Abstract
The association between the Dietary Inflammatory Index (DII) and breast cancer risk has been widely reported in recent years, but there is still controversy about whether a pro-inflammatory diet is a risk factor for breast cancer. We conducted a meta-analysis to investigate the relationship between the DII and breast cancer risk in pre-menopausal and post-menopausal women. We comprehensively searched PubMed, Embase and the Cochrane Library in January 2021 to identify articles reporting an association between the DII and breast cancer risk. A pooled analysis was conducted with 14 studies covering 312,885 participants. Overall, women in the most pro-inflammatory diet category were at greater risk for breast cancer than those in the most anti-inflammatory category (relative risk [RR]=1.37, 95% confidence interval [CI] 1.17-1.60, P<0.001). This association was strong in both pre-menopausal women (RR=1.87, 95% CI 1.17-2.99, P=0.001) and post-menopausal women (RR=1.23, 95% CI 1.08-1.40, P<0.001). Thus, a strong and independent association was observed between a pro-inflammatory diet (assessed using the DII score) and breast cancer risk, irrespective of menopausal status. Further studies will be required to determine the relationship between a pro-inflammatory diet and different subtypes of breast cancer.
Keywords: dietary inflammatory index, breast cancer
INTRODUCTION
Breast cancer has become the most common cancer among women, and the number of new cases has been increasing rapidly [1]. Other than reproductive, hormonal and genetic factors, inflammation has been reported as a potential cause of breast cancer [2]. The production of pro-inflammatory cytokines and diverse reactive oxygen and nitrogen species during inflammation facilitates cancer initiation, growth and malignant progression [3].
In addition to autoimmune diseases and infections, several risk factors related to lifestyle may lead to chronic inflammation. One such risk factor is the dietary composition. Saturated fats, refined carbohydrates and red meat may exert pro-inflammatory effects, while soy products and phytochemicals may exert anti-inflammatory effects due to their influence on oxidative stress and other pathways [4].
The Dietary Inflammatory Index (DII) was created to comprehensively investigate the inflammatory potential of the diet in the general population with adjustment for dietary differences around the world. This index was developed through an extensive review of over 6500 articles published from 1950 to 2010. Forty-five food parameters (including various macronutrients, micronutrients, flavonoids and individual food items) were evaluated, and six inflammatory biomarkers were used to calculate the inflammatory characteristics of each food parameter. A food parameter was considered pro-inflammatory if its intake was associated with significant increases in inflammatory biomarkers, or anti-inflammatory if it was associated with significant decreases in inflammatory biomarkers. A score was assigned to each food parameter based on its association with inflammatory biomarkers in the literature, and the score was weighted based on study design. The scores were then adjusted to the global standard mean intake, converted into proportions (0-1) and distributed around zero, with higher DII scores indicating pro-inflammatory effects and lower DII scores suggesting anti-inflammatory effects [5].
The relationship between the DII and breast cancer risk has been widely studied in recent years, but the results have been inconclusive. Shivappa et al. [6] reported that diets with high DII scores appeared to increase the risk of breast cancer, while Gardeazabal et al. [7] reported no association between the DII and breast cancer risk. Other researchers also have not found a significant association between the DII and breast cancer risk, but indicated that further studies with longer follow-up periods were needed to clarify the association [8, 9]. In addition, the association between the DII and breast cancer risk in different populations and breast cancer subtypes has not been fully investigated. Therefore, in this study, we conducted a systematic review and meta-analysis to explore the association between the DII and breast cancer risk using a large sample size, and to further assess this relationship in different populations and breast cancer subtypes.
RESULTS
Study characteristics
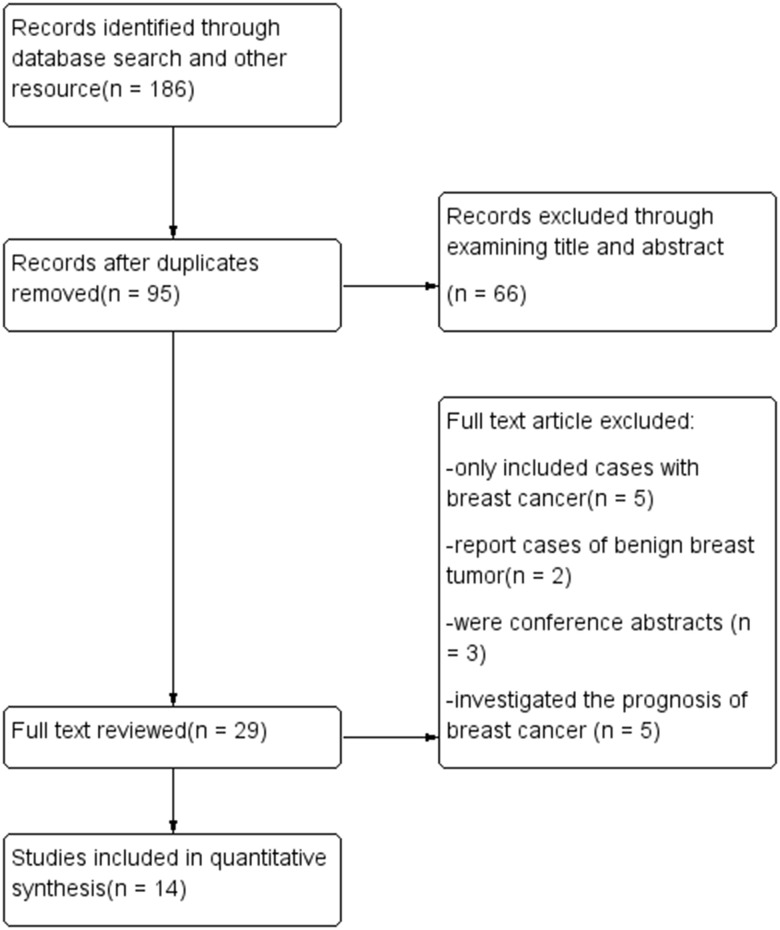
Fourteen studies were included in this meta-analysis (Figure 1), of which six were cohort studies and eight were case-control studies. In total, 312,885 participants were included in the pooled analysis. The main characteristics of the included studies are listed in Table 1. The DII was determined based on food frequency questionnaires in all the included studies, with the number of food parameters ranging from 22 to 37. The DII was calculated and adjusted using a regionally representative world dataset. Multivariate adjustment was applied based on age, body mass index, education, energy intake, family cancer history, hormone use, live birth number, menopausal status, menarche age and smoking status. Odds ratios (ORs) and relative risks (RRs) were determined between the most pro-inflammatory and most anti-inflammatory groups. In the cohort studies, the follow-up periods ranged from 10.3 to 25 years.
Figure 1.
Flow diagram.
Table 1. Characteristics of the included studies.
| Author and year | Country | Case no. | Control no. | Source of controls | Food parameters | NOS score | OR/RR with 95% CI |
| Case-Control | |||||||
| Ge 2015 | Germany | 2887 | 5512 | Community | 25 | 7 | 1.01 (0.86-1.17) |
| Huang 2016 | China | 867 | 824 | Hospital | 36 | 8 | 2.28 (1.71-3.03) |
| Shivappa 2017 | Italy | 2569 | 2588 | Community | 31 | 8 | 1.75 (1.39-2.21) |
| Jalali 2018 | Iran | 136 | 272 | Hospital | 34 | 6 | 1.30 (1.56-10.08) |
| Vahid 2018 | Iran | 145 | 148 | Hospital | 31 | 7 | 7.24 (3.14-16.68) |
| Lee 2019 | Korea | 364 | 364 | Hospital | 37 | 7 | 3.62 (2.34-5.80) |
| Santacana 2019 | Spain | 1486 | 1652 | Community | 30 | 7 | 1.22 (0.99-1.52) |
| Niclis 2020 | Argentina | 317 | 526 | Community | 22 | 6 | 1.34 (1.05-1.70) |
| Cohort | Case no. | Cohort no. | FU (yrs) | ||||
| Shivappa 2015 | Swedish | 1895 | 49,258 | 20 | 29 | 8 | 1.18 (1.00-1.39) |
| Tabung 2016 | USA | 8162 | 122,788 | 15.1 | 32 | 8 | 0.99 (0.91-1.07) |
| Graffoullere 2016 | France | 158 | 3771 | 12.6 | 36 | 8 | 0.85 (0.52-1.41) |
| Tabung 2017 | USA | 3471 | 70,998 | 16.05 | 32 | 8 | 1.03 (0.90-1.17) |
| Shivappa 2018 | USA | 1986 | 34,700 | 25 | 29 | 8 | 1.11 (1.00-1.22) |
| Gardeazabal 2018 | Spain | 100 | 10,713 | 10.3 | 28 | 8 | 1.44 (0.72-2.72) |
NOS: Newcastle-Ottawa Scale, FU: Follow-up.
Quantitative analysis
Overall, women in the most pro-inflammatory diet category were at significantly greater risk for breast cancer than those in the most anti-inflammatory category (RR=1.37, 95% confidence interval [CI] 1.17-1.60, P<0.001) (Figure 2). This significant increase in risk was also observed when the pooled analysis was restricted to case-control studies (RR=1.81, 95% CI 1.32-2.48, P<0.001), whereas a moderate increase in risk was observed when the analysis was restricted to cohort studies (RR=1.06, 95% CI 0.98-1.15, P=0.182).
Figure 2.
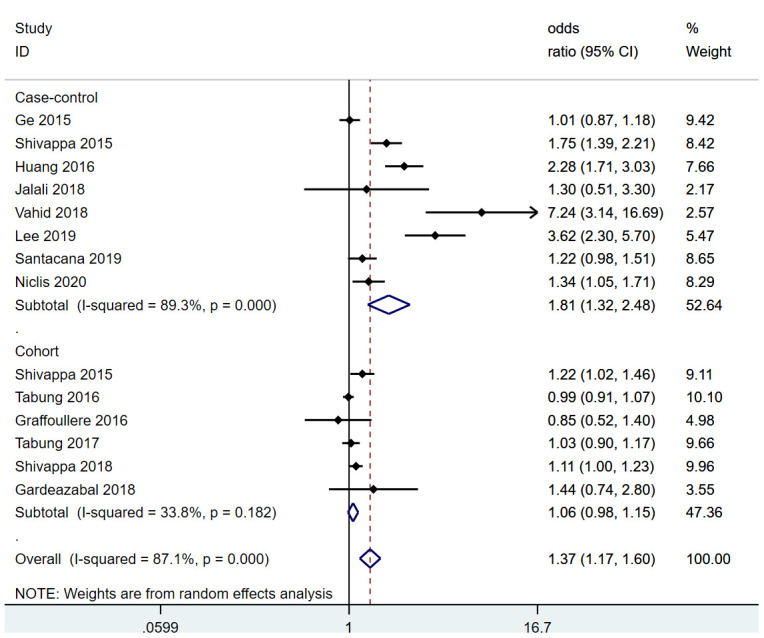
Pooled analysis of association between DII and breast cancer.
Next, a subgroup analysis was conducted based on menopausal status. In post-menopausal women, a pooled analysis including 11 studies demonstrated that the risk of breast cancer in the most pro-inflammatory diet group was significantly greater than that in the most anti-inflammatory group (RR=1.23, 95% CI 1.08-1.40, P<0.001). Likewise, in pre-menopausal women, a pooled analysis including six studies demonstrated that the risk of breast cancer in the most pro-inflammatory diet group was significantly greater than that in the most anti-inflammatory group (RR=1.87, 95% CI 1.17-2.99, P=0.001).
Seven pro-inflammatory dietary components were included in this meta-analysis: vitamin B12, carbohydrates, cholesterol, total fat, protein, saturated fat and trans-fat. Carbohydrate, cholesterol and total fat intake were assessed by all 14 studies, whereas vitamin B12 and saturated fat intake were assessed by 13 studies and protein intake was assessed by 12 studies. Subgroup analyses were conducted on studies that included particular food components. No significant difference in breast cancer risk was observed based on the inclusion of any specific food component in the DII. However, the breast cancer risk associated with the most pro-inflammatory diet was moderately higher in the subgroup analyses of studies that included vitamin B12 (RR=1.42, 95% CI 1.19-1.71) or saturated fat in the DII (RR=1.40, 95% CI 1.19-1.64) than in the subgroup analyses of studies that included the other specific components (Table 2).
Table 2. Subgroup analysis of studies that assessed different food components.
| Component | Inflammatory score based on the DII5 | Number of studies | Risk of breast cancer* |
| Vitamin B12 (ug) | 0.106 | 13 | 1.42 (1.19-1.71) |
| Carbohydrates (g) | 0.097 | 14 | 1.37 (1.17-1.60) |
| Cholesterol (mg) | 0.11 | 14 | 1.37 (1.17-1.60) |
| Total fat (g) | 0.298 | 14 | 1.37 (1.17-1.60) |
| Protein (g) | 0.021 | 12 | 1.38 (1.16-1.63) |
| Saturated fat (g) | 0.373 | 13 | 1.40 (1.19-1.64) |
| Trans-fat (g) | 0.229 | 5 | 1.30 (1.05-1.60) |
*A subgroup analysis was conducted on the association between the DII and breast cancer risk in studies that included the specified food component in the DII. The results are shown as the RR with 95% CI.
Sensitivity analysis
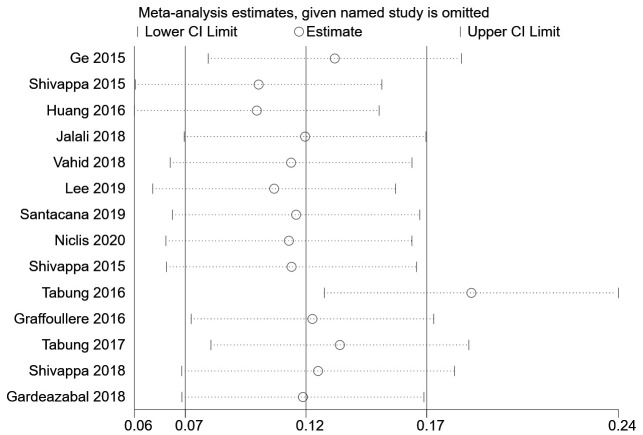
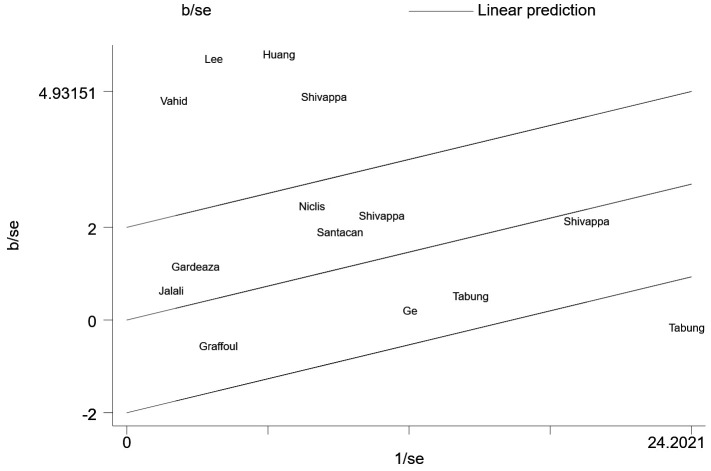
We then conducted a heterogeneity analysis, which revealed substantial heterogeneity among the included studies (I2=87.1%). A Galbraith plot (Figure 3) demonstrated that five studies were outside the 95% CI. A sensitivity analysis excluding these five studies was conducted, and the results indicated that the risk of breast cancer in the most pro-inflammatory diet group was still greater than that in the most anti-inflammatory group (RR=1.11, 95% CI 1.04-1.17, P=0.424). To further address the heterogeneity among the studies, we conducted an influence analysis in which each study was removed in turn (Figure 4). One study seemed to be the major cause of heterogeneity, and a sensitivity analysis excluding this study still revealed a strong association between the DII and breast cancer risk (RR=1.43, 95% CI 1.20-1.70, P<0.001).
Figure 3.
Galbraith plot.
Figure 4.
Influence analysis.
DISCUSSION
Chronic inflammation has been linked to various malignancies, including breast cancer [10]. Inflammatory cytokines are secreted during chronic inflammation, and numerous studies have indicated that serum interleukin (IL)-6 levels are greater in breast cancer patients than in healthy controls. Cytokines such as IL-1β and tumor necrosis factor α have also been linked to the risk and prognosis of breast cancer [11–13].
Clinical studies have indicated that pro-inflammatory dietary components may cause chronic inflammation and elevate inflammatory biomarker levels. A small number of in vitro studies have also investigated the association between food parameters and inflammation; for instance, Müller et al. [14] found that oleic acid exerted anti-inflammatory effects by attenuating inducible nitric oxide synthase, cyclooxygenase 2, tumor necrosis factor α, IL-1β and IL-6 mRNA expression in lipopolysaccharide-treated macrophages. However, there is no direct link between DII and breast cancers can be found in experimental data. Carbohydrates, cholesterol and saturated fat have been considered as pro-inflammatory food components, while pepper, caffeine, onions, green tea and garlic have been considered as anti-inflammatory. Nevertheless, people in different regions of the world vary greatly in their dietary habits and food parameters, making it difficult to assess the overall inflammatory effects of individual diets.
In order to systematically investigate the inflammatory effects of the diet, Shivappa et al. [5] developed the DII, in which each participant is standardized to the world mean and standard deviation for his/her respective food parameters. Therefore, participants from different regions of the world are standardized in each study using the DII as an assessment tool. In this meta-analysis, we evaluated the association between the DII and breast cancer risk. Seven pro-inflammatory dietary components were included: vitamin B12, carbohydrates, cholesterol, total fat, protein, saturated fat and trans-fat.
The primary finding of this study was that a pro-inflammatory diet was an independent risk factor for breast cancer after adjustment for covariates including age, body mass index, energy intake, education, menopausal status and live birth number. In some studies, additional adjustments were applied for mammography, non-steroidal anti-inflammatory drug use and oophorectomy. A subgroup analysis was conducted according to menopausal status, and a significant increase in breast cancer risk for the most pro-inflammatory diet group was found in both pre-menopausal and post-menopausal women. Major pro-inflammatory dietary components were assessed in most studies, and subgroup analyses revealed that the association between the DII and breast cancer risk was stronger in studies that included vitamin B12 and saturated fat as pro-inflammatory dietary components.
Cohort studies and case-control studies were both included in this meta-analysis [7, 15–23]. Case-control studies are more prone to bias due to their retrospective nature. The majority of the included studies reported moderate to significant associations between the DII and breast cancer risk, although two of the cohort studies (Tabung et al. [16] and Gardeazabal et al. [7]) reported no association. Accordingly, a subgroup analysis that only included the cohort studies revealed a more moderate association between the DII and breast cancer risk. Aside from the studies included in this meta-analysis, one previous study found no association between the DII and breast cancer-specific deaths [24], while Aghababayan et al. [25] reported a possible association between the DII and benign breast tumors.
A previous meta-analysis that included seven observational studies concluded that there was no significant association between the DII and breast cancer risk [8]. Our meta-analysis included 14 studies, and revealed that the most pro-inflammatory DII scores were associated with a significant increase in breast cancer risk after adjustment for covariates. This difference may have been due to the increased sample size and number of studies included in our analysis.
This study had several limitations. Due to insufficient data, we were unable to analyze the association between the DII and different subtypes of breast cancer based on hormone receptor expression and pathological characteristics. Cohort studies and case-control studies were both included in this meta-analysis, which could have caused bias; however, a subgroup analysis was conducted based on study design. Substantial heterogeneity was found among the included studies, but sensitivity and influence analyses yielded similar results to our primary findings. The strengths of this study were that the incidence of breast cancer was low, that a pooled analysis with a large sample size was conducted using the existing evidence, and that adjustments were made for other breast cancer risk factors.
In conclusion, a strong and independent association was observed between a pro-inflammatory diet (assessed using the DII score) and breast cancer risk. This association was strong in both pre-menopausal and post-menopausal women. Further studies are required to investigate the association between pro-inflammatory diets and different subtypes of breast cancer.
MATERIALS AND METHODS
Data source and search strategy
PubMed, Embase and the Cochrane Library were comprehensively searched in January 2021. The search terms included: Dietary inflammatory index, DII and breast cancer. The search terms used in PubMed were (((“diet”[MeSH Terms] OR “diet”[All Fields] OR “dietary”[All Fields] OR “dietaries”[All Fields]) AND (“inflammation”[MeSH Terms] OR “inflammation”[All Fields] OR “inflammations”[All Fields] OR “inflammation s”[All Fields]) AND (“abstracting and indexing”[MeSH Terms] OR (“abstracting”[All Fields] AND “indexing”[All Fields]) OR “abstracting and indexing”[All Fields] OR “index”[All Fields] OR “indexed”[All Fields] OR “indexes”[All Fields] OR “indexing”[All Fields] OR “indexation”[All Fields] OR “indexations”[All Fields] OR “indexe”[All Fields] OR “indexer”[All Fields] OR “indexers”[All Fields] OR “indexs”[All Fields])) OR “DII”[All Fields]) AND (“breast neoplasms”[MeSH Terms] OR (“breast”[All Fields] AND “neoplasms”[All Fields]) OR “breast neoplasms”[All Fields] OR (“breast”[All Fields] AND “cancer”[All Fields]) OR “breast cancer”[All Fields]). All articles published before January 1, 2021 were screened. Additional studies were identified from the retrieved articles.
Inclusion and exclusion criteria
The titles and abstracts of the retrieved articles were screened for relevant studies, and the full texts of studies of interest were screened by two independent researchers. Clinical trials were included if they: 1) assessed the relationship between the DII and the risk of breast cancer in humans and 2) reported quantitative data. Studies were excluded if they: 1) only included patients who had already been diagnosed with breast cancer; 2) investigated the prognosis of breast cancer; 3) did not report quantitative data or 4) were conference abstracts, animal studies, cadaveric studies, in vitro studies or articles published in a form other than a clinical trial.
Data extraction and quality assessment
Two researchers extracted the data and assessed the quality of the included studies independently. The corresponding author was sought to resolve any disagreement. Data including the sample size, study design, population characteristics, RR, OR, 95% CI and other relevant details were recorded and verified. The Newcastle-Ottawa Scale was used to assess the quality of the included studies.
Statistical analysis
Statistical analyses were performed using Stata 16 (StataCorp, College Station, TX, USA). Multivariable adjusted RRs were pooled to compare the most pro-inflammatory and most anti-inflammatory diets using a random effects model due to high heterogeneity. Because the incidence of breast cancer was low, the ORs reported in case-control studies were considered equal to RRs, in accordance with the Cochrane Handbook for Systematic Reviews. Subgroup analyses were conducted when no less than four studies could be included. Heterogeneity was assessed with I2 and a Galbraith plot. To address the high between-study heterogeneity, an influence analysis was conducted in which each study was removed in turn to examine the results of the pooled analysis.
Ethical review committee statement
Since our study is a meta-analysis, an Ethical Review Committee Statement is not required.
The analysis was mainly conducted in Guizhou Provincial People’s Hospital.
Footnotes
AUTHOR CONTRIBUTIONS: HJ Chen came up with this research topic and analysed the data. HJ Chen and YZ Gao write the manuscript. N Wei searched the database and retrieved data from included studies. KY Du searched the database and checked the included articles and retrieved data and recorded them. Q Jia did language editing and checked the statistical analysis of this article. HJ Chen revised the manuscript.
CONFLICTS OF INTEREST: The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020; 70:313. 10.3322/caac.21609 [DOI] [PubMed] [Google Scholar]
- 2.Hardman WE. Diet components can suppress inflammation and reduce cancer risk. Nutr Res Pract. 2014; 8:233–40. 10.4162/nrp.2014.8.3.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohshima H, Tazawa H, Sylla BS, Sawa T. Prevention of human cancer by modulation of chronic inflammatory processes. Mutat Res. 2005; 591:110–22. 10.1016/j.mrfmmm.2005.03.030 [DOI] [PubMed] [Google Scholar]
- 4.Islam MA, Alam F, Solayman M, Khalil MI, Kamal MA, Gan SH. Dietary Phytochemicals: Natural Swords Combating Inflammation and Oxidation-Mediated Degenerative Diseases. Oxid Med Cell Longev. 2016; 2016:5137431. 10.1155/2016/5137431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014; 17:1689–96. 10.1017/S1368980013002115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shivappa N, Blair CK, Prizment AE, Jacobs DR, Hébert JR. Prospective study of the dietary inflammatory index and risk of breast cancer in postmenopausal women. Mol Nutr Food Res. 2017; 61:10.1002. 10.1002/mnfr.201600592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardeazabal I, Ruiz-Canela M, Sánchez-Bayona R, Romanos-Nanclares A, Aramendía-Beitia JM, Shivappa N, Hébert JR, Martínez-González MA, Toledo E. Dietary inflammatory index and incidence of breast cancer in the SUN project. Clin Nutr. 2019; 38:2259–68. 10.1016/j.clnu.2018.09.030 [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Liu C, Zhou C, Zhuang J, Tang S, Yu J, Tian J, Feng F, Liu L, Zhang T, Sun C. Meta-analysis of the association between the dietary inflammatory index (DII) and breast cancer risk. Eur J Clin Nutr. 2019; 73:509–17. 10.1038/s41430-018-0196-9 [DOI] [PubMed] [Google Scholar]
- 9.Moradi S, Issah A, Mohammadi H, Mirzaei K. Associations between dietary inflammatory index and incidence of breast and prostate cancer: a systematic review and meta-analysis. Nutrition. 2018; 55–56:168–78. 10.1016/j.nut.2018.04.018 [DOI] [PubMed] [Google Scholar]
- 10.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002; 420:860–67. 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazidi M, Gao HK, Vatanparast H, Kengne AP. Impact of the dietary fatty acid intake on C-reactive protein levels in US adults. Medicine (Baltimore). 2017; 96:e5736. 10.1097/MD.0000000000005736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberg JE, Schwertfeger KL. Proinflammatory cytokines in breast cancer: mechanisms of action and potential targets for therapeutics. Curr Drug Targets. 2010; 11:1133–46. 10.2174/138945010792006799 [DOI] [PubMed] [Google Scholar]
- 13.Sheen-Chen SM, Chen WJ, Eng HL, Chou FF. Serum concentration of tumor necrosis factor in patients with breast cancer. Breast Cancer Res Treat. 1997; 43:211–15. 10.1023/a:1005736712307 [DOI] [PubMed] [Google Scholar]
- 14.Müller AK, Schmölz L, Wallert M, Schubert M, Schlörmann W, Glei M, Lorkowski S. In Vitro Digested Nut Oils Attenuate the Lipopolysaccharide-Induced Inflammatory Response in Macrophages. Nutrients. 2019; 11:503. 10.3390/nu11030503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabung FK, Steck SE, Liese AD, Zhang J, Ma Y, Caan B, Chlebowski RT, Freudenheim JL, Hou L, Mossavar-Rahmani Y, Shivappa N, Vitolins MZ, Wactawski-Wende J, et al. Association between dietary inflammatory potential and breast cancer incidence and death: results from the Women’s Health Initiative. Br J Cancer. 2016; 114:1277–85. 10.1038/bjc.2016.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tabung FK, Steck SE, Liese AD, Zhang J, Ma Y, Johnson KC, Lane DS, Qi L, Snetselaar L, Vitolins MZ, Ockene JK, Hebert JR. Patterns of change over time and history of the inflammatory potential of diet and risk of breast cancer among postmenopausal women. Breast Cancer Res Treat. 2016; 159:139–49. 10.1007/s10549-016-3925-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graffouillère L, Deschasaux M, Mariotti F, Neufcourt L, Shivappa N, Hébert JR, Wirth MD, Latino-Martel P, Hercberg S, Galan P, Julia C, Kesse-Guyot E, Touvier M. The Dietary Inflammatory Index Is Associated with Prostate Cancer Risk in French Middle-Aged Adults in a Prospective Study. J Nutr. 2016; 146:785–91. 10.3945/jn.115.225623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niclis C, Shivappa N, Hébert JR, Tumas N, Díaz MD. The Inflammatory Potential of Diet is Associated with Breast Cancer Risk in Urban Argentina: A Multilevel Analysis. Nutr Cancer. 2020; 1:1–10. 10.1080/01635581.2020.1817953 [DOI] [PubMed] [Google Scholar]
- 19.Vahid F, Shivappa N, Hatami M, Sadeghi M, Ameri F, Jamshidi Naeini Y, Hebert JR, Davoodi SH. Association between Dietary Inflammatory Index (DII) and Risk of Breast Cancer: a Case-Control Study. Asian Pac J Cancer Prev. 2018; 19:1215–21. 10.22034/APJCP.2018.19.5.1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ge I, Rudolph A, Shivappa N, Flesch-Janys D, Hébert JR, Chang-Claude J. Dietary inflammation potential and postmenopausal breast cancer risk in a German case-control study. Breast. 2015; 24:491–96. 10.1016/j.breast.2015.04.012 [DOI] [PubMed] [Google Scholar]
- 21.Lee S, Quiambao AL, Lee J, Ro J, Lee ES, Jung SY, Sung MK, Kim J. Dietary Inflammatory Index and Risk of Breast Cancer Based on Hormone Receptor Status: A Case-Control Study in Korea. Nutrients. 2019; 11:1949. 10.3390/nu11081949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obón-Santacana M, Romaguera D, Gracia-Lavedan E, Molinuevo A, Molina-Montes E, Shivappa N, Hebert JR, Tardón A, Castaño-Vinyals G, Moratalla F, Guinó E, Marcos-Gragera R, Azpiri M, et al. Dietary Inflammatory Index, Dietary Non-Enzymatic Antioxidant Capacity, and Colorectal and Breast Cancer Risk (MCC-Spain Study). Nutrients. 2019; 11:1406. 10.3390/nu11061406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang WQ, Mo XF, Ye YB, Shivappa N, Lin FY, Huang J, Hébert JR, Yan B, Zhang CX. A higher Dietary Inflammatory Index score is associated with a higher risk of breast cancer among Chinese women: a case-control study. Br J Nutr. 2017; 117:1358–67. 10.1017/S0007114517001192 [DOI] [PubMed] [Google Scholar]
- 24.Zucchetto A, Serraino D, Shivappa N, Hébert JR, Stocco C, Puppo A, Falcini F, Panato C, Dal Maso L, Polesel J. Dietary inflammatory index before diagnosis and survival in an Italian cohort of women with breast cancer. Br J Nutr. 2017; 117:1456–62. 10.1017/S0007114517001258 [DOI] [PubMed] [Google Scholar]
- 25.Aghababayan S, Sheikhi Mobarakeh Z, Qorbani M, Tiznobeyk Z, Aminianfar A, Sotoudeh G. Higher Dietary Inflammatory Index Scores are Associated with Increased Odds of Benign Breast Diseases in a Case-Control Study. J Inflamm Res. 2020; 13:61–69. 10.2147/JIR.S232157 [DOI] [PMC free article] [PubMed] [Google Scholar]