PURPOSE
Phosphatidylinositol-3-kinase (PI3K) inhibitors have shown activity in relapsed or refractory (R/R) indolent non-Hodgkin lymphoma (iNHL). PI3K inhibitors have been hampered by poor long-term tolerability and toxicity, which interfere with continuous use. Umbralisib, a dual inhibitor of PI3Kδ/casein kinase-1ε, exhibits improved selectivity for PI3Kδ compared with other PI3K inhibitors. This phase IIb trial was designed to evaluate the efficacy and safety of umbralisib in patients with R/R iNHL.
PATIENTS AND METHODS
In this multicohort, open-label, phase IIb study, 208 patients with R/R marginal zone, follicular, or small lymphocytic lymphoma (MZL, FL, or SLL) unresponsive to prior treatments (≥ 1 MZL; ≥ 2 FL/SLL), including ≥ 1 anti-CD20–based therapy, were administered umbralisib 800 mg orally once daily until disease progression, unacceptable toxicity, or study withdrawal. Primary end point is overall response rate; secondary end points include time to response, duration of response, progression-free survival, and safety.
RESULTS
The median follow-up is 27.7 months (efficacy) and 21.4 months (safety). The overall response rate was 47.1%, and tumor reduction occurred in 86.4% of patients. The median time to response was 2.7-4.6 months. The median duration of response was not reached for MZL, 11.1 months for FL, and 18.3 months for SLL. Median progression-free survival was not reached for MZL, 10.6 months for FL, and 20.9 months for SLL. At least one grade ≥ 3 treatment-emergent adverse event (TEAE) was reported in 53.4% of patients. TEAEs led to umbralisib discontinuation in 32 patients (15.4%). A total of 31 patients (14.9%) discontinued because of a treatment-related adverse event. Grade ≥ 3 TEAEs reported in ≥ 10% of patients: neutropenia (11.5%) and diarrhea (10.1%). Increased ALT/AST (grade ≥ 3) occurred in 6.7%/7.2% of patients.
CONCLUSION
Umbralisib achieved meaningful clinical activity in heavily pretreated patients with iNHL. The safety profile was manageable, with a relatively low incidence of immune-mediated toxicities and adverse event–related discontinuations.
INTRODUCTION
Non-Hodgkin lymphomas (NHLs) are a heterogeneous group of mature B-cell neoplasms.1-3 In the United States, NHL is the seventh most commonly diagnosed cancer and the most prevalent hematologic malignancy, with an estimated 77,240 new cases and 19,940 associated deaths in 2020.4,5 These lymphomas are generally classified as either indolent NHL (iNHL) or aggressive. Common iNHL subtypes include marginal zone, follicular, and small lymphocytic lymphoma (MZL, FL, and SLL).1,3 Management is usually tailored according to clinical presentation and patient characteristics. Although anti-CD20 therapy alone or in combination is standard in untreated patients, little consensus exists for patients with relapsed disease.6-9 Several treatment options available for subsequent relapses include chemotherapy and targeted agents with or without anti-CD20–directed immunotherapy. However, successive relapses are associated with declining response rates, shorter remission times, and increased cumulative toxicity risk.10-13
CONTEXT
Key Objective
Convenient, safe, and effective new drugs can afford unprecedented opportunities to manage indolent lymphomas as a chronic disease, without the need for reliance on chemotherapy. Umbralisib is a unique, potent, oral, once-daily, dual inhibitor of phosphatidylinositol-3-kinase δ-isoform and casein kinase-1ε, which has demonstrated efficacy and safety as a single agent and in combination, and may offer a novel nonchemotherapy treatment option for patients with relapsed or refractory disease.
Knowledge Generated
UNITY-NHL is a multicenter trial investigating umbralisib monotherapy in patients with relapsed or refractory marginal zone, follicular, and small lymphocytic lymphomas. These data reveal that umbralisib is associated with a manageable toxicity profile, leading to low rates of drug discontinuation and clinically meaningful efficacy in a heavily treated population.
Relevance
Available targeted treatment options for patients with relapsed or refractory disease are limited and associated with treatment-limiting toxicities. These data demonstrate that umbralisib may provide a novel targeted treatment option for previously treated patients with indolent lymphomas.
The phosphatidylinositol-3-kinase (PI3K) inhibitor drug class has emerged for treatment of relapsed B-cell malignancies. A dysregulated PI3K signaling pathway may drive abnormal cellular programming that characterizes malignant B cells. In particular, the δ-isoform (PI3Kδ), with expression restricted to cells of hematopoietic origin, is highly expressed in leukocytes and plays an essential role in B-cell development, survival, and function.14-16 Several PI3K inhibitors have shown efficacy as monotherapy in the treatment of relapsed or refractory (R/R) iNHL,11,17,18 with overall response rates (ORRs) ranging from 43.7% to 57%.11,17,18 Despite the activity of existing PI3K inhibitors, high discontinuation rates because of toxicities limit their use.11,17,18 Common serious adverse events (SAEs) include infections, hyperglycemia, transaminitis, diarrhea and/or colitis, and other immune-mediated toxicities (eg, pneumonitis).17,19-22 Although the mechanistic basis for the toxicities observed with first-generation PI3K inhibitors is not fully understood, the differential PI3K-isoform inhibition profiles of each agent23 and other specific pharmacologic properties have been implicated.24,25 For example, hyperglycemia is associated with PI3Kα inhibition,26 whereas hematologic and immune-mediated toxicities are attributed to PI3Kδ/γ inhibition.25
Umbralisib is a novel dual inhibitor of PI3Kδ and casein kinase-1ε (CK1ε), with no known clinically relevant drug-drug interactions.24,27-29 Compared with other approved PI3K inhibitors, umbralisib has a unique chemical structure, and preclinical analysis demonstrated potent PI3Kδ isoform inhibition at clinically achievable concentrations. Umbralisib exhibits more than 1,500-fold greater selectivity (Kd) for PI3Kδ over the α- and β-isoforms and approximately 225-times greater selectivity over the γ-isoform.24 PI3Kγ functions as a molecular switch between immune stimulation and suppression and, when inhibited, increases inflammation.30 In preclinical models, the loss of PI3Kδ alone was not sufficient to result in autoimmunity.31,32 However, combined loss of PI3Kγ and PI3Kδ in T cells results in severe autoimmunity and inflammation,33 suggesting that the lack of PI3Kγ inhibition by umbralisib may be advantageous.
Additionally, umbralisib is unique in that it inhibits CK1ε.29,34 This enzyme plays a pivotal role in protein translation of oncogenes (ie, MYC, BCL2, and CCND1) and has been shown to regulate elements of the β-catenin/wingless-type MMTV integration site (WNT) signaling pathway, which may influence the immunomodulatory effects of T cells.29,34,35 Therefore, improved isoform selectivity by umbralisib plus inhibition of CK1ε may account for some of the reduced immune-mediated toxicities observed.34,36
In a phase I dose escalation study, umbralisib showed activity in patients with a number of R/R hematologic malignancies, and 800 mg umbralisib (orally once daily) was identified as the recommended phase II dose.24 This phase IIb registration trial was designed to evaluate the efficacy and safety of daily, single-agent umbralisib 800 mg in patients with R/R iNHL.
PATIENTS AND METHODS
Trial Oversight
UNITY-NHL was conducted in accordance with applicable regulatory requirements, and the Protocol (online only) was approved by each study site's independent ethics committee or institutional review board. Research was conducted in accordance with updated Declaration of Helsinki and International Conference on Harmonisation Good Clinical Practice Guidelines. UNITY-NHL was designed and sponsored by TG Therapeutics (New York, NY). All patients provided written informed consent. All study investigators vouch for the accuracy and completeness of reported data and analyses and confirm the trial's adherence to the protocol (Data Supplement, online only).
Data were collected, and trial procedures were overseen by trial investigators. Data were verified by the sponsors, analyzed by sponsor statisticians, and interpreted by academic authors and sponsor representatives. The manuscript was prepared by the authors with the assistance of a medical writer funded by the sponsors. All authors had final responsibility for content and the decision to submit for publication.
Trial Design and Patient Eligibility
UNITY-NHL was a phase IIb, open-label, multicohort analysis conducted at 120 sites in nine countries (Australia, Israel, Italy, Poland, Slovakia, South Korea, Spain, United Kingdom, and United States). Patients with R/R NHL were enrolled into one of the five cohorts: MZL, FL, SLL, mantle cell lymphoma, or diffuse large B-cell lymphoma. Study procedures and efficacy and safety results for MZL, FL, and SLL cohorts of the umbralisib monotherapy arm are reported here.
Eligible patients were ≥ 18 years of age and had histologically confirmed diagnosis of B-cell iNHL, subtyped based on WHO 2008 classification criteria (MZL splenic, nodal, or extranodal; FL grade 1, 2, or 3a; or SLL).1 All patients had an Eastern Cooperative Oncology Group performance status score ≤ 2. Patients with MZL were required to have had ≥ 1 prior lines of therapy, including ≥ 1 CD20-directed regimens, with failure to achieve at least partial response (PR) or with progressive disease after the most recent systemic regimen. Patients with FL or SLL were required to have relapsed or refractory disease after ≥ 2 prior lines of systemic therapy, including an anti-CD20 monoclonal antibody and an alkylating agent. All patients were required to have evidence of ≥ 1 of the following: bulky disease (> 5 cm), high lactate dehydrogenase, B symptoms, threatened organ function, splenomegaly, cytopenias because of lymphoma, or effusions. Prophylaxis for Pneumocystis jirovecii pneumonia and antiviral therapy were mandated within 7 days before random assignment, which could later be discontinued at investigator discretion. Key exclusion criteria included prior anticancer therapy or any investigational drug within 21 days of day 1 of cycle 1 and prior hematologic stem-cell transplant, either autologous (within 6 months of study entry) or allogeneic (at any time). Study site information and additional inclusion or exclusion criteria are described online in the Data Supplement.
During the 28-day screening period, patients were required to undergo a fluorodeoxyglucose positron emission tomography (PET) with contrast-enhanced computed tomography (CT) scan of chest, abdomen, and pelvis (and neck, if clinically warranted). Treatment consisted of umbralisib 800 mg once daily with food on a 28-day cycle until disease progression, unacceptable toxicity, or study withdrawal. Umbralisib (TG Therapeutics) was supplied as 200 mg tablets. All patients were evaluated for response by CT, PET-CT, or magnetic resonance imaging at the end of cycle 3 and within 14 days before day 1 of cycles 6, 9, and 12. After cycle 12, response assessments occurred every 6 cycles or as clinically indicated. Patients with fluorodeoxyglucose-avid disease at screening were required to undergo PET-CT to confirm complete response (CR).
End Points
The primary end point was ORR, defined as the percentage of patients achieving CR or PR. Response criteria were based on the Lugano classification.37 Complete remission was defined as complete disappearance of all evidence of disease and disease-related symptoms; PR was defined as regression of measurable disease (≥ 50% decrease in the sum of the products of the diameters [SPD] of the index lesions coupled with no increase in size of other lymph nodes, liver, or spleen) and no new disease sites. Secondary end points included duration of response (DOR, defined as the time from documentation of a response to treatment to first documentation of tumor progression or death because of any cause, whichever comes first), progression-free survival (PFS, defined as the time from study entry to first documentation of tumor progression or death because of any cause, whichever comes first), and CR rate. Time to response (TTR, interval from enrollment to first documentation of CR or PR) was included as a post hoc analysis. Outcomes were assessed by an independent review committee (IRC); this analysis was considered primary. Investigator assessments for study end points were recorded.
Safety assessments included evaluation of adverse events (AEs), SAEs, and laboratory assessments. AE descriptions and grading scales were reported using the National Cancer Institute's Common Terminology Criteria for Adverse Events, version 4.0. Diagnosis of colitis was based on investigator discretion. A summary of scheduled efficacy and safety assessments is provided online (Data Supplement).
Statistical Analysis
With a minimum of 60 patients with MZL enrolled, using a one-sided test having a 2.5% false-positive error rate to assess the null hypothesis that the IRC-reviewed ORR is 25%, the trial would have 90% power against the alternative hypothesis that the ORR is 45%. With a minimum of 100 patients with FL, using a one-sided test having a 2.5% false-positive error rate to assess the null hypothesis that the IRC-reviewed ORR is 35%, the trial would have 90% power against the alternative hypothesis that the ORR is 51.5%. With a minimum of 25 patients with SLL and two-sided confidence intervals of the ORR derived using the Clopper-Pearson exact method, the 95% CI would be 34.9 to 75.6 for 14 responses. Futility was assessed at prespecified timepoints, and the study was permitted to continue to full enrollment.
Efficacy and safety analyses included all patients with MZL, FL, or SLL who received ≥ 1 dose of single-agent umbralisib. The primary end point (ORR) was estimated with 95% CI by the Clopper-Pearson method based on the binomial distribution. Secondary end points (DOR, PFS, and TTR) were summarized using the Kaplan-Meier method. Safety analyses were summarized descriptively. Continuous (nonsurvival-related) data were summarized using descriptive statistics, and frequencies and percentages were used to summarize categorical data. Other than for certain partial dates, missing data were not imputed and were treated as missing. All analyses were performed using SAS Version 9.4 or higher.
RESULTS
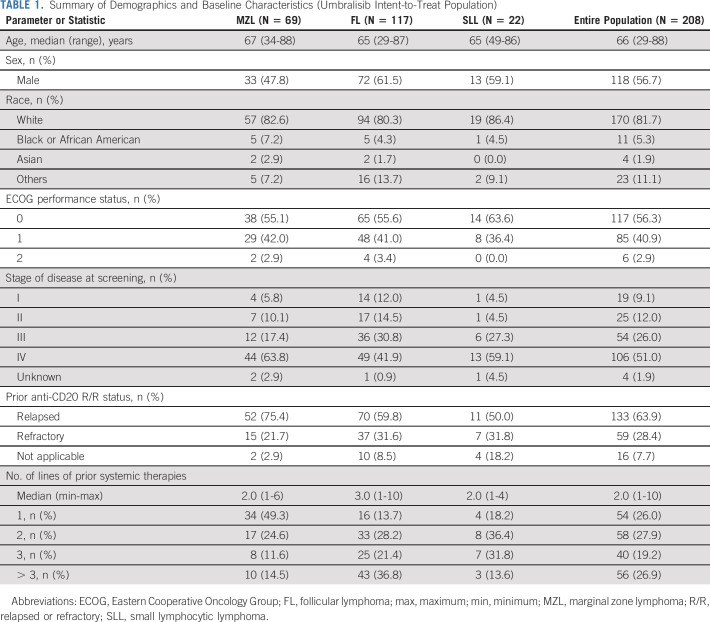
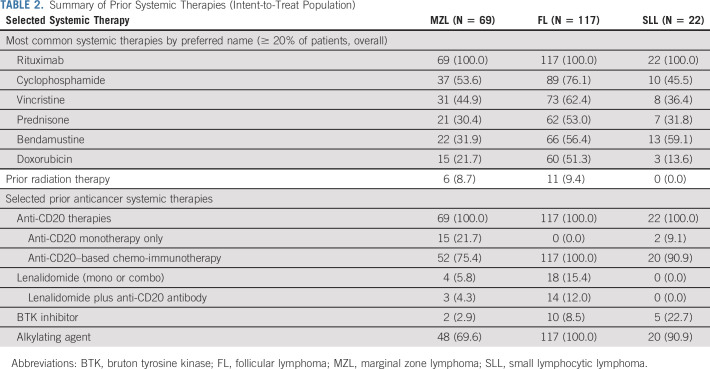
From May 2017 through September 2018, a total of 208 patients with relapsed iNHL were enrolled and treated (MZL [N = 69], FL [N = 117], and SLL [N = 22]). The intent-to-treat (ITT) cutoff date for these data was July 13, 2020. Table 1 summarizes baseline demographics and clinical characteristics for each cohort. Overall, the median age was 66 years (range, 29-88); 56.7% were male, and 81.7% were White. Of the 208 patients, 51.0% had stage IV disease; 28.4% were refractory to their last anti-CD20–based therapy. The median number of prior treatments was 2 (range, 1-10), with 46.2% of patients having received ≥ 3 prior therapies. A summary of key prior systemic therapies is shown in Table 2. All patients had received prior rituximab; the majority had received anti-CD20–based chemo-immunotherapy (75.4%, 100%, and 90.9% of patients with MZL, FL, and SLL, respectively). Some patients had received prior bruton tyrosine kinase inhibitor (2.9% MZL, 8.5% FL, and 22.7% SLL). Twelve patients in the FL cohort had undergone autologous stem cell transplant > 6 months before study entry. Additional baseline characteristics are available online (Data Supplement).
TABLE 1.
Summary of Demographics and Baseline Characteristics (Umbralisib Intent-to-Treat Population)
TABLE 2.
Summary of Prior Systemic Therapies (Intent-to-Treat Population)
Efficacy
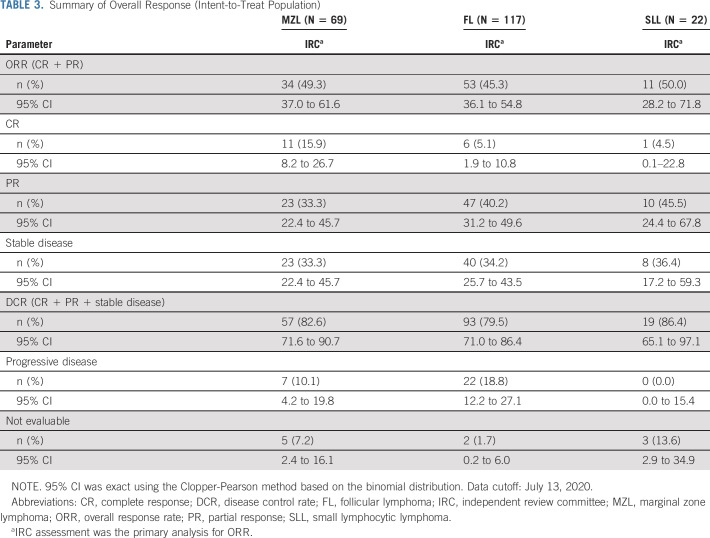
With a median follow-up of 27.7 months (MZL, 27.8 months [range, 22.4-36.1]; FL, 27.5 months [20.9-37.1]; SLL, 29.3 months [22.0-33.8]), the ORR was 47.1% for the ITT population, based on IRC assessment. For patients with MZL, FL, and SLL, respectively, ORRs were 49.3% (95% CI, 37.0 to 61.6), 45.3% (95% CI, 36.1 to 54.8), and 50.0% (95% CI, 28.2 to 71.8) (Table 3). IRC assessed ORR by subgroups available online (Data Supplement). ORR was consistent across the three subtypes of MZL. CR was seen in 11 (15.9%), 6 (5.1%), and 1 (4.5%) patient with MZL, FL, and SLL, respectively. PR and stable disease (SD) rates are shown in Table 3.
TABLE 3.
Summary of Overall Response (Intent-to-Treat Population)
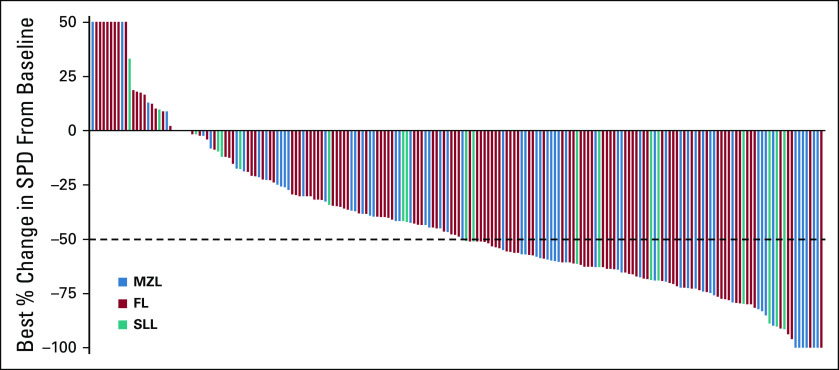
The waterfall plot depicting changes in tumor volume (data for patients with ≥ 1 postbaseline radiographic assessment [n = 198]) shows that 58 of 64 (90.6%), 96 of 115 (83.5%), and 17 of 19 (89.5%) patients with MZL, FL, and SLL experienced reduction of their disease following umbralisib, respectively (Fig 1).
FIG 1.
Response in index lesion size by independent review committee assessment (intent-to-treat population). The best percentage change in the sum of the product of the longest perpendicular dimensions (SPD) during umbralisib treatment, according to assessment by an independent review committee, is shown for patients with MZL, FL, or SLL. Data are shown only for those patients with at least one postbaseline radiographic assessment (N = 198). The majority of patients (86.4%) (with an available scan at data cutoff) showed a decrease in SPD (58 of 64 patients with MZL [90.6%], 96 of 115 patients with FL [83.5%], and 17 of 19 patients with SLL [89.5%]) as assessed by change from baseline. Data cutoff: July 24, 2020. FL, follicular lymphoma; MZL, marginal zone lymphoma; SLL, small lymphocytic lymphoma; SPD, sum of products of perpendicular diameters.
The median DOR was not reached for patients with MZL (95% CI, 10.3 to not estimable) and was 11.1 months (95% CI, 8.3 to 15.6) for patients with FL and 18.3 months (95% CI, 2.4 to not estimable) for patients with SLL (Fig 2). The median TTR (95% CI) for patients with MZL, FL, and SLL was 2.8 months (2.7 to 2.9), 4.6 months (3.0 to 5.6), and 2.7 months (2.4 to 2.8), respectively.
FIG 2.
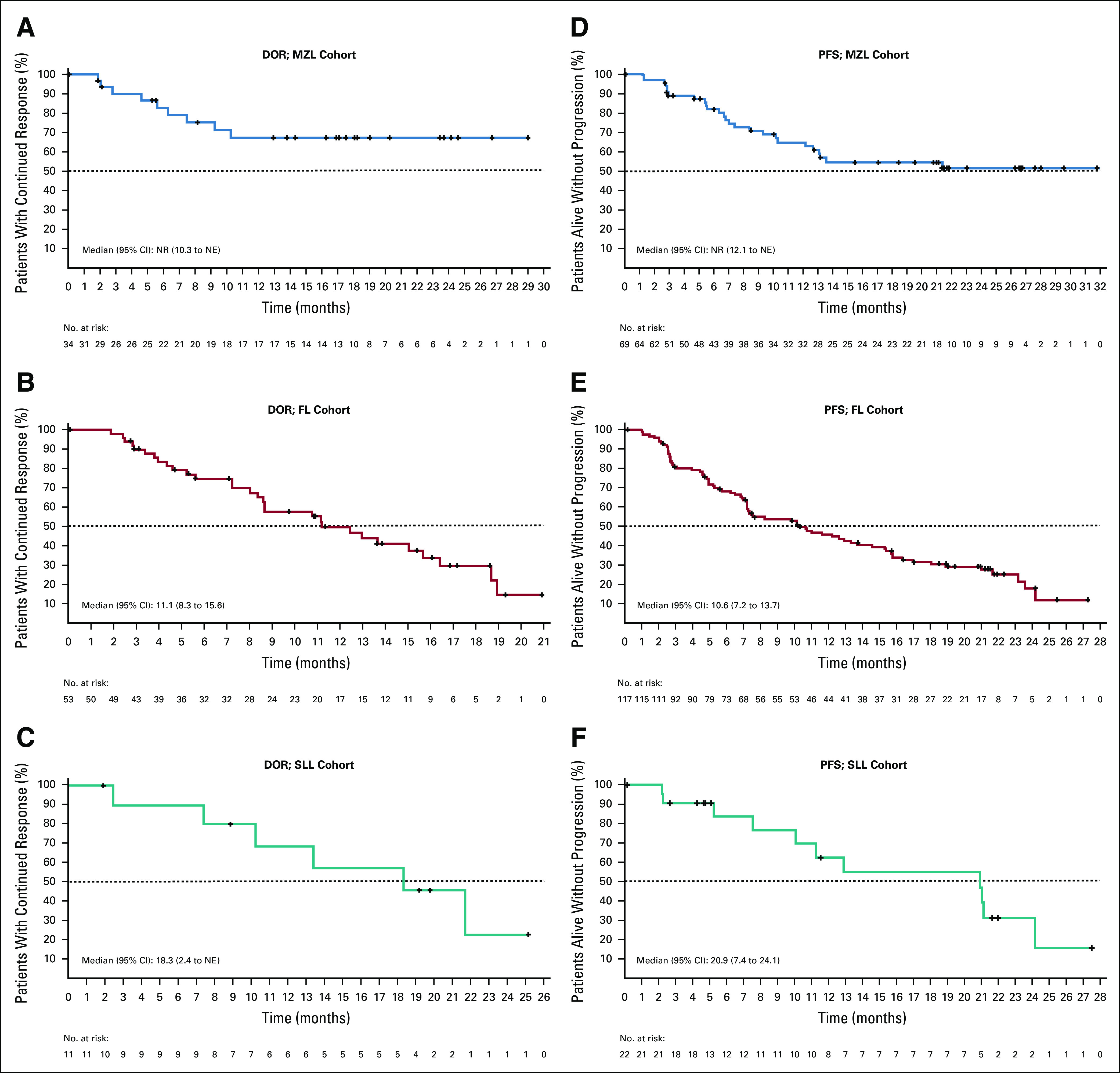
Kaplan-Meier curves for DOR and PFS by independent review committee assessment (ITT population). Kaplan-Meier curves are shown for the secondary end points of the DOR and PFS (respectively) among patients with MZL (A and D), FL (B and E), and SLL (C and F), who were treated with umbralisib (ITT population). The end points were assessed by an independent review committee. Data Cutoff: July 13, 2020. DOR, duration of response; FL, follicular lymphoma; ITT, intent-to-treat; MZL, marginal zone lymphoma; NE, not estimable; NR, not reached; PFS, progression-free survival; SLL, small lymphocytic lymphoma.
The median PFS was not reached for patients with MZL (95% CI, 12.1 to not estimable) and was 10.6 months (95% CI, 7.2 to 13.7) for patients with FL and 20.9 months (95% CI, 7.4 to 24.1) for patients with SLL (Fig 2). At 2 years, 50.5%, 18.1%, and 31.3% of patients with MZL, FL, and SLL remained progression free, respectively (Data Supplement).
Safety
Safety data were collected through January 1, 2020, corresponding to a median follow-up of 21.4 months (range, 14.6-30.8). The median duration of exposure for all patients was 8.4 months (range, 0.2-27.0). Patients with MZL, FL, and SLL had median durations of exposure of 9.8 months (range, 0.2-27.0), 7.6 months (range, 1.0-26.5), and 10.9 months (range, 0.7-25.1), respectively. At data cutoff, 60 patients (26, 27, and 7 with MZL, FL, and SLL, respectively) remained on treatment. Treatment-emergent AEs (TEAEs) occurring in ≥ 10% of patients are listed in Table 4. Overall, 207 of 208 patients (99.5%) experienced TEAEs, of which 53.4% had ≥ 1 grade ≥ 3 event. The most frequent TEAEs (any grade; ≥ 20% of patients) were diarrhea (59.1%), nausea (39.4%), fatigue (30.8%), vomiting (23.6%), and cough (20.7%). The majority of diarrhea events were grade 1 and typically manageable via dose holds; 46 patients (37%) received supportive care, which includes nine patients (7.3%) that received steroids.
TABLE 4.
TEAEs (≥ 10% Overall), System Organ Class, and Preferred Term (Intent-to-Treat Population)
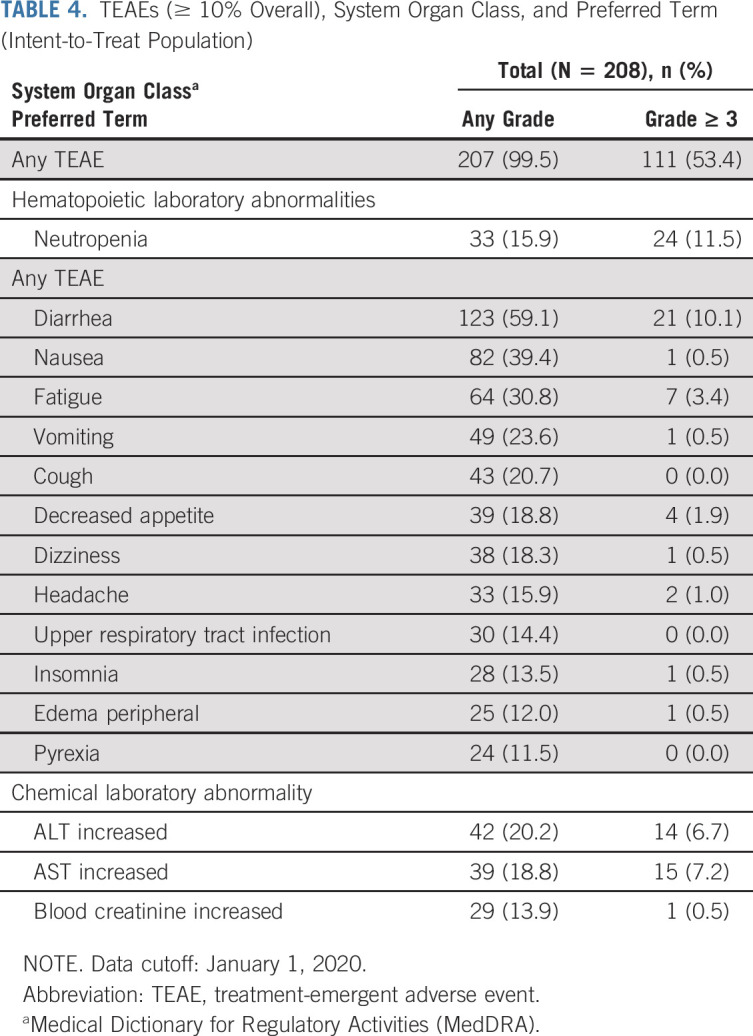
The only grade ≥ 3 AEs that occurred in ≥ 10% of patients were neutropenia (11.5%) and diarrhea (10.1%; all grade 3). Three patients experiencing grade 3 diarrhea had colonoscopies, none of which showed signs of colitis. Other grade 3 or 4 AEs of interest included opportunistic infections (7, 3.4%) and rash (4, 1.9%). Elevated levels of ALT and AST were observed in 20.2% (grade ≥ 3 in 6.7%) and 18.8% (grade ≥ 3 in 7.2%) of patients, respectively. Other AEs of interest included pneumonitis (1.4%; grade ≥ 3 1.0%) and noninfectious colitis (1.9%; grade ≥ 3 0.5%). Of the four patients experiencing noninfectious colitis, only one patient experienced overlapping diarrhea and received a colonoscopy, which revealed normal gastrointestinal morphology. Colitis resolved in three of these four patients; they remained on umbralisib.
Overall, serious TEAEs were reported in 63 patients (30.3%), with grade ≥ 3 serious TEAEs reported in 54 patients (26.0%). Serious TEAEs considered related to umbralisib were reported in 36 patients (17.3%). The most frequent serious TEAEs occurring in > 1% of patients were diarrhea (3.4%), acute kidney injury (1.4%), anemia (1.4%), dehydration (1.4%), febrile neutropenia (1.4%), pneumonia (1.4%), sepsis (1.4%), and urinary tract infection (1.4%). One patient with SLL had a fatal myocardial infarction (unrelated to umbralisib); there were no other grade 5 AEs. TEAEs led to umbralisib discontinuation in 32 patients (15.4%); 31 of these (14.9%) discontinued because of a treatment-related AE. Twenty (20 of 32) patients continued to be followed for disease progression; nine of these patients went on to develop progressive disease, which occurred at an average of 7.2 months following umbralisib cessation. The most common grade ≥ 3 TEAEs leading to discontinuation of umbralisib (≥ 5 patients) were diarrhea (6, 2.9%) and ALT/AST increase (5, 2.4%). Additional information is available online (Data Supplement). TEAEs leading to dose reductions occurred in 24 (11.5%) patients; an umbralisib-related TEAE led to a dose reduction in 9.6% of patients.
DISCUSSION
Dysregulation of the PI3K pathway plays an important role in cancer biology.14-16 Although therapeutic targeting of the pathway has produced clinical benefit for patients with iNHL, existing drugs in the class have limitations related to long-term tolerability.11,17,18 In this open-label, phase IIb study, oral once-daily umbralisib at a dose of 800 mg was effective in heavily pretreated patients with R/R iNHL. At a median follow-up of 27.7 months, primary study objective was met, with ORRs ranging from 45.3% to 50.0% for patients with MZL, FL, and SLL. The majority of patients (86.4%) experienced tumor size reduction with umbralisib. However, these results are similar to responses observed with first-generation PI3K inhibitors,11,17,18 making cross-trial comparisons limited. Notably, patients included in trials for some of the first-generation PI3K inhibitors were required to be double-refractory (to both rituximab and chemotherapy that included an alkylating agent).11,18
Umbralisib was well-tolerated, exhibiting a safety profile similar across iNHL subtypes, which was consistent with previous phase I trials evaluating umbralisib alone or in combination therapy in patients with B-cell lymphomas.24,27,38 In UNITY-NHL, there was a low incidence of umbralisib-related discontinuations and no deaths because of TEAEs. These data contrast with pivotal trials of first-generation PI3K inhibitors, in which investigators reported high TEAE-related discontinuation rates (up to 52%) and treatment-related deaths (3.9%-8.8%) despite shorter median follow-up times in most studies (6, 9.7, and 32 months).11,17,18,39
First-generation oral PI3K inhibitors are associated with fatal and/or serious immune-mediated toxicities, including diarrhea and/or colitis (idelalisib, 14% to 20% and duvelisib, 18%), pneumonitis (idelalisib, 4% and duvelisib, 5%), hepatotoxicity (idelalisib, 16% to 18% and duvelisib, 2% to 8%), and fatal and/or serious infections (idelalisib, 21% to 48% and duvelisib, 31%).21,22 The first-generation intravenous pan-PI3K inhibitor, copanlisib, is associated with fatal and/or SAEs, including hypertension 26%, hyperglycemia 41%, and infection 19%.20 In UNITY-NHL, there was a relatively low incidence of infections and immune-mediated toxicities, including ALT/AST elevations. Although the precise mechanism for the toxicities observed with first-generation PI3K inhibitors is not yet known, attention has been focused on the individual agent's differential PI3K isoform inhibition profile23 and its specific pharmacologic properties.24,25
Given the long-term continuous dosing required for PI3K inhibitors in iNHL, identifying effective agents with safety profiles that minimize toxicity-related discontinuations is essential.19 Umbralisib is an effective and well-tolerated monotherapy treatment option for patients with R/R iNHL, serving as a platform for the development of novel combination regimens not enabled by currently available PI3K inhibitors. As of July 2020, more than 1,800 patients with B-cell malignancies have been treated with umbralisib, either alone or in combination with 16 other agents.24,27,38,40
In UNITY-NHL, umbralisib showed meaningful clinical activity in patients with R/R iNHL consistent with that demonstrated by prior inhibitors of PI3K. However, the safety profile appears improved compared with first-generation PI3K inhibitors, with manageable toxicities and a relatively low number of AE-related discontinuations. These results suggest that umbralisib has a favorable benefit-risk profile in this heavily pretreated population.
ACKNOWLEDGMENT
We thank the patients who participated in this study; the investigators and coordinators at the clinical sites; those who contributed to the design, implementation, and data analyses; and ECIR Medical Communications (Linda M. Ritter, PhD, Scientific Director) for assistance in the preparation of this manuscript. Dr O'Connor is an American Cancer Society Research Professor.
Nathan H. Fowler
Employment: Bostongene
Consulting or Advisory Role: Roche/Genentech, TG Therapeutics, Verastem, Bayer, Celgene, Novartis
Research Funding: Roche, Celgene, Gilead Sciences, TG Therapeutics, Novartis, AbbVie, BeiGene
Felipe Samaniego
Consulting or Advisory Role: Astex Pharmaceuticals, ADC Therapeutics, Imbrium Therapeutics
Wojciech Jurczak
Research Funding: TG Therapeutics
Nilanjan Ghosh
Consulting or Advisory Role: Seattle Genetics, TG Therapeutics, AstraZeneca, Verastem, Pharmacyclics/Janssen, Karyopharm Therapeutics, Genmab, Bristol-Myers Squibb, Gilead Sciences, Adaptive Biotechnologies, Beigene, AbbVie, Incyte
Speakers' Bureau: AbbVie, Janssen Oncology, Kite/Gilead Sciences, Seattle Genetics, AstraZeneca, Bristol-Myers Squibb, Celgene, Pharmacyclics/Janssen, Epizyme
Research Funding: Pharmacyclics, TG Therapeutics, Genentech/Roche, Seattle Genetics, Bristol-Myers Squibb/Celgene, Gilead Sciences
Enrico Derenzini
Consulting or Advisory Role: AstraZeneca
Research Funding: TG-Therapeutics, ADC Therapeutics
James A. Reeves
Research Funding: Sarah Cannon Research Institute, Eli Lilly, Tesaro, TG Therapeutics, Genentech, Celgene, Merck, Bristol-Myers Squibb, Boston Biomedical Inc, AstraZeneca, NovoCure, Calithera Biosciences, Novartis, Guardant Health, Acerta Pharma, Rhizen Pharmaceuticals, Takeda, Onconova Therapeutics, Sanofi, CTI Biopharma, Eisai, Janssen
Chan Y. Cheah
Honoraria: Roche/Genentech, Janssen-Cilag, TG Therapeutics, Loxo/Lilly, AstraZeneca, Bristol-Myers Squibb, Gilead Sciences, Ascentage Pharma, MDS
Consulting or Advisory Role: Janssen-Cilag, Roche/Genentech, TG Therapeutics, Loxo/Lilly, Gilead Sciences, AstraZeneca, Bristol-Myers Squibb, Ascentage Pharma, MDS
Speakers' Bureau: Janssen-Cilag
Research Funding: Roche, Celgene, AbbVie
Travel, Accommodations, Expenses: Roche
Tycel Phillips
Honoraria: Seattle Genetics, Incyte, Pharmacyclics, Bayer, Gilead Sciences, Genentech
Consulting or Advisory Role: Seattle Genetics, Pharmacyclics, Incyte, Genentech, Bayer, Gilead Sciences, Curis, Kite/Gilead, Celgene, Genmab
Research Funding: AbbVie, Pharmacyclics/Janssen, Bayer
Ewa Lech-Maranda
Consulting or Advisory Role: Roche, Amgen, AbbVie, Astellas Pharma, Novartis, Janssen-Cilag, Sanofi/Aventis, Takeda, Gilead Sciences
Bruce D. Cheson
Consulting or Advisory Role: TG Therapeutics, AbbVie, Pharmacyclics/Janssen, Morphosys, Celgene, Karyopharm Therapeutics, Epizyme, Gilead Sciences, SymBio Pharmaceuticals, Parexel, Merck, Glaxo Smith Kline, Beigene, Kite, Reddy Biosimilar, Bayer, Celgene
Research Funding: TG Therapeutics, Trillium Therapeutics, Seattle Genetics, Bristol-Myers Squibb, Gilead Sciences, Pharmacyclics, AbbVie, AstraZeneca
Travel, Accommodations, Expenses: SymBio Pharmaceuticals
Paolo F. Caimi
Consulting or Advisory Role: Genentech, Seattle Genetics, TG Therapeutics, Kite Pharma, ADC Therapeutics, Bayer, Amgen, Verastem, Epizyme
Speakers' Bureau: Celgene
Research Funding: Genentech, ADC Therapeutics
Lori A. Leslie
Consulting or Advisory Role: Kite/Gilead, TG Therapeutics, Celgene/BMS, Seattle Genetics, AbbVie, PCYC/Janssen, ADC Therapeutics, BeiGene, AstraZeneca
Speakers' Bureau: Kite Pharma, Celgene/BMS, Seattle Genetics, AbbVie, PCYC/Janssen, ADC Therapeutics, BeiGene, AstraZeneca, Epizyme, Karyopharm
Julio C. Chavez
Consulting or Advisory Role: Kite/Gilead, Novartis, Karyopharm Therapeutics, MorphoSys, TeneoBio, AbbVie, Janssen
Speakers' Bureau: AstraZeneca, BeiGene, MorphoSys, Epizyme
Research Funding: Merck
Gustavo Fonseca
Honoraria: Amgen, Celgene
Consulting or Advisory Role: Amgen, Celgene, Bayer, Abvie/Pharmacyclics, Dava Oncology, Karyopharm
Speakers' Bureau: Amgen, Celgene
Research Funding: Celgene, Amgen, TG Therapeutics, Verastem Oncology
Travel, Accommodations, Expenses: Amgen, Celgene
Sunil Babu
Stock and Other Ownership Interests: Fort Wayne Medical Oncology & Hematology, Lutheran Hospital
Honoraria: Bristol-Myers Squibb, Alexion Pharmaceuticals, Lilly, Bayer, AstraZeneca
Consulting or Advisory Role: Bristol-Myers Squibb, Alexion Pharmaceuticals, AstraZeneca, argenx, Boehringer Ingelheim, Bayer, Kite Pharma, Janssen Oncology
Speakers' Bureau: Alexion Pharmaceuticals
Research Funding: Bristol-Myers Squibb, Novartis, Genentech/Roche, AstraZeneca/MedImmune, Janssen Oncology, Amgen, TG Therapeutics, AbbVie, Lilly, Alexion Pharmaceuticals, Merck, Novartis, Syndax, Nektar, Sanofi, argenx
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Alexion Pharmaceuticals, Lilly, Janssen Oncology, Genentech/Roche
Daniel J. Hodson
Research Funding: Gilead Sciences
John M. Burke
Consulting or Advisory Role: Genentech/Roche, AbbVie, Seattle Genetics, Bayer, AstraZeneca, Adaptive Biotechnologies, Verastem, MorphoSys, Kura Oncology, Epizyme, BeiGene, Kymera, Novartis
Speakers' Bureau: Seattle Genetics, Beigene
Jeff P. Sharman
Leadership: US Oncology
Consulting or Advisory Role: Pharmacyclics, Celgene, TG Therapeutics, Genentech, AbbVie, Acerta Pharma/AstraZeneca, Beigene, Pfizer
Research Funding: Pharmacyclics, Genentech, Celgene, Acerta Pharma, Gilead Sciences, Seattle Genetics, TG Therapeutics, Merck, Takeda
John M. Pagel
Consulting or Advisory Role: Gilead Sciences, AstraZeneca, Actinium Pharmaceuticals, BeiGene, Loxo
Hari P. Miskin
Employment: TG Therapeutics
Leadership: TG Therapeutics
Stock and Other Ownership Interests: TG Therapeutics
Travel, Accommodations, Expenses: TG Therapeutics
Peter Sportelli
Employment: TG Therapeutics
Leadership: TG Therapeutics
Stock and Other Ownership Interests: TG Therapeutics
Travel, Accommodations, Expenses: TG Therapeutics
Owen A. O'Connor
Employment: TG Therapeutics
Leadership: TG Therapeutics
Board Appointments, Stock, and Other Ownership Interests: TG Therapeutics, Kymera, Myeloid Therapeutics, NomoCan Pharmaceuticals
Honoraria: Mundipharma
Consulting or Advisory Role: Mundipharma Research, Astex Pharmaceuticals
Research Funding: Merck, Celgene/Bristol-Myers Squibb, Seagen, Mundipharma, Astex Pharmaceuticals, ADC Therapeutics
Michael S. Weiss
Employment: TG Therapeutics
Leadership: TG Therapeutics
Stock and Other Ownership Interests: TG Therapeutics
Travel, Accommodations, Expenses: TG Therapeutics
Pier Luigi Zinzani
Consulting or Advisory Role: Sanofi, Verastem, Celltrion, Gilead Sciences, Janssen-Cilag, Bristol-Myers Squibb, SERVIER, Sandoz, MSD, Immune Design, Celgene, Portola Pharmaceuticals, Roche, EUSA Pharma, Kyowa Hakko Kirin, TG Therapeutics, Takeda
Speakers' Bureau: Verastem, Celltrion, Gilead Sciences, Janssen-Cilag, Bristol-Myers Squibb, SERVIER, Sandoz, MSD, Immune Design, Celgene, Portola Pharmaceuticals, Roche, EUSA Pharma, Kyowa Hakko Kirin
No other potential conflicts of interest were reported.
See accompanying article on page 1671
PRIOR PRESENTATION
Presented in part at 62nd American Society of Hematology (ASH) Annual Meeting and Exposition, San Diego, CA, December 5-8, 2020.
SUPPORT
Supported by TG Therapeutics.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Nathan H. Fowler, Wojciech Jurczak, Chan Y. Cheah, Gustavo Fonseca, Jeff P. Sharman, Hari P. Miskin, Peter Sportelli, Owen A. O'Connor, Michael S. Weiss
Provision of study materials or patients: Felipe Samaniego, Enrico Derenzini, James A. Reeves, Wanda Knopińska-Posłuszny, Chan Y. Cheah, Tycel Phillips, Bruce D. Cheson, Julio C. Chavez, Gustavo Fonseca, Daniel J. Hodson, John M. Burke, Jeff P. Sharman, John M. Pagel, Owen A. O'Connor
Collection and assembly of data: Nathan H. Fowler, Wojciech Jurczak, Nilanjan Ghosh, Enrico Derenzini, James A. Reeves, Wanda Knopińska-Posłuszny, Chan Y. Cheah, Ewa Lech-Maranda, Bruce D. Cheson, Paolo F. Caimi, Sebastian Grosicki, Lori A. Leslie, Julio C. Chavez, Gustavo Fonseca, Sunil Babu, Daniel J. Hodson, Spencer H. Shao, Jeff P. Sharman, John M. Pagel, Hari P. Miskin, Peter Sportelli, Michael S. Weiss
Data analysis and interpretation: Nathan H. Fowler, Felipe Samaniego, Wojciech Jurczak, Nilanjan Ghosh, Enrico Derenzini, Chan Y. Cheah, Tycel Phillips, Bruce D. Cheson, Paolo F. Caimi, Sebastian Grosicki, Lori A. Leslie, Julio C. Chavez, Gustavo Fonseca, Sunil Babu, John M. Burke, Jeff P. Sharman, Jennie Y. Law, John M. Pagel, Hari P. Miskin, Owen A. O'Connor, Pier Luigi Zinzani
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Umbralisib, a Dual PI3Kδ/CK1ε Inhibitor in Patients With Relapsed or Refractory Indolent Lymphoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Nathan H. Fowler
Employment: Bostongene
Consulting or Advisory Role: Roche/Genentech, TG Therapeutics, Verastem, Bayer, Celgene, Novartis
Research Funding: Roche, Celgene, Gilead Sciences, TG Therapeutics, Novartis, AbbVie, BeiGene
Felipe Samaniego
Consulting or Advisory Role: Astex Pharmaceuticals, ADC Therapeutics, Imbrium Therapeutics
Wojciech Jurczak
Research Funding: TG Therapeutics
Nilanjan Ghosh
Consulting or Advisory Role: Seattle Genetics, TG Therapeutics, AstraZeneca, Verastem, Pharmacyclics/Janssen, Karyopharm Therapeutics, Genmab, Bristol-Myers Squibb, Gilead Sciences, Adaptive Biotechnologies, Beigene, AbbVie, Incyte
Speakers' Bureau: AbbVie, Janssen Oncology, Kite/Gilead Sciences, Seattle Genetics, AstraZeneca, Bristol-Myers Squibb, Celgene, Pharmacyclics/Janssen, Epizyme
Research Funding: Pharmacyclics, TG Therapeutics, Genentech/Roche, Seattle Genetics, Bristol-Myers Squibb/Celgene, Gilead Sciences
Enrico Derenzini
Consulting or Advisory Role: AstraZeneca
Research Funding: TG-Therapeutics, ADC Therapeutics
James A. Reeves
Research Funding: Sarah Cannon Research Institute, Eli Lilly, Tesaro, TG Therapeutics, Genentech, Celgene, Merck, Bristol-Myers Squibb, Boston Biomedical Inc, AstraZeneca, NovoCure, Calithera Biosciences, Novartis, Guardant Health, Acerta Pharma, Rhizen Pharmaceuticals, Takeda, Onconova Therapeutics, Sanofi, CTI Biopharma, Eisai, Janssen
Chan Y. Cheah
Honoraria: Roche/Genentech, Janssen-Cilag, TG Therapeutics, Loxo/Lilly, AstraZeneca, Bristol-Myers Squibb, Gilead Sciences, Ascentage Pharma, MDS
Consulting or Advisory Role: Janssen-Cilag, Roche/Genentech, TG Therapeutics, Loxo/Lilly, Gilead Sciences, AstraZeneca, Bristol-Myers Squibb, Ascentage Pharma, MDS
Speakers' Bureau: Janssen-Cilag
Research Funding: Roche, Celgene, AbbVie
Travel, Accommodations, Expenses: Roche
Tycel Phillips
Honoraria: Seattle Genetics, Incyte, Pharmacyclics, Bayer, Gilead Sciences, Genentech
Consulting or Advisory Role: Seattle Genetics, Pharmacyclics, Incyte, Genentech, Bayer, Gilead Sciences, Curis, Kite/Gilead, Celgene, Genmab
Research Funding: AbbVie, Pharmacyclics/Janssen, Bayer
Ewa Lech-Maranda
Consulting or Advisory Role: Roche, Amgen, AbbVie, Astellas Pharma, Novartis, Janssen-Cilag, Sanofi/Aventis, Takeda, Gilead Sciences
Bruce D. Cheson
Consulting or Advisory Role: TG Therapeutics, AbbVie, Pharmacyclics/Janssen, Morphosys, Celgene, Karyopharm Therapeutics, Epizyme, Gilead Sciences, SymBio Pharmaceuticals, Parexel, Merck, Glaxo Smith Kline, Beigene, Kite, Reddy Biosimilar, Bayer, Celgene
Research Funding: TG Therapeutics, Trillium Therapeutics, Seattle Genetics, Bristol-Myers Squibb, Gilead Sciences, Pharmacyclics, AbbVie, AstraZeneca
Travel, Accommodations, Expenses: SymBio Pharmaceuticals
Paolo F. Caimi
Consulting or Advisory Role: Genentech, Seattle Genetics, TG Therapeutics, Kite Pharma, ADC Therapeutics, Bayer, Amgen, Verastem, Epizyme
Speakers' Bureau: Celgene
Research Funding: Genentech, ADC Therapeutics
Lori A. Leslie
Consulting or Advisory Role: Kite/Gilead, TG Therapeutics, Celgene/BMS, Seattle Genetics, AbbVie, PCYC/Janssen, ADC Therapeutics, BeiGene, AstraZeneca
Speakers' Bureau: Kite Pharma, Celgene/BMS, Seattle Genetics, AbbVie, PCYC/Janssen, ADC Therapeutics, BeiGene, AstraZeneca, Epizyme, Karyopharm
Julio C. Chavez
Consulting or Advisory Role: Kite/Gilead, Novartis, Karyopharm Therapeutics, MorphoSys, TeneoBio, AbbVie, Janssen
Speakers' Bureau: AstraZeneca, BeiGene, MorphoSys, Epizyme
Research Funding: Merck
Gustavo Fonseca
Honoraria: Amgen, Celgene
Consulting or Advisory Role: Amgen, Celgene, Bayer, Abvie/Pharmacyclics, Dava Oncology, Karyopharm
Speakers' Bureau: Amgen, Celgene
Research Funding: Celgene, Amgen, TG Therapeutics, Verastem Oncology
Travel, Accommodations, Expenses: Amgen, Celgene
Sunil Babu
Stock and Other Ownership Interests: Fort Wayne Medical Oncology & Hematology, Lutheran Hospital
Honoraria: Bristol-Myers Squibb, Alexion Pharmaceuticals, Lilly, Bayer, AstraZeneca
Consulting or Advisory Role: Bristol-Myers Squibb, Alexion Pharmaceuticals, AstraZeneca, argenx, Boehringer Ingelheim, Bayer, Kite Pharma, Janssen Oncology
Speakers' Bureau: Alexion Pharmaceuticals
Research Funding: Bristol-Myers Squibb, Novartis, Genentech/Roche, AstraZeneca/MedImmune, Janssen Oncology, Amgen, TG Therapeutics, AbbVie, Lilly, Alexion Pharmaceuticals, Merck, Novartis, Syndax, Nektar, Sanofi, argenx
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Alexion Pharmaceuticals, Lilly, Janssen Oncology, Genentech/Roche
Daniel J. Hodson
Research Funding: Gilead Sciences
John M. Burke
Consulting or Advisory Role: Genentech/Roche, AbbVie, Seattle Genetics, Bayer, AstraZeneca, Adaptive Biotechnologies, Verastem, MorphoSys, Kura Oncology, Epizyme, BeiGene, Kymera, Novartis
Speakers' Bureau: Seattle Genetics, Beigene
Jeff P. Sharman
Leadership: US Oncology
Consulting or Advisory Role: Pharmacyclics, Celgene, TG Therapeutics, Genentech, AbbVie, Acerta Pharma/AstraZeneca, Beigene, Pfizer
Research Funding: Pharmacyclics, Genentech, Celgene, Acerta Pharma, Gilead Sciences, Seattle Genetics, TG Therapeutics, Merck, Takeda
John M. Pagel
Consulting or Advisory Role: Gilead Sciences, AstraZeneca, Actinium Pharmaceuticals, BeiGene, Loxo
Hari P. Miskin
Employment: TG Therapeutics
Leadership: TG Therapeutics
Stock and Other Ownership Interests: TG Therapeutics
Travel, Accommodations, Expenses: TG Therapeutics
Peter Sportelli
Employment: TG Therapeutics
Leadership: TG Therapeutics
Stock and Other Ownership Interests: TG Therapeutics
Travel, Accommodations, Expenses: TG Therapeutics
Owen A. O'Connor
Employment: TG Therapeutics
Leadership: TG Therapeutics
Board Appointments, Stock, and Other Ownership Interests: TG Therapeutics, Kymera, Myeloid Therapeutics, NomoCan Pharmaceuticals
Honoraria: Mundipharma
Consulting or Advisory Role: Mundipharma Research, Astex Pharmaceuticals
Research Funding: Merck, Celgene/Bristol-Myers Squibb, Seagen, Mundipharma, Astex Pharmaceuticals, ADC Therapeutics
Michael S. Weiss
Employment: TG Therapeutics
Leadership: TG Therapeutics
Stock and Other Ownership Interests: TG Therapeutics
Travel, Accommodations, Expenses: TG Therapeutics
Pier Luigi Zinzani
Consulting or Advisory Role: Sanofi, Verastem, Celltrion, Gilead Sciences, Janssen-Cilag, Bristol-Myers Squibb, SERVIER, Sandoz, MSD, Immune Design, Celgene, Portola Pharmaceuticals, Roche, EUSA Pharma, Kyowa Hakko Kirin, TG Therapeutics, Takeda
Speakers' Bureau: Verastem, Celltrion, Gilead Sciences, Janssen-Cilag, Bristol-Myers Squibb, SERVIER, Sandoz, MSD, Immune Design, Celgene, Portola Pharmaceuticals, Roche, EUSA Pharma, Kyowa Hakko Kirin
No other potential conflicts of interest were reported.
REFERENCES
- 1.Campo E Swerdlow S Harris NL, et al. : The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood 117:5019-5032, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galaznik A Huelin R Stokes M, et al. : Systematic review of therapy used in relapsed or refractory diffuse large B-cell lymphoma and follicular lymphoma. Future Sci OA 4:FSO322, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swerdlow SH Campo E Pileri SA, et al. : The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 127:2375-2390, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2020. CA Cancer J Clin 70:7-30, 2020 [DOI] [PubMed] [Google Scholar]
- 5.Howlader N Noone AM Krapcho M, et al. (eds): SEER Cancer Statistics Review, 1975-2017. Bethesda, MD, National Cancer Institute, https://seer.cancer.gov/csr/1975_2017/, 2020 [Google Scholar]
- 6.Denlinger NM, Epperla N, William BM: Management of relapsed/refractory marginal zone lymphoma: Focus on ibrutinib. Cancer Manag Res 10:615-624, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network : Chronic lymphocytic leukemia/small lymphocytic lymphoma (Version 2.2021). https://www.nccn.org/professionals/physician_gls/pdf/cll.pdf [Google Scholar]
- 8.National Comprehensive Cancer Network : B-Cell Lymphomas (Version 2.2021). https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf [Google Scholar]
- 9.Morrison VA Shou Y Bell JA, et al. : Treatment patterns and survival outcomes in patients with follicular lymphoma: A 2007 to 2015 Humedica database study. Clin Lymphoma Myeloma Leuk 19:e172-e183, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Kritharis A, Sharma J, Evens AM: Current therapeutic strategies and new treatment paradigms for follicular lymphoma. Cancer Treat Res 165:197-226, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Gopal AK Kahl BS de Vos S, et al. : PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med 370:1008-1018, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taverna C Martinelli G Hitz F, et al. : Rituximab maintenance for a maximum of 5 years after single-agent rituximab induction in follicular lymphoma: Results of the randomized controlled phase III trial SAKK 35/03. J Clin Oncol 34:495-500, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivas-Delgado A Magnano L Moreno-Velazquez M, et al. : Response duration and survival shorten after each relapse in patients with follicular lymphoma treated in the rituximab era. Br J Haematol 184:753-759, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Wermer M, Hobeika E, Jumaa H: Role of PI3K in the generation and survival of B cells. Immunol Rev 237:55-71, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Marshall AJ Niiro H Yun TJ, et al. : Regulation of B-cell activation and differentiation by the phosphatidylinositol 3-kinase and phospholipase Cγ pathway. Immunol Rev 176:30-46, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Liu P Cheng H Roberts TM, et al. : Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov 8:627-644, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dreyling M Santoro A Mollica L, et al. : Phosphatidylinositol 3-kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma. J Clin Oncol 35:3898-3905, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Flinn IW Miller CB Ardeshna KM, et al. : DYNAMO: A phase II study of duvelisib (IPI-145) in patients with refractory indolent non-Hodgkin lymphoma. J Clin Oncol 37:912-922, 2019 [DOI] [PubMed] [Google Scholar]
- 19.Sapon-Cousineau V, Sapon-Cousineau S, Assouline S: PI3K inhibitors and their role as novel agents for targeted therapy in lymphoma. Curr Treat Options Oncol 21:51, 2020 [DOI] [PubMed] [Google Scholar]
- 20.Aliqopa: Prescribing information. Bayer HealthCare Pharmaceuticals Inc, 2017. http://labeling.bayerhealthcare.com/html/products/pi/Aliqopa_PI.pdf [Google Scholar]
- 21.Copiktra: Prescribing information. Verastem Inc, 2018. https://copiktra.com/pdf/Copiktra-Prescribing-Information.pdf [Google Scholar]
- 22.Zydelig: Prescribing information. Gilead Sciences, Inc, 2018. https://www.gilead.com/~/media/Files/pdfs/medicines/oncology/zydelig/zydelig_pi.pdf [Google Scholar]
- 23.Curigliano G, Shah RR: Safety and tolerability of phosphatidylinositol-3-kinase (PI3K) inhibitors in oncology. Drug Saf 42:247-262, 2019 [DOI] [PubMed] [Google Scholar]
- 24.Burris HA III Flinn IW Patel MR, et al. : Umbralisib, a novel PI3Kδ and casein kinase-1ε inhibitor, in relapsed or refractory chronic lymphocytic leukaemia and lymphoma: An open-label, phase 1, dose-escalation, first-in-human study. Lancet Oncol 19:486-496, 2018 [DOI] [PubMed] [Google Scholar]
- 25.Buchanan CM, Lee KL, Shepherd PR: For better or worse: The potential for dose limiting the on-target toxicity of PI 3-kinase inhibitors. Biomolecules 9:402, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheson BD O’Brien S Ewer SM, et al. : Optimal management of adverse events from copanlisib in the treatment of patients with non-Hodgkin lymphomas. Clin Lymphoma Myeloma Leuk 19:135-141, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davids MS Kim HT Nicotra A, et al. : Umbralisib in combination with ibrutinib in patients with relapsed or refractory chronic lymphocytic leukaemia or mantle cell lymphoma: A multicentre phase 1-1b study. Lancet Haematol 6:e38-e47, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lunning M Vose J Nastoupil L, et al. : Ublituximab and umbralisib in relapsed/refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 134:1811-1820, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng C Lipstein MR Scotto L, et al. : Silencing c-Myc translation as a therapeutic strategy through targeting PI3Kδ and CK1ε in hematological malignancies. Blood 129:88-99, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaneda MM Messer KS Ralainirina N, et al. : PI3Kγ is a molecular switch that controls immune suppression. Nature 539:437-442, 2016[Erratum: Nature 542:124, 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ali K Soond DR Pineiro R, et al. : Inactivation of PI(3)K p110δ breaks regulatory T-cell-mediated immune tolerance to cancer. Nature 510:407-411, 2014[Erratum: Nature 535:580, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stark AK Davenport ECM Patton DT, et al. : Loss of phosphatidylinositol 3-kinase activity in regulatory T cells leads to neuronal inflammation. J Immunol 205:78-89, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji H Rintelen F Waltzinger C, et al. : Inactivation of PI3Kγ and PI3Kδ distorts T-cell development and causes multiple organ inflammation. Blood 110:2940-2947, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Maharaj K Powers JJ Achille A, et al. : The dual PI3Kδ/CK1ε inhibitor umbralisib exhibits unique immunomodulatory effects on CLL T cells. Blood Adv 4:3072-3084, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Loosdregt J Fleskens V Tiemessen MM, et al. : Canonical Wnt signaling negatively modulates regulatory T cell function. Immunity 39:298-310, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Lampson BL Kasar SN Matos TR, et al. : Idelalisib given front-line for treatment of chronic lymphocytic leukemia causes frequent immune-mediated hepatotoxicity. Blood 128:195-203, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheson BD Fisher RI Barrington SF, et al. : Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol 32:3059-3068, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nastoupil LJ Lunning MA Vose JM, et al. : Tolerability and activity of ublituximab, umbralisib, and ibrutinib in patients with chronic lymphocytic leukaemia and non-Hodgkin lymphoma: A phase 1 dose escalation and expansion trial. Lancet Haematol 6:e100-e109, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davids MS Kuss BJ Hillmen P, et al. : Efficacy and safety of duvelisib following disease progression on ofatumumab in patients with relapsed/refractory CLL or SLL in the DUO Crossover Extension Study. Clin Cancer Res 26:2096-2103, 2020 [DOI] [PubMed] [Google Scholar]
- 40.Davids MS Flinn IW Mato AR, et al. : Long-term integrated safety analysis of umbralisib (TGR-1202), a PI3Kδ/CK1ε inhibitor with a differentiated safety profile, in patients with relapsed/refractory lymphoid malignancies. Poster presented at 23rd Congress of the European Hematology Association (EHA), Stockholm, Sweden, June 14-17, 2018