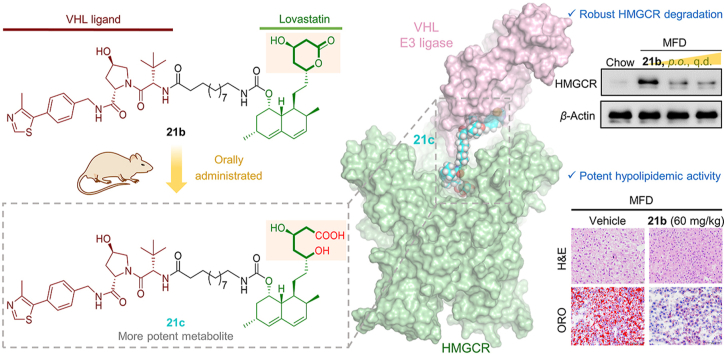
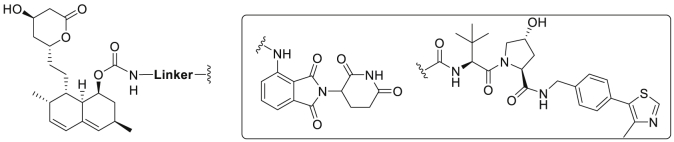
Abstract
HMG-CoA reductase (HMGCR) protein is usually upregulated after statin (HMGCR inhibitor) treatment, which inevitably diminishes its therapeutic efficacy, provoking the need for higher doses associated with adverse effects. The proteolysis targeting chimera (PROTAC) technology has recently emerged as a powerful approach for inducing protein degradation. Nonetheless, due to their bifunctional nature, developing orally bioavailable PROTACs remains a great challenge. Herein, we identified a powerful HMGCR-targeted PROTAC (21c) comprising a VHL ligand conjugated to lovastatin acid that potently degrades HMGCR in Insig-silenced HepG2 cells (DC50 = 120 nmol/L) and forms a stable ternary complex, as predicated by a holistic modeling protocol. Most importantly, oral administration of the corresponding lactone 21b reveled favorable plasma exposures referring to both the parent 21b and the conversed acid 21c. Further in vivo studies of 21b demonstrated robust HMGCR degradation and potent cholesterol reduction in mice with diet-induced hypercholesterolemia, highlighting a promising strategy for treating hyperlipidemia and associated diseases.
Key words: HMGCR, PROTACs, Oral bioavailability, Cholesterol reduction
Abbreviations: CRBN, cereblon; CVD, cardiovascular disease; DC50, half degradation concentration; ER, endoplasmic reticulum; HDAC, histone deacetylase; HMGCR, 3-hydroxy-3-methylglutaryl coenzyme A reductase; H&E, hematoxylin/eosin; LDL-C, low-density lipoprotein cholesterol; MFD, medium fat diet; ORO, oil-red O; PK, pharmacokinetic; PROTAC, proteolysis-targeting chimera; SAR, structure–activity relationship; TC, total cholesterol; TG, triglyceride; VHL, von Hippel-Lindau
Graphical abstract
The proteolysis targeting chimera (PROTAC) technology has recently emerged as a powerful approach for inducing protein degradation. A potent and orally active VHL-recruiting PROTAC was developed, displaying robust HMGCR degradation and potent hypolipidemic activity in diet-induced mice.
1. Introduction
Atherogenic dyslipidemia characterized by elevated cholesterol, especially high levels of low-density lipoprotein (LDL) cholesterol, is an important cause of cardiovascular disease (CVD)1. Pharmacological management of hypercholesterolemia has represented the most effective therapy for cardiovascular disease prevention2. 3-Hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase (HMGCR), a well-established target for hypolipidemic drugs, is the rate-limiting enzyme of the mevalonate pathway, which catalyzes the conversion of HMG-CoA to the key precursor of cholesterol, mevalonate (Fig. 1A)3, 4, 5. Statins are a class of HMGCR inhibitors with HMG-like moieties that competitively bind to the catalytic site, thereby blocking the production of mevalonate and sterols (Fig. 1A)5,6. Owing to their efficacy in reducing plasma cholesterol levels, statins remain as the main therapy for hypercholesterolemia and CVD7,8. However, there are concerns regarding poor statin adherence including insufficient dosing and high discontinuation rates, which have been documented in approximately fifty percent of patients9, 10, 11, 12. Particularly, inhibiting HMGCR function by statins usually leads to a compensatory upregulation of HMGCR protein (Fig. 1A), which has been observed in both research models13, 14, 15 and humans16 and is believed to unavoidably hamper the effectiveness of statins and limit their clinical applications17, 18, 19.
Figure 1.
(A) HMGCR inhibition by statins leads to the compensatory upregulation of HMGCR. (B) Illustration of PROTAC-mediated HMGCR degradation.
HMGCR is an endoplasmic reticulum (ER)-localized transmembrane protein whose amount under physiological conditions is regulated through multiple feedback mechanisms4,20,21. On the one hand, reduction of cholesterol synthesis activates the sterol-regulatory element binding protein (SREBP) pathway, leading to augmentation of HMGCR gene transcription4. On the other hand, less production of cholesterol and downstream intermediates maintains HMGCR protein stabilization by blocking the sterol-induced ubiquitination of HMGCR22, 23, 24, a native process of Insig-mediated HMGCR degradation25,26. Regarding statin-induced HMGCR increment, reduced HMGCR degradation was recently recognized as the predominant mechanism (Fig. 1A)27. indicating that ablating both activity and abundance of HMGCR would be a new and promising strategy to lower cholesterol levels.
As a novel chemical knockdown technology, proteolysis-targeting chimera (PROTAC)28, 29, 30 has recently emerged as a promising approach with potential to address the limitations of conventional drug development paradigms31, 32, 33, 34, 35. PROTACs are bifunctional compounds consisting of two independent ligands connected by a chemical linker, with one ligand specifically binding to target protein and the other ligand recruiting an E3 ubiquitin ligase. Upon ternary complex formation, the target protein is polyubiquitinated and subsequently degraded by the proteasome36. While this rapidly developed technique has been widely employed in the degradation of various oncogenic proteins37, 38, 39, 40, its application in CVD, the leading cause of global deaths, remains relatively less explored41. Moreover, examples of PROTACs with potent in vivo activity and favorable pharmacokinetic (PK) properties are scarce42, with most administered via injection rather than via the oral route43, 44, 45. Inspired by the native Insig-mediated degradation process, we envisioned a feasible way to eliminate HMGCR by using artificial conjugates (HMGCR-targeting PROTACs) that hijack different E3 ligases such as von Hippel-Lindau (VHL) and CRBN (Fig. 1B).
Herein, we describe the development of various HMGCR-targeted PROTACs by connecting lovastatin with either CRBN ligand pomalidomide or VHL ligand VHL231. While our work was underway, Rao group46 revealed a comprehensive structure–activity relationship (SAR) analysis of atorvastatin–CRBN conjugates, confirming that the ER-localized membrane protein HMGCR can be successfully degraded in vitro by CRBN–proteasome system. Nevertheless, attempts to validate its therapeutic efficacy in vivo need to be conducted. Furthermore, pursuing orally bioavailable PROTACs, albeit highly challenging, is of great significance particularly for hyperlipidemia, a chronic disease that usually requires long-term medication. Encouragingly, we identified a potent VHL-based PROTAC 21c that induces profound HMGCR degradation in Insig-silenced HepG2 cells through a VHL-dependent manner, a process that was further confirmed by the formation of a stable PROTAC-mediated ternary complex during in silico modeling. Most importantly, the corresponding lactone prodrug 21b has shown to afford high plasma exposures referring to the active ingredient 21c, leading to efficient HMGCR degradation and promising cholesterol-lowering potency in vivo. Overall, our work identified a first-generation, orally active VHL-based degrader of HMGCR, and proved that inducing the degradation of HMGCR by PROTACs can potently reduce cholesterol levels, providing a new strategy to prevent CVD.
2. Results and discussion
2.1. Design, synthesis, and preliminary biological evaluation
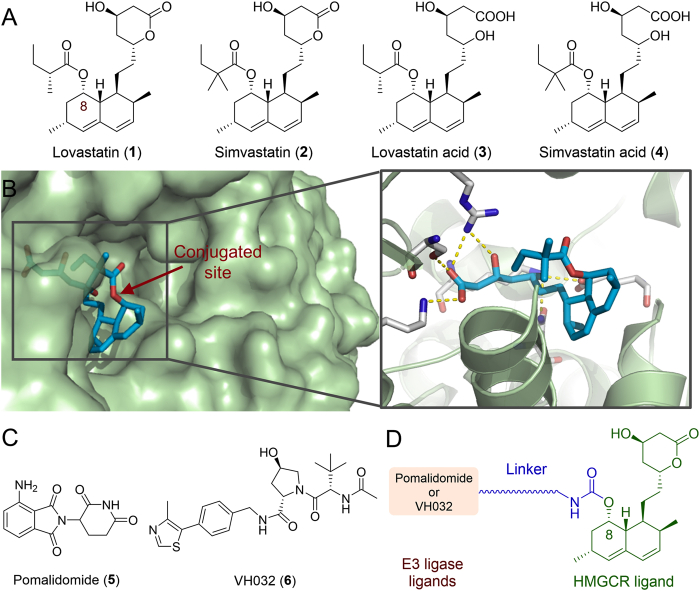
HMGCR-targeting PROTACs were designed based on the first-generation HMGCR inhibitors lovastatin/simvastatin (Fig. 2A), orally bioavailable prodrugs that are transformed to the corresponding β-hydroxyacids (active forms 3 or 4, Fig. 2A) after oral ingestion. The cocrystal structure of simvastatin acid (3) bound to HMGCR5 revealed that the crucial β-hydroxyacid moiety formed hydrogen bonds with key residues in the HMG-CoA pocket (Fig. 2B). The 8-butyrate not involved in any interactions with HMGCR was identified as a solvent exposed group, which has been verified by a previously developed dual HMGCR/HDAC inhibitor where the hydroxamic acid was attached via a carbamate linker to the C-8 oxygen atom of lovastatin47. These results indicated that the 8-butyrate of lovastatin/simvastatin may be a suitable site for the attachment of E3 ligase ligands (Fig. 2B). To probe the potential degradation of ER membrane-bound HMGCR by artificial PROTAC conjugates, the CRBN ligand pomalidomide (5) and the VHL ligand VH032 (6, Fig. 2C), two widely used E3 ligase-recruiting moieties, were examined in this study. Thus, we initially designed several HMGCR-targeting PROTAC probes by connecting E3 ligase ligands to the C-8 position of lovastatin through various carbamate linkers (Fig. 2D).
Figure 2.
(A) Structures of the HMGCR inhibitors lovastatin (1) and simvastatin (2) together with their active forms (3 and 4). (B) Cocrystal structure of HMGCR catalytic domain complexed with simvastatin acid (4) (PDB: 1HW9). The conjugated site is indicated by a red arrow. (C) Structures of the E3 ligase ligands pomalidomide (5) and VH032 (6). (D) A general scheme for the design of HMGCR-targeting PROTAC probes.
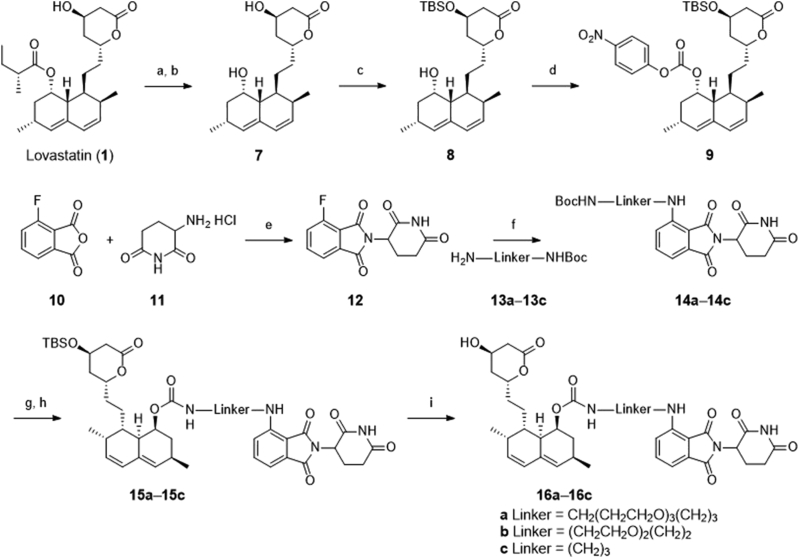
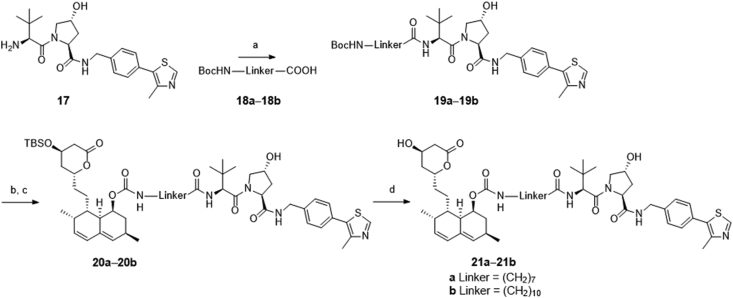
The preparation of designed compounds 16a‒16c was outlined in Scheme 1. Compound 9 was synthesized through four steps according to a previously reported route47. Treatment of the commercially available lovastatin with KOH in H2O/MeOH concurrently led to the production of ester-cleaved and lactone-opened intermediate, which was directly converted to its lactone form 7 under acidic conditions (6 mol/L HCl). Selective protection of the less-hindered hydroxyl group with a bulky TBS group provided compound 8, which subsequently reacted with p-nitrophenyl chloroformate to produce the key intermediate 9. Condensation of compound 9 with pomalidomide analogs 14a‒14c, which were synthesized according to previously published procedures48, yielded compounds 15a‒15c. Further deprotection of the TBS group led to the final CRBN-based PROTACs 16a‒16c. Similarly, as depicted in Scheme 2, the VHL-based PROTACs 21a and 21b were prepared by the condensation of the key intermediate 9 with VH032 analogs 19a and 19b49.
Scheme 1.
Synthesis of the CRBN-based compounds 16a‒16c. Reagents and conditions: (a) KOH, H2O/MeOH, reflux, 12 h; (b) 6 mol/L HCl, rt, 6 h, 45% obtained in two steps; (c) TBSCl, imidazole, CH2Cl2, rt, 6 h, 84%; (d) p-nitrophenyl chloroformate, DMAP, pyridine, rt, 16 h, 64%; (e) NaOAc, AcOH, 12 h, reflux, 70%; (f) N,N-diisopropylethylamine, DMF, 90 °C, 12 h, 35%–45%; (g) TFA, DCM, rt, 0.5 h; (h) 9, DMAP, pyridine, rt, 16 h, 60%–75%; (i) BF3·OEt2, MeCN, 0 °C, 0.5 h, 52%–65%.
Scheme 2.
Synthesis of VHL-based compounds 21a and 21b. Reagents and conditions: (a) N,N-diisopropylethylamine, HATU, DMF, rt, 12 h, 35–55%; (b) TFA, DCM, rt, 0.5 h; (c) 9, DMAP, pyridine, rt, 16 h, 45%–65%; (d) BF3·OEt2, MeCN, 0 °C, 0.5 h, 55%–60%.
According to previous studies47, lovastatin derivatives bearing linear substitutions at the C-8 position showed HMGCR inhibition that is comparable to lovastatin. Therefore, to confirm the retention of HMGCR catalytic domain binding, the inhibitory activities of synthetic compounds on HMGCR were initially examined by a cell-free enzymatic assay. As expected, conjugating the E3 recruiting ligand at C-8 position of lovastatin does not have a major effect on HMGCR inhibition. All compounds presented low micromolar potency with IC50 values (1.25–2.49 μmol/L) comparable to that of lovastatin (0.74 μmol/L, Table 1 and Supporting Information Fig. S1), implying that these 8-O-linked analogs maintained reasonable affinity for HMGCR.
Table 1.
HMGCR-targeted PROTACs derived from lovastatin with pomalidomide or VH032.
| Compd. | Linker | E3 ligase | HMGCR inhibition IC50 (μmol/L)a | Remaining HMGCR at 1 μmol/L (%)b |
|---|---|---|---|---|
| 16a | CH2(CH2CH2O)3(CH2)3 | CRBN | 1.25 | 73 ± 6 |
| 16b | (CH2CH2O)2(CH2)2 | CRBN | 1.88 | 56 ± 4 |
| 16c | (CH2)3 | CRBN | 2.49 | 89 ± 7 |
| 21a | (CH2)7 | VHL | 1.56 | 63 ± 5 |
| 21b | (CH2)10 | VHL | 1.32 | 42 ± 5 |
| Lovastatin | – | – | 0.74 | 266 ± 29 |
IC50 values for HMGCR inhibition were obtained from triplicate experiments.
Percentage HMGCR level remaining relative to the control of each compound at 1 μmol/L. The data are the means ± SD from three independent experiments. − Not applicable.
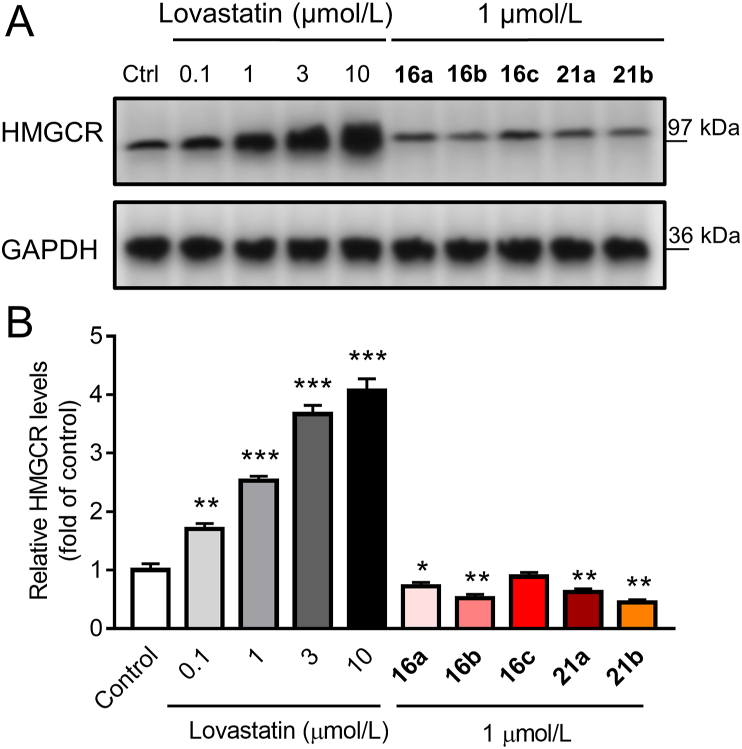
We then performed Western blot analyses to assess the ability of these analogs to prevent the compensatory upregulation of HMGCR in human hepatic HepG2 cells. As is shown in Fig. 3, consistent with previous findings27 that statins slow the native degradation of HMGCR leading to its increment, and treatment of lovastatin at a range of concentrations for 16 h remarkably upregulated HMGCR in a dose-dependent manner. In contrast, we were pleased to find that both the CRBN- and VHL-based compounds effectively attenuated the compensatory upregulation of HMGCR (Fig. 3) at a nontoxic concentration of 1 μmol/L, as measured by Cell-Counting Kit-8 assay (Supporting Information Fig. S2). Although native Insig-mediated HMGCR degradation might have disrupted the results, our data obtained by directly comparing PROTAC-treated groups with untreated controls under the same conditions (Fig. 3) clearly indicated that these compounds induced HMGCR degradation to a further extent. Compound 16b, possessing an ethylene glycol linker, was the most potent among the CRBN-based PROTACs, with 56% protein remaining relative to the untreated control, while the long carbon chain-linked VHL analog 21b exerted the most potent efficacy with 42% protein remaining (Table 1). Despite limited SAR analysis results, we reasoned that ER-bound HMGCR can be degraded by PROTACs hijacking ether the CRBN or VHL E3 ligase. However, several limitations of CRBN-based PROTACs have been previously reported including the intrinsic activity of the CRBN ligand on non-PROTAC targets and chemical instability48. The latter was also observed in CRBN-based compound 16b that showed over half decomposition in silane at 37 °C after 24 h (Supporting Information Fig. S3). With the aim of identifying a PROTAC suitable for in vivo studies, we turned our attention, therefore, to VHL-based PROTACs.
Figure 3.
Effect of lovastatin and PROTACs on HMGCR expression in HepG2 cells. (A) Cells were treated with DMSO, lovastatin (0.1, 1, 3 and 10 μmol/L) or compounds 16a‒16c, or 21a‒21b (1 μmol/L) for 16 h. Original blots are shown in Supporting Information Fig. S4. The data are represented as fold change relative to the control, means ± SD (n = 3). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 vs. DMSO-control.
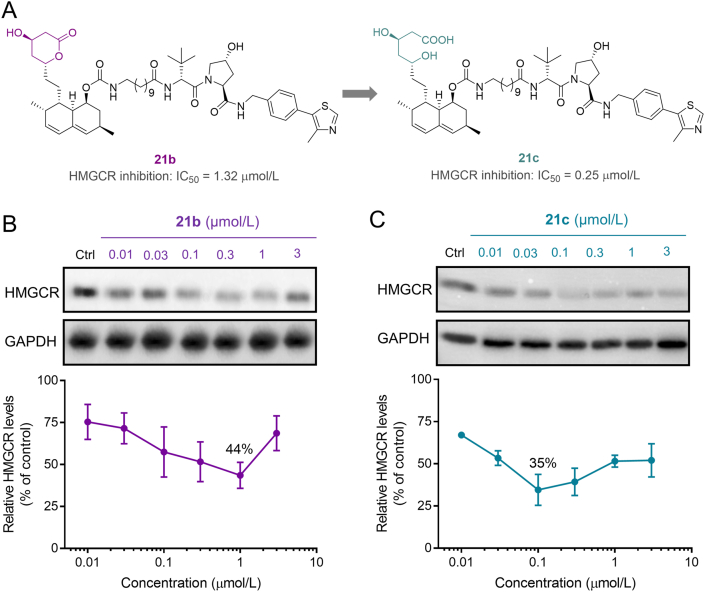
These lovastatin-derived PROTACs in the lactone form, albeit with acceptable HMGCR inhibition and degradation, were in fact prodrugs, which may produce different cellular actions. To further investigate the prodrug characteristics, we next prepared PROTAC 21c (Fig. 4A), the lactone-opened form of 21b, which in terms of the hydroxy acid pharmacophore is predicted to have better affinity for HMGCR. As expected, 21c demonstrated improved HMGCR inhibition with an IC50 value of 0.25 μmol/L (Fig. 4A). We then established dose−response protein curves for 21c and its parent lactone 21b for comparison (Fig. 4B and C). At lower doses, both PROTAC 21b and 21c attenuated the upregulation of HMGCR. Whereas higher concentrations lead to an increase in HMGCR expression, referred as the characteristic “hook effect”, indicating that these PROTACs preferentially act as HMGCR inhibitors over degraders at high doses. Additionally, another plausible explanation may be that, as HMGCR inhibition gradually dominated (particularly for 21c, Fig. 4C), greater HMGCR accumulation caused by the hindered Insig-pathway might have offset the effect of PROTACs. These results preliminarily indicated that 21c was able to inhibit HMGCR activity through the lovatstain acid moiety while promoting HMGCR degradation through the VHL moiety. Compared to the lactone 21b, which achieved a maximum degradation (Dmax) of 56% at a high dose of 1 μmol/L, the corresponding acid 21c was more efficient in inducing HMGCR degradation (Dmax = 65%, at 0.1 μmol/L). Further evaluation of lovastatin, 21b and 21c under the same conditions confirmed that 21c was a promising HMGCR degrader capable of reducing cellular cholesterol (Supporting Information Fig. S5), which was thus selected for further cellular mechanism studies.
Figure 4.
Comparison of the lactone 21b to the corresponding acid 21c in enzymatic and cellular assays. (A) Hydrolysis of the lactone ring to generate the ring-opened acid 21c with improved HMGCR inhibition. (B) and (C) HepG2 cells treated with 21b or 21c at indicated doses for 16 h, were analyzed for protein levels. The data are represented as %HMGCR remaining relative to the DMSO-control (defined as 100%), and presented as the mean ± SD from two repeated experiments shown in Fig. S4.
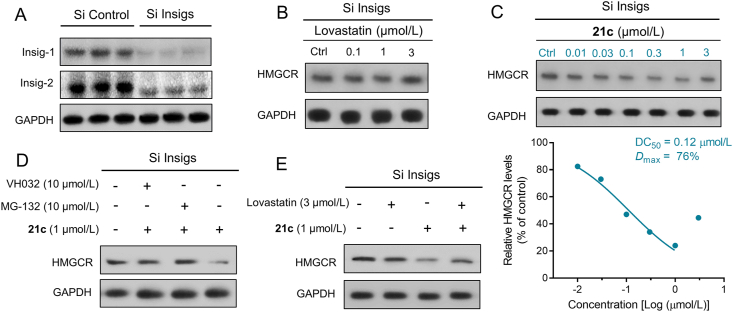
2.2. Validation of PROTAC (21c)-mediated HMGCR degradation in Insig-silenced HepG2 cells
As mentioned above, statin-induced upregulation of HMGCR was recently shown to be primarily the result of HMGCR stabilization, as the interaction between HMGCR and Insig, as well as the subsequent ubiquitination and degradation, were blocked. To exclude inherent Insig-mediated effect on HMGCR expression, we used siRNA to knockdown Insig-1 and Insig-2 in HepG2 cells (Fig. 5A) that expressed constant HMGCR levels regardless of statin treatment (Fig. 5B), allowing the direct and specific assessment of PROTAC-triggered HMGCR degradation46. First, HMGCR degradation by varying concentrations of PROTAC 21c was evaluated to assess the DC50 (concentration causing 50% HMGCR degradation). As shown in Fig. 5C, 21c effectively degraded the HMGCR protein with a DC50 of 0.12 μmol/L, and achieved a Dmax of 76% at 1 μmol/L, confirming that 21c induces PROTAC-mediated HMGCR degradation. As observed in wide-type HepG2 cells, elevated HMGCR expression was also observed in the Insig-silenced HepG2 cells at higher concentration of 3 μmol/L. However, this “hook effect” is mainly attributed to the PROTAC characteristics rather than the combinational feedback effects shown in Fig. 4C. Furthermore, a time-course study revealed that compound 21c reduced HMGCR protein level in a time-dependent manner (Supporting Information Fig. S6).
Figure 5.
Compound 21c induces HMGCR degradation through VHL-dependent ubiquitin–proteasome system in Insig-silenced HepG2 cells. (A) Knockdown efficiency of Insig-1 and Insig-2 in HepG2 cells was determined by Western blotting. (B) and (C) Insig-silenced HepG2 cells treated with lovastatin or 21c at the indicated doses (16 h), were examined. (D) and (E) Cells pretreated for 6 h with VH032 (10 μmol/L), the proteasome inhibitor MG-132 (10 μmol/L), lovastatin (3 μmol/L) or DMSO, were subsequently treated for 10 h with compound 21c (1 μmol/L).
To further explore the mechanism of 21c-induced HMGCR degradation, we treated Insig-silenced HepG2 cells with 21c, the VHL ligand (VHL032) and the proteasome inhibitor MG-132 in various concentrations. As shown in Fig. 5D, HMGCR degradation induced by 21c at 1 μmol/L was significantly blocked by the addition of VHL032 (10 μmol/L) or MG132 (10 μmol/L). Moreover, addition of lovastatin (3 μmol/L) also efficiently reduced 21c-induced HMGCR degradation (Fig. 5E). All these mechanistic data confirmed that PROTAC 21c bound simultaneously to HMGCR and VHL, and subsequently degraded HMGCR by the VHL-dependent ubiquitin–proteasome system.
2.3. In silico modeling of the PROTAC (21c)-mediated ternary complex
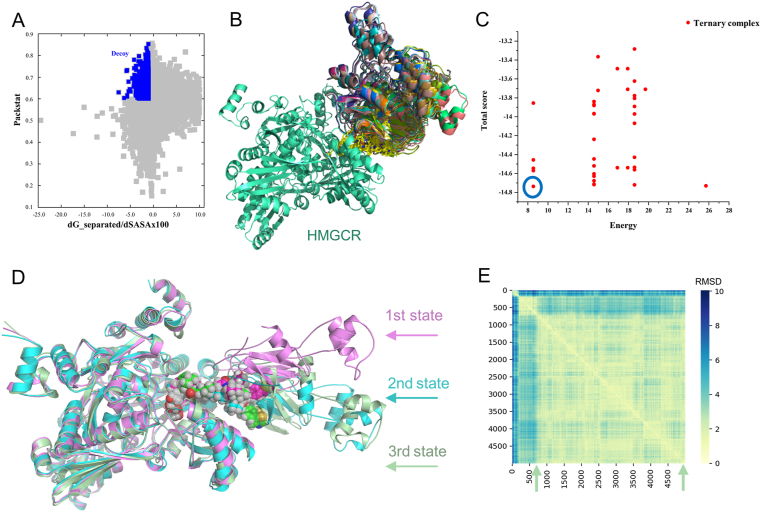
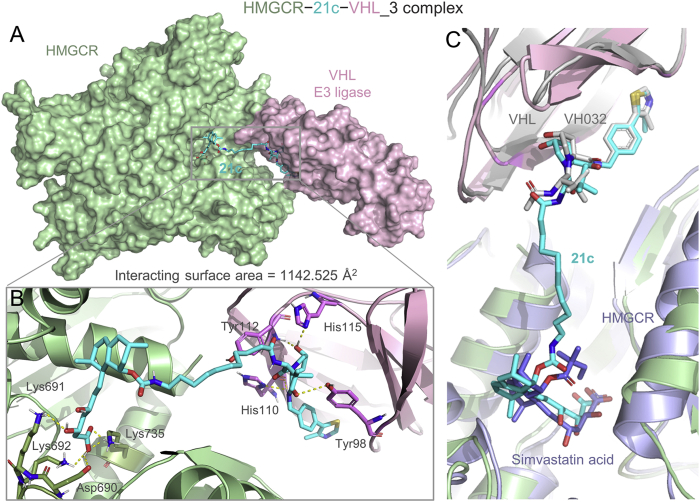
The formation of a favorable ternary complex induced by a PROTAC is considered paramount for valid degradation. To elucidate the potential ternary complex formation between 21c, HMGCR and VHL, we conducted in silico modeling, an attractive surrogate for in vitro experiments, using a holistic protocol50,51 including several consecutive steps described in detail in Supporting Information Fig. S7. Initially, the Rosetta protein−protein docking framework52 was used to build a global HMGCR–VHL interaction modes. Among them, the decoy with packstat score ≥0.5 and binding energy ≤−1.0 (Fig. 6A) was selected to further generate ternary complexes through linker conformer alignment, which outputs a set of feasible ternary modes (Fig. 6B). Then, the pose with the lowest protein docking score and ligand conformer energy (the one in the blue circle, Fig. 6C) was advanced for a 500 ns molecular dynamics simulation to verify whether it could maintain a stable ternary conformation. As is shown in Fig. 6D and E, the conformation ensemble can be separated into three parts with initial steady state lasting through approximately 100 frames (10 ns), a second state lasting from 100 to 800 frames, and the last conformation stabilizing during the rest of the simulation time, which clearly indicated a stable ternary conformation (3rd state, named HMGCR−21c−VHL_3). A detailed analysis revealed that the β-hydroxyacid moiety of 21c fit well into the catalytic pocket of HMGCR forming hydrogen bonds with key residues, and the VH032 moiety engaged the catalytic tunnel of VHL (Fig. 7A and B). In addition, VHL interacted with the catalytic domain of HMGCR, generating an interface area of 1142.525 Å2 (Fig. 7A). Taken together, these modeling results indicated that 21c can form a stable ternary conformation with HMGCR and VHL, and their interactions closely resembled those observed in the respective crystal structures (Fig. 7C). Furthermore, these results establish compound 21c as a suitable VHL-recruiting PROTAC targeting HMGCR for degradation, providing structural insights into the mechanism of PAOTAC (21c)-mediated degradation that can facilitate further optimization. Additionally, we also performed molecular modeling of the shortest linker containing compound 16c within HMGCR (PDB: 1HW9) and CRBN (PDB: 4CI3), which failed to provide any ternary complex due to the limit of linker length. Thus, we speculated that the weak degradation of HMGCR by 16c might be mediated by other mechanisms that needs further investigations.
Figure 6.
Representative modeling results. (A) Symmetric protein–protein docking energy and packstat score landscape for the interaction between the HMGCR–simvastatin acid complex (PDB: 1HW9) and the VHL–VH032 complex (PDB: 4W9H) through Rosetta docking. The decoy (packstat score ≥0.5 and binding energy ≤−1.0) is shown in blue as insert. (B) Superposition of the ternary mode output after linker conformer alignment. HMGCR, VHL and 21c poses are shown in green, multicolor and yellow, respectively. (C) Symmetric docking score and ligand conformer energy landscape for the ternary mode output. The pose with the lowest score and energy was selected, as indicated by the blue circle. (D) A 500 ns molecular dynamics simulation of the selected ternary complex revealed three relatively stable HMGCR/21c/VHL states, shown as violet (1st state), bright blue (2nd state) and green (3rd state) cartoons, respectively, with 21c are shown as spheres. (E) Symmetric pair RMSD value landscape of the above three states generated from the 500 ns molecular dynamics simulation. The third state stabilized during the rest of the simulation time (from 800 to 5000 frames) was selected as the most stable conformation (named HMGCR−21c−VHL_3) and was used for further analysis.
Figure 7.
Structure of the most stable model (3rd state, named HMGCR−21c−VHL_3) from molecular dynamics simulation of HMGCR/21c/VHL ternary complexes is shown. (A) Surface representation of the HMGCR−21c−VHL_3 ternary complex: HMGCR (green), VHL (violet) and 21c (blue stick). (B) Close-up of interactions between HMGCR (green cartoon), VHL (violet cartoon) and 21c (blue stick). Yellow dotted lines represent H-bond interactions. (C) Superposition of HMGCR−21c−VHL_3 complex with the VHL E3 ligase (gray cartoon) bound to VH032 (gray stick) (PDB: 4W9H) and HMGCR (light blue cartoon) bound to simvastatin acid (light blue stick) (PDB: 1HW9).
2.4. Pharmacokinetic (PK) studies
Initially, the in vitro metabolic stability of lovastatin 21b and 21c was evaluated in mouse liver microsomes. As is shown in Table 2, possibly due to the unstable esters (8-butyrate and lactone), lovastatin was metabolized quickly with an extremely high clearance rate. The introduction of the carbamate-linked VHL ligand remarkably enhanced the metabolic stability, with the lactone-opening compound 21c being the least susceptible to phase I metabolism. Calculation (Supporting Information Table S1), however, suggested that the lactone 21b has better permeability and intestinal absorption than 21c and would be less problematic in terms of oral delivery. Furthermore, as lovastatin is an orally bioavailable prodrug, we sought to directly compare the in vivo potency of HMGCR inhibitor with that of a degrader; therefore, we selected the lactone form PROTAC 21b for the following in vivo studies. To verify whether oral administration of 21b can achieve a therapeutically effective concentration, we then conducted pharmacokinetic studies of 21b in mice and analyzed the PK parameters of both 21b and its ring-opening metabolite 21c. Surprisingly, a single oral dosing of 21b at 60 mg/kg afforded good drug exposure in plasma, resulting in desirable Cmax and AUC values for both the parent 21b and active ingredient 21c (Table 3). Furthermore, the plasma levels of 21b and its metabolite 21c reached maximum concentrations after 8 and 4 h (time to peak concentration, Tmax), respectively, which are longer than those of lovastatin and lovastatin acid previously reported in mice (Tmax = 2 and 1.5 h, respectively)53. Consistent with in vitro metabolic stability, 21b exhibited a slower clearance rate than lovastatin53 in mice. These encouraging observations indicated that prodrug 21b, albeit non-adherence to the classic “Rule of Five” (Table S1), exhibited favorable absorption properties and oral bioavailability44, allowing it to be deployed in mouse disease models.
Table 2.
In vitro metabolic stability of 21b, 21c and lovastatin in mouse liver microsomes.
The data are mean of duplicate. Additional data are shown in the Supporting Information Table S21 and Fig. S8.
Table 3.
Pharmacokinetic profile of 21b and metabolite 21c in mice.
| PK parameter |
21b at 60 mg/kg p.o.a |
|
|---|---|---|
| 21bb | 21cc | |
| t1/2 (h) | 5.1 ± 0.4 | 6.2 ± 0.6 |
| Tmax (h) | 8.0 | 4.0 |
| Cmax (μmol/L) | 0.47 ± 0.04 | 0.29 ± 0.03 |
| AUC0−24 (μmol·h/L) | 5.0 ± 0.41 | 3.3 ± 0.16 |
| AUC0−∞ (μmol·h/L) | 5.4 ± 0.47 | 3.6 ± 0.18 |
Prodrug 21b was dosed via a single p.o. route at indicated concentrations.
PK parameters of the parent 21b.
PK parameters of the metabolite 21c. All data are mean ± SD, n = 5. Additional data are shown in the Supporting Information Fig. S9 and Table S3.
2.5. In vivo efficacy of compound 21b in mice with MFD-Induced hypercholesterolemia
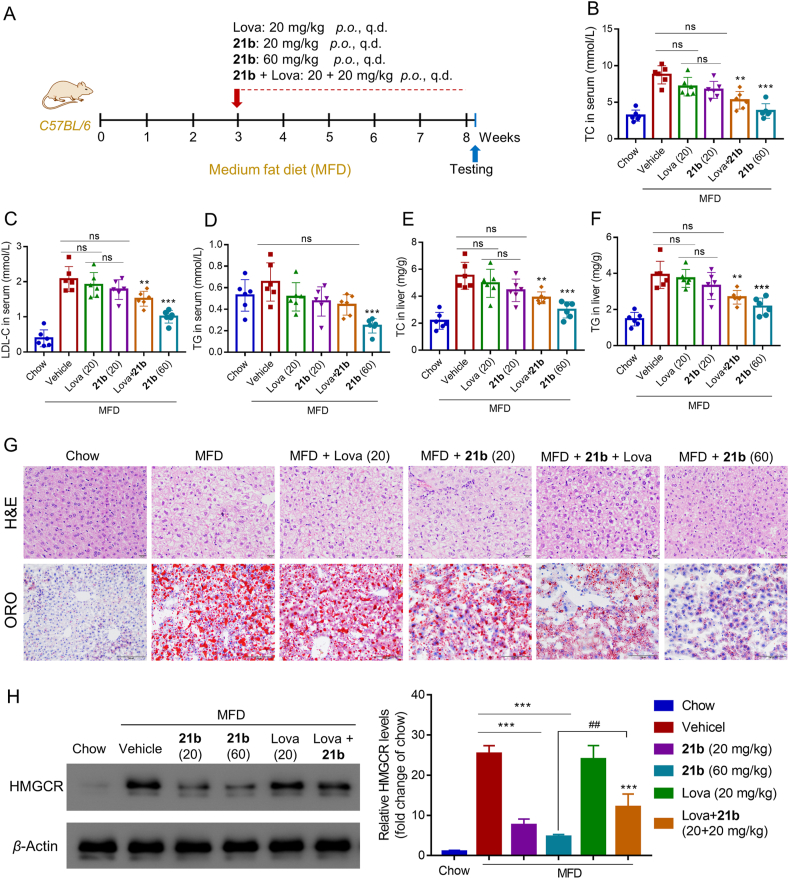
Encouraged by the pharmacokinetic data, we evaluated the effect of compound 21b on a medium fat diet (MFD)-induced mouse model of hypercholesterolemia. After a 3-week induction of hypercholesterolemia, compound 21b and lovastatin were administered orally once a day for 5 weeks (Fig. 8A). Notably, compound 21b was well tolerated, with body weight and food intake comparable to those of the mice treated with lovastatin or MFD vehicle (Supporting Information Fig. S10). As depicted in Fig. 8B‒D, compound 21b at a single oral dose of 20 mg/kg demonstrated similar effects to lovastatin, leading to a moderate decrease of total cholesterol (TC), LDL-C and triglyceride (TG) in serum of mice fed on MFD. Combinations of 21b and lovastatin enhanced the reduction in serum lipid levels. Moreover, this cholesterol-lowering activity was dose-dependent for 21b: at a higher dose of 60 mg/kg, serum TC and cholesteryl esters were all significantly reduced to lower levels. Consistently, compound 21b reduced hepatic TC and TG in a dose-dependent manner (Fig. 8E and F), and ameliorated MFD-induced steatosis and lipid deposition in liver sections, as determined by histochemical staining (Fig. 8G).
Figure 8.
Compound 21b effectively degraded HMGCR and lowered cholesterol in mice with MFD-induced hypercholesterolemia. (A) Schematic representation of the experimental protocol. C57BL/6 male mice (n = 6) on a normal diet or MFD were orally administered lovastatin (20 mg/kg/day), 21b (20 or 60 mg/kg/day) or combination of 21b and lovastatin (20 + 20 mg/kg) for 5 weeks. Sixteen hours after the final gavage, livers and plasma were collected and analyzed for serum total cholesterol (TC) (B), serum LDL-C (C), serum TG (D), hepatic TC (E) and hepatic TG (F). Data are the means ± SD (n = 6), ∗∗P < 0.01, ∗∗∗P < 0.001 vs. untreated MFD vehicle. (G) Photomicrograph of livers stained with H&E (scale bar, 20 μm) and oil red O (ORO) (scale bar, 100 μm). (H) Hepatic HMGCR levels were examined through Western blot experiment. Data are presented as HMGCR fold relative to the chow-control (defined as 1), and as the means ± SD (3 mice per group, Supporting Information Fig. S10), ∗∗∗P < 0.001 vs. untreated MFD vehicle; ##P < 0.01 (21b group vs. 21b/lovastatin cotreatment group).
To further clarify the mechanism of 21b, Western blotting was performed to determine the HMGCR expression in liver. As shown in Fig. 8H, robust HMGCR degradation was induced by 21b at 16 h after final gavage even at a low dose of 20 mg/kg, while lovastatin group retained high HMGCR level. These findings demonstrate that 21b is a highly potent and orally active HMGCR degrader. In consistent with above cellular results, addition of lovastatin to 21b significantly impaired its HMGCR degradation (##P < 0.01). It is worth noting that cotreatment of 21b with lovastatin, albeit attenuating HMGCR degradation, was more effective in lowering total lipids (TC, cholesteryl ester and TG) than respective single drug group. These results suggest that although 21b at a low dose alone was able to suppress de novo cholesterol synthesis via HMGCR degradation, synergetic statin therapy and HMGCR degradation may provide greater benefit for promoting excretion of redundant lipids absorbed daily from an MFD27. Therefore, considering the pleiotropic effects of statin therapy in the lipid metabolism, combining 21b with statins could be a potential strategy to produce optimal therapeutic effect that warrants further investigations using respective other kinds of statins and in vivo models.
3. Conclusions
The use of PROTACs, as an emerging small-molecule knockdown strategy, has gained considerable attention in both academia and the pharmaceutical industry as means of expanding therapeutic landscapes not accessible to conventional drugs31, 32, 33, 34, 35. To date, this rapidly developed technique has been successfully employed for the degradation of various proteins involved in cancer37, 38, 39, 40 and neurodegenerative diseases54,55, including nuclear receptors56,57, kinases58, 59, 60, epigenetic readers61, 62, 63 and transcription factors64. On the other hand, statin-induced compensatory upregulation of HMGCR (an ER transmembrane protein), a common phenomenon13, 14, 15, 16, decreases statin sensitivity and leads to higher dose requirements that unavoidably cause high risks of side effects17,18. Fueled by recent progress in accelerating Insig-mediated HMGCR degradation as a potential strategy27, we sought to probe HMGCR degradation by artificial PROTACs that hijack different E3 ligases, which would be an extension of PROTAC to less-explored cardiovascular diseases.
Lovastatin, an orally bioavailable prodrug, was selected as the HMGCR-recognizing ligand since a previous study confirmed that liner substitutions at the C-8 position maintained HMGCR binding for inhibition47, making it a reasonable choice for conjugating E3 ligase ligands at this site. Initial screens with HepG2 cells led to VHL-based 21b as a potent PROTAC achieving the least remaining HMGCR. However, since the HMGCR level is physiologically regulated by Insig-mediated degradation and because blockade of this degradation is the predominant cause of statin-induced HMGCR increment, the results from wide-type HepG2 cells might not truly reflect the potency of PROTAC-mediated degradation, particularly for the acid 21c, as indicated by more protein remaining at higher treatment concentrations. A plausible explanation might be that HMGCR degradation induced by 21c via the VHL moiety was compromised by HMGCR increment induced by 21c via the lovastatin acid moiety. Subsequent evaluation in Insig-silenced cells, a suitable model for evaluating PROTAC-mediated degradation46, provided unbiased evidence that 21c does promote HMGCR degradation via the VHL-dependent ubiquitin–proteasome system. Nevertheless, due to this harsh requirement for an Insig-deficient cell model, extensive screens and systematic SAR studies would be impeded. Therefore, we used a combined computational method to evaluate the suitability of 21c for stable ternary complex formation, providing structural insights to facilitate further optimization.
To directly compare PROTAC with lovastatin, we then selected the lactone form 21b for further animal studies, which achieved surprisingly good oral PK properties for both parent 21b and active ingredient 21c, translating to efficient HMGCR degradation in mice with MFD-induced hypercholesterolemia. The safety profile of 21b was confirmed when no apparent change of body weight and food intake after long-term treatment (5 weeks). Collectively, as the first-generation VHL-based HMGCR-PROTAC, 21b has already displayed favorable oral bioavailability and great promise for promoting HMGCR degradation and cholesterol reduction in vivo, and can be a promising strategy alone or synergetic with statin therapy for the treatment of hyperlipidemia. Moreover, advances in this work demonstrate that favorable oral PK properties for PROTACs with challenging physicochemical property can be regularly achievable44, which paves the way for the development of more orally bioavailable PROTACs in the future.
4. Experimental
4.1. Chemistry
Reactions monitorization was conducted by precoated silica gel plates (GF/UV 254) under UV light. To obtain purified compounds, silica gel column (200–300 mesh) was used. EI-MS was collected on Shimadzu GCMS-2010 instruments. High resolution mass spectra (HRMS) were determined by Agilent Technologies 6520 Accurate-Mass Q-TOF MS instruments. Bruker Avance 400 MHz spectrometer was used to determine 1H NMR and 13C NMR. Tetramethylsilane (TMS) was employed as an internal standard. Purity was determined by HPLC: Discovery® 504971 column (C18, 250 mm × 4.6 mm, 5 μm); temperature, 25 °C; injection volume, 5 μL; isocratic flow, rate, 1 mL/min; solvent, 90% MeCN in H2O, and the purity of target compounds are greater than 95%. Synthesis of intermediate 9 and 15a‒15c are shown in the Supporting Information.
4.1.1. (1S,3R,7S,8S,8aR)-8-(2-((2R,4R)-4-Hydroxy-6-oxotetrahydro-2H-pyran-2-yl)ethyl)-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl (3-(2-(2-(3-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)amino)propoxy)ethoxy)ethoxy)propyl)carbamate (16a)
Compound 15a (0.17 g, 0.18 mmol) in anhydrous CH3CN was added boron trifluoride etherate (0.031 mL, 0.22 mmol) at 0 °C, and continue to stir for 30 min. After completion, the reaction group was washed with saturated Na2CO3 solution, and extracted with EA, then purified through column chromatography to give green solid 16a (0.076 g, 52% yield). 1H NMR (400 MHz, CDCl3) δ 8.97 (d, J = 3.2 Hz, 1H), 7.53–7.38 (m, 1H), 7.06 (d, J = 7.1 Hz, 1H), 6.92 (d, J = 8.6 Hz, 1H), 6.46 (d, J = 4.9 Hz, 1H), 5.94 (d, J = 9.6 Hz, 1H), 5.76 (dd, J = 9.3, 6.1 Hz, 1H), 5.49 (s, 1H), 5.23 (d, J = 32.2 Hz, 1H), 4.92 (dd, J = 9.7, 5.4 Hz, 1H), 4.61 (d, J = 3.2 Hz, 1H), 4.29 (s, 1H), 3.78–3.31 (m, 15H), 3.24 (d, J = 6.0 Hz, 2H), 2.92–2.28 (m, 7H), 2.23 (d, J = 11.2 Hz, 1H), 2.10 (d, J = 7.0 Hz, 1H), 2.01–1.50 (m, 11H), 1.34 (d, J = 8.7 Hz, 1H), 1.06 (d, J = 7.4 Hz, 3H), 0.87 (d, J = 6.9 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 171.7, 170.9, 169.4, 167.8, 156.6, 146.9, 136.1, 133.4, 132.5, 131.9, 129.7, 128.3, 116.7, 111.3, 109.8, 70.5, 70.1, 69.2, 68.9, 68.5, 62.3, 60.4, 59.7, 53.5, 48.8, 40.2, 38.5, 37.3, 36.7, 36.1, 32.8, 32.6, 31.4, 30.9, 29.7, 29.2, 27.4, 23.8, 22.8, 22.7, 13.9. MS (ESI) m/z: 823.1 [M+H]+. HRMS (ESI): m/z, Calcd. for C43H58N4O12 [M+H]+, 823.4131, Found 823.4131. Compounds 16b and 16c were synthesized by similar procedure.
4.1.2. (1S,3R,7S,8S,8aR)-8-(2-((2R,4R)-4-Hydroxy-6-oxotetrahydro-2H-pyran-2-yl)ethyl)-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl (2-(2-(2-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)amino)ethoxy)ethoxy)ethyl)carbamate (16b)
Yellow solid (0.08 g, 60% yield). 1H NMR (400 MHz, CDCl3) δ 7.50 (t, J = 8.0 Hz, 1H), 7.12 (d, J = 5.6 Hz, 1H), 6.89 (d, J = 8.5 Hz, 1H), 6.52 (s, 1H), 5.96 (d, J = 9.5 Hz, 1H), 5.78 (dd, J = 9.5, 4.8 Hz, 1H), 5.51 (s, 1H), 5.24 (s, 2H), 4.93 (s, 1H), 4.60 (d, J = 23.0 Hz, 1H), 4.27 (m, 1H), 3.80–2.99 (m, 14H), 2.93–2.08 (m, 10H), 2.01–1.48 (m, 9H), 1.34 (dd, J = 25.9, 17.4 Hz, 3H), 1.07 (d, J = 7.3 Hz, 3H), 0.88 (d, J = 4.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 170.7, 169.4, 167.7, 146.7, 136.2, 133.6, 132.6, 131.8, 129.7, 128.2, 116.8, 111.8, 77.4, 77.2, 77.0, 76.7, 76.3, 70.8, 70.3, 69.2, 69.0, 68.8, 62.6, 53.5, 50.2, 48.8, 42.2, 40.8, 38.4, 37.3, 36.8, 32.7, 31.3, 30.9, 29.6, 29.3, 27.4, 23.9, 23.0, 22.7, 13.9, 1.0, 0.01. HRMS (ESI): m/z, Calcd. for C39H50N4O11 [M+H]+, 751.3551, Found 751.3554.
4.1.3. (1S,3R,7S,8S,8aR)-8-(2-((2R,4R)-4-Hydroxy-6-oxotetrahydro-2H-pyran-2-yl)ethyl)-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl (3-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)amino)propyl)carbamate (16c)
Yellow solid (0.06 g, 65% yield). 1H NMR (400 MHz, CDCl3) δ 9.18 (s, 1H), 7.46 (m, 1H), 7.06 (dd, J = 7.0, 2.4 Hz, 1H), 6.86 (d, J = 8.6 Hz, 1H), 6.43 (s, 1H), 5.96 (d, J = 9.8 Hz, 1H), 5.83–5.58 (m, 1H), 5.51 (s, 1H), 5.36–5.13 (m, 2H), 5.01–4.83 (m, 1H), 4.61 (s, 1H), 4.24 (s, 1H), 3.50 (s, 1H), 3.29 (s, 4H), 2.92–2.68 (m, 3H), 2.60 (dt, J = 29.7, 11.2 Hz, 2H), 2.47–2.16 (m, 4H), 2.09 (s, 1H), 1.77 (m, 8H), 1.44–1.29 (m, 2H), 1.07 (d, J = 7.3 Hz, 3H), 0.88 (m, 3H). 13C NMR (101 MHz, CDCl3) δ 172.1, 171.2, 169.4, 169.3, 167.7, 156.9, 146.7, 136.2, 133.5, 132.5, 132.0, 129.7, 128.3, 116.6, 111.5, 109.9, 75.8, 68.9, 62.4, 60.4, 48.9, 39.6, 38.5, 37.3, 35.9, 32.6, 31.4, 30.9, 29.6, 27.4, 22.7, 21.1, 14.2, 13.9. HRMS (ESI): m/z, Calcd. for C36H44N4O9 [M+H]+, 677.3109, Found 677.3183.
4.1.4. tert-Butyl (8-(((S)-1-((2S,4R)-4-hydroxy-2-((4-(4-methylthiazol-5-yl)benzyl)carbamoyl)pyrrolidin-1-yl)-3,3-dimethyl-1-oxobutan-2-yl)amino)-8-oxooctyl)carbamate (19a)
Compound 17 (0.4 g) was added to a solution of compound 18a (0.93 mmol), HATU (0.35 g, 0.93 mmol) and DIEA (1.86 mmol) in 10 mL DMF at rt and stirred for another 12 h. After completion, reaction was washed with H2O and extracted with EA. Purified by column to afford compound 19a as a gray solid (0.22 g, 35% yield). MS (ESI) m/z: 672.1 [M+H]+. Compound 19b was synthesized according to the step for 19a.
4.1.5. tert-Butyl (11-(((S)-1-((2S,4R)-4-hydroxy-2-((4-(4-methylthiazol-5-yl)benzyl)carbamoyl)pyrrolidin-1-yl)-3,3-dimethyl-1-oxobutan-2-yl)amino)-11-oxoundecyl)carbamate (19b)
Gray solid (1.8 g, 55% yield). MS (ESI) m/z: 714.1 [M+H]+.
4.1.6. (1S,3R,7S,8S,8aR)-8-(2-((2R,4R)-4-((tert-Butyldimethylsilyl)oxy)-6-oxotetrahydro-2H-pyran-2-yl)ethyl)-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl (8-(((S)-1-((2S,4R)-4-hydroxy-2-((4-(4-methylthiazol-5-yl)benzyl)carbamoyl)pyrrolidin-1-yl)-3,3-dimethyl-1-oxobutan-2-yl)amino)-8-oxooctyl)carbamate (20a)
To a solution of carbamate 19a (0.22 g, 0.33 mmol) in 4 mL DCM was added 2 mL TFA, which was stirred for 30 min at rt. After evaporation, the resultant crude was dissolved in anhydrous pyridine (2 mL), and was added carbonate 9 (0.09 g, 0.15 mmol), and DMAP (0.07 g, 0.6 mmol). The reaction was stirred at rt for 16 h. After completion, pyridine was removed, and the residue was extracted with EA and washed with 1 mol/L HCl, dried by anhydrous Na2SO4, and then purified by column using CH2Cl2/MeOH to give white solid 20a (0.1 g). MS (ESI) m/z: 1032.1 [M+H]+. Compounds 20b (white solid, 45% yield). MS (ESI) m/z: 1074.1 [M+H]+ was obtained according to the step for 20a.
4.1.7. (1S,3R,7S,8S,8aR)-8-(2-((2R,4R)-4-Hydroxy-6-oxotetrahydro-2H-pyran-2-yl)ethyl)-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl (8-(((S)-1-((2S,4R)-4-hydroxy-2-((4-(4-methylthiazol-5-yl)benzyl)carbamoyl)pyrrolidin-1-yl)-3,3-dimethyl-1-oxobutan-2-yl)amino)-8-oxooctyl)carbamate (21a)
According to the synthesis of 16a, compound 21a was obtained as a white solid (55% yield). 1H NMR (400 MHz, CDCl3) δ 8.68 (s, 1H), 7.55 (d, J = 5.0 Hz, 1H), 7.35 (s, 4H), 6.49 (d, J = 9.0 Hz, 1H), 5.95 (d, J = 9.7 Hz, 1H), 5.84–5.67 (m, 1H), 5.49 (s, 1H), 5.18 (s, 1H), 4.98 (s, 1H), 4.74–4.45 (m, 6H), 4.43–4.19 (m, 3H), 4.01 (d, J = 11.1 Hz, 1H), 3.65 (d, J = 13.5 Hz, 1H), 3.25–2.93 (m, 2H), 2.67 (dd, J = 23.2, 18.1 Hz, 4H), 2.50 (s, 3H), 2.45–2.28 (m, 3H), 2.20 (t, J = 16.2 Hz, 4H), 1.85 (dd, J = 37.6, 30.5 Hz, 4H), 1.75–1.48 (m, 5H), 1.47–1.11 (m, 13H), 1.06–0.91 (m, 12H), 0.87 (d, J = 7.0 Hz, 4H). 13C NMR (101 MHz, CDCl3) δ 174.0, 171.2, 156.6, 150.43, 148.3, 138.3, 133.5, 131.9, 131.7, 130.8, 130.7, 129.7, 129.4, 128.0, 76.2, 69.9, 68.5, 62.5, 60.4, 58.9, 57.5, 56.9, 43.1, 40.7, 38.6, 37.3, 36.7, 36.2, 36.1, 35.4, 33.1, 32.6, 30.9, 29.8, 28.5, 27.4, 26.4, 26.1, 25.4, 23.9, 22.7, 21.1, 16.0, 14.2, 13.9. HRMS (ESI): m/z, Calcd. for C50H71N5O9S [M+H]+, 918.5045, Found 918.5048.
4.1.8. (1S,3R,7S,8S,8aR)-8-(2-((2R,4R)-4-Hydroxy-6-oxotetrahydro-2H-pyran-2-yl)ethyl)-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl (11-(((S)-1-((2S,4R)-4-hydroxy-2-((4-(4-methylthiazol-5-yl)benzyl)carbamoyl)pyrrolidin-1-yl)-3,3-dimethyl-1-oxobutan-2-yl)amino)-11-oxoundecyl)carbamate (21b)
According to the synthesis of 16a, compound 21b was obtained as a white solid (0.19 g, 60% yield). 1H NMR (400 MHz, CDCl3) δ 8.66 (s, 1H), 7.56 (s, 1H), 7.33 (s, 4H), 6.45 (d, J = 8.6 Hz, 1H), 5.94 (d, J = 9.7 Hz, 1H), 5.75 (dd, J = 9.2, 6.2 Hz, 1H), 5.48 (s, 1H), 5.16 (s, 1H), 4.99 (s, 1H), 4.57 (ddd, J = 21.5, 15.6, 6.8 Hz, 6H), 4.37–4.19 (m, 3H), 3.99 (d, J = 11.1 Hz, 1H), 3.65 (d, J = 7.8 Hz, 1H), 3.09 (d, J = 6.2 Hz, 2H), 2.63 (d, J = 3.7 Hz, 2H), 2.48 (s, 3H), 2.43–2.04 (m, 7H), 1.97 (s, 1H), 1.80 (d, J = 9.2 Hz, 2H), 1.74–1.47 (m, 5H), 1.36 (m, 4H), 1.29–1.15 (m, 14H), 0.94 (s, 8H), 0.86 (d, J = 6.8 Hz, 4H). 13C NMR (101 MHz, CDCl3) δ 173.8, 171.5, 171.3, 171.2, 156.6, 150.5, 148.3, 138.3, 133.4, 131.93, 131.7, 130.7, 129.7, 129.4, 128.2, 127.9, 77.3, 76.4, 69.9, 68.4, 62.2, 60.4, 58.9, 57.4, 56.9, 43.1, 40.8, 38.7, 37.3, 36.6, 36.4, 35.9, 35.4, 32.9, 32.7, 30.9, 29.9, 29.2, 29.0, 27.4, 26.5, 26.4, 25.6, 23.8, 22.7, 21.1, 16.0, 14.12, 13.9. HRMS (ESI): m/z, Calcd. for C53H77N5O9S [M+H]+, 960.5509, Found 960.5514.
4.1.9. (3R)-3,5-Dihydroxy-7-((1S,2S,6R,8S,8aR)-8-(((5-(((S)-1-((2S,4R)-4-hydroxy-2-((4-(4-methylthiazol-5-yl)benzyl)carbamoyl)pyrrolidin-1-yl)-3,3-dimethyl-1-oxobutan-2-yl)amino)-5-oxopentyl)carbamoyl)oxy)-2,6-dimethyl-1,2,6,7,8,8a-hexahydronaphthalen-1-yl)heptanoic acid (21c)
Compound 21b (40 mg, 0.04 mmol) was dissolved in THF/H2O (0.5 mL/0.5 mL), LiOH (1 mg, 0.04 mmol) was added, then the mixture was stirred at rt for 0.5 h. Purification using preparative TLC chromatography provided compound 21c as a white solid (24 mg, 60% yield). 1H NMR (400 MHz, DMSO) δ 8.98 (d, J = 6.1 Hz, 1H), 8.62–8.50 (m, 1H), 7.81 (t, J = 9.7 Hz, 1H), 7.53–7.34 (m, 5H), 6.90 (d, J = 20.0 Hz, 1H), 5.89 (t, J = 12.5 Hz, 1H), 5.76 (dd, J = 20.3, 11.0 Hz, 1H), 5.43 (d, J = 18.0 Hz, 1H), 5.36–5.27 (m, 1H), 5.03 (s, 1H), 4.53 (t, J = 12.1 Hz, 1H), 4.48–4.32 (m, 4H), 4.30–4.18 (m, 1H), 3.99 (s, 1H), 3.72–3.61 (m, 5H), 3.00–2.84 (m, 2H), 2.45 (s, 5H), 2.41–2.14 (m, 11H), 2.14–1.95 (m, 14H), 1.95–1.77 (m, 13H), 1.64 (s, 1H), 1.54–1.39 (m, 8H), 1.35 (d, J = 8.7 Hz, 5H), 1.04 (d, J = 7.1 Hz, 6H), 0.85 (dd, J = 16.5, 6.6 Hz, 9H). 13C NMR (101 MHz, DMSO) δ 172.6, 170.2, 151.9, 148.2, 146.7, 139.9, 132.6, 131.6, 130.1, 129.7, 129.1, 128.7, 127.9, 70.3, 69.3, 66.6, 59.2, 56.8, 44.8, 43.1, 42.1, 38.4, 37.4, 36.4, 35.7, 35.6, 35.4, 35.1, 32.7, 31.7, 31.6, 30.9, 30.3, 29.9, 29.5, 29.5, 29.4, 29.4, 29.3, 29.3, 29.3, 29.2, 29.0, 27.5, 27.0, 26.8, 26.6, 25.9, 25.6, 24.5, 22.8, 22.54, 16.4, 14.4, 14.3. HRMS (ESI): m/z, Calcd. for C53H79N5O10S [M+H]+, 978.5620, Found 978.5626.
4.2. Pharmacology
4.2.1. HMG-CoA reductase activity assay
The inhibition of indicated compounds on HMGCR activity was evaluated by the HMGCR kit according to manufacturer's instructions with minor modifications (Biovision, Catalog # K588-100). Briefly, lovastatin or test compounds (5 μL) dissolved in DMSO were incubated with recombinant HMGCR protein (2 μL) in assay buffer at rt for 10 min, then HMG-CoA (5 μL), NADPH (2 μL) were added, then the mix was incubated for 10 min in water bath (37 °C). The absorbance was detected using a Multiskan Sky (Thermo Scientific), fitted with a 340 nm excitation filter. Sample absorbance was measured against a blank, containing no HMGCR. The IC50 values for the test compounds were calculated using Graphpad Prism software.
4.2.2. Cell culture
Human hepatic HepG2 cells purchased from ATCC (MD, USA) were maintained in EMEM (Gibco, NY, USA) that contains 10% FBS (fetal bovine serum, Gibco), 100 μg/mL streptomycin sulfate and 100 units/mL penicillin (Sigma, St. Louis, MO, USA). Si-HepG2 cells (Insig-1 and Insig-2 silenced HepG2 cells) were self-made and maintained in DMEM (Gibco C11995500BT) with 10% FBS (Gibco), 100 μg/mL streptomycin sulfate and 100 units/mL penicillin.
4.2.3. Insig-1 and Insig-2 knockdown
Sequence information of siRNA duplexes used in this work are shown in the following: siRNA-Insig-1 (sc-44432, Santa Cruz), 5ʹ-AGGACGACAGTTAGCTATGGGTG-3ʹ; siRNA-Insig-2 (sc-45781, Santa Cruz), 5ʹ-GGCUUUCACUUAAGAACUUTT-3ʹ; NC-siRNA, 5ʹ-UUCUCCGAACGUGUCACGUTT-3ʹ. According to the manufacturer's protocols of Lipofectamine RNAimax (11668-0194, Invitrogen), the siRNA sequences of respective duplexes were transfected into HepG2 and incubated for 48 h, then analyzed by immunoblotting.
4.2.4. Western blotting assay
Cells plated into 6- or 12-well plates were treated with indicated compounds at varying doses. Whole cell lysates were collected by RIPA Lysis Buffer (Solarbio) with protease inhibitor. The determination of protein concentrations was conducted by BCA assay (Beyotime), Equal cell lysates were electrophoresed through 10% SDS-polyacrylamide gels and transferred onto polyvinylidene difluoride membranes and blotted against different target antibodies at 4 °C overnight. Primary antibodies include Anti-HMGCR (ab174830, Abcam), Anti-Insig-1 (ab112248, Abcam) and Anti-Insig-2 (ab86145, Abcam).
4.2.5. Cellular cholesterol assay
Cellular cholesterol content in HepG2 cells was determined using Amplex Red Cholesterol Assay Kit (Invitrogen, Catalog No. A12216), according to the manufacturer's instructions. Briefly, HepG2 cells seeded in 12-well plates were incubated with tested compounds for 24 h. Cellular free cholesterol were extracted with lysis buffer, the mixture was then centrifuged (2000×g, 5 min), and the supernatant was added to glass tube containing working liquid for the cholesterol assay. Free cholesterol is oxidized by cholesterol oxidase to yield hydrogen peroxide that then reacts with Amplex Red reagent to produce fluorescent resorufin, which is measured in a fluorescence microplate reader at 550 nm (excitation) and 590 nm (emission).
4.2.6. Ternary complex modeling
Protein−protein docking were performed by Rosetta software suite (www.rosettacommons.org)50. RDKit, an open-source cheminformatics software, version 2030.03, was employed to generate conformers. To model the ternary structure for HMGCR, VHL and PROTAC, co-structures (HMGCR−simvastatin complex: 1HW9 and VHL−VH032 complex: 4W9H) were downloaded from PDB. Rosetta docking_protocol.mpi_linuxgccrelease program was used to generate 10,000 initial protein−protein interaction results which were then analyzed by Interface-Analyzer.mpi.linuxgccrelease. RDKit was used to generate 10,000 linker conformations with threshold value larger than 1.5. The code used in RDKi is shown in attached file: Linker conformation generator.py. Then, a custom python script was employed to predict ternary models through RMSD values. Finally, kinetics of selected ternary complex were evaluated by molecular dynamics simulation. Detailed computational methods and procedures are detailed in Supporting Information.
4.2.7. In vitro metabolic stability assay
The metabolic stability assay in mouse liver microsomes was conducted in Shanghai ChemPartner Co., Ltd. (Shanghai, China) with the approval from Animal Committee of Shanghai ChemPartner Co., Ltd. The assay incubation system contained microsomes (0.5 mg/mL, Corning), test compounds (1 μmol/L) and NADPH regeneration system (6 mmol/L) in phosphate buffer (1.0 mmol/L EDTA) at pH 7.4. Then 15 μL of NADPH stock solution (6 mmol/L) was added to the plates to start the reaction. At 5, 15, 30, and 45 min, 135 μL of ACN containing internal standard was added, respectively, to stop the reaction. The mixture was shaken on the vibrator (IKA, MTS 2/4) for 10 min (600 rpm) and then centrifuged at 5594×g for 15 min (Thermo Multifuge × 3R). Transfer 50 μL of the supernatant from each well into a 96-well sample plate containing 50 μL of ultra pure water (Millipore, ZMQS50F01) for LC−MS/MS analysis.
4.2.8. Animals
For pharmacokinetic studies, C57BL/6 mice were purchased from Hangzhou Subsource Experimental Animal Technology Co., Ltd. (SCXK: 2019-0004, Hangzhou, China). For hypercholesterolemia models, male C57BL/6 mice (20–24 g) were obtained from Nanjing Qinglongshan Animal Company (Nanjing, China). Mice were maintained under standard conditions with ad libitum access to water. In the study of hypercholesterolemia, C57BL/6 mice were fed with MFD (medium-fat containing 12% fat, 0.5% sodium cholate and 1.25% cholesterol) for 8 weeks. Mice were handled with the approval from Animal Committee of China Pharmaceutical University, Nanjing, China.
4.2.9. Pharmacokinetic studies
Compound 21b was dissolved in saline containing 0.5% CMC-Na and given orally at a single dose of 60 mg/kg (n = 5 per group, two groups), respectively; three animals received the vehicle (saline containing 5% CMC-Na). After administration, blood samples (50 μL/time) were collected via the lateral vein at different times (first group at 0.25, 0.5, 1, and 2 h; second group at 4, 8, 12, and 24 h). The blood samples were mixed with 20 μL internal standard and 600 μL MeOH containing 0.1% formic acid and centrifuged (12,000 rpm, 5 min). The supernatants (600 μL) were collected and dried under nitrogen (Organomation, HSC-24A) then dissolved in 50 μL 50% MeOH. After centrifugation, supernatants (5 μL) were collected for LC−MS analysis. The pump flow rate of HPLC (LC-30AD, Shimadzu) was 0.5 mL/min, and the compounds were separated on an Agilent Eclipse plus C18 (4.6 mm × 150 mm, 3.5 μm). MeOH (A) and 0.1% formic acid water (B) are gradient elutions: 0–1.5 min, 15%–5% A; 1.5–3 min, 5%–60% A; 3–5 min, 60% A; 5–8 min, 60%–5% A. MS (ESI) spectrometry (AB API4000) equipped with an electrospray ionization source was used for detection. Generic parameter set: ion-transfer capillary temperature 500 °C, capillary voltage 4.5 kV, dwell time 100 ms, collision gas 8 psi of argon, GS1 40 psi of argon, GS2 60 psi of argon, and CUR 20 psi. Standard cures for 21b and 21c are Y = 0.0035X + 0.0018 (R = 0.9986, LLOQ = 2.24 pg/mL) and Y = 0.0043X + 0.0064 (R = 0.9994, LLOQ = 1.28 pg/mL), respectively. Analytes were performed by using multiple-reaction monitoring (MRM) mode. Retention time for internal standard, 21b and 21c are 2.84, 1.95 and 1.83 min, respectively. Pharmacokinetic parameters were calculated by noncompartmental methods using Phoenix WinNonlin.
4.2.10. Analysis of hypercholesterolemia models
Randomly grouped mice (n = 6) fed with medium fat diet (MFD) were treated by gavage once daily with compound 21b (20 or 60 mg/kg) or 20 mg/kg lovastatin or combinations (20 mg/kg + 20 mg/kg) for 5 weeks. At 16 h post last gavage, blood (600 μL) was obtained by retro-orbital puncture, which was then centrifuged for 10 min (2000 rpm) to prepare serum for quantification of levels of serum TC, LDL-C and TG measured by automatic biochemical analyzer (C16000, Abbott). For liver collection, mice were sacrificed by cervical dislocation after blood collection. A small faction of livers was fixed with 10% formaldehyde saline for H&E staining (n = 3 per group) and Oil Red O. Meantime, another liver faction from randomly selected mice (n = 3 per group) were ground into nitrogen, then lysed for Western blot analysis as described detailed in the above section. The rest livers (n = 6 per group) were homogenized for the analysis of TC and TG levels. Survival, body weight and food intake were recorded weekly.
4.2.11. Statistical analysis
GraphPad Prism 7 was employed to perform all statistical analysis related to this work. Data are analyzed by one-way ANOVA multiple comparisons tests and expressed as the mean ± SD.
Acknowledgments
This work was supported by grants from Postdoctoral Research Foundation of China (2019M662007) and National Natural Science Foundation of China (81874286).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supporting information to this article can be found online at https://doi.org/10.1016/j.apsb.2020.11.001.
Contributor Information
Lizhe Zhu, Email: zhulizhe@cuhk.edu.cn.
Hua Xiang, Email: xianghua@cpu.edu.cn.
Author contributions
Hua Xiang and Guoshun Luo obtained the funding, designed the research and superintended the whole study. Guoshun Luo, Zhenbang Li and Xin Lin carried out the experiments and performed data analysis. Lizhe Zhu, Xinyu Li and Kun Xi designed and performed the molecular modeling. Yu Chen, Maoxu Xiao and Hanlin Wei participated part of the experiments. Guoshun Luo wrote the manuscript. Hua Xiang and Lizhe Zhu revised the manuscript. All the authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Virani S.S., Alonso A., Benjamin E.J., Benjamin E.J., Bittencourt M.S., Callaway C.W. Heart disease and stroke statistics-2020 update: a report from the american heart association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Leopold J.A., Loscalzo J. Emerging role of precision medicine in cardiovascular disease. Circ Res. 2018;122:1302–1315. doi: 10.1161/CIRCRESAHA.117.310782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein J.L., Brown M.S. A century of cholesterol and coronaries: from plaques to genes to statins. Cell. 2015;161:161–172. doi: 10.1016/j.cell.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein J.L., Brown M.S. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 5.Istvan E.S., Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2002;292:1160–1164. doi: 10.1126/science.1059344. [DOI] [PubMed] [Google Scholar]
- 6.Stancu C., Sima A. Statins: mechanism of action and effects. J Cell Mol Med. 2001;5:378–387. doi: 10.1111/j.1582-4934.2001.tb00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sirtori C.R. The pharmacology of statins. Pharmacol Res. 2014;88:3–11. doi: 10.1016/j.phrs.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Agouridis A.P., Elisaf M.S., Nair D.R., Mikhailidis D.P. All for statins and statins for all; an update. Curr Pharmaceut Des. 2016;22:18–27. doi: 10.2174/1381612822666151109111511. [DOI] [PubMed] [Google Scholar]
- 9.Alonso R., Cuevas A., Cafferata A. Diagnosis and management of statin intolerance. J Atherosclerosis Thromb. 2019;26:207–215. doi: 10.5551/jat.RV17030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gislason G.H., Rasmussen J.N., Abildstrøm S.Z., Gadsbøll N., Buch P., Friberg J. Long-term compliance with beta-blockers, angiotensin-converting enzyme inhibitors, and statins after acute myocardial infarction. Eur Heart J. 2006;27:1153–1158. doi: 10.1093/eurheartj/ehi705. [DOI] [PubMed] [Google Scholar]
- 11.Ellis J.J., Erickson S.R., Stevenson J.G., Bernstein S.J., Stiles R.A., Fendrick A.M. Suboptimal statin adherence and discontinuation in primary and secondary prevention populations. J Gen Intern Med. 2004;19:638–645. doi: 10.1111/j.1525-1497.2004.30516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blackburn D.F., Dobson R.T., Blackburn J.L., Wilson T.W., Stang M.R., Semchuk W. Adherence to statins, beta-blockers and angiotensin-converting enzyme inhibitors following a first cardiovascular event: a retrospective cohort study. Can J Cardiol. 2005;21:485–488. [PubMed] [Google Scholar]
- 13.Schonewille M., de Boer J.F., Mele L., Wolters H., Bloks V.W., Wolters J.C. Statins increase hepatic cholesterol synthesis and stimulate fecal cholesterol elimination in mice. J Lipid Res. 2016;57:1455–1464. doi: 10.1194/jlr.M067488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ness G.C., Chambers C.M., Lopez D. Atorvastatin action involves diminished recovery of hepatic HMG-CoA reductase activity. J Lipid Res. 1988;39:75–84. [PubMed] [Google Scholar]
- 15.Burnett J.R., Wilcox L.J., Telford D.E., Kleinstiver S.J., Barrett P.H., Newton R.S. The magnitude of decrease in hepatic very low density lipoprotein apolipoprotein B secretion is determined by the extent of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibition in miniature pigs. Endocrinology. 1999;140:5293–5302. doi: 10.1210/endo.140.11.7150. [DOI] [PubMed] [Google Scholar]
- 16.Reihner E., Rudling M., Stahlberg D., Berglund L., Ewerth S., Bjorkhem I. Influence of pravastatin, a specific inhibitor of HMG-CoA reductase, on hepatic metabolism of cholesterol. N Engl J Med. 1990;323:224–228. doi: 10.1056/NEJM199007263230403. [DOI] [PubMed] [Google Scholar]
- 17.Armitage J. The safety of statins in clinical practice. Lancet. 2007;370:1781–1790. doi: 10.1016/S0140-6736(07)60716-8. [DOI] [PubMed] [Google Scholar]
- 18.Rosenson R.S., Baker S.K., Jacobson T.A., Kopecky S.L., Parker B.A. An assessment by the statin muscle safety task force: 2014 update. J Clin Lipidol. 2014;8:S58–S71. doi: 10.1016/j.jacl.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Brown M.S., Faust J.R., Goldstein J.L., Kaneko I., Endo A. Induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts incubated with compactin (ML-236B), a competitive inhibitor of the reductase. J Biol Chem. 1978;253:1121–1128. [PubMed] [Google Scholar]
- 20.Luo J., Yang H.Y., Song B.L. Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol. 2020;21:225–245. doi: 10.1038/s41580-019-0190-7. [DOI] [PubMed] [Google Scholar]
- 21.Brown M.S., Goldstein J.L. Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J Lipid Res. 1980;21:505–517. [PubMed] [Google Scholar]
- 22.DeBose-Boyd R.A. Feedback regulation of cholesterol synthesis: sterol-accelerated ubiquitination and degradation of HMG CoA reductase. Cell Res. 2008;18:609–621. doi: 10.1038/cr.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song B.L., DeBose-Boyd R.A. Ubiquitination of 3-hydroxy-3-methylglutaryl-CoA reductase in permeabilized cells mediated by cytosolic E1 and a putative membrane bound ubiquitin ligase. J Biol Chem. 2004;279:28798–28806. doi: 10.1074/jbc.M402442200. [DOI] [PubMed] [Google Scholar]
- 24.Chen L., Ma M.Y., Sun M., Jiang L.Y., Zhao X.T., Fang X.X. Endogenous sterol intermediates of the mevalonate pathway regulate HMGCR degradation and SREBP-2 processing. J Lipid Res. 2019;60:1765–1775. doi: 10.1194/jlr.RA119000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song B.L., Javitt N.B., DeBose-Boyd R.A. Insig-mediated degradation of HMG CoA reductase stimulated by lanosterol, an intermediate in the synthesis of cholesterol. Cell Metabol. 2005;1:179–189. doi: 10.1016/j.cmet.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Johnson B.M., DeBose-Boyd R.A. Underlying mechanisms for sterol-induced ubiquitination and ER-associated degradation of HMG CoA reductase. Semin Cell Dev Biol. 2018;81:121–128. doi: 10.1016/j.semcdb.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang S.Y., Li H., Tang J.J., Wang J., Luo J., Liu B. Discovery of a potent HMG-CoA reductase degrader that eliminates statin-induced reductase accumulation and lowers cholesterol. Nat Commun. 2018;9:5138. doi: 10.1038/s41467-018-07590-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pettersson M., Crews C.M. Proteolysis targeting chimeras (PROTACs)—past, present and future. Drug Discov Today Technol. 2019;31:15–27. doi: 10.1016/j.ddtec.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neklesa T.K., Winkler J.D., Crews C.M. Targeted protein degradation by PROTACs. Pharmacol Ther. 2017;174:138–144. doi: 10.1016/j.pharmthera.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 30.Toure M., Crews C.M. Small-molecule PROTACS: new approaches to protein degradation. Angew Chem Int Ed Engl. 2016;55:1966–1973. doi: 10.1002/anie.201507978. [DOI] [PubMed] [Google Scholar]
- 31.Konstantinidou M., Li J.Y., Zhang B.D., Wang Z.F., Shaabani S., Brake T.F. PROTACs—a game-changing technology. Expet Opin Drug Discov. 2019;14:1255–1268. doi: 10.1080/17460441.2019.1659242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun X., Gao H.Y., Yang Y.Q., He M., Wu Y., Song Y.G. PROTACs: great opportunities for academia and industry. Signal Transduct Target Ther. 2019;4:64. doi: 10.1038/s41392-019-0101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burslem G.M., Crews C.M. Proteolysis-targeting chimeras as therapeutics and tools for biological discovery. Cell. 2020;181:102–114. doi: 10.1016/j.cell.2019.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao H., Sun X., Rao Y. PROTAC technology: opportunities and challenges. ACS Med Chem Lett. 2020;11:237–240. doi: 10.1021/acsmedchemlett.9b00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu J., Hu B., Wang M., Xu F., Miao B., Yang C.Y. Discovery of ERD-308 as a highly potent proteolysis targeting chimera (PROTAC) degrader of estrogen receptor (ER) J Med Chem. 2019;62:1420–1442. doi: 10.1021/acs.jmedchem.8b01572. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y., Loh C., Chen J., Mainolfi N. Targeted protein degradation mechanisms. Drug Discov Today Technol. 2019;31:53–60. doi: 10.1016/j.ddtec.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Li X., Song Y. Proteolysis-targeting chimera (PROTAC) for targeted protein degradation and cancer therapy. J Hematol Oncol. 2020;13:50. doi: 10.1186/s13045-020-00885-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qian M., Yan F., Yuan T., Yang B., He Q., Zhu H. Targeting post-translational modification of transcription factors as cancer therapy. Drug Discov Today. 2020;25:1502–1512. doi: 10.1016/j.drudis.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Khan S., He Y., Zhang X., Yuan Y.X., Pu S.Y., Kong Q.P. Proteolysis targeting chimeras (PROTACs) as emerging anticancer therapeutics. Oncogene. 2020;39:4909–4924. doi: 10.1038/s41388-020-1336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu J., Ma J., Liu Y., Xia J., Li Y.Y., Wang Z.P. PROTACs: a novel strategy for cancer therapy. Semin Canc Biol. 2020;67:171–179. doi: 10.1016/j.semcancer.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Winzker M., Friese A., Koch U., Janning P., Ziegler S., Waldmann H. Development of a PDEδ-targeting PROTACs that impair lipid metabolism. Angew Chem Int Ed Engl. 2020;59:5595–5601. doi: 10.1002/anie.201913904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng J.F., Li Y., Wang X., Dong G.Q., Sheng C.Q. Discovery of novel PDEδ degraders for the treatment of KRAS mutant colorectal cancer. J Med Chem. 2020;63:7892–7905. doi: 10.1021/acs.jmedchem.0c00929. [DOI] [PubMed] [Google Scholar]
- 43.Cantrill C., Chaturvedi P., Rynn C., Petrig Schaffland J., Walter I., Wittwer M.B. Fundamental aspects of DMPK optimization of targeted protein degraders. Drug Discov Today. 2020;25:969–982. doi: 10.1016/j.drudis.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 44.Pike A., Williamson B., Harlfinger S., Martin S., McGinnity D.F. Optimising proteolysis-targeting chimeras (PROTACs) for oral drug delivery: a drug metabolism and pharmacokinetics perspective. Drug Discov Today. 2020;25:1793–1800. doi: 10.1016/j.drudis.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 45.Churcher I. Protac-induced protein degradation in drug discovery: breaking the rules or just making new ones? J Med Chem. 2018;61:444–452. doi: 10.1021/acs.jmedchem.7b01272. [DOI] [PubMed] [Google Scholar]
- 46.Li M.X., Yang Y.Y., Zhao Q.Y., Wu Y., Song L., Yang H.Y. Degradation versus inhibition: development of proteolysis-targeting chimeras for overcoming statin-induced compensatory upregulation of 3-Hydroxy-3-methylglutaryl coenzyme A reductase. J Med Chem. 2020;63:4908–4928. doi: 10.1021/acs.jmedchem.0c00339. [DOI] [PubMed] [Google Scholar]
- 47.Chen J.B., Chern T.R., Wei T.T., Chen C.C., Lin J.H., Fang J.M. Design and synthesis of dual-action inhibitors targeting histone deacetylases and 3-hydroxy-3-methylglutaryl coenzyme A reductase for cancer treatment. J Med Chem. 2013;56:3645–3655. doi: 10.1021/jm400179b. [DOI] [PubMed] [Google Scholar]
- 48.Li Y.B., Yang J.J., Aguilar A., McEachern D., Przybranowski S., Liu L. Discovery of MD-224 as a first-in-class, highly potent, and efficacious proteolysis targeting chimera murine double minute 2 degrader capable of achieving complete and durable tumor regression. J Med Chem. 2019;62:448–466. doi: 10.1021/acs.jmedchem.8b00909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng L.J., Zhang Z.S., Lei C., Li S., Zhang Z., Ren X.M. Identification of new small-molecule inducers of estrogen-related receptor α (ERRα) degradation. ACS Med Chem Lett. 2019;10:767–772. doi: 10.1021/acsmedchemlett.9b00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bai N., Kirubakaran P., Karanicolas J. Rationalizing PROTAC-mediated ternary complex formation using Rosetta. bioRxiv. 2020 doi: 10.1016/j.semcancer.2020.02.006. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drummond M.L., Williams C.I. InSilico modeling of PROTAC-mediated ternary complexes: validation and application. J Chem Inf Model. 2019;59:1634–1644. doi: 10.1021/acs.jcim.8b00872. [DOI] [PubMed] [Google Scholar]
- 52.Sircar A., Chaudhury S., Kilambi K.P., Berrondo M., Gray J.J. A generalized approach to sampling backbone conformations with RosettaDock for CAPRI rounds 13-19. Proteins. 2010;78:3115–3123. doi: 10.1002/prot.22765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feng D., Ge C., Tan Z.Y., Sun J.G., Xie Y. Isoflavones enhance pharmacokinetic exposure of active lovastatin acid via the upregulation of carboxylesterase in high-fat diet mice after oral administration of Xuezhikang capsules. Acta Pharmacol Sin. 2018;39:1804–1815. doi: 10.1038/s41401-018-0039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y., Jiang X., Feng F., Liu W., Sun H. Degradation of proteins by PROTACs and other strategies. Acta Pharm Sin B. 2020;10:207–238. doi: 10.1016/j.apsb.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tomoshige S., Ishikawa M. Recent progress in PROTACs and other chemical protein degradation technologies for the treatment of neurodegenerative disorders. Angew Chem Int Ed Engl. 2021;60:3346–3356. doi: 10.1002/anie.202004746. [DOI] [PubMed] [Google Scholar]
- 56.Lin X., Xiang H., Luo G.S. Targeting estrogen receptor α for degradation with PROTACs: a promising approach to overcome endocrine resistance. Eur J Med Chem. 2020;206:112689. doi: 10.1016/j.ejmech.2020.112689. [DOI] [PubMed] [Google Scholar]
- 57.Han X., Zhao L., Xiang W., Qin C., Miao B., Xu T. Discovery of highly potent and efficient PROTAC degraders of androgen receptor (AR) by employing weak binding affinity VHL E3 ligase ligands. J Med Chem. 2019;62:11218–11231. doi: 10.1021/acs.jmedchem.9b01393. [DOI] [PubMed] [Google Scholar]
- 58.Sun Y., Zhao X., Ding N., Gao H., Wu Y., Yang Y. PROTAC-induced BTK degradation as a novel therapy for mutated BTK C481S induced ibrutinib-resistant B-cell malignancies. Cell Res. 2018;28:779–781. doi: 10.1038/s41422-018-0055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Su S., Yang Z., Gao H., Yang H., Zhu S., An Z. Potent and preferential degradation of CDK6 via proteolysis targeting chimera degraders. J Med Chem. 2019;62:7575–7582. doi: 10.1021/acs.jmedchem.9b00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crew A.P., Raina K., Dong H., Qian Y., Wang J., Vigil D. Identification and characterization of von Hippel-Lindau-recruiting proteolysis targeting chimeras (PROTACs) of TANK-binding kinase 1. J Med Chem. 2018;61:583–598. doi: 10.1021/acs.jmedchem.7b00635. [DOI] [PubMed] [Google Scholar]
- 61.Qin C., Hu Y., Zhou B., Fernandez-Salas E., Yang C.Y., Liu L. Discovery of QCA570 as an exceptionally potent and efficacious proteolysis targeting chimera (PROTAC) degrader of the bromodomain and extra-terminal (BET) proteins capable of inducing complete and durable tumor regression. J Med Chem. 2018;61:6685–6704. doi: 10.1021/acs.jmedchem.8b00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang H., Lv W., He M., Deng H., Li H., Wu W. Plasticity in designing PROTACs for selective and potent degradation of HDAC6. Chem Commun. 2019;55:14848–14851. doi: 10.1039/c9cc08509b. [DOI] [PubMed] [Google Scholar]
- 63.Bai L., Zhou B., Yang C.Y., Ji J., McEachern D., Przybranowski S. Targeted degradation of BET proteins in triple-negative breast cancer. Canc Res. 2017;77:2476. doi: 10.1158/0008-5472.CAN-16-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou H., Bai L., Xu R., Zhao Y., Chen J., McEachern D. Structure-based discovery of SD-36 as a potent, selective, and efficacious PROTAC degrader of STAT3 protein. J Med Chem. 2019;62:11280–11300. doi: 10.1021/acs.jmedchem.9b01530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.