Abstract
In response to the COVID-19 pandemic, we developed and launched a student-led telemedicine program in Chelsea. From April to November 2020, over 200 student volunteers contacted over 1,000 patients to assess COVID-19 symptoms, provide counseling, and triage patients. Through a retrospective cohort study, we determined that student triage decision was associated with patient outcomes, including hospitalization status, COVID-19 test administration, and COVID-19 test result. These results quantify the outcomes of a student-led telemedicine clinic to combat the ongoing pandemic and may serve as a model for implementation of similar clinics to alleviate mounting healthcare system burden.
Keywords: COVID-19, medical student, telemedicine, triage, Chelsea
Introduction:
As the number of COVID-19 cases in the United States exceeds 20 million, there is an increasing need to develop public health interventions capable of both easing the burden of the pandemic on the healthcare infrastructure and decreasing disease transmission and mortality rates. Mounting evidence supports the observation that members of black, Asian, and minority ethnic communities contract and die from COVID-19 at increased rates1–6. Chelsea is a predominantly Hispanic community that has the highest coronavirus infection rate in Massachusetts and continues to have a rolling average incidence rate that is more than two times greater than the state average7,8. In response to this pandemic, we developed and launched a student-led telemedicine clinic with the Massachusetts General Hospital’s Respiratory Illness Clinic (MGH RIC) in Chelsea to assess patients with acute respiratory symptoms and provide appropriate COVID-19 counseling.
Since April 2020, student volunteers from Harvard Medical School (HMS) and the MGH Institute of Health Professions (IHP) have contacted over 1,000 patients in the Chelsea community, providing basic assessment and counseling for COVID-19 symptom management and home isolation. As similar hotspots continue to develop over the course of this pandemic9,10, it is important to determine whether similar student-led telemedicine clinics might be beneficial, particularly in majority Black, Asian, or minority ethnic communities. Lessons learned from our clinic might be applied to similar implementations in order to maximize both efficiency and benefit on patients and communities. We hypothesized that triage decisions made by student volunteers were associated with subsequent patient healthcare engagement. To this end, we performed a retrospective cohort study to measure the patient outcomes after contact by our telemedicine clinic on the Chelsea community.
Methods:
Student-led telemedicine clinic
The MGH Chelsea Healthcare Center opened an in-person Respiratory Illness Clinic (RIC) on April 1, 2020. The RIC was established to evaluate and manage patients with acute respiratory symptoms and risk factors for severe illness, triaging those requiring a higher level of care to emergency departments. COVID-19 testing criteria evolved over the course of RIC operation, with tests initially only administered to symptomatic patients with underlying comorbidities. After visiting the RIC, patients received follow-up calls 2, 5, and 8 days after COVID-19 screening to assess symptoms, identify red flag symptoms, and provide social support and resources. We recruited students to make these phone calls in order to alleviate burden on healthcare workers at MGH Chelsea. Student volunteers began performing these follow-up phone calls on April 18, 2020.
Over 200 student volunteers were recruited from Harvard Medical School (HMS) and the MGH Institute of Health Professions (IHP), including medical, nurse practitioner, and physician assistant students. Volunteers were assigned to one of two possible roles: junior clinicians (JCs) who performed phone calls and senior clinicians (SCs) who managed workflow, supervised JCs, and contacted the supervising clinician when necessary. Three 4-hour shifts were created per day, each staffed by one SC and 3–5 JCs; telemedicine outreach occurred 7 days per week. Students were supervised by the MGH RIC clinician on call, who was available to answer clinical questions, address concerns and emergencies, and reviewed all clinic notes written by student volunteers.
At the beginning of each shift, SCs assigned patients to JCs from the MGH Chelsea RIC patient list generated using electronic medical record (EMR)-generated reports. JCs performed chart review via remote access to medical records, then made phone calls using Doximity Dialer (Doximity; San Francisco, CA). Telephone-based interpreter services were used when the indicated patient preferred language was not English. Through the telemedicine encounter, student volunteers assessed patient symptoms and provided counseling, following standardized scripts and guidelines11. JCs documented the telemedicine encounter using a standardized patient note, which included structured history questions and determination of patient triage to the ED, RIC, home quarantine, or removal from the RIC follow-up list (because the infection had resolved, the patient was inadvertently on the list, the patient was instructed to follow up with their PCP, or the patient was hospitalized). At the end of each shift, SCs contacted the attending physician about any patients that required further discussion, and the attending physician reviewed student triaging and documentation. Before volunteering, all student volunteers received virtual training in the triage, assessment, and documentation procedures.
Study design
The MGH Partners Institutional Review Board approved this retrospective cohort study (2020P002838) as minimal risk using data collected during routine clinical care and waived the requirement for informed consent. Protected health information was handled in accordance with HIPAA regulations. MGH Chelsea Healthcare Center primary care patients flagged for COVID-19 follow-up between April 18, 2020 and November 18, 2020 were included.
Patient lists were generated using EMR-generated reports and patient data was obtained from the Partners Research Patient Data Registry (RPDR) service. The date of the earliest encounter at an MGH RIC after April 18, 2020 was labeled as the index date. Time to COVID-19 test was determined by the number of days between the index date and the earliest recorded COVID-19 test date. Number of encounters was determined by the number of unique inpatient or outpatient encounters within 30 days after the index date. Encounter type was noted for each patient, and for patients with multiple encounters of different types, inpatient encounters were noted over emergency department encounters, which were noted over outpatient encounters. Additional outcomes included whether a patient was tested for COVID-19 and its result, which was extracted from laboratory results. Test positivity rate was calculated only among patients who were tested for COVID-19 after their index date. Mortality was determined by vital status information present in the patients’ charts.
Cohorts of patients triaged by student volunteers to the ED, RIC, home quarantine, or removed from the list were identified by EMR-generated reports. Patient demographics, including age, gender, race, and ethnicity were obtained from outpatient records. Comorbidities were annotated by searching each patient’s past medical history by ICD-10-CM code for recognized risk factors for COVID-196,12–16. These comorbidities included asthma (J45), chronic obstructive pulmonary disease (J44), chronic kidney disease (N18), cancer (C00-C96), hypertension (I10-I16), diabetes (E08-E13), coronary artery disease (I25), and obesity (E66). ICD-10-CM designations were determined by CDC coding guidelines17(p10).
Statistical analysis
Categorical variables are presented as frequency rates and percentages, and continuous variables are presented as mean and interquartile range. To compare patient healthcare engagement outcomes among the student triage categories, comparisons between categorical variables were calculated as odds ratios and p-values were calculated by Chi-squared test. Comparisons between student triage category and numerical variables such as patient age and number of encounters were performed by one-way ANOVA. P-values reported in tables reflect the null hypothesis that triage decision was independent of the indicated variable. Kaplan-Meier curves measuring time to positive COVID-19 result were compared using the log-rank test. To account for confounding variables, sensitivity analyses were performed using a binomial or multinomial generalized linear model with iteratively reweighted least squares for multivariate regression. All analyses were performed in R (version 3.6.1). Kaplan-Meier plot was created using survminer (version 0.4.8) and survival (version 3.2–7).
Results:
A total of 1,286 patients were contacted and evaluated by our student-led telemedicine clinic between April 18, 2020 and November 18, 2020 (Table 1). The median age was 45 years (IQR, 35–58 years) and 846 (66%) were female. Of these patients, 306 (24%) were Hispanic, 285 (22%) were white, 73 (6%) were Black, 18 (1%) were Asian, and 570 (44%) were other. Common comorbidities included hypertension (335; 26%), diabetes (257; 20%), obesity (259; 20%), and cancer (192; 15%) (Table 2). As of December 5, 2020, 1,090 (85%) patients had ever been tested for COVID-19 and 230 (18%) had ever tested positive for COVID-19 (Table 3). In the 30 days following initial RIC visit, the median number of encounters (including telemedicine phone calls) was 8 (IQR, 5–12) encounters and 49 (4%) patients required hospitalization, 78 (6%) visited the emergency department, and 843 (66%) had outpatient encounters. Overall, 6 patients died after their RIC visit.
Table 1.
Demographics of MGH RIC patients
| Total | ED | Isolate | RIC | Cleared | P-value | |
|---|---|---|---|---|---|---|
| (N=1286) | (N=8) | (N=478) | (N=107) | (N=693) | ||
| Gender | 0.824* | |||||
| Female | 846 (66 %) | 4 (50 %) | 315 (66 %) | 71 (66 %) | 456 (66 %) | |
| Male | 440 (34 %) | 4 (50 %) | 163 (34 %) | 36 (34 %) | 237 (34 %) | |
| Age | 45 (35 – 58) | 44 (36 – 50) | 42 (33 – 53) | 52 (39 – 64) | 47 (36 – 60) | <0.001† |
| Race | 0.113* | |||||
| Black | 73 (6 %) | 2 (25 %) | 27 (6 %) | 6 (6 %) | 38 (5 %) | |
| Hispanic | 306 (24 %) | 1 (12 %) | 124 (26 %) | 22 (21 %) | 159 (23 %) | |
| Other | 570 (44 %) | 3 (38 %) | 228 (48 %) | 47 (44 %) | 292 (42 %) | |
| White | 285 (22 %) | 2 (25 %) | 84 (18 %) | 29 (27 %) | 170 (25 %) | |
| American Indian | 1 (0 %) | 0 (0 %) | 1 (0 %) | 0 (0 %) | 0 (0 %) | |
| Asian | 18 (1 %) | 0 (0 %) | 3 (1 %) | 2 (2 %) | 13 (2 %) | |
| Missing | 33 (2.6%) | 0 (0%) | 11 (2.3%) | 1 (0.9%) | 21 (3.0%) |
ED: emergency department; RIC: respiratory illness clinic.
Chi-squared p-value,
one-way ANOVA p-value
Table 2.
Comorbidities of MGH RIC patients
| Total | ED | Isolate | RIC | Cleared | P-value* | |
|---|---|---|---|---|---|---|
| (N=1286) | (N=8) | (N=478) | (N=107) | (N=693) | ||
| Comorbidity (>=1) | 740 (58 %) | 5 (62 %) | 240 (50 %) | 78 (73 %) | 417 (60 %) | <0.001 |
| Asthma | 158 (12 %) | 0 (0 %) | 43 (9 %) | 28 (26 %) | 87 (13 %) | <0.001 |
| COPD | 44 (3 %) | 1 (12 %) | 3 (1 %) | 10 (9 %) | 30 (4 %) | <0.001 |
| CKD | 57 (4 %) | 2 (25 %) | 14 (3 %) | 6 (6 %) | 35 (5 %) | 0.00925 |
| Cancer | 58 (5 %) | 1 (12 %) | 16 (3 %) | 8 (7 %) | 33 (5 %) | 0.174 |
| Hypertension | 335 (26 %) | 2 (25 %) | 98 (21 %) | 39 (36 %) | 196 (28 %) | 0.00148 |
| Diabetes | 257 (20 %) | 2 (25 %) | 71 (15 %) | 27 (25 %) | 157 (23 %) | 0.00479 |
| CAD | 91 (7 %) | 1 (12 %) | 20 (4 %) | 18 (17 %) | 52 (8 %) | <0.001 |
| Obesity | 259 (20 %) | 2 (25 %) | 93 (19 %) | 28 (26 %) | 136 (20 %) | 0.425 |
ED: emergency department; RIC: respiratory illness clinic; COPD: chronic obstructive pulmonary disease; CKD: chronic kidney disease; CAD: coronary artery disease.
Chi-squared p-value
Table 3.
Outcomes of MGH RIC patients
| Total | ED | Isolate | RIC | Cleared | P-value | |
|---|---|---|---|---|---|---|
| (N=1286) | (N=8) | (N=478) | (N=107) | (N=693) | ||
| COVID-19 test (ever) | 1090 (85 %) | 8 (100 %) | 433 (91 %) | 97 (91 %) | 552 (80 %) | <0.001* |
| Positivity | 230 (21 %) | 2 (25 %) | 139 (32 %) | 5 (5 %) | 84 (15 %) | <0.001* |
| COVID-19 test (post-RIC) | 451 (35 %) | 5 (62 %) | 163 (34 %) | 52 (49 %) | 231 (33 %) | 0.00625* |
| Positivity | 46 (10 %) | 0 (0 %) | 25 (15 %) | 1 (2 %) | 20 (9 %) | 0.0187* |
| COVID-19 positive | 230 (18 %) | 2 (25 %) | 139 (29 %) | 5 (5 %) | 84 (12 %) | <0.001* |
| Total Encounters (post-RIC) | 8.0 (5.0 – 12) | 12 (7.3 – 15) | 8.0 (6.0 – 11) | 8.0 (5.0 – 13) | 8.0 (5.0 – 13) | 0.141† |
| Encounter Type | <0.001* | |||||
| Inpatient | 49 (4 %) | 2 (25 %) | 12 (3 %) | 9 (8 %) | 26 (4 %) | |
| Outpatient | 843 (66 %) | 4 (50 %) | 364 (76 %) | 63 (59 %) | 412 (59 %) | |
| Emergency | 78 (6 %) | 0 (0 %) | 25 (5 %) | 12 (11 %) | 41 (6 %) | |
| Missing | 316 (24.6%) | 2 (25.0%) | 77 (16.1%) | 23 (21.5%) | 214 (30.9%) | |
| Mortality | <0.001* | |||||
| Alive | 1280 (100 %) | 7 (88 %) | 476 (100 %) | 107 (100 %) | 690 (100 %) | |
| Dead | 6 (0 %) | 1 (12 %) | 2 (0 %) | 0 (0 %) | 3 (0 %) |
ED: emergency department; RIC: respiratory illness clinic.
Chi-squared p-value,
one-way ANOVA p-value
After each telemedicine encounter, patients had four possible triage outcomes: referred to the ED, referred back to the RIC, instructed to home quarantine, or removed from the list by student volunteers (Figure 1). Of the 1,286 patients contacted, 693 (54%) were ultimately removed from the list, 478 (37%) were instructed to quarantine, 107 (8.3%) were referred to the RIC, and 8 (0.6%) were referred to the ED. Student triage decision was independent of patient gender and race, but not age (Table 1). Patients referred back to the RIC had a median age of 52 years (IQR 39–64 years), whereas patients instructed to isolate had a median age of 42 years (IQR 33–53 years). Most comorbidities were associated with student triage decision, including a history of asthma, COPD, cancer, hypertension, diabetes, and coronary artery disease (Table 2). These results suggest that patient age and comorbidity status influenced student triage decisions.
Figure 1.
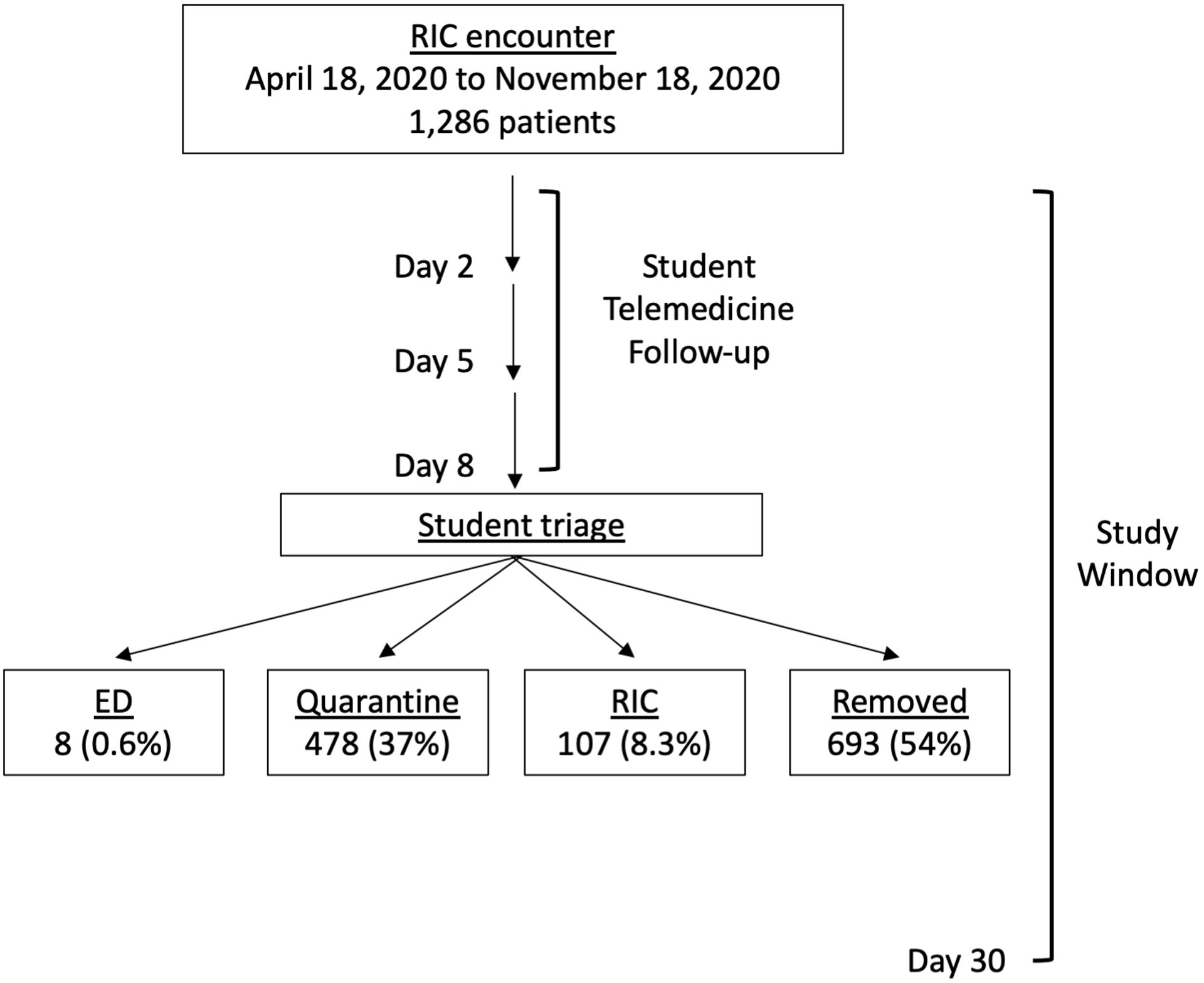
Diagram of student-led telemedicine clinic patient flow
After visiting the MGH Respiratory Illness Clinic (RIC), patients received follow up calls from student volunteers 2, 5, and 8 days after COVID-19 screening to assess symptoms, identify red flag symptoms, and provide social support and resources. Students then triaged patients to the ED, to the RIC, to home quarantine, or removed them from the list.
To quantify the result of student triage after telemedicine encounters, we compared clinical outcomes among patients triaged by student volunteers. Number of encounters in the 30 days after initial RIC visit, but not encounter type, was independent of student triage (Table 3). Subsequent hospitalization was most frequent in patients who were referred to the ED (25%), subsequent outpatient encounters were most frequent in patients who were instructed to isolate (76%), and the absence of any subsequent encounter was most common in patients removed from the list (31%). Whether a patient was tested for COVID-19 after their initial RIC visit (p=0.0063) and whether these patients tested positive for COVID-19 (p=0.019) were both associated with student triage (Table 3). These results suggest that student triage decisions were associated with healthcare engagement by patients in the 30 days following their initial RIC visit.
To account for the association between student triage decision and age and comorbidity status, we performed sensitivity analysis. We built a multivariate logistic regression model to predict outcomes of COVID-19 patients based on student triage decision, gender, age, race, and comorbidities. After adjusting for gender, age, race, and comorbidities, student triage decision was not a significant predictor of post-RIC encounter type (p=0.64), post-RIC testing status (p=0.84), or post-RIC test result (p=0.13). These results suggest that student triage decision and its association with patient outcomes was not independent of the association between patient demographic information and comorbidity status on outcomes.
To quantify the cumulative risk of testing positive for COVID-19 after initial RIC visit, we performed Kaplan-Meier analysis of post-RIC COVID-19 test result using date of initial RIC encounter and positive COVID-19 test date (Figure 2). Among the 46 (4%) patients who tested positive for COVID-19 after their initial RIC visit, the median time to COVID-19 diagnosis was 27.5 days (IQR 12–99 days). As the incubation period of COVID-19 ranges from 4–14 days after exposure18–20, post-RIC COVID-19 positive cases likely included patients who were not infected at the time of initial presentation to the RIC and student triage. Log rank testing revealed that student triage decision was associated with risk of testing positive at any time after RIC visit among patients who were tested for COVID-19 after their RIC visit (p = 0.014). Patients who were referred to home quarantine were most at risk for receiving a positive COVID-19 result after their initial RIC visit. These results suggest that student triaging was predictive of a future COVID-19 diagnosis.
Figure 2.
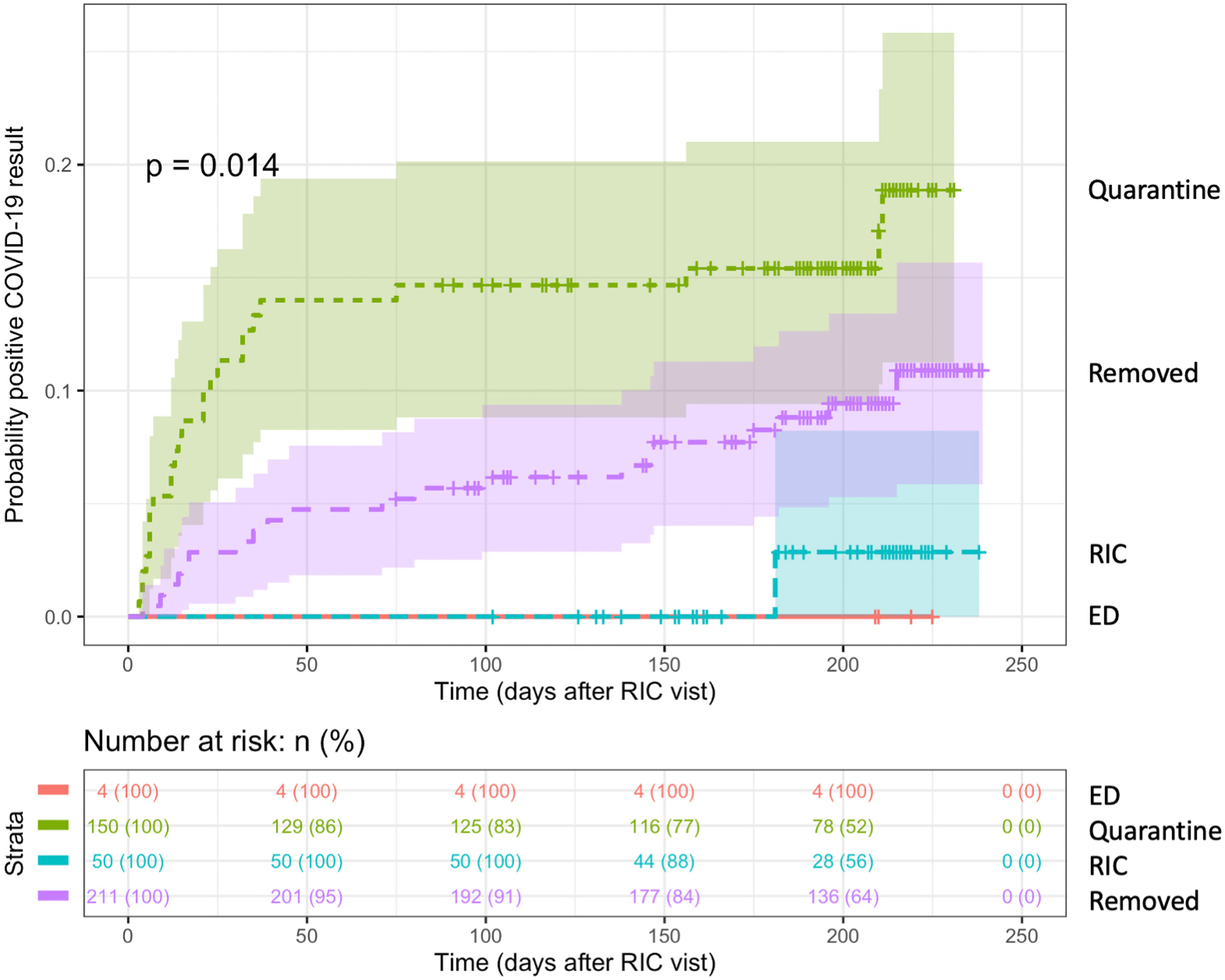
Student triage is associated with risk of COVID-19 diagnosis in patients tested after RIC visit.
Cumulative Kaplan-Meier plot of risk of positive post-RIC COVID-19 test result stratified by student triage decision. Log rank p-value indicated on plot.
Discussion:
Chelsea, MA is a predominantly Hispanic community that experienced a surge of COVID-19 cases in March 2020, which stressed both the healthcare infrastructure and the local community21. This study offers an assessment of a targeted approach to address this hotspot through a student-led telemedicine clinic. Over the course of seven months, our student-led telemedicine clinic contacted and triaged 1,286 patients. Student triage decision was associated with patient age and comorbidities, but not race or gender. We also found that student triage was associated with future outcomes, such as patient hospitalization status, whether a patient was tested for COVID-19 after their visit, and whether a patient tested positive for COVID-19 after their visit.
Patients who were referred to the ED or the RIC were more likely to be tested for COVID-19 and were more likely to be hospitalized, suggesting that student triaging was associated with patient interaction with the healthcare system. As this was a retrospective cohort study of associations with COVID-19 outcomes, it remains unclear whether the student triage decisions influenced COVID-19 outcomes or were a consequence of underlying disease. This association was not significant after adjusting for patient age and comorbidity status, suggesting that student triage decision may have incorporated these variables as known risk factors of COVID-19. Indeed, student triage decision itself was associated with patient age and comorbidity status. These results suggest that the association between student triage decision and patient outcomes was dependent on underlying COVID-19 risk, and reinforces the well-documented association between age and comorbidity status on COVID-19 outcomes6,12–16,22.
We also observed that patients who were instructed to quarantine at home or were removed from the list had increased risk of testing positive for COVID-19, whereas patients who were referred to the ED or RIC were less likely to test positive. This difference in test positivity rate is likely due to the increased frequency of testing in the ED and RIC referral cohorts, reflecting a lower clinical threshold for deciding to test for COVID-19 in patients requiring ED or RIC referral. Alternatively, the ED and RIC referral cohorts might have lower rates of post-visit COVID-19 positivity because they had already been infected with and cleared their SARS-CoV-2 infection, putting them at decreased risk of re-infection23,24. Indeed, this study is limited in our ability to determine if eventual COVID-19 diagnosis is related to symptoms present at initial presentation, or due to SARS-CoV-2 infection that occurred after visiting the RIC. Furthermore, system-wide testing criteria evolved with testing availability and SARS-CoV-2 infection rates over the course of our follow up window7. We are also unable to capture information from patients who were triaged by our clinic but sought follow up care elsewhere. To address these concerns, post-visit hospitalization status was determined by encounters at any MGH healthcare facility occurring in the 30 days after initial RIC visit, which we deemed most likely to be related to symptoms present at the time of student triage25–27. Regardless of whether eventual COVID-19 diagnosis was due to symptoms at initial presentation, risk of COVID-19 positivity and student triage decision are both likely associated with underlying risk factors unrelated to symptoms present at initial RIC visit.
Student triage decision was determined by smart phrase designation in patient notes. However, all patient notes were routed to the attending physician on call, and therefore our analysis is unable to capture student triage decisions that were ultimately rejected or amended by the supervising physician. Throughout this study a single attending physician was able to oversee all student volunteers for each shift while meeting their own clinical demands, suggesting that physician oversight of the student clinic was not unduly burdensome on the MGH Chelsea workflow. Furthermore, this study is limited to a single student-led telemedicine clinic, and therefore the generalizability of our findings from this clinic to other clinics with or without student volunteers remains unknown. Measurement of the alleviation of the burden on the MGH Chelsea healthcare system, effect of student volunteers on RIC workflow, and educational benefit provided to student volunteers is needed. Comparisons of patient outcomes from this RIC to others is difficult, as the patient population served by MGH Chelsea is distinct, most notably by its socioeconomic and racial make-up28, and the Chelsea community was a COVID-19 hotspot during the outbreak of the pandemic7. Nevertheless, our ability to engage over 1,000 patients with the healthcare system over the course of the beginning of the COVID-19 pandemic exceeds patient volumes of typical student-led clinics29–32.
Through this retrospective cohort study, we describe outcomes of patients served by our student-led telemedicine clinic. Implementation of a volunteer student clinic for a predominantly minority ethnic community in a COVID-19 hotspot was associated with substantial healthcare engagement and patient outcomes. These findings not only provide lessons for clinic improvement for future implementation, but also demonstrate the utility of similar clinics to address future COVID-19 outbreaks. As COVID-19 imposes increasing strain on the healthcare infrastructure, there is increasing interest in using student volunteers to provide much-needed support33–37. We hope our clinic may serve as a model for this support.
Acknowledgements:
The authors wish to thank all student volunteers who participated in the telemedicine clinic.
Funding/Support: E.A.G. was supported by NIH grant T32GM007753.
Footnotes
Other disclosures: J.C. has financial interests in Pfizer, Johnson & Johnson, Cisco Systems Inc, and Boston Scientific Corp. G.K. has financial interests in Dimagi Inc. The authors declare no other conflicts of interest.
Ethical approval: The MGH Partners Institutional Review Board approved this retrospective cohort study (2020P002838) as minimal risk using data collected during routine clinical care and waived the requirement for informed consent.
References:
- 1.Gold JAW, Rossen LM, Ahmad FB, et al. Race, Ethnicity, and Age Trends in Persons Who Died from COVID-19 - United States, May-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(42):1517–1521. doi: 10.15585/mmwr.mm6942e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore JT, Ricaldi JN, Rose CE, et al. Disparities in Incidence of COVID-19 Among Underrepresented Racial/Ethnic Groups in Counties Identified as Hotspots During June 5–18, 2020 – 22 States, February-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(33):1122–1126. doi: 10.15585/mmwr.mm6933e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and Mortality among Black Patients and White Patients with Covid-19. N Engl J Med. 2020;382(26):2534–2543. doi: 10.1056/NEJMsa2011686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gold JAW, Wong KK, Szablewski CM, et al. Characteristics and Clinical Outcomes of Adult Patients Hospitalized with COVID-19 - Georgia, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(18):545–550. doi: 10.15585/mmwr.mm6918e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garg S, Kim L, Whitaker M, et al. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 - COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458–464. doi: 10.15585/mmwr.mm6915e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naranbhai V, Chang CC, Beltran WFG, et al. High Seroprevalence of Anti-SARS-CoV-2 Antibodies in Chelsea, Massachusetts. J Infect Dis. 2020;222(12):1955–1959. doi: 10.1093/infdis/jiaa579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.weekly-covid-19-public-health-report-12-31-2020.pdf | Mass.gov. Accessed January 5, 2021. https://www.mass.gov/doc/weekly-covid-19-public-health-report-december-31-2020
- 9.Oster AM. Transmission Dynamics by Age Group in COVID-19 Hotspot Counties — United States, April–September 2020. MMWR Morb Mortal Wkly Rep. 2020;69. doi: 10.15585/mmwr.mm6941e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dasgupta S, Bowen VB, Leidner A, et al. Association Between Social Vulnerability and a County’s Risk for Becoming a COVID-19 Hotspot — United States, June 1–July 25, 2020. Morb Mortal Wkly Rep. 2020;69(42):1535–1541. doi: 10.15585/mmwr.mm6942a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Integrating Health Professions Students Into Telehealth Efforts During the COVID-19 Pandemic in Chelsea, Massachusetts. Accessed January 13, 2021. https://icollaborative.aamc.org/resource/11119/
- 12.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 13.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet Lond Engl. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CDC COVID-19 Response Team. Preliminary Estimates of the Prevalence of Selected Underlying Health Conditions Among Patients with Coronavirus Disease 2019 - United States, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):382–386. doi: 10.15585/mmwr.mm6913e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison SL, Fazio-Eynullayeva E, Lane DA, Underhill P, Lip GYH. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: A federated electronic medical record analysis. PLoS Med. 2020;17(9):e1003321. doi: 10.1371/journal.pmed.1003321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ICD-10-CM Official Guidelines for Coding and Reporting. https://www.cdc.gov/nchs/data/icd/10cmguidelines-FY2020_final.pdf
- 18.Chan JF-W, Yuan S, Kok K-H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet Lond Engl. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staff MGG, April 7 U, 2020, Comments32 3:48 p m Email to a Friend Share on Facebook Share on TwitterPrint this Article View. Chelsea, city of the working Latino immigrant, emerges as a COVID-19 hotspot - The Boston Globe BostonGlobe.com. Accessed January 5, 2021. https://www.bostonglobe.com/2020/04/07/opinion/chelsea-city-working-latino-immigrant-emerges-covid-19-hotspot/
- 22.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abu-Raddad LJ, Chemaitelly H, Malek JA, et al. Assessment of the risk of SARS-CoV-2 reinfection in an intense re-exposure setting. Clin Infect Dis. 2020;(ciaa1846). doi: 10.1093/cid/ciaa1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomassini S, Kotecha D, Bird PW, Folwell A, Biju S, Tang JW. Setting the criteria for SARS-CoV-2 reinfection – six possible cases. J Infect. Published online August 12, 2020. doi: 10.1016/j.jinf.2020.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen PA, Hall LE, John JN, Rapoport AB. The Early Natural History of SARS-CoV-2 Infection: Clinical Observations From an Urban, Ambulatory COVID-19 Clinic. Mayo Clin Proc. 2020;95(6):1124–1126. doi: 10.1016/j.mayocp.2020.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.U.S. Census Bureau QuickFacts: Chelsea city, Massachusetts; United States. Accessed January 5, 2021. https://www.census.gov/quickfacts/fact/table/chelseacitymassachusetts,US/PST045219 [Google Scholar]
- 29.Pelly FE, Wiesmayr‐Freeman T, Tweedie J. Student placement adaptability during COVID‐19: Lessons learnt in 2020. Nutr Diet. Published online June 23, 2020. doi: 10.1111/1747-0080.12625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pellegrini WR, Danis DO, Levi JR. Medical Student Participation in Otolaryngology Telemedicine Clinic During COVID-19: A Hidden Opportunity. Otolaryngol Neck Surg. Published online November 10, 2020:0194599820970964. doi: 10.1177/0194599820970964 [DOI] [PubMed] [Google Scholar]
- 31.Belzer A, Olamiju B, Antaya RJ, et al. A novel medical student initiative to enhance provision of teledermatology in a resident continuity clinic during the COVID‐19 pandemic: a pilot study. Int J Dermatol. Published online November 23, 2020. doi: 10.1111/ijd.15322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischbein R, Gardner-Buckshaw S, Loucek A, Ravichandran S, Eicher M, Boltri JM. Pandemic Productivity: Student-Run Free Clinic Integrates Behavioral Health in the Wake of COVID-19. Acad Psychiatry. Published online November 17, 2020. doi: 10.1007/s40596-020-01368-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soled D, Goel S, Barry D, et al. Medical Student Mobilization During a Crisis: Lessons From a COVID-19 Medical Student Response Team. Acad Med. Published online April 27, 2020. doi: 10.1097/ACM.0000000000003401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller DG, Pierson L, Doernberg S. The Role of Medical Students During the COVID-19 Pandemic. Ann Intern Med. 2020;173(2):145–146. doi: 10.7326/M20-1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khamees D, Brown CA, Arribas M, Murphey AC, Haas MRC, House JB. In Crisis: Medical Students in the COVID-19 Pandemic. AEM Educ Train. 2020;4(3):284–290. doi: 10.1002/aet2.10450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chandratre S Medical Students and COVID-19: Challenges and Supportive Strategies. J Med Educ Curric Dev. 2020;7:2382120520935059. doi: 10.1177/2382120520935059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed H, Allaf M, Elghazaly H. COVID-19 and medical education. Lancet Infect Dis. 2020;20(7):777–778. doi: 10.1016/S1473-3099(20)30226-7 [DOI] [PMC free article] [PubMed] [Google Scholar]


