Supplemental Digital Content is available in the text.
Keywords: anesthesia, intravenous, patient outcome assessment, propofol, ketamine, etomidate, rapid sequence induction and intubation
Abstract
IMPORTANCE:
Propofol, ketamine, and etomidate are common anesthetic agents for induction of anesthesia in the ICU. The choice between these agents is complex and may not depend solely upon severity of illness.
OBJECTIVES:
To evaluate the association between the administration of propofol, ketamine, and etomidate and ICU, hospital mortality, and length of stay.
DESIGN, SETTING, AND PARTICIPANTS:
Retrospective single-center cohort study. ICUs in a tertiary medical center, between January 01, 2012, and December 31, 2017. Critically ill adult patients given a single IV anesthetic for intubation.
MAIN OUTCOME AND MEASURES:
Primary outcomes were ICU and hospital mortality. Secondary outcomes were ICU- and hospital-free days through 28 days. An inverse probability of treatment weighed approach was used. The propensity score was estimated using a generalized logit model as a function of patient characteristics, admission source, ICU type, readmission status, length of ICU stays prior to intubation, and acute physiology score. Mortality outcomes were assessed with weighted logistic regression and -free days assessed by weighted linear regression with Bonferroni correction for pairwise comparisons.
RESULTS:
Of 2,673 patients, 36% received propofol, 30% ketamine and 34% etomidate. Overall ICU and hospital mortality were 19% and 29%, respectively. Patients given ketamine had higher odds of ICU mortality (1.45; [95% CI, 1.07–1.94]; p = 0.015) and patients given etomidate had higher odds of ICU mortality (1.87; 1.40–2.49; p < 0.001), hospital mortality (1.43; 1.09–1.86; p = 0.009), and less ICU-free days (–2.10; –3.21 to –1.00; p < 0.001) than those given propofol. Patients given ketamine and etomidate had similar odds of hospital mortality (1.06; 0.80–1.42; p = 0.761) and similar hospital-free days (0.30; –0.81 to 1.40; p = 0.600).
CONCLUSIONS AND RELEVANCE:
Compared with ketamine and etomidate, propofol was associated with better outcome in critically ill patients undergoing anesthesia for intubation. Even after adjusting for severity of illness prior to intubation, residual confounders cannot be excluded.
Intubation in the ICU is a high-risk procedure (1). Propofol, ketamine, and etomidate are common IV anesthetic agents used for induction of general anesthesia prior to rapid sequence intubation in critically ill patients (2). The choice between them is variable, complex, and multifactorial and not always adapted to the clinical condition. In a recent multicenter, observational, cross-sectional study of adult and PICU and emergency department patients in the United States, practices varied among providers and medications were often used inappropriately based on patient hemodynamics and contraindications (3). The choice of drug may also depend on the preference and background of the providers. In a survey of emergency physicians and anesthesiologists on their choice of drug for rapid sequence intubation among trauma patients, propofol was more frequently used by anesthesiologists and etomidate more frequently used by emergency physicians (4). Emergency physicians would also prefer ketamine or etomidate in unstable patients (4).
Each of these anesthetic agents has advantages and disadvantages (5), and one is not necessarily superior to the other. Propofol (2,6-Bis [1-methylethyl] phenol), a sedative and hypnotic, may decrease systemic blood pressure, especially in hypovolemic patients, the elderly, and those with reduced left ventricular function (6, 7). In trauma patients, the use of propofol has been associated with more hypotension than with nonpropofol agents without long-term consequences (8), and the occurrence of hypotension may depend on the dose of propofol used (9). Ketamine (2-[2-Chlorophenyl]-2-[methylamino]-cyclohexanone hydrochloride), a sedative, analgesics, and N-Methyl-d-aspartate receptor antagonist, has a favorable hemodynamic profile but may cause hypotension in case of catecholamine depletion or by direct negative inotropic effect (2, 3). Etomidate (1-[1-Phenylethyl]-1H-imidazole-5-carboxylic acid ethyl ester), a sedative and hypnotic, has also a favorable hemodynamic profile but has been associated with high rate of transient adrenal insufficiency and mortality especially in patients with sepsis (10, 11). Eventually, any drug, when properly administered, may be adequate. Among patients undergoing rapid sequence intubation in the emergency department for various medical and surgical emergencies, the choice of the anesthetic agent did not influence outcomes when adjusted for the severity of illness (12). However, there has been a wide variability in the usage of these drugs, as recently demonstrated in children with trauma; propofol was commonly used and potentially associated with poorer outcomes (13). Therefore, the objective of this study was to evaluate the association between the use of any of these induction agents and ICU and hospital outcomes (mortality and length of stay). Our hypothesis was that hospital mortality would be lower with propofol or etomidate than with ketamine when used as an induction agent for intubation of critically ill patients in the ICU.
MATERIALS AND METHODS
We performed a retrospective single-center cohort study of consecutive critically ill adult patients who were given a single IV anesthetic agent (propofol, ketamine, or etomidate) for endotracheal intubation in adult ICUs in two hospital campuses of a tertiary medical center between January 01, 2012, and December 31, 2017. The study enrolled only those who had a research authorization on file that would allow their medical record to be reviewed. We excluded patients who received a combination of IV anesthetic agents. During that study period, the ICU team followed a preprocedural checklist for intubation. We retrieved the data from the intubation note in the electronic medical record (© 1979-2020 Epic Systems Corporation) which was standardized with prefilled items including indication for intubation (multiple indications were possible), drugs used for the anesthesia, technical aspects of the intubation, and immediate complications after intubation. It had limited free text for clarification only and included “hard stops” that prevented missing items. As per standard of care and institution policy, the note needed to be completed immediately after the procedure by the operator (residents, fellows, or advanced practice providers) and cosigned by the attending physician. The procedure was performed under the attending physician’s direct supervision, with an automatic backup by an on-call anesthesiologist for a difficult airway. The choice of drugs and their combination (anesthetic agent, other sedative, analgesic, and neuromuscular blocking agent) were left to the discretion of the care team. The dosage of drugs was also left to the discretion of the clinicians but followed the usual standard of care. The intensivists were primarily board-certified in Internal Medicine, Pulmonary Disease and Critical Care, Internal Medicine and Critical Care, or Anesthesiology and Critical Care. Data collected included demographic characteristics (age, sex, body mass index), source of admission, type of ICU where the patients were admitted, indication for intubation, complications following intubation, and outcomes through 28 days after hospital discharge. The study was approved by the Mayo Clinic Institutional Review Board (18-003292).
Primary outcomes were ICU and hospital mortality. Secondary outcomes were ICU- and hospital-free days through 28 days post ICU admission. Continuous variables were summarized as median (quartile 1, quartile 3), categorical variables were summarized as n (%). Pairwise p values were from Pearson chi-square tests for categorical variables and Kruskal-Wallis rank-sum tests for continuous variables with Bonferroni correction for pairwise comparisons. The comparisons did not account for multiple observations per subject.
An inverse probability of treatment weighed approach was used to compare treatment groups. A multinomial regression model estimated the probabilities of each treatment (propensity scores) using predictors age, sex, body mass index, admission source, ICU location, readmission status, length of ICU stay prior to intubation, and Acute Physiology Score (Acute Physiology and Chronic Health Evaluation [APACHE] III score minus chronic conditions) (14). Stabilized inverse probability of treatment weights was created from the estimated propensity scores. The distribution of propensity scores was assessed for overlap among the treatment groups, and the distribution of weights assessed to detect extreme weights; no major concerns arose in this assessment. Baseline characteristics were compared by treatment group unweighted and weighted using standardized differences. For each variable, standardized differences were calculated for the three pairwise comparisons. Standardized differences less than 0.1 were considered adequate balance in the weighted sample. Mortality outcomes were assessed with weighted logistic regression and –free days assessed by weighted linear regression, all fitted using generalized estimating equations with a robust sandwich covariance. For –free days outcomes, the distribution of residuals was assessed visually by quantile-quantile plots and residuals versus predicted to evaluate normality and homoscedasticity assumptions; no violations were detected.
Missing data were present for many physiologic measures that are components of the acute physiology score. Multiple imputation of 20 imputed datasets using fully conditional specification was performed. Within the fully conditional specification approach, linear regression methods were used to impute continuous variables and logistic models used for categorical variables, using cumulative logit and multinomial models where appropriate for ordinal and nominal variables. Missing data were imputed as a function of other baseline covariates and outcomes. Continuous variables were transformed prior to imputation for normality assumptions when appropriate and back transformed prior to other analyses. Results reflected the combined analysis using Rubin’s rules. Pairwise treatment comparisons were made by adjusting multiple comparisons with a Bonferroni correction. For each pairwise comparison (ketamine vs propofol, etomidate vs propofol, etomidate vs ketamine), statistical significance was defined by p < 0.05/3 = 0.017. Descriptive tables, including standardized differences, and model assumptions described previously were assessed using only the first imputed dataset. Data were analyzed using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
We identified 3,368 adult patients who were intubated in an ICU during the study period. We excluded 695 patients who received combined anesthetic agents, leaving 2,673 patients who received a single IV anesthetic agent for analysis: 962 (36%) received propofol, 792 (30%) received ketamine, and 919 (34%) received etomidate (Fig. 1). Patient characteristics, source of admission, and ICU type are summarized in Table 1S-A (http://links.lww.com/CCX/A637). Patients could have one or multiple indications for intubation. The most common indications for intubation were acute respiratory failure and neurologic failure (i.e., airway protection, unresponsiveness, airway compromise) (Table 1). Cardiopulmonary arrest (3%) and cardiovascular failure (< 1%) were almost never reported as a reason for intubation in the procedure note. Patients intubated for neurologic failure more often received propofol or etomidate than ketamine. Patients who received ketamine or etomidate generally were slightly older and had higher severity scores (APACHE III and Sequential Organ Failure Assessment [SOFA] score on day 1) than patients who received propofol (Table 1S-B, http://links.lww.com/CCX/A637).
Figure 1.
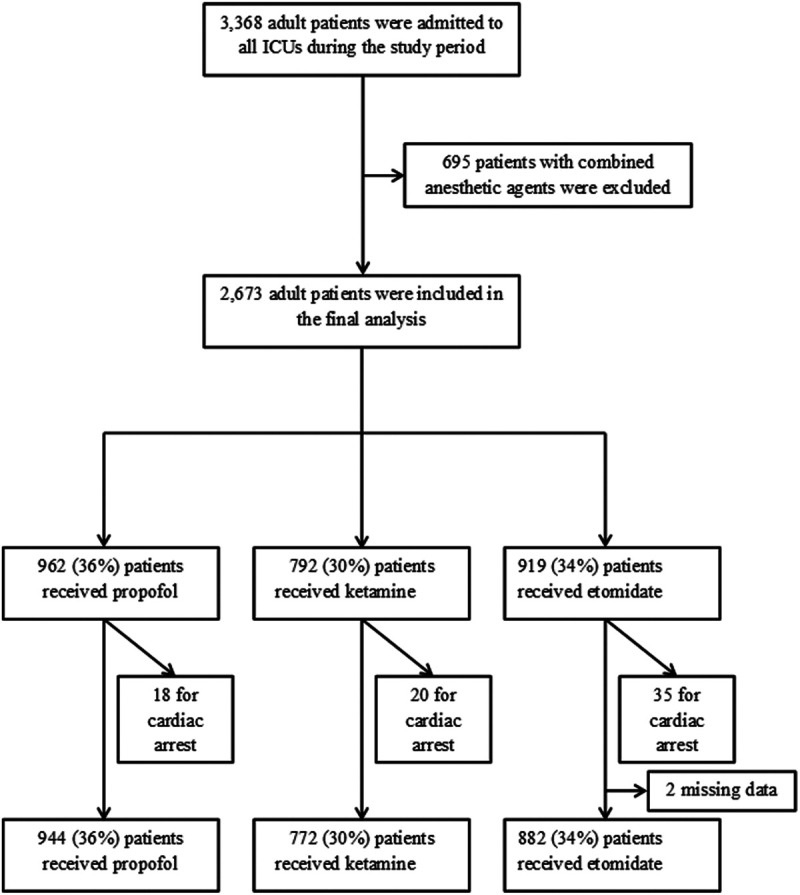
Flow diagram of critically ill patients who received propofol, ketamine, or etomidate as a single anesthetic agent.
Table 1.
Indication for Intubation by Single IV Anesthetic Agent Used in All Patients
| Indications for Intubation, n (% Anesthetic Agent) | Overall (N = 2,673) | Propofol (N = 962) | Ketamine (N = 792) | Etomidate (N = 919) | p |
|---|---|---|---|---|---|
| Cardiac arrest | 74 (3) | 18 (2) | 20 (3) | 35 (4) | 0.0330 |
| Single indication per patient (except cardiac arrest) | (N = 2,598) | (N = 944) | (N = 772) | (N = 882) | |
| Acute respiratory failure | 1,564 (60) | 484 (51) | 508 (66)b | 572 (65)c | < 0.0001 |
| Airway protection | 1,162 (45) | 462 (49) | 310 (40)b | 390 (44) | 0.0012 |
| Unresponsiveness | 649 (25) | 244 (26) | 156 (20)b | 249 (28)d | 0.0006 |
| Procedure | 442 (17) | 190 (20) | 135 (17) | 117 (13)c | 0.0005 |
| Airway compromise | 133 (5) | 51 (5) | 40 (5) | 42 (5) | 0.8212 |
| Endotracheal tube exchange | 20 (1) | 18 (2) | 0 (0)b | 2 (< 1)c | < 0.0001a |
| Miscellaneous | 31 (1) | 7 (1) | 10 (1) | 14 (2) | 0.2389 |
| Hemodynamic instability | 6 (< 1) | 0 (0) | 5 (< 1) | 1 (< 1) | 0.0092a |
| Ventricular tachycardia | 6 (< 1) | 1 (< 1) | 1 (< 1) | 4 (< 1) | 0.3367a |
| Shock | 3 (< 1) | 2 (< 1) | 1 (< 1) | 0 (0) | 0.5197a |
| Other | 16 (< 1) | 4 (< 1) | 3 (< 1) | 9 (1) | 0.1670 |
Except for those intubated for cardiopulmonary arrest, indications may be multiple per patient. For comparison between the three treatments, χ2 of independence was used, or aFisher exact test as appropriate, with significance level set to 0.05. For each of the pairwise comparisons, χ2 was used, or Fisher exact test as appropriate, with statistical significance defined by p < 0.017 after Bonferroni correction (bketamine vs propofol, cetomidate vs propofol, detomidate vs ketamine).
After excluding patients intubated during cardiopulmonary resuscitation for cardiopulmonary arrest (n = 73) without return of spontaneous circulation at the time of intubation, and two cases with missing data, we were left with 2,598 patients. Comedications were frequently given, mainly neuromuscular blocking agents (81%), analgesics (66%), and other sedatives (49%) (Table 2S, http://links.lww.com/CCX/A637). An amnesic drug (midazolam) was more frequently added when propofol, which has also amnesic property, was not given. The rate of use of an analgesic agent was identical in each group. A neuromuscular blocking agent was more frequently used with ketamine and less often used with etomidate than with propofol. The rate of immediate complication post intubation reported in the procedure note was very low (7%). There was no difference in immediate complications between patients who received propofol, ketamine, and etomidate (Table 3S, http://links.lww.com/CCX/A637). Although the prevalence of cardiac arrest immediately after intubation was extremely low (5 cases), there were more cardiac arrests within 2 hours post intubation or any time after intubation in patients who received either ketamine or etomidate than in those who received propofol (Table 3S, http://links.lww.com/CCX/A637). There was also more sustained hypoxia and cardiovascular collapse in patients who received ketamine and etomidate compared with those who received propofol.
Overall ICU and hospital mortality were 19% and 29%, respectively (Table 2). Compared with those given propofol, patients who received ketamine had higher odds of ICU mortality (odds ratio; 95% CI; p value) (1.45; 1.07–1.94; p = 0.015) and those who received etomidate had also higher odds of ICU mortality (1.87; 1.40–2.49; p < 0.001) and higher odds of hospital mortality (1.43; 1.09–1.86; p = 0.009) (Table 3). There was insufficient evidence to conclude differences between ketamine and propofol with respect to ICU-free days (–1.24; –2.41 to –0.06; p = 0.039) and hospital-free days (–1.21; –2.37 to –0.05; p = 0.041) after Bonferroni correction for pairwise comparisons. Compared with those given propofol, patients who received etomidate had also less ICU-free days (–2.10; –3.21 to –1.00; p < 0.001) but not hospital-free days (–0.92; –1.97 to 0.13; p = 0.087). Outcomes did not differ significantly between patients who received ketamine and etomidate with similar odds of hospital mortality (1.06; 0.80–1.42; p = 0.761) and similar hospital-free days (0.30; –0.81 to 1.40; p = 0.600).
Table 2.
Summary of Primary and Secondary Outcomes According to Medication in All Patients
| Outcomes | Propofol (n = 962) | Ketamine (n = 792) | Etomidate (n = 919) | p |
|---|---|---|---|---|
| Ventilator duration (hr), median (quartile 1, quartile 3) | 35.9 (12.7–95.1) | 35.6 (11.9–85.8) | 40.3 (15.5–90.3)b,c | < 0.001 |
| Hospital length of stay (d), median (quartile 1, quartile 3) | 13.9 (7.3–25.5) | 13.9 (6.7–27.8)a | 11.9 (6.2–21.5) | 0.003 |
| Hospital mortality, n (%) | 219 (23) | 251 (32)a | 297 (32)b | < 0.001 |
| ICU length of stay (d), median (quartile 1, quartile 3) | 4.5 (2.0–9.1) | 4.2 (2.0–9.3)a | 4.7 (2.2–9.0)b | < 0.001 |
| ICU mortality, n (%) | 125 (13) | 177 (22)a | 208 (23)b | < 0.001 |
Pairwise p values are from Pearson χ2 tests for categorical variables and Kruskal-Wallis rank-sum tests for continuous variables with statistical significance defined by p < 0.017 after Bonferroni correction (aketamine vs propofol, betomidate vs propofol, cetomidate vs ketamine). The comparisons do not account for multiple observations per subject. p values for ventilator duration, hospital, and ICU length of stay are from the analysis of ventilator-, hospital-, and ICU-free days (of 7, 28, and 28 d, respectively).
Table 3.
Results of Inverse Probability of Treatment Weighted Analyses
| Outcomes | Ketamine vs Propofol | Etomidate vs Propofol | Etomidate vs Ketamine | |||
|---|---|---|---|---|---|---|
| Estimates (95% CI) | p | Estimates (95% CI) | p | Estimates (95% CI) | p | |
| Hospital mortality | 1.34 (0.98–1.84) | 0.070 | 1.43 (1.09–1.86)b | 0.009 | 1.06 (0.80–1.42) | 0.671 |
| Hospital-free days (28) | –1.21 (–2.37 to –0.05) | 0.041 | –0.92 (–1.97–0.13) | 0.087 | 0.30 (–0.81 to 1.40) | 0.600 |
| ICU mortality | 1.45 (1.07–1.94)a | 0.015 | 1.87 (1.40–2.49)b | < 0.001 | 1.29 (0.99–1.68) | 0.057 |
| ICU-free days (28) | –1.24 (–2.41 to –0.06) | 0.039 | –2.10 (–3.21 to –1.00)b | < 0.001 | –0.87 (–2.09 to 0.35) | 0.164 |
Results from inverse probability of treatment weighted models using generalized estimating equations with robust variance estimates to account for the weighted analysis approach. For each outcome, model results from 20 imputed datasets were combined to estimate the pairwise treatment effects. Estimates for mortality endpoints are odds ratios. Estimates for hospital- and ICU-free days represent the increased number of hospital- or ICU-free days associated with the given drug such that estimates below 1 indicate poorer outcomes compared with the reference group. For each of the pairwise comparisons, statistical significance was defined by p < 0.017 after Bonferroni correction (aketamine vs propofol, betomidate vs propofol, etomidate vs ketamine [not observed]).
DISCUSSION
Our study indicates that, in this large cohort of patients intubated in multiple ICUs, the use of propofol was associated with better ICU mortality than the use of either ketamine or etomidate and better hospital mortality and reduced ICU length of stay than etomidate, even after adjusting for severity of illness. Our study does not show evidence of a difference between ketamine and etomidate with respect to ICU and hospital mortality and length of stay.
Could the use of propofol be associated with a better outcome than the use of ketamine or etomidate, or was propofol used in less critically ill patients? Patients who received propofol had a lower severity of illness (APACHE III and SOFA score) and higher mean arterial pressure prior to intubation than those who received ketamine or etomidate. They were less often intubated for acute respiratory failure and more often for neurologic failure. Cardiorespiratory failure as an indication for intubation was infrequent and similar between propofol, ketamine, and etomidate. After adjustment for severity of illness, ICU mortality remained lower in the propofol group. Although immediate complications were rare and similar between the groups, there was less delayed cardiac arrest, less sustained hypotension, and less sustained hypoxia in the propofol group than in the ketamine and etomidate groups.
Propofol, ketamine, and etomidate can all induce hypotension. With propofol, hypotension is predictable and usually short-lived and depends on the dosage of the drug, the degree of hypovolemia, and underlying heart failure. It is possible that clinicians are familiar with the hemodynamic effects of propofol and thus carefully plan their intubations and periintubation interventions to prevent significant hypotension. It is also possible that dose adjustments may mitigate some of the hemodynamic effects of propofol. In a recent retrospective study in the medical ICU, the use of propofol for urgent endotracheal intubation was safe with limited, transient, and reversible adverse events (15). With etomidate, hypotension can be related to transient adrenal insufficiency (16) which may have contributed to the higher risk of mortality and prolonged ICU length of stay in our study. In noncardiac surgery, the use of etomidate has been associated with higher 30-day mortality and cardiovascular morbidity than with propofol (17). In septic patients, the deleterious effect of etomidate remains unclear (18). A single dose of etomidate was not associated with increased mortality in ICU patients in a large cohort of septic patients (19). The coadministration of hydrocortisone with etomidate was associated with decreased risk of mortality in septic shock (20). With ketamine, hypotension is less predictable; this is a rare and less well-recognized phenomenon that may have also contributed to the higher risk of ICU mortality in our study. Ketamine is typically associated with a transient increase in blood pressure and heart rate. Clinicians accustomed to the positive hemodynamic effects of ketamine may not anticipate the risk of paradoxical hypotension in certain unstable patients. In one prospective study on the use of ketamine to facilitate prehospital intubation, which included mainly adult patients with medical illnesses, hypotension occurred in 7% of the cases (21). In a prospective observational study of patients undergoing rapid sequence intubation with ketamine in the out-of-hospital setting, hypotension was observed in up to 24% in patients with high shock index, whereas patients with low shock index had sustained increase in blood pressure and heart rate (22). In a prospective multicenter study of intubation in hemodynamically unstable patients, ketamine exposure was associated with 15% postintubation hypotension albeit less than the 25% seen with midazolam-propofol combination (23). So, the assumption that ketamine is safe to use in hemodynamically unstable patients may be misleading as some patients may experience potentially sustained paradoxical hypotension.
How do propofol, ketamine, and etomidate compare pairwise in the literature? In a randomized study comparing the use of propofol and ketamine for procedural sedation in the emergency department, ketamine was associated with more subclinical respiratory depression and longer time to regain basal mental status (24). In patients undergoing major abdominal surgery, the incidence of hypotension was higher with propofol than with etomidate, even after reduced dose of propofol (25). In patients undergoing elective surgery, intubating conditions were superior with etomidate than propofol (26). In patients with coronary artery disease and reduced left ventricular function undergoing elective coronary artery bypass graft surgery, both propofol and etomidate were associated with decreased heart rate and blood pressure (27). In a randomized controlled trial comparing ketamine to etomidate for emergent intubation of patients with identical SOFA score and intubating conditions (i.e., in the field, the emergency department, or the ICU), the incidence of adrenal insufficiency was higher in the etomidate group (28). In another series of critically ill patients in a medical ICU, the use of etomidate was associated with more hypotension than with the use of ketamine (29). Recent guidelines for the management of septic shock in children, based on adult and children data, recommend not using etomidate when intubating children and suggest that ketamine and fentanyl may be associated with a better hemodynamic profile (30). In a series of trauma patients undergoing rapid sequence intubation, hospital mortality, ICU-free days, and ventilator-free days were similar for patients who received etomidate and ketamine, a finding similar to our own (31). Eventually, more than the choice of a drug, the process related to procedure itself may impact outcome, from the use of a preprocedure checklist to preoxygenation, the choice and use of sedative, analgesic, and neuromuscular blocking agents, the type of laryngoscopy, the number of intubation attempts, and post intubation care (e.g., recruitment maneuver) (32). It is possible that our protocolized process of care could explain, at least in part, our findings, that is the process of the intubation may matter more that the choice of drug itself.
This study has several limitations. First, it is retrospective and inherently exposed to recollection and hidden bias such as indication bias and others and missing data. To limit this, we enrolled all consecutive patients (except those with no research authorization) during the study period and used a standardized intubation note as the main source document. In compliance with our institution policy, this note needed to be completed immediately after the procedure in the electronic medical record. Although the immediate complications were documented as per clinical judgment, and we have no reason to believe that the recording of complications would differ between groups, we cannot exclude that the rate of complications might have been underreported or minimized. Missing data were taken into account in the statistical analysis. Second, we did not address the dose of the induction agent used nor the influence of the combination of comedications such as analgesics, benzodiazepines, and neuromuscular blocking agents, although we reported their use. We can only assume that the use of any of those comedications reflected their pharmacologic property such as induction of amnesia for the sedatives or facilitator of intubation for the neuromuscular blocking agents. Third, we did not provide data on the use of vasopressors (pushes or infusions) prior or at the time of intubation nor did we assess for a potential transient adrenal insufficiency in those who received etomidate. We provided the APACHE III score which incorporates mean arterial pressure, oxygenation, Glasgow Coma Scale, and other clinical and biological variables (minus chronic conditions) to stratify by severity. We also provided the SOFA score and its subclasses that incorporates different organ derangements observed at the time of intubation and not limited to cardiovascular failure which was rarely reported as an indication for intubation. However, we did not report granular data of the hemodynamic profile in the periintubation period. Fourth, this study was from a single center, but it included data from two campuses with a mix of medical, surgical, and mixed ICU and providers from diverse specialties. Fifth, propensity scoring was used to adjust for severity, but residual confounders may remain. Although our findings may prevent its generalizability, its broad scale including multiple ICUs renders its results exploratory and hypothesis generating.
CONCLUSIONS
In a large cohort of adult critically ill patients who underwent anesthesia induction for intubation in various ICUs, the use of propofol was associated with better ICU mortality when compared with ketamine. It was also associated with better ICU and hospital mortality and better ICU length of stay when compared with etomidate. In contrast, the use of ketamine showed no difference with respect to ICU and hospital mortality and length of stay when compared with etomidate. Even after adjusting for severity of illness that incorporated, among other things, mean arterial blood pressure, oxygenation, and Glasgow Coma Scale prior to intubation, residual confounders cannot be excluded. Those results can only be exploratory and hypothesis generating.
ACKNOWLEDGMENT
We thank John G. Park for his review and precious input regarding this article.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Supported, in part, by a small grant from the Research Subcommittee of the Critical Care Independent Multidisciplinary Program at Mayo Clinic, Rochester, MN.
This work was performed at Mayo Clinic, Rochester, MN.
REFERENCES
- 1.Lapinsky SE. Endotracheal intubation in the ICU. Crit Care. 2015; 19:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stollings JL, Diedrich DA, Oyen LJ, et al. Rapid-sequence intubation: A review of the process and considerations when choosing medications. Ann Pharmacother. 2014; 48:62–76 [DOI] [PubMed] [Google Scholar]
- 3.Groth CM, Acquisto NM, Khadem T. Current practices and safety of medication use during rapid sequence intubation. J Crit Care. 2018; 45:65–70 [DOI] [PubMed] [Google Scholar]
- 4.Wahlen BM, El-Menyar A, Asim M, et al. Rapid sequence induction (RSI) in trauma patients: Insights from healthcare providers. World J Emerg Med. 2019; 10:19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferguson I, Bell A, Treston G, et al. Propofol or ketofol for procedural sedation and analgesia in emergency medicine-The POKER Study: A randomized double-blind clinical trial. Ann Pharmacother. 2014; 48:62–76 [DOI] [PubMed] [Google Scholar]
- 6.Khoi CS, Wong JJ, Wang HC, et al. Age correlates with hypotension during propofol-based anesthesia for endoscopic retrograde cholangiopancreatography. Acta Anaesthesiol Taiwan. 2015; 53:131–134 [DOI] [PubMed] [Google Scholar]
- 7.Bovill JG. Intravenous anesthesia for the patient with left ventricular dysfunction. Semin Cardiothorac Vasc Anesth. 2006; 10:43–48 [DOI] [PubMed] [Google Scholar]
- 8.Dietrich SK, Mixon MA, Rogoszewski RJ, et al. Hemodynamic effects of propofol for induction of rapid sequence intubation in traumatically injured patients. Am Surg. 2018; 84:1504–1508 [PubMed] [Google Scholar]
- 9.Zettervall SL, Sirajuddin S, Akst S, et al. Use of propofol as an induction agent in the acutely injured patient. Eur J Trauma Emerg Surg. 2015; 41:405–411 [DOI] [PubMed] [Google Scholar]
- 10.Albert SG, Ariyan S, Rather A. The effect of etomidate on adrenal function in critical illness: A systematic review. Intensive Care Med. 2011; 37:901–910 [DOI] [PubMed] [Google Scholar]
- 11.Chan CM, Mitchell AL, Shorr AF. Etomidate is associated with mortality and adrenal insufficiency in sepsis: A meta-analysis*. Crit Care Med. 2012; 40:2945–2953 [DOI] [PubMed] [Google Scholar]
- 12.Baird CR, Hay AW, McKeown DW, et al. Rapid sequence induction in the emergency department: Induction drug and outcome of patients admitted to the intensive care unit. Emerg Med J. 2009; 26:576–579 [DOI] [PubMed] [Google Scholar]
- 13.Mudri M, Williams A, Priestap F, et al. Comparison of drugs used for intubation of pediatric trauma patients. J Pediatr Surg. 2020; 55:926–929 [DOI] [PubMed] [Google Scholar]
- 14.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991; 100:1619–1636 [DOI] [PubMed] [Google Scholar]
- 15.Koenig SJ, Lakticova V, Narasimhan M, et al. Safety of propofol as an induction agent for urgent endotracheal intubation in the medical intensive care Unit. J Intensive Care Med. 2015; 30:499–504 [DOI] [PubMed] [Google Scholar]
- 16.Cuthbertson BH, Sprung CL, Annane D, et al. The effects of etomidate on adrenal responsiveness and mortality in patients with septic shock. Intensive Care Med. 2009; 35:1868–1876 [DOI] [PubMed] [Google Scholar]
- 17.Komatsu R, You J, Mascha EJ, et al. Anesthetic induction with etomidate, rather than propofol, is associated with increased 30-day mortality and cardiovascular morbidity after noncardiac surgery. Anesth Analg. 2013; 117:1329–1337 [DOI] [PubMed] [Google Scholar]
- 18.Tekwani KL, Watts HF, Rzechula KH, et al. A prospective observational study of the effect of etomidate on septic patient mortality and length of stay. Acad Emerg Med. 2009; 16:11–14 [DOI] [PubMed] [Google Scholar]
- 19.McPhee LC, Badawi O, Fraser GL, et al. Single-dose etomidate is not associated with increased mortality in ICU patients with sepsis: Analysis of a large electronic ICU database. Crit Care Med. 2013; 41:774–783 [DOI] [PubMed] [Google Scholar]
- 20.Jung B, Clavieras N, Nougaret S, et al. Effects of etomidate on complications related to intubation and on mortality in septic shock patients treated with hydrocortisone: A propensity score analysis. Crit Care. 2012; 16:R224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sibley A, Mackenzie M, Bawden J, et al. A prospective review of the use of ketamine to facilitate endotracheal intubation in the helicopter emergency medical services (HEMS) setting. Emerg Med J. 2011; 28:521–525 [DOI] [PubMed] [Google Scholar]
- 22.Miller M, Kruit N, Heldreich C, et al. Hemodynamic response after rapid sequence induction with ketamine in out-of-hospital patients at risk of shock as defined by the shock index. Ann Emerg Med. 2016; 68:181-188.e2 [DOI] [PubMed] [Google Scholar]
- 23.Ishimaru T, Goto T, Takahashi J, et al. ; Japanese Emergency Medicine Network Investigators. Association of ketamine use with lower risks of post-intubation hypotension in hemodynamically-unstable patients in the emergency department. Sci Rep. 2019; 9:17230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miner JR, Gray RO, Bahr J, et al. Randomized clinical trial of propofol versus ketamine for procedural sedation in the emergency department. Acad Emerg Med. 2010; 17:604–611 [DOI] [PubMed] [Google Scholar]
- 25.Möller Petrun A, Kamenik M. Bispectral index-guided induction of general anaesthesia in patients undergoing major abdominal surgery using propofol or etomidate: A double-blind, randomized, clinical trial. Br J Anaesth. 2013; 110:388–396 [DOI] [PubMed] [Google Scholar]
- 26.Ko YK, Kim YH, Park SI, et al. Comparison of etomidate and propofol on intubating conditions and the onset time associated with cisatracurium administration. Korean J Anesthesiol. 2015; 68:136–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soleimani A, Heidari N, Habibi MR, et al. Comparing hemodynamic responses to diazepam, propofol and etomidate during anesthesia induction in patients with left ventricular dysfunction undergoing coronary artery bypass graft surgery: A double-blind, randomized clinical trial. Med Arch. 2017; 71:198–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jabre P, Combes X, Lapostolle F, et al. ; KETASED Collaborative Study Group. Etomidate versus ketamine for rapid sequence intubation in acutely ill patients: A multicentre randomised controlled trial. Lancet. 2009; 374:293–300 [DOI] [PubMed] [Google Scholar]
- 29.Van Berkel MA, Exline MC, Cape KM, et al. Increased incidence of clinical hypotension with etomidate compared to ketamine for intubation in septic patients: A propensity matched analysis. J Crit Care. 2017; 38:209–214 [DOI] [PubMed] [Google Scholar]
- 30.Weiss SL, Peters MJ, Alhazzani W, et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med. 2020; 21:e52–e106 [DOI] [PubMed] [Google Scholar]
- 31.Upchurch CP, Grijalva CG, Russ S, et al. Comparison of etomidate and ketamine for induction during rapid sequence intubation of adult trauma patients. Ann Emerg Med. 2017; 69:24–33.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cabrini L, Landoni G, Baiardo Redaelli M, et al. Tracheal intubation in critically ill patients: A comprehensive systematic review of randomized trials. Crit Care. 2018; 22:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


