Abstract
Background
The kinetics of the antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) needs to be evaluated since long-term duration of antibody remains largely unknown, particularly in infected healthcare workers (HCW).
Methods
Prospective study, evaluating the longitudinal profile of anti-SARS-CoV-2 antibody titers in a random sample of 331 seropositive healthcare workers (HCW) of Spanish Hospitals Group. Serial measurements of serum IgG-anti-SARS-CoV-2 were obtained at baseline (April-May,2020), and in 2 follow-up visits. Linear mixed models were used to investigate antibody kinetics and associated factors.
Results
A total of 306 seropositive subjects (median age: 44.7years;69.9% female) were included in the final analysis. After a median follow-up of 274 days between baseline and final measurement, 235(76.8%) maintained seropositivity. Antibody titers decreased in 82.0%, while remained stable in 13.1%. Factors associated with stability of antibodies over time included age≥45 years, higher baseline titers, severe/moderate infection and high-grade exposure to COVID-19 patients. In declining profile, estimated mean antibody half-life was 146.3 days(95%CI:138.6–154.9) from baseline. Multivariate models show independent longer durability of antibodies in HCW with high-risk exposure to COVID-19 patients (+14.1 days;95%CI:0.6–40.2) and with symptomatic COVID-19 (+14.1 days;95%CI:0.9–43.0). The estimated mean time to loss antibodies was 375(95% CI:342–408) days from baseline.
Conclusions
We present the first study measuring the kinetics of antibody response against SARS-CoV-2 in HCW beyond 6 months. Most participants remained seropositive after 9 months but presented a significant decline in antibody-titers. Two distinct antibody dynamic profiles were observed (declining vs. stable). Independent factors associated with longer durability of antibodies were symptomatic infection and higher exposure to COVID-19 patients.
Kewords: SARS-CoV-2, COVID-19, Antibodies, Antibody kinetics, Immune-response, Durability, Persistence, Healthcare Workers, Healthcare Personnel
Introduction
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has led to an unprecedented health emergency, causing over 125 million confirmed cases and over 2.5 million deaths worldwide as of March 2021 [1]. Understanding the long-term immunological response in infected individuals will be central in defeating the COVID-19 pandemic, and understanding humoral kinetics, timing, and persistence of SARS-CoV-2 antibodies after natural infection, will be essential to ensure an appropriate vaccination strategy.
Reports studying the immunological response in previous coronaviruses outbursts have shown that after infection, a specific IgG response is elicited and sustained for 1–2 years declining thereafter, [2,[3] but available data for SARS-CoV-2 is very limited beyond 4 months [4], especially in health care workers (HCW), a population particularly exposed to SARS-CoV-2 with potential high risk for reinfection. Indeed, the scarce published longitudinal data on seroprevalence of HCW shows a wide variability on the percentage of subjects who remain seropositive after 4 months of infection, ranging from 42 to 96% in predominantly small follow up cohorts [5], [6], [7], [8]. Furthermore, there is almost no data about asymptomatic infected HCW.
To the best of our knowledge, this is the first study to evaluate the durability and to characterize the longitudinal profile of anti-SARS-CoV-2 antibody levels beyond 6 months in a large cohort of seropositive health personnel. In this context, we conducted a 10-month follow-up serological quantitative study to determine the kinetics of the humoral response against SARS-CoV-2 infection in a representative sample of over 300 seropositive HCW of Grupo HM Hospitales (GHM), a chain of 17 Hospitals across 4 regions of Spain. Furthermore, we analyzed different variables to identify clinical and demographic factors associated with stability/declining of antibody levels and seroreversion.
The current work expands on a transversal study of seroprevalence of the entire population of workers of Grupo HM-Hospitales, over 6000 HCW, to identify both symptomatic and asymptomatic individuals who presented anti-SARS-CoV-2 antibodies, the results of which have been recently published [9].
Methods
Study design, population, setting and procedures
Prospective study, measuring the evolution of serum IgG anti-SARS-CoV-2 titers among employees of the GHM who presented a positive result for SARS-CoV-2 antibodies after the first COVID-19 pandemic wave in Spain. The baseline study was carried out between April-June,2020, including 6038 employees (mean age:43.8;71%female) and 662(11.0%) presented IgG anti-SARS-CoV-2 at baseline (39% asymptomatic), the results of which have been published [9].
A sample corresponding to 50% of all seropositive HCW was randomly selected by simple random sampling from the baseline study, and its longitudinal profile of anti–SARS-CoV-2 antibodies was evaluated (n = 331). Serial blood measurements were collected at baseline (April13th–May28th,2020) and in 2 follow-up visits (last measurement: January12th-February9th,2021). Those HCW with a significant increase in the antibody titer during the follow-up were specifically studied to rule out reinfection by evaluating clinical data and performing PCR test.
In January 2021, the COVID-19 vaccination of all healthcare personnel was implemented in all Grupo HM-Hospitales workers and, as a result, 185 (55.4%) workers of the study received the first dose before the last serological extraction, with a median (IQR) time between the vaccination (first dose) and the third serological sample of 11 (8–13) days, without no impact in antibody titers waning (slopes between the last two measurements were −0.49[−0.71,−0.21]A.U./month for not vaccinated and −0.34[−0.71,−0.19]A.U./month for vaccinated individuals [Mann-Whitney U test, p = 0.17]; Figure S1 in the Supplement).
Quantification of antibodies against SARS-CoV-2
We used the Enzyme Immunoassay (ELISA) developed by DIA.PRO to measure serum anti-nucleocapsid (anti-N) and anti-spike (anti-S) protein IgG antibody titers against SARS-CoV-2 in a venous blood sample for all the measurements of the study. This serum test has a clinical sensitivity estimated at 98%−100% and a specificity >98% (Enzyme immunoassay for the determination of IgG antibodies to COVID-19 in human serum and plasma DIA.PRO IFU). Serum IgG titers were considered negative (non-reactive) with a result less than 0.90 arbitrary units (AU)/mL (<0.90 AU/mL), positive (reactive) with a result greater than 1.10 AU/mL (>1.10AU/mL) and indeterminate with a result in the interval between 0.900 and 1.10(0.90≤x ≤ 1.10AU/mL).
The ELISA test used detects IgG antibodies against specific subunits of spike and nucleocapsid proteins with a stablished proportion in the solid phase. This specificity would explain in part why the antibody titers are not affected in vaccinated subjects as compared with other available kits.
Statistical analysis
Individuals were classified according to their antibody profile over time as: stable (n = 40) when the variation in antibody titers were lower than 25%, declining (n = 251) when the loss of antibody titers was higher than 25% overtime and others (n = 15) for those profiles that show an increase of more than 25% in antibody titer at some time point.
Summary statistics were performed as absolute and relative frequencies (%). Chi-squared tests (or Fisher tests when needed) were used to study the dependence between stable and declining profiles and age (categorized by median), sex, symptoms, infection category, grade of exposure to COVID-19, receiving SARS-CoV-2 vaccine or not and baseline antibody titer (categorized by terciles). Those variables with a p value <0.2 were considered for univariable and multivariable logistic regression with the antibody profile (stable or declining) as dependent variable.
Linear mixed models were used to investigate antibody waning for those individuals with declining profile. Assuming antibody levels fell exponentially, the natural log transformed titers were modelled over time. Correlated random intercept and slope were allowed. The univariable effect of covariates, including age, sex, grade of exposure to COVID-19, infection category and symptoms, on the fixed effect intercept and slope was analysed. A multivariable model including all the analysed covariates was performed to study independent effects on antibody waning. Analyses were performed using R 4.0.4 and the lme4 library [10].
Seroreversion was defined as the loss of antibody titers below the diagnostic threshold of 1.1A.U. The association of the different variables with seroreversion was analysed using Chi-squared test or Fisher test if required.
Results
Three hundred and six HCW were included in the final analysis (25 subjects were excluded due to incomplete data). Serological testing was performance between 13-April 2020 and 9-February 2021, with a median (IQR) [range] time from the baseline to last measurement of 274 (265–280) [156–294] days. Median age was 44.7 years (IQR 34.9–53.2; range 21.3 to 80.2 years) and 69.9% were females. Demographic and clinical characteristics are summarized in Table 1 .
Table 1.
Demographic and clinical characteristics, by antibody dynamic profile.
| All 1 (n = 306) | Declining (n = 251) | Stable (n = 40) | P value | ||
|---|---|---|---|---|---|
| Age (years) | < 45 years | 156 (51.0%) | 135 (88.2%) | 9 (5.8%) | 0.0004 |
| ≥45 years | 150 (49.0%) | 111 (75.5%) | 31 (20.7%) | ||
| Sex | Female | 214 (69.9%) | 183 (85.5%) | 23 (10.7%) | 0.071 |
| Male | 92 (30.1%) | 68 (73.9%) | 17 (18.5%) | ||
| Baseline IgG anti-SARS-CoV-2 Antibody Titer | T1 | 102 (33.3%) | 88 (86.3%) | 2 (2.0%) | < 0.0001 |
| T2 | 101 (33.0%) | 92 (91.1%) | 6 (5.9%) | ||
| T3 | 103 (33.7%) | 71 (68.9%) | 32 (31.1%) | ||
| Exposure Risk | Non-high grade | 94 (30.7%) | 84 (89.4%) | 6 (6.4%) | 0.031 |
| High grade | 212 (69.3%) | 167 (78.8%) | 34 (16.0%) | ||
| COVID-19 Symptoms | No | 128 (41.8%) | 110 (85,9%) | 12 (9.4%) | 0.14 |
| Yes | 178 (58.2%) | 141 (79,2%) | 28 (15.7%) | ||
| Infection Category | Asymptomatic/ Mild | 281 (91.8%) | 235 (83,6%) | 31 (11,0%) | 0.003 |
| Moderate/Severe | 25 (8.2%) | 16 (64,0%) | 9 (36,0%) | ||
| Vaccinated | No | 121 (39.5%) | 95 (78,5%) | 21 (17,4%) | 0.11 |
| Yes | 185 (60.5%) | 156 (84,3%) | 19 (10,3%) |
COVID-19: Coronavirus Disease 2019; SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; IgG: Immunoglobulin G.
Fifteen subjects (4.9%) showed a significant increase (>25%) in antibody titer and were excluded from the analysis.
Dynamic antibody profile
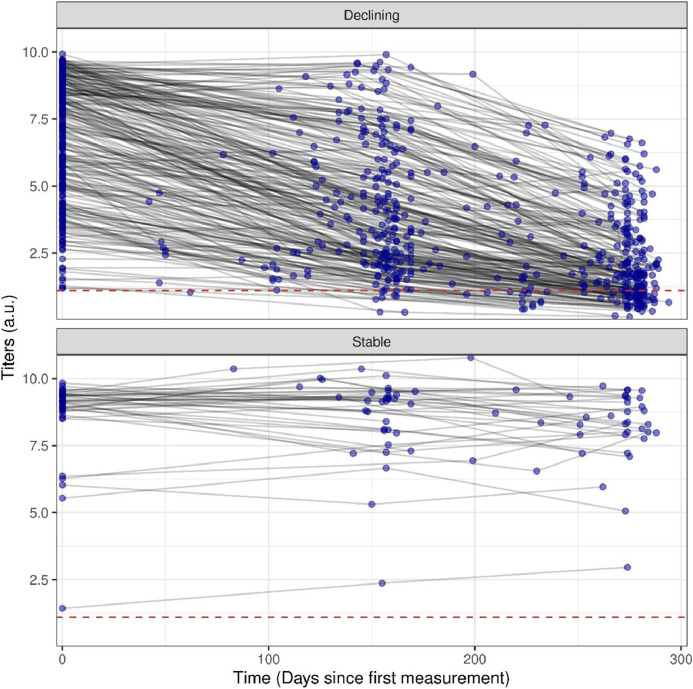
We identified two distinct antibodies dynamic profiles according to the quantitative antibody concentration kinetics, which we have categorized as: declining, which included those subjects who presented a significant decrease in antibody titers (defined as a decline > 25% between the first and the last serological testing) (n = 251; 82.0%); and stable, for those who presented a variation in antibody titers less than 25% throughout the study period (n = 40; 13.1%). Fig. 1 shows the tendency of IgG antibody levels over time, from baseline visit (April-June 2020) to last follow-up visit (January-February 2021), while Table 1 shows the demographic and clinical characteristics according to antibody dynamic profile.
Fig. 1.
Declining and Stable dynamic profiles, according to the rate of change IgG anti SARS-CoV-2 antibody levels over time, from baseline visit (April-June 2020) to last follow-up visit (January-February 2021).
Fifteen subjects (4.9%) showed a significant increase (>25%) in antibody titer (Figure S2 in the Supplement shows their dynamic profile). We believe this increase probably represents either baseline measurement being carried out during the increasing phase for IgG or possible re-infection (these HCW were specifically evaluated to detect reinfection), and these subjects were therefore excluded from the analysis.
Factors associated with persistence/declining anti-SARS-CoV-2 IgG antibody levels are shown in Table 2 , highlighting that stable profile was observed more frequently in the third tercile of baseline levels of antibody titers (p <0.0001), age > 45 (p = 0.0004), high-grade risk exposure (p = 0.031), and moderate/severe infection (p = 0.003). Univariate logistic regression models identified age ≥ 45 years (OR:4.1; 95% CI: 1.9 - 9.4; p = 0.0005), third tercile of baseline antibody level (OR= 19.8; 95% CI: 5.7 - 125; p<0.0001), high-risk exposure (OR:2.9; 95% CI:1.2–7.8; p = 0.024) and moderate/severe infection (OR:4.3;95% CI: 1.7–10.3; p = 0.001) as variables significantly associated with stable/non-declining antibody titers. The multivariate logistic regression model showed that age, baseline antibody titer and exposure risk are independently and positively associated with the stable antibody profile.
Table 2.
Univariate and multivariate logistic regression models for identification factors associated with a stable profile.
| Univariate Model | P value | Multivariate Model | P value | ||
|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | ||||
| Age (years) | < 45 years | Ref. | – | Ref. | – |
| ≥45 years | 4.1 (1.9 - 9.4) | 0.0005 | 3.1 (1.3 - 8.0) | 0.012 | |
| Sex | Female | Ref. | – | Ref. | – |
| Male | 2.0 (1.0 - 3.9) | 0.05 | 1.7 (0.7 - 3.8) | 0.23 | |
| Baseline IgG anti-SARS-CoV-2 Antibody Titer | T1 | Ref. | – | Ref. | – |
| T2 | 2.9 (0.6 - 19.9) | 0.20 | 2.5 (0.5 - 17.8) | 0.28 | |
| T3 | 19.8 (5.7 - 125.0) | < 0,0001 | 13.0 (3.6 - 84.1) | 0.0008 | |
| Exposure Risk | Non-high grade | Ref. | – | Ref. | – |
| High grade | 2.9 (1.2 - 7.8) | 0.024 | 2.8 (1.1 - 8.3) | 0.037 | |
| Infection Category | Asymptomatic/ Mild | Ref. | – | Ref. | – |
| Moderate/Severe | 4.3 (1.7 - 10.3) | 0.001 | 2.2 (0.7 - 6.9) | 0.16 | |
| COVID-19 Symptoms | No | Ref. | – | Ref. | – |
| Yes | 1.8 (0.9 - 3.9) | 0.10 | 1.4 (0.6 - 3.5) | 0.51 | |
| Vaccinated | No | Ref. | – | Ref. | – |
| Yes | 0.55 (0.28 - 1.1) | 0.082 | 0.66 (0.30 - 1.4) | 0.29 |
COVID-19: Coronavirus Disease 2019; SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; IgG: Immunoglobulin G.
Kinetics on declining of antibodies
We considered the antibody titer of the first measurement as the baseline antibody levels. It is worth mentioning that 75 individuals reported a positive PCR with median (IQR) of 28 (22 - 37) days prior to the first measurement.
The mean trajectory of IgG antibody levels in each individual with declining profile was modelled using a linear mixed model (Fig. 2 A, Table 3 , Table S1). Estimated mean antibody half-life was 146.3 (95%CI: 138.6–154.9) days from baseline. The estimated mean baseline antibody titer was 6.55 (95% CI: 6.20 - 6.93) arbitrary units (A.U.). Initial antibodies titer were correlated with longer estimated antibody half-lives (Fig. 2B, Spearman's rank R2 = 0.55, p < 0.0001).
Fig. 2.
Linear mixed model for the SARS-CoV-2 IgG antibodies decline. A) Overall mean trajectory from the baseline observed level. Mean trajectory and 95% confidence interval are shown as solid line and dashed area. The dashed grey line represents the limit of the observed period of time and the dashed red line represents the titer threshold of 1.1 A.U. B) Comparison of baseline observed titers and the estimated antibody half-life per subject.
Table 3.
Model to estimate mean half-life of antibodies and its variation considering demographic and clinical characteristics.
| Univariate model | Multivariate model | ||||
|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | ||
| Baseline model | Initial titer | 6.55 | (6.20 - 6.93) | 6.05 | (5.31 - 6.89) |
| half-life | 146.3 | (138.6 - 154.9) | 121.4 | (109.1 - 136.6) | |
| Age model | Initial titer: < 45 years | 6.49 | (6.03 - 6.98) | – | – |
| half-life: < 45 years | 141.1 | (131.4 - 152.2) | – | – | |
| Δ Initial titer: > 45 years | 0.36 | (−0.32 - 1.23) | 0.33 | (−0.30 - 1.23) | |
| Δ half-life: > 45 years | 10.1 | (−5.0 - 35.5) | 10.5 | (−1.4 - 33.2) | |
| Sex model | Initial titer: Male | 6.81 | (6.12 - 7.57) | – | – |
| half-life: Male | 146.2 | (132.3 - 163.5) | – | – | |
| Δ Initial titer: Female | −0.34 | (−0.99 - 0.57) | 0.35 | (−0.35 - 1.36) | |
| Δ half-life: Female | 0.1 | (−13.3 - 26.6) | −0.5 | (−9.7 - 17.5) | |
| Exposure Risk model | Initial titer: Low risk | 6.48 | (5.89 - 7.12) | – | – |
| half-life: Low risk | 135.8 | (124.7 - 149.1) | – | – | |
| Δ Initial titer: High risk | 0.12 | (−0.56 - 1.03) | 0.22 | (−0.41 - 1.12) | |
| Δ half-life: High risk | 16.3 | (−0.1 - 46.4) | 14.1 | (0.6 - 40.2) | |
| Infection Category model | Initial titer: Asymptomatic/Mild | 6.53 | (6.17 - 6.92) | – | – |
| half-life: Asymptomatic/Mild | 145.6 | (137.7 - 154.4) | – | – | |
| Δ Initial titer: Moderate/ Severe | 0.36 | (−0.97 - 2.24) | 0.07 | (−1.01 - 1.82) | |
| Δ half-life: Moderate/ Severe | 11.3 | (−17.2 - 70.5) | 3.1 | (−13.9 - 43.0) | |
| COVID-19 Symptoms model | Initial titer: No | 6.26 | (5.76 - 6.80) | – | – |
| half-life: No | 138.0 | (127.9 - 149.8) | – | – | |
| Δ Initial titer: Yes | 0.54 | (−0.16 - 1.45) | 0.34 | (−0.30 - 1.25) | |
| Δ half-life: Yes | 15.4 | (−0.5 - 43.1) | 14.1 | (0.9 - 39.8) | |
COVID-19: Coronavirus Disease 2019; SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; IgG: Immunoglobulin G.
The effect of demographic and other covariates on the antibody's trajectories was marginal for longer half-lives for age, high-risk exposure and previous COVID-19 symptoms (Table 3; Table S1 and Figure S3–4 in the Supplement). Multivariate model showed that high-risk exposure to COVID-19 patients (+14.1 days; 95%CI:0.6–40.2) and previous COVID-19 symptoms (+14.1 days; 95%CI: 0.9 −39.8) were independently associated with higher antibodies durability. Age ≥ 45 years (+10.5 days;95%CI:−1.4–33.2) showed marginal evidence of association with longer half-life of the antibodies.
Table S2 in the Supplement shows the median (IQR) for baseline, last follow-up and change in IgG antibody titer, considering exposure risk to COVID-19 patients, infection category and baseline levels of antibodies.
Seroreversion
The percentage of participants who experienced seroreversion (defined as an antibody titer >1.1 AU/mL at baseline and ≤1.1AU/mL at the follow-up visits) was 23.2% (71/306).
Median (IQR) time to seroreversion was 275 (224–280) days, as most seroreversion events were observed at the third visit. Seroreversion percentages, depending on demographics and other covariates are summarized in Table 4 .
Table 4.
Demographic and clinical characteristics, by seroreversion status.
| Non-seroreversion (n = 235) | Seroreversion (n = 71) | P value | ||
|---|---|---|---|---|
| Age (years) | < 45 years | 112 (71.8%) | 44 (28.2%) | 0.045 |
| ≥45 years | 123 (82.0%) | 27 (18.0%) | ||
| Sex | Female | 161 (75.2%) | 53 (24.8%) | 0.4 |
| Male | 74 (80.4%) | 18 (19.6%) | ||
| Baseline IgG anti-SARS-CoV-2 Antibody Titer | T1 | 49 (48.0%) | 53 (52.0%) | < 0.0001 |
| T2 | 83 (82.2%) | 18 (17.8%) | ||
| T3 | 103 (100.0%) | 0 (0.0%) | ||
| Exposure Risk | Non-high grade | 70 (74.5%) | 24 (25.5%) | 0.62 |
| High grade | 165 (77.8%) | 47 (22.2%) | ||
| COVID-19 Symptoms | No | 96 (75.0%) | 32 (25.0%) | 0.62 |
| Yes | 139 (78.1%) | 39 (21.9%) | ||
| Infection Category | Asymptomatic/ Mild | 211 (75.1%) | 70 (24.9%) | 0.034 |
| Moderate/Severe | 24 (96.0%) | 1 (4.0%) | ||
| Vaccinated | No | 99 (81.8%) | 22 (18.2%) | 0.12 |
| Yes | 156 (84,3%) | 19 (10,3%) |
Statistical inference was performed using Chi-squared test or Fisher test when needed.
COVID-19: Coronavirus Disease 2019; SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; IgG: Immunoglobulin G.
In order to have a projection of the time to loss of antibodies, we used a linear mixed baseline model in order to extrapolate the mean time to cross the threshold of 1.1A.U. (mean time to loss antibodies), finding that, on average, subjects will have detectable concentrations of IgG anti SARS-COV-2 up to 375 (95%CI:342–408) days from baseline (Fig. 2A).
Reinfection
Fifteen subjects did not present a declining or stable longitudinal antibody profile. Evaluating their profile and available clinical data, we found one probable case of PCR-documented reinfection (Table S3 in the Supplement).
Discussion
The aim of the current study was to investigate the immunological response in antibody levels over time (mean follow up over nine months) in a large and representative cohort of more than 300 HCW who tested positive for IgG anti-SARS-CoV-2 antibodies after the first wave of COVID-19 outbreak in Spain. To the best of our knowledge, this is the first report evaluating the kinetics of SARS-CoV-2 antibodies in HCW beyond 6 months. These results provide further insight into the nature of post-infection immunity.
The results showcase a high percentage of healthcare personnel maintaining detectable antibody levels beyond 9 months after infection (median follow-up 274 days) with more than 75% of subjects remaining seropositive, but with a significant decline in antibody levels in most of them. Only 13.1% of the population presented stable concentrations of antibodies in the study follow up period. A recent work carried out in HCW in Britain estimated a mean antibody half-life of 85 days from the maximum positive IgG titer, after a median follow-up time of 4 months [8]. The results of our study expand on this observation and demonstrate a significant longer durability of antibodies with a mean half-life of 146 days from the first detectable measurement, after a mean follow-up higher than 9 months.
Our results are inconsistent with previous works in HCW which included significantly shorter follow up periods. Some studies reported a rapid loss of SARS-CoV-2 specific antibodies within the first three months after infection, alerting about a short-lasting humoral protection [6,7]. These differences may reflect, among other issues, differences in baseline characteristics of the study population (symptomatic category of SARS-CoV2 infection, age, baseline antibody titers…) and, more importantly, different sensitivity of the immunoassays used to detect specific antibodies against SARS-CoV-2. One of the strengths of the current work is that the same immunoassay (from the same company, with same badge of production) was used in the complete population throughout the study testing for both serum anti-nucleocapsid (anti-N) and anti-spike (anti-S) protein IgG antibodies.
Here, we report a novel finding about the dynamic profile of the humoral response against SARS-CoV-2. While most subjects present a declining profile, a subgroup shows a stable (non-declining) antibody titer profile during at least the first 10 months after infection. Further characterization of this stable profile shows a significant correlation with high baseline antibody levels, but other factors as well. Interestingly, a higher degree of exposure to COVID-19 patients is associated to stability in antibody levels, with stable profile being observed more predominantly in first line HCW. This thought-provoking observation may be signaling a potential mechanism of viral re-exposure in maintaining antibody levels in HCW directly involved with the care of COVID-19 patients.
Information of antibody titers beyond 4 months after first positivity is also scarce in the general population. Most of the cohorts with up to 5–6 months follow-up have showed high percentages in persistence of seropositivity (80% or higher) [11], [12], [13], [14], [15], [16]. However, the majority of them have also reported a relevant decrease in antibody titers over time, with one of them estimating a 2-fold decrease of IgG antibodies time of 158 days [15]. In our cohort, the model estimated a mean time to loss antibodies of 375 days from baseline. This finding is in the line of studies in previous SARS-CoV and MERS-CoV outbrakes in which specific IgG antibodies were shown to persist for 1–2 years post-infection and decline thereafter [2,3].
Factors associated with stability in antibody titers
Higher baseline antibody titers have been associated with an increased probability to sustain detectable antibody levels over time compared to lower baseline antibody levels [6], [7], [8]. In the current study, baseline antibody levels were positively correlated with half-lives of antibody and HCW who presented baseline antibody levels in the third tercile showed a significant longer half-life of antibodies (Fig. 2B). This finding has been associated with the severity of SARS-CoV-2 infection. Previous reports have described that levels of antibodies against SARS-CoV-2 in the acute phase correlate to clinical disease severity and then decline after 3 months since onset of illness [17], [18], [19]. While a recent study reported that the antibodies are persistent for at least 6 months in severe COVID-19 patients,[20] reports in asymptomatic infection and pauci-symptomatic disease have showed a weak and non-sustained humoral immunity response against SARS-CoV-2 [21,22].
Our findings, expanding previous reports, suggest that moderate/severe COVID-19 patients present significantly higher levels of baseline antibodies, but no significant difference in the decreasing rate over time, compared to subjects with mild COVID-19 (half-life of antibodies was significantly longer in symptomatic compared with asymptomatic COVID-19, but not in moderate/severe compared to asymptomatic/ mild infection). In contrast, HCW with high-grade risk exposure to COVID-19 patients showed not only significantly more frequency of stable dynamic antibody response over time, but also longer antibody half-life and lower estimated monthly decrease in antibody titers compared to HCW with low-grade exposure (Table S2). This finding adds validity to the previously mentioned mechanism that viral re-exposure could be playing a role in this more stable dynamic antibody profile in first line HCW.
Finally, in our study, age above 45 was also independently associated with stable dynamic antibody profile and longer antibody half-life. This finding is consistent with a previous study reporting that increasing age was associated with longer durability of anti-SARS-CoV-2 antibodies [8].
Reinfection
Whether the rate of decline in anti-SARS-CoV-2 antibodies is associated with an increased risk of reinfection and disease remains to be determined. This issue has special implications in first line HCW, since their inherent particular risk due to close contact with COVID-19 patients. In the current work we detected a single case of possible reinfection. This is in the line with the findings of Lumley et al. who reported that the presence of anti-SARS-CoV-2 antibodies is associated with a significant reduction in risk of SARS-CoV-2 reinfection in the ensuing 6 months, with only 2 asymptomatic cases of re-infection and no symptomatic infection detected in seropositive subjects [23].
Implications
The results of the current study suggest an important role for serological screening and follow-up and may help in our understanding of the humoral response in HCW exposed to SARS-COV-2. These in turn may have potential implications in the current vaccination strategy. Results from ongoing long-term studies will help better understand the kinetics of the humoral response and their association to clinical manifestations of the disease.
Limitations
The current study has several limitations. One of the main is that we have non-acute information on timing of infection onset to evaluate antibody kinetics. However, our study is based on a real-world HCW cohort with a wide range of symptoms, including 40% of asymptomatic infected HCW, and in this setting does not allow to accurately obtain this information. Another limitation that needs to be pointed out is that the methodology used quantitatively measures antibodies against the spike and nucleocapsid proteins of SARS-CoV-2 (anti-S and anti-N antibodies) but does not identify the presence of neutralizing antibodies. The measurement of anti-S and anti-N antibodies is much more accessible in a general clinical setting, and supporting this strategy, several reports have shown that anti–SARS-CoV-2 antibodies to the spike protein correlates with neutralizing antibodies and are responsible for neutralizing activity against SARS-CoV-2 [24,25].
Conclusions
In conclusion, our results demonstrate that most of infected HCW maintain seropositivity levels of IgG anti-SARS-CoV-2 antibodies after 9 months, but with a significant decline in levels. A subgroup of subjects shows a stable, sustained (non-declining) dynamic profile over time, associated to high baseline antibody titers, higher age and high-grade exposure to COVID-19 patients. A longer durability in half-life of antibodies was associated with symptomatic infection and high-risk exposure to COVID-19 patients. Finally, based on the current results, we were able to extrapolate a time to loss of detectable antibody titers of 375 days, in line with the immunological response demonstrated in previous coronarviridae outbrakes.
Ethics approval
The protocol was approved by the Ethics Committee of HM Group (GHM) (Comité Ético de Investigación con Medicamentos de HM Hospitales) (ref. no. 20.04.1611/1640-GHM).
Funding
This work has not received any funding
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author
Author contributions
All authors conceptualized and designed the study, J.F.V., R.M. and J.M.C. drafted the manuscript and made final revisions, and all authors critically revised, read and approved the final manuscript.
Declaration of Competing Interest
Declared No Conflict of Interest
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ejim.2021.05.028.
Appendix. Supplementary materials
References
- 1.World Health Organization (WHO). Coronavirus disease (COVID-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- 2.Lin Q., Zhu L., Ni Z., Meng H., You L. Duration of serum neutralizing antibodies for SARS-CoV-2: lessons from SARS-CoV infection. J Microbiol Immunol Infect. 2020;53(5):821–822. doi: 10.1016/j.jmii.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O Murchu E., Byrne P., Walsh K.A., et al. Immune response following infection with SARS-CoV-2 and other coronaviruses: a rapid review. Rev Med Virol. 2021;31(2):e2162. doi: 10.1002/rmv.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arkhipova-Jenkins I., Helfand M., Armstrong C., et al. Antibody Response After SARS-CoV-2 Infection and Implications for Immunity: a Rapid Living Review. Ann Intern Med. 2021 Mar 16 doi: 10.7326/M20-7547. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel M.M., Thornburg N.J., Stubblefield W.B., et al. Change in antibodies to SARS-CoV-2 over 60 days among health care personnel in Nashville, Tennessee. JAMA. 2020;324:1781–1782. doi: 10.1001/jama.2020.18796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Self W.H., Tenforde M.W., Stubblefield W.B., et al. Decline in SARS-CoV-2 antibodies after mild infection among frontline health care personnel in a multistate hospital network—12 States, April–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1762. doi: 10.15585/mmwr.mm6947a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marot S., Malet I., Leducq V., et al. Rapid decline of neutralizing antibodies against SARS-CoV-2 among infected healthcare workers. Nat Commun. 2021;12:1–7. doi: 10.1038/s41467-021-21111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lumley S.F., Wei J., O’Donnell D., et al. The duration, dynamics and determinants of SARS-CoV-2 antibody responses in individual healthcare workers. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varona J.F., Madurga R., Peñalver F., et al. Seroprevalence of SARS-CoV-2 antibodies in over 6000 healthcare workers in Spain. Int J Epidemiol. 2021;50(2):400–409. doi: 10.1093/ije/dyaa277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bates D., Mächler M., Bolker B., Walker S. Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw. 2015;67(1):1–48. [Google Scholar]
- 11.Wajnberg A., Amanat F., Firpo A., et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370(6521):1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sherina N., Piralla A., Du L.W., et al. Persistence of SARS-CoV-2-specific B and T cell responses in convalescent COVID-19 patients 6-8 months after the infection. Med (N Y) 2021;2(3):281–295. doi: 10.1016/j.medj.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pradenas E., Trinité B., Urrea, et al. Stable neutralizing antibody levels 6 months after mild and severe COVID-19 episodes. Med (N Y) 2021;2(3):313–320. doi: 10.1016/j.medj.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dan J.M., Mateus J., Kato Y., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.den Hartog G., Vos E.R., van den Hoogen L.L., et al. Persistence of antibodies to SARS-CoV-2 in relation to symptoms in a nationwide prospective study. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He Z., Ren L., Yang J., et al. Seroprevalence and humoral immune durability of anti-SARS-CoV-2 antibodies in Wuhan, China: a longitudinal, population-level, cross-sectional study. Lancet. 2021 Mar 20;397(10279):1075–1084. doi: 10.1016/S0140-6736(21)00238-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeewandara C., Jayathilaka D., Gomes L., et al. SARS-CoV-2 neutralizing antibodies in patients with varying severity of acute COVID-19 illness. Sci Rep. 2021;11:1–7. doi: 10.1038/s41598-021-81629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Legros V., Denolly S., Vogrig M., et al. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cell Mol Immunol. 2021;18(2):318–327. doi: 10.1038/s41423-020-00588-2. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seow J., Graham C., Merrick B., et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020;5:1598–1607. doi: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han Y., Liu P., Qiu Y., et al. Effective virus-neutralizing activities in antisera from the first wave of survivors of severe COVID-19. JCI insight. 2021;6(4):e146267. doi: 10.1172/jci.insight.146267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anichini G., Gandolfo C., Terrosi C., et al. Antibody response to SARS-CoV-2 in infected patients with different clinical outcome. J Med Virol. 2021;93(4):2548–2552. doi: 10.1002/jmv.26789. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long Q.-.X., Tang X.-.J., Shi Q.-.L., et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 23.Lumley S.F., O’Donnell D., Stoesser N.E., et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384:533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zost S.J., Gilchuk P., Case J.B., et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature. 2020;584:443–449. doi: 10.1038/s41586-020-2548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ju B., Zhang Q., Ge J., et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584:115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author