Abstract
Background
There are few studies demonstrating how the effectiveness of various extents of non-pharmaceutical interventions (NPIs) before and after vaccination periods. The study aimed to demonstrate such an effectiveness in the alteration of the epidemic curves from theory to practice.
Methods
The empirical data on the daily reported COVID-19 cases were extracted from open source. A computer simulation design in conjunction with the susceptible-exposed-infected-recovered (SEIR) type model was applied to evaluating confinement measures in Italy with adjustment for underreported cases; isolation and quarantine in Taiwan; and NPIs and vaccination in Israel.
Results
In Italy scenario, the extents of confinement measures were 34% before the end of March and then scaled up to 70% after then. Both figures were reduced to 22–69% after adjusting for underreported cases. Approximately 44% of confinement measures were implemented in the second surge of pandemic in Italy.
Fitting the observational data on Taiwan assuming the initial outbreak similar to Wuhan, China, 44% of isolation and quarantine were estimated before March 23rd, 2020. Isolation and quarantine were scaled up to 90% and at least 60% to contain community-acquired outbreaks from March 24th, 2020 onwards. Given 15% monthly vaccination rate from December 2020 in Israel, the effectiveness estimates of reducing the infected toll were 36%, 56%, and 85% for NPIs alone, vaccination alone, and both combined, respectively.
Conclusion
We demonstrated how various NPIs stamp out and delay the epidemic curve of COVID-19. The optimal implementation of these NPIs has to be planned before wide vaccine uptake worldwide.
Keywords: COVID-19, Epidemic, Non-pharmaceutical interventions, Vaccination, SEIR model
Introduction
The third novel coronavirus, coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged in Wuhan, China since December 2019. As of April 15, 2021, approximately 120 million people were infected with COVID-19 and the death toll was more than 2.65 million.1 Following the accompanying article is the same special issue, the first epidemic wave of COVID-19 hit China. The second epidemic wave hit South Korea, Italy and Iran. Only about one month later, the outbreak of COVID-19 rapidly swept through most European countries followed by the United States and Africa. The efforts made to completely contain the spread from hotspot to hotspot to avert pandemic have failed. In Italy, which was one of the countries with a large outbreak, confirmed cases doubled from 41,035 to 86,498 in just one week (from March 19 to March 27, 2020) during the first surge period on region level (from February 1, 2020) in Lombardy. In the second surge, COVID-19 swept Italy entirely from October 1, 2020 onwards in the wake of the emerging UK variant (B.1.1.7) that dominated the origin lineage (D614G).2 The rapid growth of confirmed cases has crowded some hospitals, enforcing emergency rooms to be closed to avoid newly diagnosed patients. The capacity of health care system, including medical crews as doctors and nurses as well as basic medical equipment like respirator masks, gown and mechanical ventilators, was insufficient. In Italy, about 100,000 student doctors were permitted to omit their final exams and rushed into service to support the struggling health care system.3 The lack of medical resources partially led to the case-fatality rate as relatively high as 12.7% in Italy comparing with other countries.1 , 4 Note that such a case-fatality rate may be biased as underreported cases were very likely and should be adjusted. The devastating situation also occurred in Hubei province of China. The mortality rate of COVID-19 in Hubei was significantly higher than that of other regions in China.5 As the situation in Italy showed that the rate at which people contracted COVID-19 was crucial to whether the medical infrastructure was sufficient to cope with the outbreak.
These fatal sequelae of COVID-epidemic called for the necessity of implementing non-pharmaceutical interventions (NPIs), such as confinement measures by lockdown as seen in Italy, and isolation and quarantine as seen in many countries like Taiwan during pandemic period and Israel even in the period of the roll-out of vaccine.
In the theory of infectious epidemiology, the provision of NPIs would flatten the epidemic curve, leading to a lower rate of spread to buy time for developing mitigation strategies such as social distancing and preparedness for personal protection and hygiene in order to stamp out the subsequent epidemic wave during the prolonged period of epidemic curve after initial outbreak. The curve refers to the projected number of people who would be contracted by COVID-19 over a period of time. The flatter curve also means the slower transmission rate. The implementation of these interventions can slow down the spread rate of virus so that fewer infected people seek medical advice simultaneously and the health care system can afford to take care of the sick. The slower transmission rate can avoid the overload of health care system beyond its capacity. The implementation of NPIs is also important for the country with the roll-out of vaccine like nationwide mass vaccination campaigns using newly developed vaccines with emergency use authorization initiated against COVID-19 since December, 2021 in Israel.6
Here, we applied a computer simulation study design to simulate three scenarios with various NPIs before and after vaccination period in Italy, Taiwan and Israel and demonstrated how confinement measures and isolation and quarantine with and without vaccination flatten the epidemic curve and evaluate the corresponding results of effectiveness by using a Susceptible-Exposed-Infected-Recovered (SEIR) model.
Materials and methods
The computer simulated study design with various NPIs
The reproductive numbers of Italy and Israel were estimated before the confinement measures such as lockdown implemented. A computer simulation design was developed to compare the epidemic curves of various scenarios of confinement measures compared with the theoretical epidemic curve without intervention.
The theoretical epidemic curve for the scenario without the traditional containment measures of quarantine and isolation used in Taiwan scenario assume that there would have been an initial outbreak of COVID-19 had there been lacking of these containment measures. The purported outbreak would be similar to the first outbreak in Wuhan, China that is supposed to have been spread to Taiwan through importation cases before border control.
Furthermore, the joint effect of confinement measures and vaccination was demonstrated by using Israel scenario based on the simulated study design.
Data collection
The data, including daily number of infected, recovered and death cases in Italy, Taiwan and Israel, were extracted from the open database, Coronavirus Resource Center built by Johns Hopkins University and Medicine.1 The collected duration of Italy was form January 26, which was the reported date of the first case, to February 20, 2021; that of Israel was from February 20, 2020 to February 20, 2021. The data between January, 2020 and April, 2020 in Taiwan was extracted from the database of Taiwan Centers for Diseases Control.7 The detailed information of source, intervention and model assumption for three countries were described in Table 1 .
Table 1.
Data source, intervention and model assumptions for Italy, Taiwan and Israel.
| Country | Source of information | Intervention | Assumptions |
|---|---|---|---|
| Italy | Open data from Coronavirus Resource Center (Johns Hopkins University) | Confinement measures | The extents of confinement measures are the same across areas in Italy |
| Taiwan | Open data from Coronavirus Resource Center (Johns Hopkins University) and Taiwan Centers for Diseases Control | Isolation and quarantine | The transmission rate was the same as Wuhan, China |
| Israel | Open data from Coronavirus Resource Center (Johns Hopkins University) | Confinement measures and vaccination | The extents of confinement measures are the same across areas in Israel |
Definitions of NPIs
Confinement measures (Italy)
The confinement measures such as lockdown was implemented in north and middle Italy since March 8, 2020. The main measures included the restriction for stores, restaurants and amusement building. The residents lived in the affected area were asked to stay at home and the students were asked to attend the online course at home rather than gathering at school.
Conventional containment of quarantine and isolation (Taiwan)
We focused on the impacts of the two NPIs, isolation and quarantine, on the change of epidemic curve in Taiwan. The definition of isolation is the separation of symptomatic cases from non-infected persons and the objective is to interrupt transmission to susceptible persons. The definition of quarantine is restriction of asymptomatic cases in order to reduce transmission from asymptomatic cases to susceptible persons. The percentage of asymptomatic cases was set 20% and the transmission probability was assumed only 35% of symptomatic cases based on the previous studies.8 To simulate the effects of these interventions, we created synthetic contact matrices for each intervention scenario from these building block matrices.
We considered the following scenarios: First, neither isolation nor quarantine is introduced namely natural spread. Second, under the background with 30% of symptomatic cases isolated (the extent of isolation is 30%), Third, under the background with 50% of symptomatic cases isolated (the extent of isolation is 50%), incorporation with different extents of quarantine are observed (the extents of quarantine are 0%, 30%, 60% and 90%, respectively) and under the background with 70% and 90% of symptomatic cases isolated. Under the framework of the SEIR model, we estimated the extents of these two NPIs achieved to contain the viral spread by fitting the data of daily reported in Taiwan during January, 2020 to April, 2020.
Vaccine (Israel)
We further investigated the various vaccination rates to contain the epidemic under the situations with and without confinement measures in Israel. The vaccination coverage rate was assumed 70% and compared to natural spread situation, the 10%, 15% and 20% of people vaccinated per month since December 19, 2020 in Israel were conducted to evaluate the efficacy of vaccination.6
Statistical analysis
The theoretical epidemic curves in Italy, Taiwan and Israel to evaluate the different NPIs such as confinement measures and traditional containments and vaccination were simulated by using a deterministic compartment SEIR type model. The whole population were categorized into four compartments based on their infection status, including susceptible (S), exposed (E), infected (I), and recovered (R). Susceptible individuals might contract the infection at a given rate (α) when they contacted with an infectious person and got into the exposed state. Then, they progressed to infected state (the rate from the exposed state to infected state was β) followed by the state of recovered with the rates of β (transmission coefficient) and σ (recovery rate). We assumed that the population in Italy, Israel, and Taiwan was a closed system with a constant population size through the whole course of the epidemic (i.e. S(t)+E(t)+I(t)+R(t) = N). The infected state was separated into the contributions of 80% symptomatic cases and 20% asymptomatic cases.8
Let S(t), E(t), I(t), and R(t) denote the numbers of susceptible, exposed, infected, and resistant with time. To incorporate the symptomatic and asymptomatic cases into our model, the Is(t), Ias(t), Rs(t) and Ras(t) denote the numbers of symptomatic and asymptomatic of infected and resistant cases. The instantaneous change of the four states with time can be derived by the following ordinary differential equations:
The parameters of transmission rate (α), progression rate (β) and resistant rate (σ) from the infected status can be estimated by the solution of the four-state differential equations regarding the empirical data on the observed COVID-19 cases in Italy, Taiwan and Israel. Where transmission rate (α) was estimated for Italy by the daily case number in Italy at the early phase of the outbreak from January 26 to the end of February, 2020, for Taiwan by the daily case number in Wuhan, China before lockdown measures and for Israel by the daily case number in the first and second surge of the outbreak (Table 2 ).
Table 2.
The parameters applied for SEIR model of COVID-19.
| Parameters | Estimates | Reference |
|---|---|---|
| The proportion of asymptomatic case | 0.20 | Buitrago et al.,8 |
| Progression rate (β, 1/incubation period) | 0.1428 | Backer et al.,9 |
| Resistant rate (σ, 1/infectious period) | 0.20 | Woelfel et al.,10 |
| Transmission rate (α) | ||
| Italy (First period) | 10−8 (R0 = 6.37) | Estimated |
| Italy (Second period) | 10−9 (R0 = 3.04) | |
| Wuhan, China (First period)a | 10−8 (R0 = 5.07) | |
| Israel (First period) | 10−7 (R0 = 6.80) | |
| Israel (Second period) | 10−8 (R0 = 2.40) | |
| Relative transmission rate of asymptomatic case compared to symptomatic case | 0.35 | Buitrago et al.,8 |
Abbreviations: S, susceptible; E, exposed; I, infected; R, recovered.
The parameter was used for simulate the dynamic of SEIR in Taiwan.
Progression rate (β) is the daily probability of an exposed individual becoming infected status with being the inverse of incubation period.9 Recovery rate (σ) consists of Rs and Ras represents the daily intensity for an infected subject to individual becoming resistant status like recovery or death, which is the inverse of the average time of infection and contributed from symptomatic (Is) and asymptomatic (Ias) cases.10 The details of individual parameter are listed in Table 2. The estimated results are further used to calculate the projection of four infectious states by time. The calibration of SEIR model for underreported COVID-19 cases using the Lombardy outbreak as an illustration.
All statistical analyses were conducted using MATLAB (version R2017b, The MathWorks Inc., Natick, Massachusetts, USA).
Results
Theoretical part of effectiveness of non-pharmaceutical interventions with three scenarios
Confinement measures of COVID-19 epidemic curve
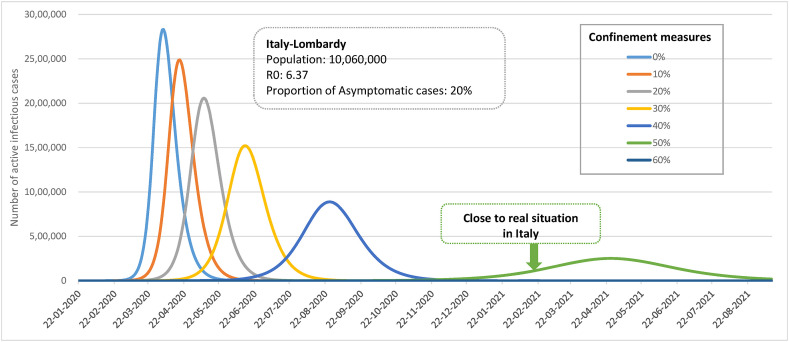
Fig. 1 shows epidemic curves of 10%–60% confinement measures as opposed to the theoretical epidemic curve without confinement measures. Note that the latter is a reflection of the initial outbreak of COVID-19 in Lombardy region, Italy. Such a model provides a benchmark for evaluation of effectiveness of various extents of confinement measures during lockdown as seen in Italy after this initial outbreak and other European countries.
Figure 1.
Simulated effect of confinement measures of COVID-19 epidemic curve in Italy.
Isolation-quarantine-oriented containment of COVID-19 epidemic curve
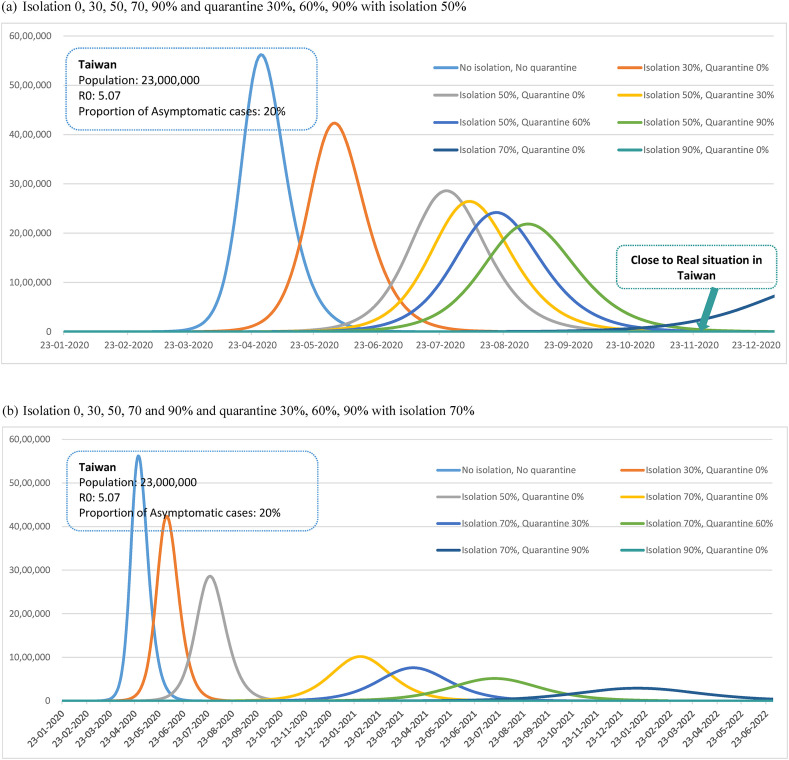
Fig. 2 (a)-(b) show the comparisons of epidemic curves between various traditional scenarios of isolation and quarantine and the theoretical epidemic curve without these containment measures using Taiwan as a theoretical example. As mentioned earlier, the theoretical epidemic curve used here would have been similar to the initial outbreak (R0 = 5.07) of COVID-19 in Wuhan, China had been lacking of these containment measures.
Figure 2.
Simulated effect of isolation and quarantine of COVID-19 epidemic curve in Taiwan. (a) Isolation 0, 30, 50, 70, 90% and quarantine 30%, 60%, 90% with isolation 50%. (b) Isolation 0, 30, 50, 70 and 90% and quarantine 30%, 60%, 90% with isolation 70%.
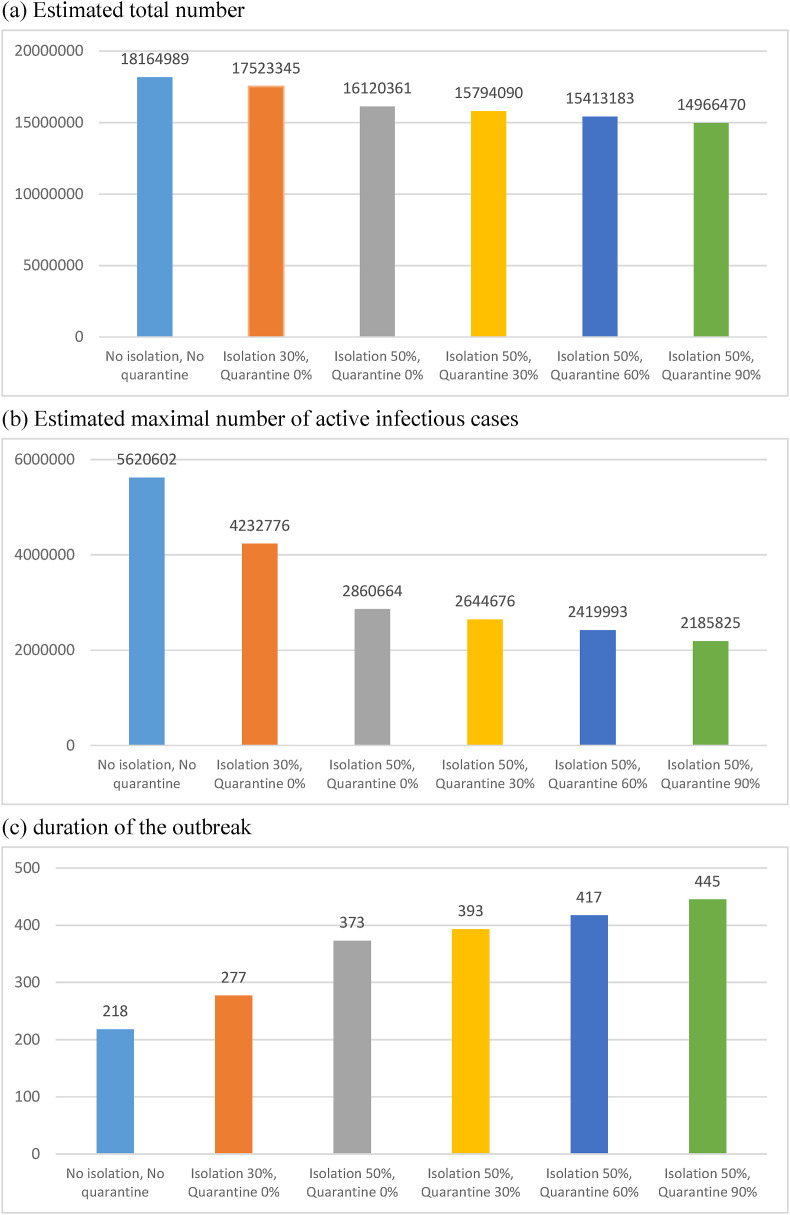
Under natural spread situation without isolation and quarantine, the total infected number of cases and the peak of actively infected cases would be 18, 164,989 and 5,620,602, respectively, and the duration of the outbreak would be 218 days (Figure 2, Figure 3 ). Under the circumstance with 30% of symptomatic cases isolated, the total infected number of cases and the peak of actively infected cases would be reduced to 17, 523,345 and 4,232,776 and the duration of the outbreak would be prolonged to 277 days. With 50% of symptomatic cases isolated in combination with different extents of quarantine as 0%, 30%, 60% and 90%, the peak of infected cases would be reduced from 2,860,664 persons to 2,185,825 persons accordingly, as well as the duration of the outbreak prolonged from 373 days to 445 days, respectively. While the extent of isolation can be expanded to more than 90% no subsequent outbreak would be expected. Figure 2, Figure 3 shows the similar results based on the scenario of 70% isolation.
Figure 3.
Estimated total number, maximal number of active infectious cases, and duration of outbreak in scenarios with different extent of isolation and quarantine. (a) Estimated total number. (b) Estimated maximal number of active infectious cases. (c) Duration of the outbreak.
Vaccine effect on COVID-19 epidemic curve
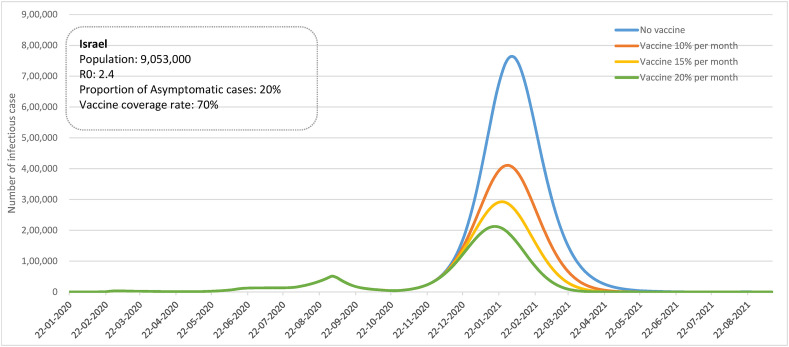
Under natural spread situation without confinement measures, isolation and quarantine, the nationwide mass vaccination campaign was implemented in Israel. Assume the 70% coverage rate of vaccination, compared to natural spread situation, 10%, 15% and 20% of people vaccinated per month led to reduce the peak of actively infected cases from 764,475 to 410,914, 292,635, 212,618, respectively and also shorten the duration of outbreak (Fig. 4 ).
Figure 4.
Simulated effect of vaccination of COVID-19 epidemic curve in Israel.
Empirical results with applications to three scenarios
First and second surge in Italy
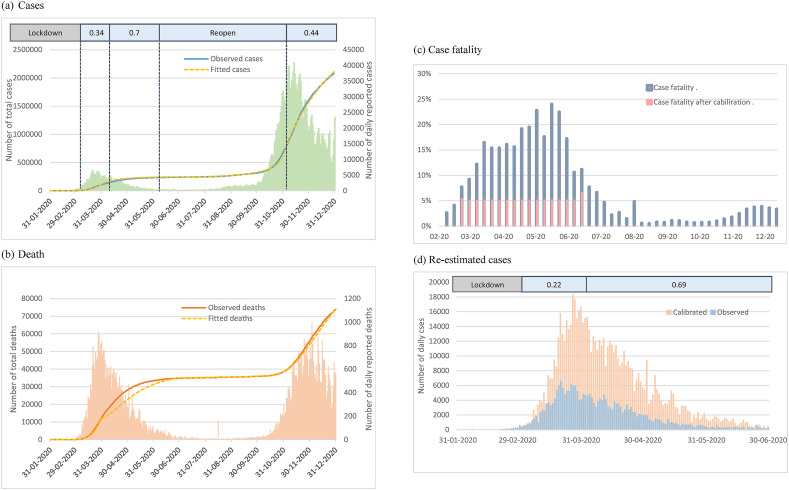
We fitted the observational data of cumulative case number since January 26, 2020 until the end of 2020 to estimate the extents of confinement measures in Italy. During the first outbreak period, the transmission rate was reduced by increasing the extent of confinements from 34% to 70% (see the upper panel of Fig. 5 (a)). After around the reopening period of five months, 44% of confinement measures was re-operated to respond the second surge from early November until the end of 2020 (Fig. 5(a)).
Figure 5.
Empirical results of confinement measures of COVID-19 epidemic curve in Italy. (a) Cases (b) Death (c) Case fatality (d) Re-estimated cases.
Calibration of underreporting in the first surge of epidemic in Italy
However, the early period of reported cases in Italy might be under-estimated. On the basis of the rate of disease progression from the mild respiratory disease to pneumonia (72.9%), from pneumonia to ARDS (16.2%), and from ADRS to death (42.6%) derived from the early period of Italy from the previous study as indicated in the method section,11 the total expected frequencies of death would have 4652, which was substantially fewer than the reported number of 10,023 by a factor of 0.46, implying the possibility of underreporting. We thus calibrated the proposed SEIR-type model by using this factor. After the calibration, the case fatality was reduced from around 20%–5% (Fig. 5(c)) and the re-estimated extents of confinement measures was therefore reduced from 34-70% to 22–69% (Fig. 5(b) and (d)).
Empirical results of Taiwan scenario
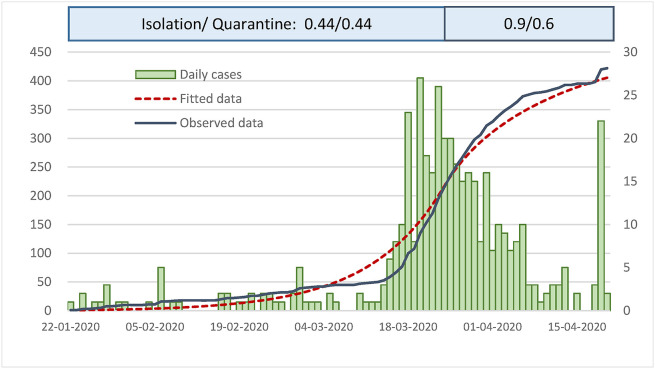
We applied the initial R0 of 5.07 in Wuhan, China to fit the observational data of Taiwan. Before March 23, 2020, 44% of isolation and quarantine was estimated during the early phase and isolation and quarantine were scaled up to 90% and 60% to contain the epidemic from March 24, 2020 onwards (Fig. 6 ).
Figure 6.
Empirical results of Isolation and quarantine of COVID-19 epidemic curve in Taiwan.
Empirical results of vaccination in Israel
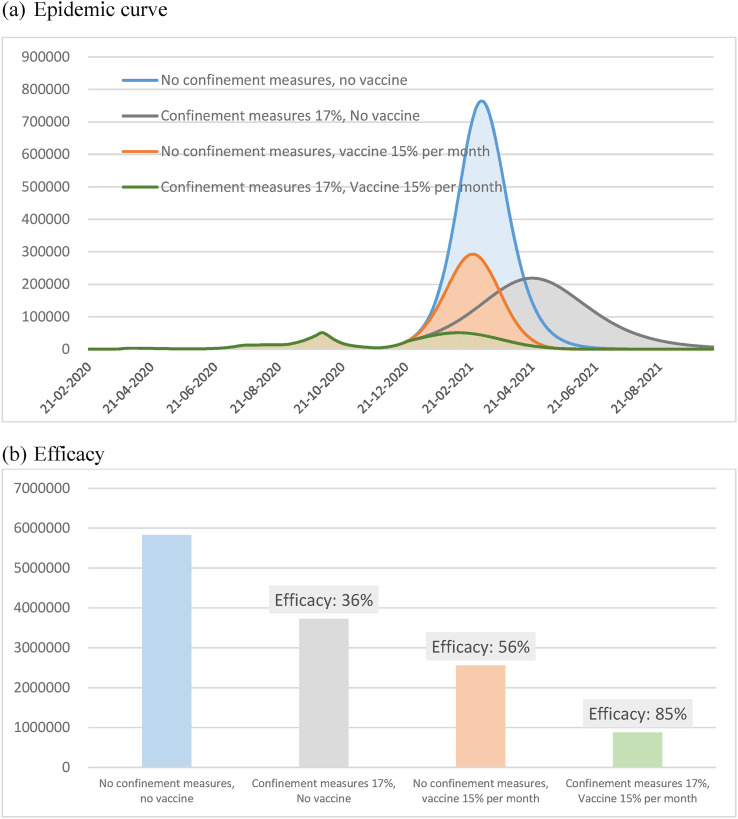
Nationwide mass vaccination campaign with 15% monthly was conducted since December 19, 2920 in Israel.12 In order to investigate whether the continuation of confinement measures are necessary or not after the roll out of vaccine, we simulated the epidemic curve in Israel under different extents of confinement measures with or without vaccination. Under the situation without vaccination, the daily case peaked at nearly 764,475 if no confinement measures was adopted, but they were expected to be reduced to 218,758 if current confinement measures continued (17%). Under the situation with 15% vaccination rate per month, the daily case peaked at 292,637 if no NPI was conducted, but they were expected to be reduced to 50,732 while current confinement measures continued (Fig. 7 (a)).
Figure 7.
Empirical results of vaccination of COVID-19 epidemic curve in Israel. (a) Epidemic curve. (b) Efficacy.
Taking the circumstance without vaccination nor confinement measures as the reference group, the efficacy of confinement measures alone, vaccination alone, and the combined vaccination with confinement measures in reducing the infected cases were 36%, 56%, and 85%, respectively (Fig. 7(b)).
Discussion
In the study, we demonstrated how the epidemic curves of COVID-19 were re-shaped by three scenarios of different NPIs from the period before pandemic, through pandemic period without vaccination, and the pandemic period with the roll-out of vaccine using three countries as illustrations, Italy, Taiwan, and Israel. Using the simulated study design, the theoretical epidemic curve of each scenario was first simulated by using the SEIR model to represent the natural spread of SRAS-COV-2 in the absence of NPIs. The differences of the peak of confirmed case and the whole duration of the outbreak were modelled by comparing that of the natural spread with those of interventions with various extent of confinement measures, isolation, quarantine, in combination with the roll-out of vaccine. The results of three scenarios show that the NPIs such as confinement measures, isolation and quarantine were key factors responsible for the temporal changes in transmission rates of the epidemic wave even if mass vaccination has been initiated. The stricter the interventions were enforced, the peak of active case number and the infected toll was reduced while the whole period of the outbreak was prolonged. According to our simulation, the second epidemic wave of Italy would have been contained if isolation and quarantine had been adopted as seen in the pilot study13 conducted in Vò, a small town in Veneto, Italy, showing that testing and identifying infected people for isolation and tracking the contact history can stop the spread of COVID-19 even though variant stain with B.1.1.7 emerged. However, the second surge of pandemic was extended to the entire Italy, the extents of confinement measures might be different from region to region. The heterogeneity of lockdown in Italy may not be well studied due to the limitation of data acquisition on policies and socioeconomic in different areas. In addition, based on the disease progression model, proposed by Hsu et al.,11 the reported cases in the first surge might be under-estimated in Italy. The issue of under-report of COVID-19 outbreak also has been addressed by a comparison study of excess mortality attributed to COVID-19.14
The lacking of community-acquired epidemic in Taiwan was also attributed to very strict containment measures on isolation and quarantine scaled up to 90% isolation or 70% isolation in combination with 90% quarantine. Accordingly, the epidemic has been contained effectively in Taiwan in the presence of cluster infection derived from nosocomial or household infections even though there were frequent contacts between Taiwan and China on the grounds of economics and business. In Israel, continuation of NPIs with 17% of confinement measures is recommended to facilitate the epidemic control after the implementation of nationwide mass vaccination. In comparison with the situation without any NPIs, the peak of daily case reported reduced from approximately 218,758 to 50,732 while conducting nationwide mass vaccination campaign.
Several NPIs implemented in face of emerging infectious diseases worldwide. During 1918 flu pandemic, a lot of public health measures were implemented for the containment of Spanish flu in U.S., including case isolation, closure of schools and churches, ban on gathering and regulation of face mask etc. However, the outbreak patterns were significantly different across the United States cities. In some cities, only one epidemic wave was observed, such as Atlanta, Baltimore and Philadelphia etc., whereas at least two epidemic waves were observed in other cities, including New York, San Francisco and St. Louis etc. The death rates among these cites considerably varied. This was because rapid implementation of these interventions at an early phase brought more effectiveness on influenza prevention.15 For example, in Philadelphia, the mayor ignored the warning from infectious disease experts that flu virus had invaded the community and still allowed the parade for supporting World War I. In the followings 48–72 h, the confirmed cases jumped around the Philadelphia regions and the death toll was up to 16,000 within six months. In contrast, within two days of the first few cases reported, the officials of St. Louis promptly introduced measures like isolation of the sick, case tracing and quarantine of the people with contact history.16 Finally, the death rate of St. Louis was only 50 per 100,000 persons, but this of Philadelphia was high as 250 per 100,000 persons. Such an empirical evidence shows how and how rapid to implement public health measures is important for containment of infectious disease.
During March to July of 2003, the outbreak of severe acute respiratory syndrome (SARS) spread from Taipei to Kaohsiung in Taiwan. The epidemic caused 346 confirmed cases and claimed 73 lives, including two young physicians and five critical care nurses.17 In order to fight against SARS, multifaceted containment measures were implemented, including active surveillance of all inbound travelers, exposed healthcare workers and contacts of patients, quarantine, isolation and integrated nosocomial infection control strategy.18 , 19 , 20 Taiwan spent only forty-six days to be removed from the list of areas with recent local transmission of SARS by the World Health Organization (WHO).21 Based on the previous anti-SARS experience, the government of Taiwan took action against COVID-19 promptly. News about the cluster of unexplained pneumonia in Wuhan announced on PTT, the biggest one of bulletin board systems in Taiwan, sharpened the vigilance of medical officers in Taiwan Centers for Disease Control and the government of Taiwan initiated active surveillance for all travellers of arriving flights from Wuhan since December 31, 2019.22 Otherwise, most Taiwanese people realized the importance of NPIs and kept social distancing indeed. In order to stabilize the provision of face mask for everyone, the real-name registration through the app was carried out by the government since February 6, 2020. Moreover, the smart contact tracing-based mobile sensor data via the mobile geopositioning method was developed by the government to identify potential contact persons rapidly and send automated alert messaging for self-restriction promptly.23 As a result, the epidemic is contained effectively in Taiwan with strong containments even though the geographic location of Taiwan is close to China and the airplane trafficking is frequent.
It should be noted that the implementation of NPIs would not only alter the natural spread but also affect the medical capacity of accommodating COVID-19 epidemic. The human-to-human transmission of COVID-19 is airborne route.24 Rapid identification and isolation of the sick followed by quarantining the contacts could effectively reduce the interaction between the healthy people and the potential spreaders. The direct consequence would slow the transmission rate of the virus, which was reflected by the change of reproductive number and the epidemic curve.25
Our study indicates that in the outbreak of COVID-19 under different scenarios, case isolation and contact tracing with quarantine can reduce the overall size of the outbreak and make it controlled over a longer period. In the circumstance, people still get infected but at a rate that health care system can catch up with. Nevertheless, isolation and quarantine alone is not sufficient to control the outbreak because asymptomatic proportion of COVID-19 is relatively high and complete quarantine is impossible in the real-world scenario.25 , 26 , 27 Although 44% of confinement measures were estimated to be achieved during the second epidemic wave of Italy, the extents of NPIs was so insufficient that the predicted cumulative case number will incessantly increase until August, 2021. As a result, further mitigation measures, including enhancing public awareness,28 assessing the capacity of health care system from primary to tertiary level,29 the integration of services in the health system and across other associated sectors,30 and the establishment of therapeutic and triage strategies, should be taken action promptly.
Policy-makers must have trade-offs between infection control and standard medical principles, and adapt the strategies immediately while more information and resources are available. Furthermore, the development of rapid test to accommodate more screening and vaccine as preventive measures could be taken into consideration.31 , 32 Our study also demonstrated that NPIs should be continued before herd immunity achieved through vaccination and natural spread.
However, isolation and quarantine are serious public health concerns for the elderly due to high risk of cardiovascular, neurocognitive and mental health problems. Even though necessary supplies and medications are provided, social disconnection puts the elderly at risk of anxiety, anger, confusion, depression and post-traumatic stress symptoms.32 The elderly population are the most vulnerable to COVID-19 infection and they often suffer from more complications and higher case-fatality rate than the young- and middle-aged groups.4 , 33 In many countries affected by COVID-19, a large number of old people are in isolation or quarantine, in order to protect over-burden health care system.34 Social support via online technologies, telephone contacts and community outreach projects can minimize affective disorders and improved mental wellbeings.35 , 36 On the other hand, health officials should declare clear rationale of isolation and quarantine, and give information about protocol.37
The strength of the study is to demonstrate and quantify the impacts of confinement measures, isolation and quarantine even the roll-out of vaccination on the spread of COVID-19 across the overall population by using a mathematical model. This provides strong evidence on the importance of NPIs for COVID-19 prevention. The model also gives a clue for health policy-makers to the timing of lifting that is pivotal in averting subsequent waves of a COVID-19 epidemic.
However, several weaknesses are inevitable. First, quarantine at home may give a way to speed the household transmission and increase the probability of household cases.38 Nevertheless, clustering events of household are not taken into account in our model but has been modelled in the accompanying article of this special issue.39 Second, the model cannot capture heterogeneity in contacts under different situations, such as super spreaders, nosocomial infection or long-term care facilities. Third, the epidemiological characteristics of COVID-19 remain uncertain in terms of incubation period, the proportion of asymptomatic cases and the variation of the time needed to be recovery. In case more and more studies are available, more precise information can be extracted for further analysis.
In conclusion, NPIs such as confinement measures, isolation and quarantine can delay the spread of COVID-19 so that new cases would not be propagated in a short period, but may be prolonged through the longer course. Then, health care system can reorient resources to provide mitigation strategies such social distancing and personal protection such as facial masks and hand-washing in order to contain community-acquired epidemic. Even though vaccination has been initiated, the NPIs should be continued to accelerate the containment of the epidemic.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgements
This study was supported by Ministry of Science and Technology, Taiwan (MOST 109-2327-B-002-009).
References
- 1.Coronavirus COVID-19 global cases by the center for systems science and engineering (CSSE) at Johns Hopkins university (JHU) John Hopkins University; 2020. [Google Scholar]
- 2.Calistri P., Amato L., Puglia I., Cito F., Di Giuseppe A., Danzetta M.L. Infection sustained by lineage B.1.1.7 of SARS-CoV-2 is characterised by longer persistence and higher viral RNA loads in nasopharyngeal swabs. Int J Infect Dis. 2021;105:753–755. doi: 10.1016/j.ijid.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angelo Amante C.B. Italy rushes new doctors into service as coronavirus deaths rise above 2,500. REUTERS. 2020 March 17:2020. [Google Scholar]
- 4.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. J Am Med Assoc. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 5.Ji Y., Ma Z., Peppelenbosch M.P., Pan Q. Potential association between COVID-19 mortality and health-care resource availability. Lancet Glob Health. 2020;8 doi: 10.1016/S2214-109X(20)30068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coronavirus (COVID-19) vaccinations. 2021. https://ourworldindata.org/covid-vaccinations 2021/03/21; Available from: [Google Scholar]
- 7.Reported cases of COVID-19 in Taiwan. 2021. https://www.cdc.gov.tw/ 2021/03/31; Available from: [Google Scholar]
- 8.Buitrago-Garcia D., Egli-Gany D., Counotte M.J., Hossmann S., Imeri H., Ipekci A.M. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Backer J.A., Klinkenberg D., Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20-28 January 2020. Euro Surveill. 2020;25:2000062. doi: 10.2807/1560-7917.ES.2020.25.5.2000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woelfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Mueller M.A. Clinical presentation and virological assessment of hospitalized cases of coronavirus disease 2019 in a travel-associated transmission cluster. medRxiv. 2020 doi: 10.1101/2020.03.05.20030502. [DOI] [Google Scholar]
- 11.Hsu C.Y., Lai C.C., Yeh Y.P., Chan C.C., Chen H.H. Progression from pneumonia to ARDS as a predictor for fatal COVID-19. J Infect Public Health. 2021;14:504–507. doi: 10.1016/j.jiph.2020.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A. BNT162b2 mRNA covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crisanti A., Cassone A. 2020. In one Italian town, we showed mass testing could eradicate the coronavirus. The Guardian.https://www.theguardian.com/commentisfree/2020/mar/20/eradicated-coronavirus-mass-testing-covid-19-italy-vo [Google Scholar]
- 14.2021. https://www.scienzainrete.it/articolo/italia-inghilterra-dallo-tsunami-di-uscita-da-covid-19/eugenio-paci/2021-04-30 access available on April 30, 2021.
- 15.Bootsma M.C., Ferguson N.M. The effect of public health measures on the 1918 influenza pandemic in U.S. cities. Proc Natl Acad Sci U S A. 2007;104:7588–7593. doi: 10.1073/pnas.0611071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatchett R.J., Mecher C.E., Lipsitch M. Public health interventions and epidemic intensity during the 1918 influenza pandemic. Proc Natl Acad Sci U S A. 2007;104:7582–7587. doi: 10.1073/pnas.0610941104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yen M.Y., Lin Y.E., Su I.J., Huang F.Y., Huang F.Y., Ho M.S. Using an integrated infection control strategy during outbreak control to minimize nosocomial infection of severe acute respiratory syndrome among healthcare workers. J Hosp Infect. 2006;62:195–199. doi: 10.1016/j.jhin.2005.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Twu S.J., Chen T.J., Chen C.J., Olsen S.J., Lee L.T., Fisk T. Control measures for severe acute respiratory syndrome (SARS) in Taiwan. Emerg Infect Dis. 2003;9:718–720. doi: 10.3201/eid0906.030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsieh Y.H., King C.C., Chen C.W., Ho M.S., Lee J.Y., Liu F.C. Quarantine for SARS, Taiwan. Emerg Infect Dis. 2005;11:278–282. doi: 10.3201/eid1102.040190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J.W., Lu S.N., Chen S.S., Yang K.D., Lin M.C., Wu C.C. Epidemiologic study and containment of a nosocomial outbreak of severe acute respiratory syndrome in a medical center in Kaohsiung, Taiwan. Infect Control Hosp Epidemiol. 2006;27:466–472. doi: 10.1086/504501. [DOI] [PubMed] [Google Scholar]
- 21.WHO . 2009. Removes Taiwan from SARS list - 2003-07-05.https://www.voanews.com/archive/who-removes-taiwan-sars-list-2003-07-05 Available from: [Google Scholar]
- 22.2021. https://www.cdc.gov.tw/En/Bulletin/Detail/p6Rh9tOURU-S2K9vd5Avmg?typeid=158
- 23.Chen C.M., Jyan H.W., Chien S.C., Jen H.H., Hsu C.Y., Lee P.C. Containing COVID-19 among 627,386 persons in contact with the diamond princess cruise ship passengers who disembarked in Taiwan: big data analytics. J Med Internet Res. 2020;22 doi: 10.2196/19540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao Y., Torok M.E. Taking the right measures to control COVID-19. Lancet Infect Dis. 2020;20:523–524. doi: 10.1016/S1473-3099(20)30152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prem K., Liu Y., Russell T.W., Kucharski A.J., Eggo R.M., Davies N. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5:e261–e270. doi: 10.1016/S2468-2667(20)30073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hellewell J., Abbott S., Gimma A., Bosse N.I., Jarvis C.I., Russell T.W. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob Health. 2020;8:e488–e496. doi: 10.1016/S2214-109X(20)30074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai Q., Huang D., Ou P., Yu H., Zhu Z., Xia Z. COVID-19 in a designated infectious diseases hospital outside Hubei province, China. Allergy. 2020;75:1742–1752. doi: 10.1111/all.14309. [DOI] [PubMed] [Google Scholar]
- 28.Bradley D.T., Mansouri M.A., Kee F., Garcia L.M.T. A systems approach to preventing and responding to COVID-19. EClin Med. 2020;21:100325. doi: 10.1016/j.eclinm.2020.100325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacDonald G., Moen A.C., St Louis M.E. The national inventory of core capabilities for pandemic influenza preparedness and response: an instrument for planning and evaluation. Influenza Other Respir Viruses. 2014;8:189–193. doi: 10.1111/irv.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Legido-Quigley H., Asgari N., Teo Y.Y., Leung G.M., Oshitani H., Fukuda K. Are high-performing health systems resilient against the COVID-19 epidemic? Lancet. 2020;395:848–850. doi: 10.1016/S0140-6736(20)30551-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brueck H. A mix-and-match coronavirus-testing strategy has allowed New York to screen 32,000 people — far more than any other state. BUSSINESS INSIDER. March 20, 2020 https://www.businessinsider.com/new-york-more-coronavirus-testing-than-the-cdc-any-state-2020-3 [Google Scholar]
- 32.Ahmed S.F., Quadeer A.A., McKay M.R. Preliminary identification of potential vaccine Targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020;12:254. doi: 10.3390/v12030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. J Am Med Assoc. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coronavirus Isolation for over-70s 'within weeks. BBC. March 15, 2020 https://www.bbc.co.uk/news/uk-51895873 [Google Scholar]
- 35.Newman M.G., Zainal N.H. The value of maintaining social connections for mental health in older people. Lancet Public Health. 2020;5:e12–e13. doi: 10.1016/S2468-2667(19)30253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Käll A., Jägholm S., Hesser H., Andersson F., Mathaldi A., Norkvist B.T. Internet-based cognitive behavior Therapy for loneliness: a pilot randomized controlled trial. Behav Ther. 2020;51:54–68. doi: 10.1016/j.beth.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Brooks S.K., Webster R.K., Smith L.E., Woodland L., Wessely S., Greenberg N. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395:912–920. doi: 10.1016/S0140-6736(20)30460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Potter G.E., Handcock M.S., Longini I.M., Jr., Halloran M.E. Estamating within-household contact networks from egocentric data. Ann Appl Stat. 2011;5:1816–1838. doi: 10.1214/11-aoas474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu C.Y., Wang J.T., Huang K.C., Fang C.H., Yeh Y.P., Chen L.S. Household transmission but without the community-acquired outbreak of COVID-19 in Taiwan. J Formos Med Assoc. 2021 doi: 10.1016/j.jfma.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]