Abstract
Background:
The American Diabetes Association (ADA) recommends sodium-glucose cotransporter-2 (SGLT2) inhibitors as the second medication to be started, after metformin, for patients with chronic kidney disease (CKD). Sodium-glucose cotransporter-2 inhibitors may cause volume, blood pressure, and electrolyte disturbances; consequently, frequent monitoring and adjustments to other diabetes, blood pressure, and/or diuretic medications may be necessary.
Objective:
To evaluate the safety and efficacy of an interprofessional clinic model partnering nephrologists and pharmacists for the initiation and monitoring of SGLT2 inhibitors.
Methods:
A clinical pharmacist was embedded within the nephrology clinic to provide patient education, telephone follow-up, and to work collaboratively with the nephrologists. Diabetes, hypertension, and diuretic regimens were adjusted as needed after empagliflozin initiation. Diabetes regimens were adjusted to adhere to the 2019 ADA guidelines that promote agents with CKD and atherosclerotic cardiovascular disease benefit.
Results:
Fourteen patients were initiated on empagliflozin during the study period. Urine albumin-to-creatinine ratio (UACR) improved (mean % change −12% ± 61%); the mean percentage change was greater in patients with a higher baseline UACR. The mean change in hemoglobin A1c was 0.3% ± 0.6%. Common adverse reactions were observed and improved over time; no serious adverse drug reactions occurred. Finally, empagliflozin initiation necessitated adjustments to diabetes, hypertension, and diuretic regimens in almost all patients (n = 13, 93%).
Conclusion:
The implementation of an innovative, interprofessional care model within a nephrology clinic for the initiation and monitoring of empagliflozin in patients with DKD demonstrated clinical benefit with minimal safety concerns.
Keywords: chronic kidney disease, SGLT-2 inhibitors, collaborative care, pharmacist, nephrology
Introduction
In developed countries, diabetic kidney disease (DKD) is the primary cause of end-stage renal disease (ESRD), accounting for 50% of cases.1,2 DKD is characterized by elevated urinary albumin excretion in the presence or absence of a decreased estimated glomerular filtration rate (eGFR).3 Proteinuric DKD is associated with rapid eGFR decline and progression to ESRD.4,5 The most common cause of death in patients with chronic kidney disease (CKD) is cardiovascular disease, and the proportion of cardiovascular deaths increases with declining eGFR.6 As the incidence of ESRD in the United States is projected to rise by 18% in the next 15 years, there have been calls for interventions focused on preventing the progression of CKD to ESRD.7 Glycemic control is one of the key factors in the prevention of DKD progression, and a novel class of antidiabetic medications, sodium-glucose cotransporter-2 (SGLT2) inhibitors, has emerged as a preferred treatment option in patients with comorbid diabetes and CKD.8 SGLT2 inhibitors lower blood glucose by reducing glucose and sodium reabsorption; they provide renoprotective effects by reducing glomerular hyperfiltration.1 In clinical trials, SGLT2 inhibitors decreased the incidence and progression of albuminuria, slowed eGFR decline, and reduced the need for renal replacement therapy in the DKD population.9–11 SGLT2 inhibitors also significantly decreased the risk of all-cause mortality in participants having DKD with an eGFR as low as 30 mL/min/1.73 m2.9–17
SGLT2 inhibitors are now recommended as the second medication to be started, after metformin, for diabetes management in patients with CKD, atherosclerotic cardiovascular disease (ASCVD), or heart failure (HF).2,18–20 However, current guidelines and studies do not provide practical guidance for implementation in the real-world setting. A recent population-based study noted that patients seen by nephrologists are the most complex among different specialties21; in the real-world setting, patients with DKD have multiple comorbidities and a high medication burden.22,23 SGLT2 inhibitors can cause volume, blood pressure, and electrolyte disturbances beyond their glycemic effects, and after initiation patients may require adjustments to their diabetes, blood pressure, and/or diuretic regimens.1,9,12,24–27 Appropriate patient education and close monitoring for adverse effects are extremely important,28 and yet the current literature lacks guidance to support an implementation strategy of SGLT2 inhibitors, specifically the resources for patient education, management of other medications, and patient monitoring in the real-world setting.29–31
To align patient care with current guidelines,3,18,32 the outpatient nephrology clinic at the VA Boston Medical Center created an interprofessional clinic model utilizing nephrologists and pharmacists for initiation and monitoring of SGLT2 inhibitors in patients with DKD. Our aim was to assess the clinical, safety, and feasibility outcomes of our care model during the 3-month initiation and 3-month monitoring period (6 months total) of empagliflozin, our formulary SGLT2 inhibitor, in patients with DKD in a real-world setting.
Our rationale for the development of this collaborative clinic stems from evidence demonstrating that collaborative practice interventions utilizing pharmacists promotes patient education and closer monitoring, leading to improved clinical and health system outcomes in both diabetes and CKD.33–39 Within our model, the nephrology providers are poised to manage changes in volume status or blood pressure, electrolyte derangements (ie, hyperkalemia), and metabolic syndromes that may occur within the first 3 months of empagliflozin initiation while clinical pharmacists are well positioned to conduct collaborative diabetes management including medication adjustments, monitoring, and patient education (Figure 1). Our clinical model was approved by the internal Research and Development Committee at the VA as a quality improvement initiative and was exempt from further Institutional Review Board oversight.
Figure 1.
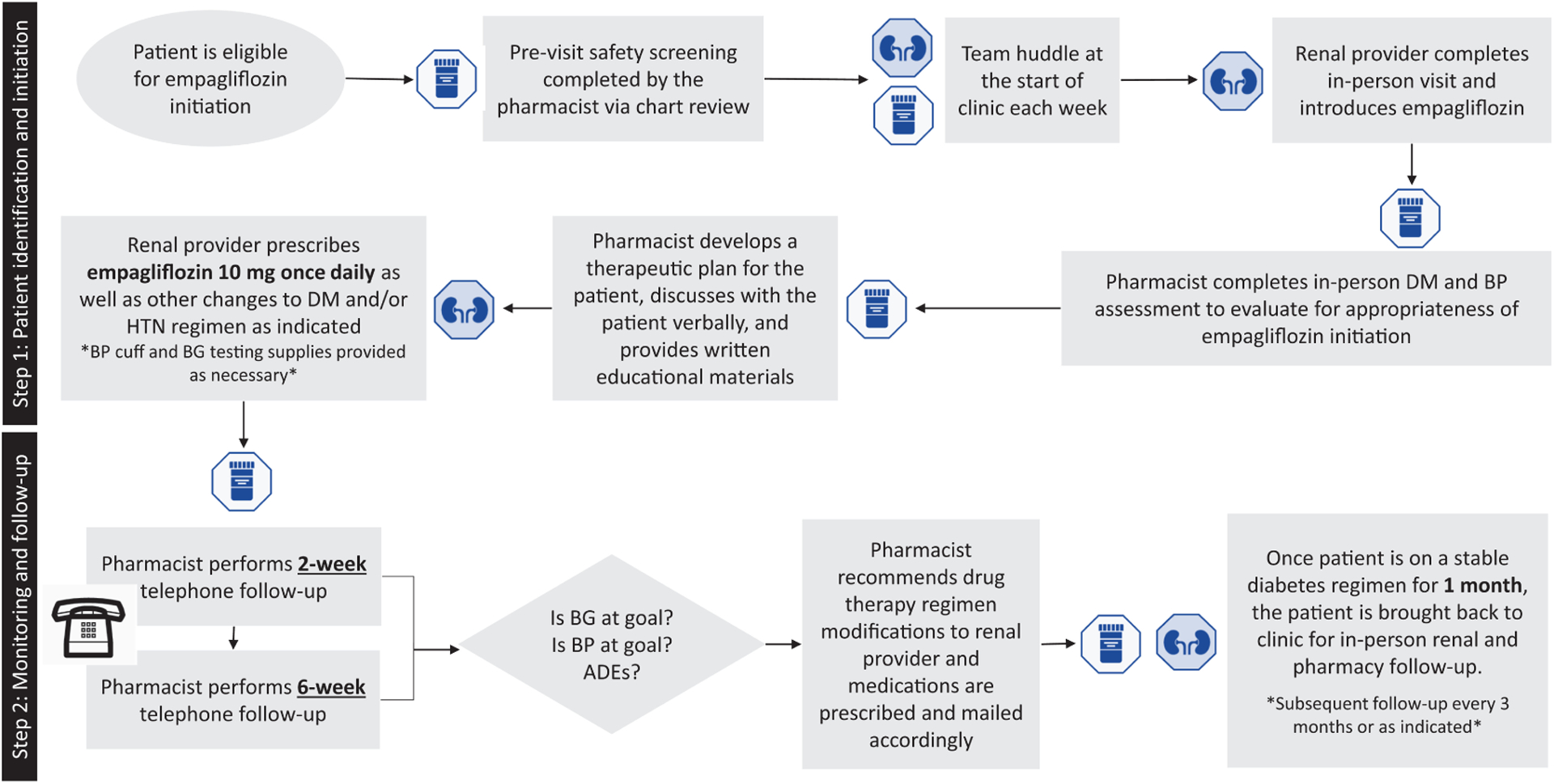
Process flow diagram of interprofessional model for the initiation and monitoring of empagliflozin within an interdisciplinary nephrology clinic. ADEs indicates adverse drug events; BP, blood pressure; BG, blood glucose; DM, diabetes mellitus; HTN, hypertension.
Methods
Patients
Patients were targeted for inclusion if they had a history of type 2 diabetes mellitus (T2DM), urine albumin-to-creatinine ratio (UACR) >300 mg/g at least once within the past 12 months prior to screening, and an eGFR >35 mL/min/1.73 m2 at the time of visit (Figure 2). The inclusion criteria intended to select complex patients at high risk for kidney disease progression. The UACR cutoff was chosen based on criteria in published trials that featured participants with an eGFR as low as 30 mL/min/1.73 m2 and demonstrated the greatest benefit in proteinuria reduction and eGFR preservation among participants with UACR >300 mg/g.9–12 After candidates were identified, the pharmacist completed an additional previsit screening via review of the electronic medical record (EMR) to select patients who were likely to adhere to instructions and have low risk for common side effects of SGLT2 inhibitors (Supplemental Table 1).
Figure 2.
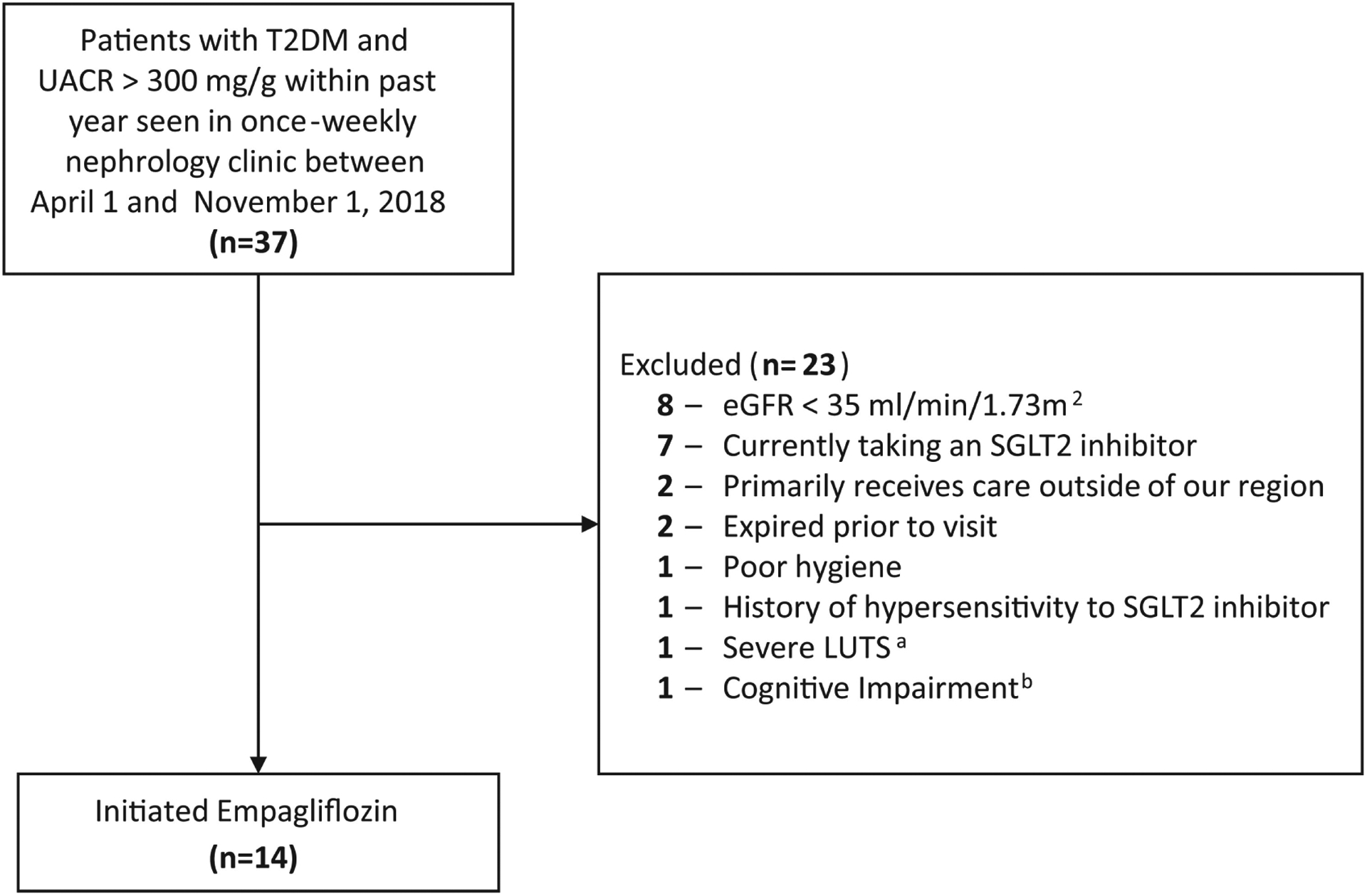
Participant enrollment.
Abbreviations: T2DM, type 2 diabetes mellitus; UACR, urine albumin to creatinine ratio; eGFR, estimated glomerular filtration rate; SGLT2i, sodium-glucose cotransporter-2 inhibitor; LUTS, lower urinary tract symptoms.
aThis patient had severe LUTS indicated by an International Prostate Symptom Score (I-PSS) of 20 out of 35.
bThis patient had a Montreal Cognitive Assessment score of 22 out of 30 indicating mild cognitive impairment.
Study Design and Clinic Workflow
Prior to each clinic session, the interprofessional team conducted a huddle where pharmacists and nephrology providers discussed eligible empagliflozin candidates with scheduled clinic visits that day. A candidate patient was seen initially by the nephrology provider who introduced the patient to the potential benefits and risks of empagliflozin initiation. If the patient was interested in initiating empagliflozin, the patient subsequently had an in-person visit with the pharmacist in the same clinic space. The pharmacist completed diabetes and blood pressure assessments as well as a patient interview, with a guided note template, to collect the following key information:
current diabetes and blood pressure medication regimen;
home monitoring of blood sugar and blood pressure;
hypoglycemic events in the last year and hypoglycemic management; and
medication management (ie, adherence, assistance with medications, etc)
The pharmacist then provided the patient with a written clinical plan outlining empagliflozin use and a description of the mechanism of action in relation to side effects and monitoring parameters. The written clinical plan also noted any changes made to the patient’s current diabetes, hypertension, or diuretic regimens; the date of the patient’s next in-person nephrology clinic visit; and the date and time of the pharmacist’s first follow-up phone call with the patient. The written clinical plan was reviewed verbally and given to the patient, and the patient was provided the opportunity to ask questions. Finally, the pharmacist documented the clinical assessment and plan in a templated note in the EMR. The nephrology provider prescribed empagliflozin 10 mg by mouth once daily and any additional prescriptions or monitoring devices recommended during the visit. The patient’s primary care provider and diabetes management team were notified via secure e-mail that the patient had been initiated on empagliflozin and would receive intensive pharmacist follow-up for a 6-month period. The e-mail included the plan for pharmacist phone follow-up and contact information for the pharmacist and attending nephrologist for any questions or concerns that might arise.
Two weeks after initiation, the clinical pharmacist completed a telephone follow-up assessment to monitor the efficacy and safety of empagliflozin and documented the assessment in the EMR. If the patient was tolerating the regimen and blood glucose and blood pressure readings were appropriate, no changes were made. If the clinical pharmacist determined that adjustments were required, these recommendations were discussed with the nephrology provider; the nephrology provider then placed prescription orders for any diabetes, hypertension, or diuretic medications as necessary. The pharmacist coordinated the mailing of prescriptions as well as a new written clinical plan each time medication changes were made. A second telephone follow-up adhering to the same process was performed by the pharmacist at 6 weeks. The pharmacist performed telephone follow-up every 2 to 4 weeks thereafter as necessary based on clinical judgement. Once the patient was on a stable diabetes regimen for at least 1 month without adverse events (ie, hypoglycemia, hypotension, polyuria), the pharmacist discontinued telephone follow-up calls. Instead, the pharmacist conducted in-clinic follow-up as needed during scheduled nephrology clinic visits which occurred every 3 to 6 months. Additional in-clinic visits, earlier than 3 months, were arranged if needed. In-clinic follow-up consisted of a visit with the nephrology provider for assessment of kidney disease, laboratory monitoring, and a visit with the pharmacist for medication reconciliation as well as diabetes management. We collected data for every patient over at least 6 months; however, some patients may have required intensive pharmacist follow-up beyond 6 months due to further required changes to their diabetes regimens (Supplemental Table 1).
Outcomes
The primary outcome was efficacy of empagliflozin and included changes to renal parameters such as UACR and eGFR and changes to the hemoglobin A1c (HbA1c). Secondary outcomes included medication changes to diabetes, hypertension and/or diuretic regimens, safety, and feasibility. Safety outcomes included the incidence of hypoglycemia, hypotension, polyuria, genitourinary infection, acute renal failure (ARF), bone fractures, amputations, ketoacidosis, and/or ketonuria. Hypoglycemia was defined as a blood glucose level <70 mg/dL or symptoms of hypoglycemia requiring self-treatment per patient self-report. Hypotension was defined as a blood pressure <90/60 mm Hg or symptoms of hypotension at baseline which included incidence within the past month per patient self-report. The definition of ARF was based on the Standardized MedDRA Queries or an increase in serum creatinine greater than or equal to 0.5 mg/dL and an increase in blood urea nitrogen greater than or equal to 10 mg/dL.9 Laboratory monitoring was completed at the time of empagliflozin initiation, at 3 months, and at 6 months (±1 month) after empagliflozin initiation. Feasibility was determined by time spent by the pharmacist during initial and follow-up visits as well as the number of patients who were on a stable regimen at 6 months post empagliflozin initiation. All data were collected through chart review of clinical notes, VA prescription refill data, and laboratory results. A Fisher’s exact test was performed to compare the incidence of adverse effects at 3 and 6 months in those with an eGFR <45 mL/min/1.73 m2 compared to ≥45 mL/min/1.73 m2.
Results
Patients
Fourteen patients met the inclusion criteria and were initiated on empagliflozin (Table 1). Patients had a heavy comorbidity burden, polypharmacy,40 and received outpatient medications from multiple prescribers.
Table 1.
Baseline Characteristics.a
| Age, mean years ± SD | 67 ± 8 |
| Male, n (%) | 14 (100) |
| Number of medications, mean ± SD | 17 ± 6 |
| Number of outpatient prescribers in the last year, mean ± SD | 14 ± 6 |
| Incidence of hyperkalemiab | 8 (57) |
| Comorbid conditions, n (%) | |
| Hypertension | 14 (100) |
| Dyslipidemia | 14 (100) |
| Clinical ASCVD | 8 (57) |
| 10-year ASCVD risk score > 10%c | 6 (43) |
| Obese (body mass index > 30 kg/m2) | 7 (50) |
| Atrial fibrillation | 4 (29) |
| Heart failure | 2 (14) |
| Patients on a diabetes medication, n (%) | 13 (93) |
| Insulin, basal only | 5 (36) |
| Insulin, basal-bolus regimen | 4 (29) |
| Metformin | 8 (57) |
| Sulfonylurea | 4 (29) |
| DPP-4 inhibitor | 1 (7) |
| GLP-I receptor agonist | 1 (7) |
| Patients on an antihypertensive medication, n (%) | 14 (100) |
| Beta blocker | 11 (79) |
| ACE inhibitor/ARB | 9 (64) |
| Calcium channel blocker | 8 (57) |
| Direct vasodilator (hydralazine) | 1 (7) |
| Alpha blocker | 1 (7) |
| Patients on a diuretic medication, n (%) | 6 (43) |
| Loop diuretics | 3 (21) |
| Thiazide diuretics | 3 (21) |
ACE, Angiotensin converting enzyme inhibitor; ARB, Angiotensin receptor blocker; ASCVD, atherosclerotic cardiovascular disease; DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1; SD, standard deviation.
n = 14.
Defined as a serum potassium >5.5 mEq/L, baseline included incidence within the past 5 years.
Renal Parameters
At 6 months, the mean UACR improved on average from 1838 mg/g to 1138 mg/g (mean%change −12% ± 61%). Almost half of the included patients (n = 6, 43%) achieved greater than 30% reduction in UACR at both 3 and 6 months after empagliflozin initiation. The mean % change was greater in patients with a higher baseline UACR. The eGFR at 6 months declined slightly in half of the patients (n = 7, 50%) by an average of −2 ± 8mL/min/1.73 m2 at 3 months and an average of −0 ± 12 mL/min/1.73 m2 at 6 months. Three patients had an eGFR decrease to below 35 mL/min/1.73 m2 within the initial 3-month monitoring period; however, the eGFRreturned to above 35mL/min/1.73m2 in all 3 patients following adjustments to other implicated medications (ie, diuretics or other antihypertensives; Table 2).
Table 2.
Results: Changes From Baseline After 3 and 6 Months of Empagliflozin Therapy.a
| Baseline | 3 Months | Mean % Change ± SD, Baseline to 3 Months | 6 Months | Mean % Change ± SD, Baseline to 6 Months | |
|---|---|---|---|---|---|
| Renal Results: UACR | |||||
| UACR, mg/g, n (%) | |||||
| <300b | 3 (21) | 4 (29) | −11 ± 24 | 5 (36) | −23 ± 43 |
| 300–999 | 4 (29) | 5 (36) | −6 ± 42 | 3 (21) | −4 ± 85 |
| 1000–3499 | 5 (36) | 4 (29) | −4 ± 88 | 6 (43) | +3 ± 52c |
| >3500 | 2 (14) | 1 (7) | −43 ± 20 | 0 (0) | - |
| Overall mean UACR, ± SD | 1838 ± 2013 | 1270 ± 1236 | −12 ± 60 | 1138 ± 1023 | −12 ± 61 |
| Achieved reduction > 30%, n (%) | - | 6 (43) | - | 6 (43) | - |
| Achieved reduction > 50%, n (%) | - | 4 (29) | - | 5 (36) | - |
| Renal Results: eGFR | |||||
| eGFR, mL/min/l.73 m2, n (%) | |||||
| >60 | 4 (28.5) | 3 (21) | −5 ± 14 | 2 (14) | +13 ± 9 |
| 45–60 | 4 (28.5) | 5 (36) | −3 ± 4 | 7 (50) | −1 ± 14 |
| 35–44 | 6 (43) | 6 (43) | +0 ± 5.0 | 5 (36) | −3 ± 3 |
| Mean eGFR, mL/min/1.73 m2, ± SD | 56 ± 21 | 54 ± 22 | −2 ± 8 | 55 ± 25 | +0 ± 12 |
| Glycemic results | |||||
| Hemoglobin A1c (%), n (%) | |||||
| < 7.0 | 9 (65) | 8 (57) | +0.2 ± 0.4 | 5 (36) | +0.2 ± 0.4 |
| 7.1–8.0 | 3 (21) | 4 (29) | −0.2 ± 0.3 | 6 (43) | +0.4 ± 0.6 |
| 8.1–9.1 | 2 (14) | 2 (14) | −0.1 ± 0.1 | 3 (21) | +0.5 ± 0.7 |
| Mean hemoglobin A1c (%), ± SD | 7.0 ± 0.8 | 7.1 ± 0.7 | +0.1 ± 0.4 | 7.4 ± 0.9 | +0.3 ± 0.6 |
| At or below hemoglobin A1c goal, n (%)d | 11 (79) | 11 (79) | - | 8 (57) | - |
| Average weight, kg, mean ± SD | 95.7 ± 15.6 | 89.9 ± 14.1 | −2.7 ± 2.2 | 92.1 ± 16.1 | −3.6 ± 4.5 |
| Adverse Events | Baseline | 3 Months | 6 Months | ||
| History of hypoglycemia, n (%)e | 3 (21) | 5 (36) | - | 4 (29) | - |
| Incidence of hypotension, n (%)f | 5 (33) | 6 (43) | - | 2 (14) | - |
| Incidence of polyuria, n (%) | 2 (14) | 7 (50) | - | 4 (29) | - |
| Incidence of acute renal failure, n (%)g | 2 (14) | 2 (14) | - | 1 (7) | - |
Abbreviations: UACR, urine albumin-to-creatinine ratio; eGFR, estimated glomerular filtration rate; ACE inhibitor, Angiotensin converting enzyme inhibitor; ARB, Angiotensin receptor blocker; SD, standard deviation.
n = 14.
For study inclusion, patients were required to have a UACR >300 mg/g in the past year; however, some patients may have subsequently updated laboratory test results and therefore had a UACR <300 at baseline due to other agents that lower UACR (ie, ACE inhibitors or ARBs).
One patient experienced an acute kidney injury at 6 months due to over-diuresis (+103% increase in UACR), thus skewing the data.
Per self-report, a blood glucose <70 mg/dL or symptoms of hypoglycemia requiring self-treatment, baseline included incidence within the past year.
Per self-report, a blood pressure <90/60 mm Hg or symptoms of hypotension, baseline included incidence within the past month.
As defined by the KDIGO Guidelines,36 baseline included incidence within the past year.
Glycemic Control
After 3 months of empagliflozin therapy, the same number of patients had an HbA1c at goal compared to baseline2,18 (n = 11, 79%), with an overall mean change in HbA1c of +0.1% ± 0.4%. However, at 6 months, less patients had an HbA1c at or below goal (n = 8, 57%). Patients reported an average weight loss of 3.6 kg (range: +7 to −12 kg) over 6 months (Table 2).
Medication Changes
Post empagliflozin initiation, almost all patients required adjustments to their diabetes, hypertension, or diuretic regimens (n = 13, 93%) over the 6-month period. Multiple adjustments took place over the course of the 6 months including uptitration and downtitration of various medications. There were 9 changes to noninsulin diabetes medications. All patients on insulin at baseline required dose adjustments; the total daily dose of insulin was reduced on average by 35% at 6 months. Diabetic medication changes were predominantly deprescribing of agents with high hypoglycemia risk.2,18 There were 9 changes to antihypertensive agents and 4 changes to diuretics over 6 months. After 3 months of empagliflozin therapy, patiromer, a medication to decrease serum potassium, was discontinued in 2 of 2 patients who were taking the medication at baseline, and they were able to remain off of this medication at 6 months (Supplemental Table 2).
Adverse Drug Reactions
At 3 months, 5 (36%) patients reported hypoglycemia, 6 (43%) reported hypotension, and 7 (50%) reported polyuria. The incidence of all 3 decreased at 6 months to 4 (29%), 2 (14%), and 4 (29%). ARF occurred in 2 (14%) patients within 3 months. The first case of ARF occurred in a patient with an eGFR greater than 60 mL/min/1.73 m2 in the setting of a pneumonia infection. The second occurred in a patient with an eGFR between 35 to 44 mL/min/1.73 m2 as a result of overdiuresis, prompting the need for a diuretic dose reduction. ARF occurred in 1 (7%) patient within 6 months; this incidence of ARF occurred following a bilateral nephrostomy tube exchange. In all patients who experienced ARF, kidney function returned to baseline. There were no significant differences in the incidence of hypoglycemia, hypotension, polyuria, or ARF in those with eGFR <45 mL/min/1.73 m2 compared to those with eGFR >45 mL/min/1.73 m2 at 3 or 6 months (P values all >.05). Genital infections, bone fractures, amputations, ketoacidosis, and ketonuria were not observed for any of the included patients (Table 2).
Feasibility
On average, the pharmacists spent 101 minutes per patient for the initial in-clinic visit: 20 minutes for previsit safety screening, 36 minutes spent in clinic with the patient, and 45 minutes for documentation and provider communication. Subsequent telephone follow-up encounters (average 2.3 calls per patient, range 1–4 calls) required less time (average 27 minutes spent with patient and 30 minutes for documentation and communication). In-clinic follow-up required an average of 56 minutes per patient: 36 minutes with patient and 20 minutes for documentation and communication. For all pharmacist activities over the initial 3-month period, each patient required an average of 4.8 hours of pharmacist time (total time for n = 14 patients, 67 hours over 3 months). At 3 months post empagliflozin initiation, 9 (64%) patients were on a stable diabetes regimen without hypoglycemia and did not require additional intensive pharmacist phone follow-up beyond that time point. This increased to 10 (71%) patients at 6 months.
Discussion
To our knowledge, our model is the first to describe specific strategies for initiation and monitoring of SGLT2 inhibitors in the DKD population outside clinical trials. Our interprofessional model provided safe and effective empagliflozin initiation in a group of real-world patients with DKD and complex medical conditions. Our model aligns with the 2019 American Diabetes Association (ADA) guidelines that recommend SGLT2 inhibitors as the second medication to be added, after metformin, in patients with diabetes and established ASCVD or CKD.2,18 In our cohort, 57% had clinical ASCVD,41,42 and the remaining 43% had an estimated 10-year ASCVD risk score >10%41,42; all patients had CKD. The need for changes to diabetes, hypertension, and diuretic regimens during the 6-month period following empagliflozin initiation emphasized the importance of systematic clinical monitoring. Based on our observations, we attempt to provide insights into best practices for how to safely initiate and monitor SGLT2 inhibitors in this vulnerable population.
A 6-month time period may not have been long enough to detect meaningful improvements in HbA1c as seen in other models.37–39 In line with the ADA guidelines, our model promoted initiation of agents with cardiovascular (CV) or renal benefit and discontinuation of agents without benefit, even in patients with an HbA1c at or below goal.2,18 Particularly, we were able to deprescribe sulfonylureas, which are the second most commonly prescribed diabetes medication in CKD43 but have a high risk of hypoglycemia,44 lack renal benefits, and confer increased cardiovascular mortality.45,46 Prescription of empagliflozin also led to discontinuation of bolus insulin, further reducing the risk of hypoglycemia.18,47 While patients required individualized insulin dose reductions depending on their baseline insulin requirements and blood glucose control, the results seen in our cohort suggest a preemptive 20% to 30% dose reduction in total daily insulin requirements may help prevent hypoglycemia when initiating empagliflozin.
UACR reduction greater than 30% in a 2-year period is associated with 20% to 30% relative risk reduction of incident ESRD and more than 1% absolute risk reduction in 10-year risk of ESRD.48 We can reasonably assume that our observation of proteinuria reduction in a short period of time will likely improve future renal outcomes. Clinical trials also demonstrate that patients with an eGFR between 30 and 45 mL/min/1.73 m2 experience similar renal and cardiac benefits without an increase in adverse events compared to those without kidney disease. Our cohort included 6 patients with an eGFR between 35 and 44 mL/min/1.73m2 at baseline; these patients had the greatest magnitude of UACR reduction in the 6-month period without significant differences in the incidence of adverse effects.
In line with results from clinical trials, our patients required changes to their antihypertensive medications including dose reductions and discontinuation.10,49 SGLT2 inhibitors cause natriuresis resulting in reduced blood pressure. SGLT2 inhibitors also induce diuretic synergy with loop diuretics resulting in potential overdiuresis. In our patients, the mean reduction in loop diuretics at 6 months was 77% (range −50% to −100%). We propose close monitoring and consideration for an empiric 50% dose reduction in loop diuretics to minimize the risk of overdiuresis.50 The diuretic synergy of loop diuretics and SGLT2 inhibitors may be beneficial in those with some degree of diuretic resistance and warrants further investigation.50 Also noteworthy, empagliflozin decreases UACR without raising serum potassium. This may enable reinitiation and tolerability of an ACE inhibitor or ARB for further proteinuria reduction and also may allow for deprescribing of patiromer in patients with hyperkalemia.9,12
All patients tolerated empagliflozin throughout the 6-month initiation and monitoring period. The most common adverse event was polyuria which may be anticipated and managed with close monitoring and proper patient education.28 Many patients who experienced polyuria anecdotally reported that the polyuria was transient and manageable. Due to the glucose-dependent mechanism of action of SGLT2 inhibitors, there is little to no risk for hypoglycemia when used as mono-therapy; however, in patients on other diabetes medications, including agents with a high risk of hypoglycemia, the risk increases.1 In our cohort, we observed an increase in the incidence of hypoglycemia at 3 and 6 months, highlighting the importance of prudent blood sugar monitoring and downtitration of antidiabetic agents with a high risk of hypoglycemia, both activities completed by the clinical pharmacist in our model. Four (80%) of 5 patients who experienced hypoglycemia were also on insulin. Most cases of hypoglycemia were singular, asymptomatic blood glucose readings below 70 mg/dL observed in the early stages of empagliflozin initiation while implementing insulin dose reductions. There was a small increase in the incidence of hypotension which subsequently prompted reductions or discontinuation of blood pressure medications; this was in line with the current literature.29 Our results generally support the safety of using empagliflozin in patients with eGFR between 35 and 44 mL/min/1.73m2 while emphasizing the need for careful monitoring.
At 6 months, 4 (29%) patients continued to require pharmacist follow-up for diabetes management, all due to insulin titration. We did not conduct a cost–benefit analysis for our model. However, we propose the following financial considerations. We spent approximately 67 hours of pharmacist time to initiate 14 patients with DKD on empagliflozin, which costs $63 per hour (national average hourly pay rate of clinical pharmacists in 2017) 67 hours = $4221. According to the EMPA-REGOUTCOME [ESRD] trial, the number needed to treat was 14 for the renal composite outcome.9,12 We argue that by conservative estimation, we could prevent one CKD3 to CKD4 progression, which translates to an annual cost saving of $9300 in 2010 US dollars,51 in addition to the potential for other cost savings by preventing hypoglycemia requiring emergency department visits, HF admissions, and incident ESRD.7 Our model defines the role and demonstrates the feasibility of clinical pharmacists in the CKD clinic and suggests the great potential for cost savings by preventing the progression of DKD to ESRD.7 These types of initiatives are being called for on a national level within the nephrology community.7
Our pilot study was limited by a small sample size and short follow-up duration which limits the ability to draw long-term conclusions on the cardiovascular and renal protective effects of SGLT2 inhibitors.16 We carefully screened patients for study inclusion to reduce the risk of SGLT2 adverse effects. Future studies should be conducted in larger populations with comparator groups to study scalability and comparative efficacy. Finally, a formal cost–benefit analysis of our care model has yet to be defined.
Nonetheless, the complexity of caring for patients with DKD was underscored by our small group of patients who required intensive follow-up and multiple medication changes to ensure patient safety.29 The patients in our pilot may represent the most suitable candidates for SGLT2 initiation by the nephrologist in the CKD clinic. The nature of our collaborative model supported the successful initiation of SGLT2 inhibitors, allowing for frequent follow-up and monitoring which may have been too time intensive for nephrology providers alone. We believe that nephrologists and pharmacists offer a unique partnership for SGLT2 inhibitor initiation in patients with DKD, monitoring for medication adverse effects and performing subsequent adjustments to diabetes, hypertensive, and diuretic regimens. We hope that our results provide insights for guiding how to safely initiate and monitor SGLT2 inhibitors in patients who may benefit greatly from the renal and cardiovascular protective effects.
Conclusion
The implementation of an innovative, interprofessional care model within a nephrology clinic for the initiation and monitoring of empagliflozin over 6 months in patients with DKD demonstrated clinical benefit with minimal safety concerns. This model is a feasible approach for providers interested in partnering with pharmacists to align with current diabetes guidelines and improve patient care. While SGLT2 inhibitors remain highly efficacious in preventing and improving renal outcomes, they remain complex medications that require thorough monitoring and follow-up to ensure safe use.
Supplementary Material
Acknowledgments
The authors are grateful for the Nephrology Clinic Team including Susan C. McGinnis, NP, Bernard F Kelley, Jr. LPN, and Ramon Bonegio, MD, PhD. The authors would also like to thank the Endocrinology team including Dhiren Patel, PharmD, CDE, BC-ADM, BCACP. Finally, we would like to recognize Kay Li, PharmD Candidate Class of 2020, for her assistance with data collection. Finally, we would like to recognize Kay Li, PharmD Candidate Class of 2020, for her assistance with data collection.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr. Li is supported by the Ben J. Lipps Research Fellowship from the American Society of Nephrology. Dr. Paik is supported by a NIH award DK100447.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental Material
Supplementary material for this article is available online.
References
- 1.Neumiller JJ, Alicic RZ, Tuttle KR. Therapeutic considerations for antihyperglycemic agents in diabetic kidney disease. J Am Soc Nephrol. 2017;28(8):2263–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018; 41(12):2669–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association: 11. Microvascular complications and foot care: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(suppl 1):S124–S38. [DOI] [PubMed] [Google Scholar]
- 4.Koye DN, Magliano DJ, Reid CM, et al. Risk of Progression of Nonalbuminuric CKD to end-stage kidney disease in people with diabetes: the CRIC (Chronic Renal Insufficiency Cohort) study. Am J Kidney Dis. 2018;72(5):653–661. [DOI] [PubMed] [Google Scholar]
- 5.Koye DN, Shaw JE, Reid CM, et al. Incidence of chronic kidney disease among people with diabetes: a systematic review of observational studies. Diabet Med. 2017;34(7):887–901. [DOI] [PubMed] [Google Scholar]
- 6.Thompson S, James M, Wiebe N, et al. Cause of death in patients with reduced kidney function. J AmSoc Nephrol. 2015;26(10): 2504–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCullough KP, Morgenstern H, Saran R, et al. Projecting ESRD incidence and prevalence in the United States through 2030. J Am Soc Nephrol. 2019;30(1):127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mima A. Renal protection by sodium-glucose cotransporter 2 inhibitors and its underlying mechanisms in diabetic kidney disease. J Diabetes Complications. 2018;32(7):720–725. [DOI] [PubMed] [Google Scholar]
- 9.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in Type 2 diabetes. N Engl J Med. 2016;375:323–334. [DOI] [PubMed] [Google Scholar]
- 10.Cherney DZI, Zinman B, Inzucchi SE, et al. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5(8):610–621. [DOI] [PubMed] [Google Scholar]
- 11.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular outcomes, and mortality in Type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. [DOI] [PubMed] [Google Scholar]
- 12.Wanner C, Lachin JM, Inzucchi SE, et al. Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation. 2018;137(2):119–129. [DOI] [PubMed] [Google Scholar]
- 13.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in Type 2 diabetes. N Engl J Med. 2017;377(7):644–657. [DOI] [PubMed] [Google Scholar]
- 14.Perkovic V, Zeeuw D, Mahaffey KW, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6(9):691–704. [DOI] [PubMed] [Google Scholar]
- 15.Jardine MJ, Mahaffey KW, Neal B, et al. The canagliflozin and renal endpoints in diabetes with established nephropathy clinical evaluation (CREDENCE) study rationale, design, and baseline characteristics. Am J Nephrol. 2017;46(6):462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–39. [DOI] [PubMed] [Google Scholar]
- 17.Neuen BL, Ohkuma T, Neal B, et al. Cardiovascular and renal outcomes with canagliflozin according to baseline kidney function. Circulation. 2018;138(15):1537–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Diabetes Association: 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(suppl 1):S90–S102. [DOI] [PubMed] [Google Scholar]
- 19.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in Type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. [DOI] [PubMed] [Google Scholar]
- 20.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in Type 2 diabetes. N Engl J Med. 2019; 380(4):347–357. [DOI] [PubMed] [Google Scholar]
- 21.Tonelli M, Wiebe N, Manns BJ, et al. Comparison of the complexity of patients seen by different medical subspecialists in a universal health care system. JAMA Netw Open. 2018;1(7):e184852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Triantafylidis LK, Hawley CE, Perry LP, et al. The role of deprescribing in older adults with chronic kidney disease. Drugs Aging. 2018;35(11):973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whittaker CF, Miklich MA, Patel RS, et al. Medication safety principles and practice in CKD. Clin J Am Soc Nephrol. 2018; 13(11):1738–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Lullo L, Mangano M, Ronco C, et al. The treatment of type 2 diabetes mellitus in patients with chronic kidney disease: what to expect from new oral hypoglycemic agents. Diabetes Metab Syndr. 2017;11(suppl 1): S295–S305. [DOI] [PubMed] [Google Scholar]
- 25.Torimoto K, Okada Y, Koikawa K, et al. Early effects of sodium-glucose co-transporter 2 inhibitors in type 2 diabetes: study based on continuous glucose monitoring. Diabetol Metab Syndr. 2017;9(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heerspink HJL, Kosiborod M, Inzucchi SE, et al. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int. 2018;94(1):26–39. [DOI] [PubMed] [Google Scholar]
- 27.Muskiet MHA, van Bommel EJ, van Raalte DH. Antihypertensive effects of SGLT2 inhibitors in type 2 diabetes. Lancet Diabetes Endocrinol. 2016;4(3):188–189. [DOI] [PubMed] [Google Scholar]
- 28.Fitchett DH. Empagliflozin and cardio-renal outcomes in patients with type 2 diabetes and cardiovascular disease—implications for clinical practice. Eur Endocrinol. 2018;14(2):40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez-Peralta F, Abreu C, Lecube A, et al. Practical approach to initiating SGLT2 inhibitors in Type 2 diabetes. Diabetes Ther. 2017;8(5):953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolge SC, Flores NM, Huang S, Cai J. Health care provider experience with canagliflozin in real-world clinical practice: favorability, treatment patterns, and patient outcomes. Int J Gen Med. 2017;10:177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Bommel EJ, Muskiet MH, Tonneijck L, et al. SGLT2 Inhibition in the diabetic kidney-from mechanisms to clinical outcome. Clin J Am Soc Nephrol. 2017;12(4):700–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies A, Macleod R, Bennett-Britton I, et al. E-learning and near-peer teaching in electrocardiogram education: a randomised trial. Clin Teach 2016;13(3):227–230. [DOI] [PubMed] [Google Scholar]
- 33.Bayliss EA, Bhardwaja B, Ross C, et al. Multidisciplinary team care may slow the rate of decline in renal function. Clin J Am Soc Nephrol. 2011;6(4):704–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooney D, Moon H, Liu Y, et al. A pharmacist based intervention to improve the care of patients with CKD: a pragmatic, randomized, controlled trial. BMC Nephrol. 2015;16(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderegg MD, Gums TH, Uribe L, et al. pharmacist intervention for blood pressure control in patients with diabetes and/or chronic kidney disease. Pharmacotherapy. 2018;38(3):309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Migliozzi DR, Zullo AR, Collins C, et al. Achieving blood pressure control among renal transplant recipients by integrating electronic health technology and clinical pharmacy services. Am J Health Syst Pharm. 2015;72(22):1987–1992. [DOI] [PubMed] [Google Scholar]
- 37.Proia KK, Thota AB, Njie GJ, et al. Team-based care and improved blood pressure control: a community guide systematic review. Am J Prev Med. 2014;47(1):86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirsch JD, Bounthavong M, Arjmand A, et al. Estimated cost-effectiveness, cost benefit, and risk reduction associated with an endocrinologist-pharmacist diabetes intense medical management “Tune-Up” clinic. J Manag Care Spec Pharm. 2017;23(3):318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu J, Shah BM, Ip EJ, et al. A Markov model of the cost-effectiveness of pharmacist care for diabetes in prevention of cardiovascular diseases: evidence from Kaiser Permanente Northern California. J Manag Care Pharm. 2013;19(2):102–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017; 17(1):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73(24):3168–3209. [DOI] [PubMed] [Google Scholar]
- 42.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889–2934. [DOI] [PubMed] [Google Scholar]
- 43.Boye KS, Botros FT, Haupt A, et al. Glucagon-like peptide-1 receptor agonist use and renal impairment: a retrospective analysis of an electronic health records database in the U.S. Population. Diabetes Ther. 2018;9(2):637–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monami M, Dicembrini I, Kundisova L, et al. A meta-analysis of the hypoglycaemic risk in randomized controlled trials with sulphonylureas in patients with type 2 diabetes. Diabetes, Obes Metab. 2014;16(9):833–840. [DOI] [PubMed] [Google Scholar]
- 45.O’Brien MJ, Karam SL, Wallia A, et al. Association of second-line antidiabetic medications with cardiovascular events among insured adults with type 2 diabetes. JAMA Netw Open. 2018;1(8):e186125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Azoulay L, Suissa S. Sulfonylureas and the risks of cardiovascular events and death: a methodological meta-regression analysis of the observational studies. Diabetes Care. 2017;40(5):706–714. [DOI] [PubMed] [Google Scholar]
- 47.American Diabetes Association: 12. Older adults: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(suppl1):S139–S147. [DOI] [PubMed] [Google Scholar]
- 48.Coresh J, Heerspink HJL, Sang Y, et al. Change in albuminuria and subsequent risk of end-stage kidney disease: an individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol. 2019;7(2):115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petrykiv S, Sjostrom CD, Greasley PJ, et al. Differential effects of dapagliflozin on cardiovascular risk factors at varying degrees of renal function. Clin J Am Soc Nephrol. 2017;12(5):751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilcox CS, Shen W, Boulton DW, et al. Interaction between the sodium-glucose-linked transporter 2 inhibitor dapagliflozin and the loop diuretic bumetanide in normal human subjects. J AmHeart Assoc. 2018;7(4):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Honeycutt AA, Segel JE, Zhuo X, et al. Medical costs of CKD in the Medicare population. J Am Soc Nephrol. 2013;24(9): 1478–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


