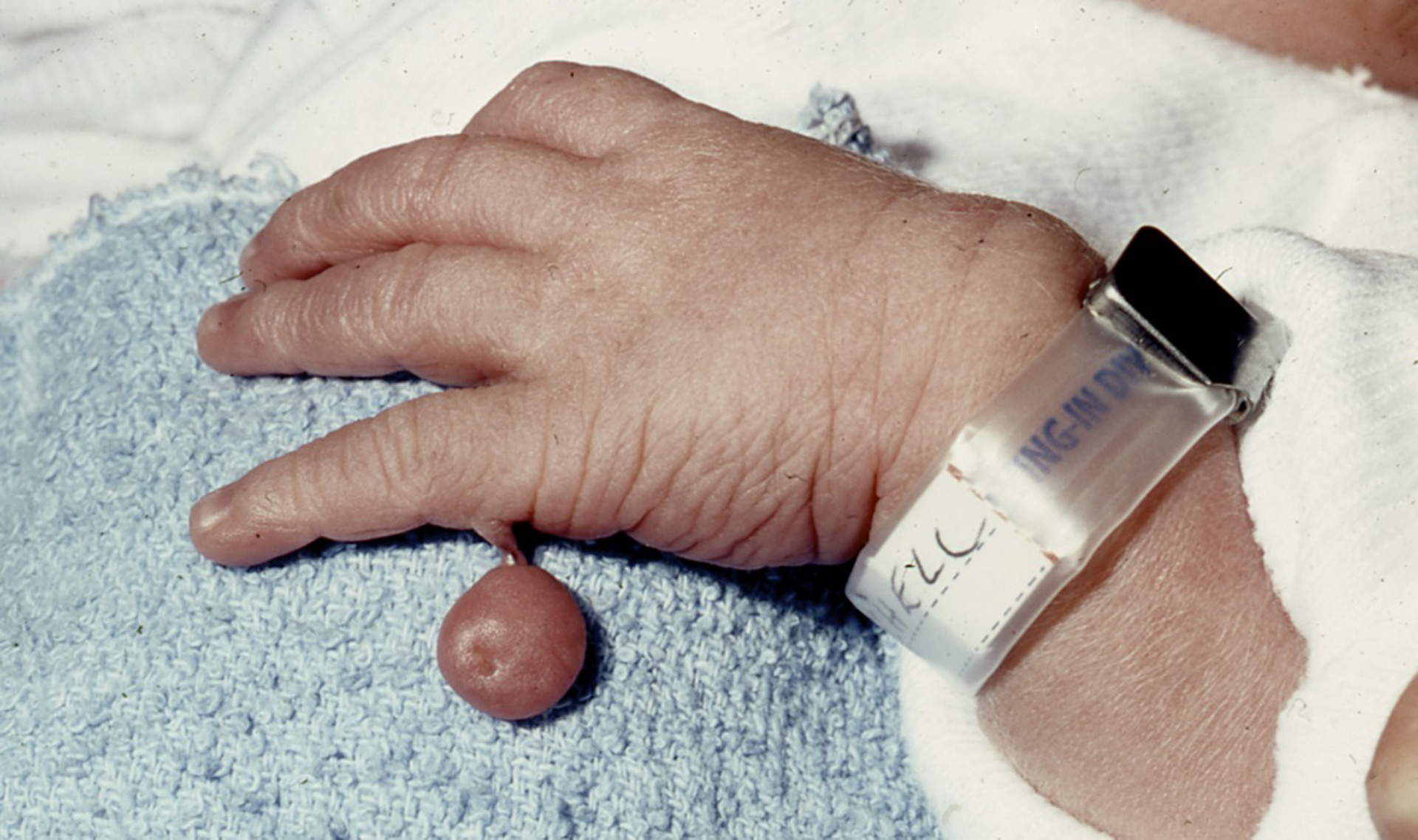
Postaxial polydactyly, type B: a digit-like structure that dangles usually from the level of the proximal or middle phalanges of the fifth finger or toe; the pedicle contains blood vessels and nerves and is typically ligated or removed surgically soon after the infant’s birth (19).
The time window used in a low-resource setting is the first 24 hours of an infant’s life, as discharge by 24 hours (or sooner) after delivery is common (Zash, 2019). If signs or symptoms of an external abnormality, such as cleft lip or syndactyly, were identified between birth and discharge (or up to 5 days of age) in a surface examination in the Active Malformations Surveillance Program in Boston, it was considered likely to be identified by the nurse midwife in the low-resource setting. In addition, some internal anomalies, such as cyanotic heart defects and esophageal atresia, were considered likely to be identified. While the nurse midwife would not auscultate the infant’s chest to identify murmurs that could reflect heart defects, the presence of a heart defect might be suspected when an infant’s lips were blue. This infant could be referred for further evaluation, where the heart defects would be identified. Therefore, the infants with cyanotic heart defects, such as pulmonary or tricuspid atresia, hypoplastic left heart syndrome or truncus arteriosus (Mahle, 2009), were considered to be detected by the limited surface examination. Likewise, esophageal atresia could be detected by the nurse midwife, because the excessive secretions and the infant’s inability to swallow breast milk or infant formula would be identified within the first 24 hours of life. These adjustments for infants with esophageal atresia identified by the Active Malformations Surveillance Program in Boston were based on the descriptions of the affected infants’ symptoms and the physical features described in the notes of the examining pediatricians in the affected infants’ medical records.
The reliability of the findings by the nurse midwife is increased by the photographs taken by cellphone of each abnormality identified. These photographs are forwarded for independent review by a birth defects specialist who is unaware of the infant’s exposure status.
RESULTS
The survey of 289,365 infants and fetuses in the Active Malformations Surveillance Program identified 5,941 (2.05%) with one or more malformations (Table 1). The limitations of the surface examination by the nurse midwives meant that they did not identify abnormalities within the mouth, non-cyanotic heart defects, peripheral vascular abnormalities, undescended testes, inguinal hernias, hip dysplasia and internal anomalies. Specifically, this means they would have missed identifying 67 infants with natal teeth or mouth cysts; 70, cleft palate; 219, non-cyanotic heart defects; 16, peripheral vascular abnormalities; 548, undescended testes; 11, inguinal hernia; 229 internal anomalies and 239, hip dysplasia. In addition, 88 infants with multiple malformations would not be identified because of the limited nature of the physical examination, such as an infant with a heart defect and cleft palate. The total number of malformations identified after subtracting those missed by the limited nature of the physical examination would be 4,454 (1.5%). After excluding the infants (n=575) with isolated postaxial polydactyly, type B, the total prevalence of malformations identified would be 3,879 or 1.3% of the 289,365 births surveyed.
TABLE 1:
MALFORMATIONS NOT IDENTIFIED BY LIMITED SURFACE EXAMINATION
| TOTAL NUMBER OF INFANTS WITH MALFORMATIONS IDENTIFIED AMONG 289,365 BIRTHS | NUMBER 5,941 | PERCENTAGE OF TOTAL NUMBER EXAMINED 2.05% |
|---|---|---|
| A. MALFORMATIONS MISSED: | ||
| NATAL TEETH/MOUTH CYSTS | 67 | 0.02 |
| CLEFT PALATE ALONE | 70 | 0.02 |
| *HEART DEFECTS | 219 | 0.08 |
| **PERIPHERAL VASCULAR ABNORMALITIES | 16 | 0.01 |
| ***INTERNAL ANOMALIES | 229 | 0.08 |
| UNDESCENDED TESTES | 548 | 0.19 |
| INGUINAL HERNIA | 11 | 0.00 |
| HIP DYSPLASIA | 239 | 0.08 |
| ****MULTIPLE MALFORMATIONS | 88 | 0.03 |
| *****SUBTOTAL | 1,487 | 0.51% |
| ESTIMATED TOTAL NUMBER OF MALFORMED INFANTS IDENTIFIED IN LIMITED SURFACE EXAMINATION | 4,454 | 1.54% |
Excludes premature infants with physiologic findings, such as patent ductus arteriosus and patent foramen ovale, as well as infants with cyanotic congenital heart defects;
Includes coarctation of aorta (n=9) and abnormalities of vein of Galen (n=7);
Internal anomalies, such as polycystic kidney disease and the bowel atresias;
Infants with two or more malformations, none of which would be identified, such as an infant with a non-cyanotic heart defect and inguinal hernia;
The 0.51% is based on: 1,487/289,365.
DISCUSSION
The limited surface examination can be used in hospitals and clinics in resource-limited settings where there are not enough physicians to carry out complete surface examinations. This approach makes it possible to monitor newborn infants for the presence of birth defects in regions where pregnant women are taking medications of concern to treat serious infections, like HIV (human immunodeficiency virus), malaria, tuberculosis or parasitic infections (Zash, 2019; Mehta, 2012)). Although less comprehensive than a more complete examination by a pediatrician, the limited surface exam identifies the majority of malformations and increases the likelihood of identifying unrecognized problems (as occurred with the recent concern for an association between dolutegravir at conception and neural tube defects) [Zash, 2019; Raesima, 2019].
A potential disadvantage of relying on the limited surface examination is that photographs will not always be possible. The mother must give her permission for a picture to be taken, and she may refuse. Another factor in the detection and documentation of abnormalities is the short stay of the mothers postpartum in low-resource settings, which is typically 24 hours or less.
Some malformations were identified on days 2 to 5 of life of the surveyed newborns in the Active Malformations Surveillance Program in Boston (Holmes, 2012; Toufaily, 2018; Holmes, 2018c)) and the comparison should be made for only those abnormalities identified in the first 24 hours of the infants’ lives. However, data on the detection only on the first day of life were not available, a limitation of this study. One study with related data is the Metropolitan Atlanta Congenital Birth Defects (MACDP) Program, in which the prevalence rate was 2.09% identified between birth and 7 days of age and 2.48% for infants between ages 8 days to 6 months of age (Correa, 2007). These data suggest that the prevalence rate of malformations identified in the newborn period could increase slightly between the first and fifth days of life.
The many limitations in establishing diagnoses for malformations in resource-limited settings include the lack of: 1) examinations by physicians with more expertise limits the ability to establish diagnoses, such as Down Syndrome; 2) diagnostic testing, such as chromosome karyotypes and microarray and mutation analysis; 3) imaging by radiography or CT (computerized tomography); 4) autopsies, for example, to confirm the presumed diagnosis of hydrocephalus. (As was noted above in the list of exclusions, genetic disorders and chromosome abnormalities would be excluded from the number of malformations potentially associated with the exposure to a teratogen.)
This estimate of the malformations missed by the limited nature of the surface examination is approximate. Heart defects are the most common malformations, but are not detected because the nurse midwives do not listen with a stethoscope for heart murmurs. In conversations with these nurses, we learned that they occasionally identify a newborn with a heart defect when the infant’s lips are blue. These infants with blue lips are transferred to a referral center where examinations by physicians and imaging studies can identify the presence of heart defects. These findings are added to the findings by the nurse midwives. Another example of the imprecision is the fact that, while the nurses do not examine the inside of each infant’s mouth, she could see a natal tooth, when the affected infant was crying. A third example of the imprecision is the fact that, while the nurses do not palpate for the absence of a descended testicle, they could see a significant asymmetry of the scrotum and record absence of a descended testicle as a finding.
We have observed the use of a limited surface examination described in Botswana, a low-resource setting in which the occurrence of malformations is being monitored in an on-going study of the potential teratogenicity of antiretroviral drugs used to treat HIV. Since the nurse midwives were delivering the infants, they were the logical individuals to teach to perform the surface examination. In another setting, a neonatal nurse could be trained to carry out this surface examination of each newborn. Likewise, the person carrying out the examination could be taught additional steps in the examination, such as how to examine the inside of the infant’s mouth or how to identify an inguinal hernia or an undescended testicle. Ideally, there would be a series of duplicate examinations of the same infant by a non-physician examiner and a pediatrician to establish the rate of agreement on the major findings.
The lower prevalence rate of malformations to be identified, specifically 1.3% in the limited surface examination (Table 1), after subtracting the infants with postaxial polydactyly, type B, impacts the sample sizes that will be needed to provide statistical significance for any abnormalities identified in the exposed newborns in comparison to the unexposed infants. Using 1.3% as the limited surface exam prevalence rate of malformations and a 1:1 ratio of exposed to unexposed infants, power calculations indicate that 1,398 exposed infants would be needed to identify a 2-fold increase in this prevalence rate, while a 4-fold increase, a much larger effect, would require 255 exposed infants. If the ratio of exposed to unexposed infants was increased to 1:4, 810 exposed infants would be needed to identify a 2-fold increase in the prevalence rate, while 136 exposed infants would be needed to identify a 4-fold increase (Faul, 2009).
The prevalence rate for the number of infants with one or more malformations of 2% identified in the Active Malformations Surveillance Program in Boston, is to be expected, when a full surface examination is carried out by a physician, based on the inclusion/exclusion criteria being used (Holmes, 2011)). A similar prevalence rate has been reported for two large population-based surveys of newborn infants (Correa, 2007; Feldkamp, 2017). For example, in a five-county area including Atlanta, Georgia the Metropolitan Atlanta Congenital Defects Program (MACDP) has used multiple ascertainment sources to identify all infants and fetuses with birth defects since 1968. The inclusion criteria are that the affected fetus must be at least 20 weeks of gestation and has a major structural or chromosomal defect present at birth that adversely affects health or development. As was noted above, the prevalence rate was 2.09% among infants from birth to 7 days of age (Correa, 2007)). In a second study in 2005–2009 in Utah, 2.03% of the infants were born with birth defects (Feldkamp, 2017). Birth defects were defined as “any structural or functional anomaly with measurable effects on physical, intellectual and social well being.” Their exclusions included muscular ventricular septal defects and distal hypospadias, two findings excluded, also, in the Active Malformations Surveillance Program in Boston (Holmes, 2012; Toufaily, 2018; Holmes, 2018c).
In summary, a limited surface exam can be used to determine the frequency of external malformations among newborn infants exposed to potential human teratogens. This approach offers a feasible method to identify potential teratogenic exposures in low-resource settings.
ACKNOWLEDGEMENTS
We thank the many parents, nurses, pediatricians, obstetricians, pathologists, consultants and laboratory directors whose cooperation and expertise supported the diagnoses established in this malformations surveillance program.
Funding:
Supported by the Birth Defects Registry of the Massachusetts Department of Public Health, which is part of the National Birth Defects Prevention Study, a project of the Birth Defects and Developmental Disabilities Center of the Centers for Disease Control, Atlanta. Also supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).
Footnotes
CONFLICTS OF INTEREST:
None
DATA AVAILABILITY STATEMENT:
The data that support the findings in this analysis are available from the corresponding author upon reasonable request.
Contributor Information
Lewis B. Holmes, Medical Genetics and Metabolism Unit, MassGeneral Hospital for Children, Boston
Hanah Z. Nasri, Medical Genetics and Metabolism Unit, MassGeneral Hospital for Children, Boston
Anne-Therese Hunt, Hunt Consulting Associates, Chapel Hill.
Rebecca Zash, Division of Infectious Diseases, Beth Israel Deaconess Medical Center, Boston.
Roger L. Shapiro, Division of Infectious Diseases, Beth Israel Deaconess Medical Center, Boston
REFERENCES
- Cohen MJ, Meador KJ, May R, Loblein H, Conrad T, Baker GA, et al. Fetal antiepileptic drug exposure and learning and memory functioning at 6 years of age: the NEAD prospective observational study. Epilepsy Behav. 2019; 92:154–164. doi: 10.1016/j.yebeh.2018.12.031. [DOI] [PubMed] [Google Scholar]
- Cooper WO, Hernandez-Diaz S, Arbogast PG, Dudley JA, Dyer S, Gideon PS, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med. 2006; 354:2443–51. doi: 10.1056/NEJMoa055202. [DOI] [PubMed] [Google Scholar]
- Correa A, Cragan JD, Kucik JE, Alverson CJ, Gilboa SM, Balakrishnan R…Chitra J. Metropolitan Atlanta Congenital Defects Program (MACDP) 40th Anniversary Edition Surveillance Report. Report birth defects surveillance data 1968–2003. Birth Defects Res A Clin Mol Teratol. 2007; 79:65–186. doi: 10.1002/bdra.20350. [DOI] [PubMed] [Google Scholar]
- Crawshaw P The new BPA classification. Arch Dis Child. 1995; 73:563–7. doi: 10.1136/adc.73.6.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analysis using G Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods. 2009; 41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Feldkamp ML, Carey JC, Byrne JLB, Krikov S, Botto LD. Etiology and clinical presentation of birth defects: population based study. BMJ. 2017; 357:j2249. doi: 10.1136/bmj.j2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Díaz S, McElrath TF, Pennell PB, Hauser WA, Yerby M, Holmes LB. Fetal growth and premature delivery in pregnant women on antiepileptic drugs. Ann Neurol. 2017; 82:457–465. doi: 10.1002/ana.25031. [DOI] [PubMed] [Google Scholar]
- Holmes LB. Teratogen-induced limb malformations. Am J Med Genet. 2002; 112:297–303. doi: 10.1002/ajmg.10781. [DOI] [PubMed] [Google Scholar]
- Holmes LB. Common Malformations, Oxford University Press, New York: 2012. [Google Scholar]
- Holmes LB, Harvey EA, Coull BA, Huntington KB, Khoshbin S, Hayes AM, et al. The teratogenicity of anticonvulsant drugs. N Engl J Med. 2001; 344:1132–8. doi: 10.1056/NEJM200104123441504. [DOI] [PubMed] [Google Scholar]
- Holmes LB, Westgate M-N. Inclusion and exclusion criteria for malformations in newborn infants exposed to potential teratogens. Birth Defects Res A Clin Mol Teratol. 2011; 91:807–12. doi: 10.1002/bdra.20842. [DOI] [PubMed] [Google Scholar]
- Holmes LB, Nasri H, Beroukhim R, Hunt AT, Roberts DJ, Toufaily MH, Westgate M-N. Stillborn infants: associated malformations. Birth Defects Res. 2018; 110:114–121. doi: 10.1002/bdr2.1097. [DOI] [PubMed] [Google Scholar]
- Holmes LB, Nasri H, Hunt AT, Toufaily MH, Westgate MN. Polydactyly, postaxial, type B. Birth Defects Res. 2018; 110:134–141. doi: 10.1002/bdr2.1184. [DOI] [PubMed] [Google Scholar]
- Holmes LB, Nasri H, Westgate MN, Toufaily MH, Lin AE. The Active Malformations Surveillance Program, Boston in 1972–2012: methodology and demographic characteristics. Birth Defects Res. 2018; 110:148–156. doi: 10.1002/bdr2.1156. [DOI] [PubMed] [Google Scholar]
- Johansson MA, Mier-y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the risk of microcephaly. N Engl J Med. 2016; 375:498. doi: 10.1056/NEJMp1605367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Moore CA, Li Z, Berry RJ, Gindler J, Hong SX, et al. A population-based birth defects surveillance system in the People’s Republic of China. Paediatr Perinat Epidemiol. 2003; 17:287–93. doi: 10.1046/j.1365-3016.2003.00478.x. [DOI] [PubMed] [Google Scholar]
- Mahle WT, Newburger JW, Matherne GP, Smith FC, Hoke TR, Koppel R, et al. Role of pulse oximetry in examining newborns for congenital heart disease. Circulation. 2009; 120:447–58. doi: 10.1161/CIRCULATIONAHA.109.192576. [DOI] [PubMed] [Google Scholar]
- Marden PM, Smith DW, McDonald MJ. Congenital anomalies in the newborn infant, including minor variations. J Pediatr. 1964; 64:357–71. doi: 10.1016/s0022-3476(64)80188-8. [DOI] [PubMed] [Google Scholar]
- Mehta U, Clerk C, Allen E, Yore M, Sevene E, Singlovic J, et al. Protocol for a drug exposure pregnancy registry for implementation in resource-limited settings. BMC Pregnancy Childbirth. 2012; 12:89. doi: 10.1186/1471-2393-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raesima MM, Ogbuabo CM, Thomas V, Forhan SE, Gokatweng G, Dintwa E, et al. Dolutegravir use at conception – additional surveillance data from Botswana. N Engl J Med. 2019; 381:885–887. doi: 10.1056/NEJMc1908155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard TH, Fantel AG, Fitzsimmons J. Congenital defect rates among spontaneous abortuses: twenty years of monitoring. Teratology. 1989; 39:325–31. doi: 10.1002/tera.1420390404. [DOI] [PubMed] [Google Scholar]
- Thomas EG, Higgins C, Westgate MN, Lin AE, Anderka M, Holmes LB. Malformations Surveillance: Comparison between Findings at Birth and Age 1 Year. Birth Defects Res. 2018; 110:142–147. doi: 10.1002/bdr2.1096. [DOI] [PubMed] [Google Scholar]
- Toufaily MH, Westgate M-N, Lin AE, Holmes LB. Causes of Congenital malformations. Birth Defects Res. 2018; 110:87–91. doi: 10.1002/bdr2.1105. [DOI] [PubMed] [Google Scholar]
- Zash R, Holmes L, Diseko M, Jacobson DL, Brummel S, Mayondi G, et al. Neural-tube defects and antiretroviral treatment regimens in Botswana. N Engl J Med. 2019; 381:827–840. doi: 10.1056/NEJMoa1905230. [DOI] [PMC free article] [PubMed] [Google Scholar]


