Abstract
Background
Vaccine is supposed to be the most effective means to prevent COVID-19 as it may not only save lives but also reduce productivity loss due to resuming pre-pandemic activities. Providing the results of economic evaluation for mass vaccination is of paramount importance for all stakeholders worldwide.
Methods
We developed a Markov decision tree for the economic evaluation of mass vaccination against COVID-19. The effectiveness of reducing outcomes after the administration of three COVID-19 vaccines (BNT162b2 (Pfizer-BioNTech), mRNA-1273 (Moderna), and AZD1222 (Oxford-AstraZeneca)) were modelled with empirical parameters obtained from literatures. The direct cost of vaccine and COVID-19 related medical cost, the indirect cost of productivity loss due to vaccine jabs and hospitalization, and the productivity loss were accumulated given different vaccination scenarios. We reported the incremental cost-utility ratio and benefit/cost (B/C) ratio of three vaccines compared to no vaccination with a probabilistic approach.
Results
Moderna and Pfizer vaccines won the greatest effectiveness among the three vaccines under consideration. After taking both direct and indirect costs into account, all of the three vaccines dominated no vaccination strategy. The results of B/C ratio show that one dollar invested in vaccine would have USD $13, USD $23, and USD $28 in return for Moderna, Pfizer, and AstraZeneca, respectively when health and education loss are considered. The corresponding figures taking value of the statistical life into account were USD $176, USD $300, and USD $443.
Conclusion
Mass vaccination against COVID-19 with three current available vaccines is cost-saving for gaining more lives and less cost incurred.
Keywords: Cost-benefit analysis, Cost-utility analysis, COVID-19, Vaccine, Value of statistical life
Introduction
The outbreak of the novel Coronavirus disease 2019 (COVID-19) since December 2019 has overwhelmed health systems around the world. It has claimed more than 2.7 million deaths as of the end of March 2021.1 The high contagiousness of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the pathogen of COVID-19, has accumulated more than 125 million COVID-19 confirmed cases and forced authorities to issue strong containment measures, including stay home order, closure of stores, restaurants, schools and airports, lockdown of cities, and border quarantine in almost every country globally. In addition to the public health impact, the pandemic of COVID-19 also caused an enormous economic loss due to costly medical expenditure and loss of production capacity resulted from mitigation strategies. The total cost of the COVID-19 pandemic was estimated to be 90% annual gross domestic product in the US.2 One-month lockdown in Tokyo would result in an 86% reduction of the daily production in Japan.3
The development of novel vaccine against SARS-CoV-2 is anticipated to be the most useful tool to curb the rampage of the disease. As a matter of fact, the duration from the development of COVID-19 vaccines, the implementation of phase 1 to phase 4 randomized controlled trials, to the authority approval for market use is less than one year, even including the next-generation vaccine platforms for COVID-19,4 much shorter than the traditional development of other vaccines, such as Ebola, polio, and influenza in history.5 , 6 As of the end of March 2021, there have been 13 vaccines approved, given the emergency use authorization issued by the Food and Drug Administration (FDA) in different countries. The UK is the first country to deploy a mass immunization campaign to the public, in which the first dose was delivered to a person on 8th December 2020. Until now, >328 million subjects have received at least one dose in 158 countries. Among them, Israel takes the lead in vaccinations around the world. The epidemic curve of COVID-19 in Israel started to decline two weeks after the vaccination program launched on 20th December 2020.7 The more investment from government has been propagandized with an expected return of a global benefit of USD $17.4 trillion from an installation of capacity for 3 billion annual vaccine courses based on a vaccine market design.8
Given the promising hope from vaccination, the cost-effectiveness would be of great interest to health decision-makers and governments worldwide. In addition to the incremental cost to save one additional person year widely used in cost-effectiveness analysis (CEA), one would like to know which factors and how much the magnitude these factors would influence the results of cost-effectiveness. Besides cost-effectiveness analysis, it is also very interesting to report cost-benefit analysis (CBA) to answer the question of “how much benefit (e.g. monetary value) would be returned later given one unit price (dollar) spent in vaccine earlier?” with benefit (B)/Cost (C) ratio by considering direct and indirect cost from single payer viewpoint or societal viewpoint, respectively.
In this study, we aimed to develop a Markov decision model to evaluate the cost-effectiveness for COVID-19 vaccines. Take Israel as our role model, we simulated the epidemic curve since 1st November 2020 when they had a resurge from the first wave of epidemic, implemented a vaccination program at day 51, and followed the cohort until day 180 with the built-in susceptible-infectious-recovery (abbreviated as SIR) model. The developed algorithm was also applied to the scenario if Israel would have not been administered with vaccination program ever. Compared with no vaccination strategy, the cost-effectiveness analysis was performed not only for the major brand of Israel's use, BNT162b2 (Pfizer-BioNTech) but also for two other major COVID-19 vaccines of mRNA-1273 (Moderna) and AZD1222 (Oxford-AstraZeneca) used worldwide. Factors relevant to the transmissibility of COVID-19, vaccination, and the disease-related medical expense in the sensitivity analyses would be tested for the robustness of the cost-effectiveness analyses. Finally, CBA was also performed to estimate B/C ratios for three vaccines.
Materials and methods
Study design with a Markov decision tree
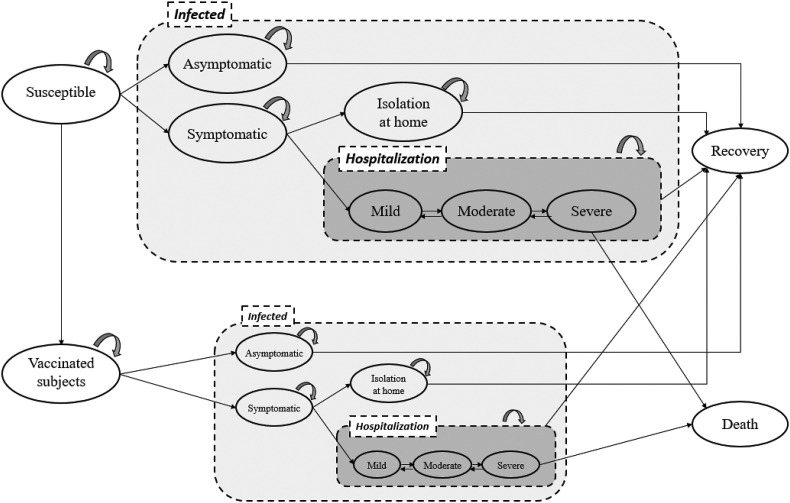
Fig. 1 shows a Markov decision model structure of COVID-19 disease with and without the administration of vaccine. This model gets involved with the disease transmission model from susceptible, infected, and recovery and the clinical evolution model for COVID-19 cases (shaded panel of “Infected” in Fig. 1). The compartment model of Susceptible (the “Susceptible” node)-Infected (the shaded square marked by “Infected”)-Recovery (the “Recovery” node) (abbreviated as SIR) was used for depicting the dynamic of COVID-19 transmission in community.9 , 10 For the prevention strategy with vaccination program, susceptible subjects will be moved to the vaccinated group (the “Vaccinated subjects” node) according to the vaccination schedule. The vaccinated subjects follow the same structure of SIR and disease progression but with lower risks of being infected, depending on the efficacy of vaccine under consideration.
Figure 1.
The structure of the Markov decision tree for the cost-effectiveness analysis for COVID-19 vaccination.
Extended from the conventional SIR model, subjects being infected by SARS-CoV-2 can be symptomatic or asymptomatic with the asymptomatic proportion of 17%.11 Regarding the symptomatic COVID-19 cases, some of them would be recovered after a period of self-isolation (the node of “Isolation at home”), whereas 15% of these subjects were required to be treated with hospitalization, which was estimated from the reported data in Italy by using a Queue model with the methods detailed in this special issue.12 For the hospitalized COVID-19 patients, we applied a COVID-19 clinical evolution model detailed as follows.
COVID-19 evolution model
Fig. 1 (shaded panel of “Hospitalization”) summaries the evolution of hospitalized COVID-19 patients. The daily probabilities of transitions between the three transient states of low- (without supplemental oxygenation or low-flow oxygen), medium- (high-flow oxygen with non-invasive ventilator), and high- (invasive ventilator or Extracorporeal Membrane Oxygenation (ECMO)) risk until the two outcomes of recovery and death were projected from the results of a five-state Markov model reported in the previous study by Jen et al.13 The parameters governing the progression of hospitalized COVID-19 patients were abstracted from the estimated result of Jen et al. (2021) by using the empirical information of the standard care group of a randomized controlled trial for treating hospitalized patients affected by COVID-19.14 , 15 Table 1 shows the values of daily transition probabilities from three disease states.
Table 1.
Base-case estimates for cost-effectiveness analysis.
| Variables | Base-case estimate | Distribution | Reference/source |
|---|---|---|---|
| Initial probability of state | |||
| Initial probability of asymptomatic | 0.000263798 | ||
| Initial probability of symptomatic | 0.001055192 | ||
| Proportion of asymptomatic | 17% | Beta(111,552) | Byambasuren et al., 2020 |
| Transition probability of state | |||
| Transmission duration (days) | 7 | ||
| Proportion of hospitalization | 15% | Jen et al., 2021 | |
| COVID-19 Clinical progression during hospitalization | |||
| Low risk | Jen et al., 2021 | ||
| Recovery | 12.2% | Dirichlet∗ (776,88,14,122,0.2) | |
| Medium risk | 8.8% | ||
| High risk | 1.4% | ||
| Death | 0.02% | ||
| Medium risk | |||
| Recovery | 2.6% | Dirichlet∗ (267,516,187,2 6,4) | |
| Low risk | 26.7% | ||
| High risk | 18.7% | ||
| Death | 0.4% | ||
| High risk | |||
| Recovery | 0.2% | Dirichlet∗(17,76,871,2,34) | |
| Low risk | 1.7% | ||
| Medium risk | 7.6% | ||
| Death | 3.4% | ||
| Efficacy of vaccine (%) | |||
| For symptomatic cases | |||
| Moderna | 94.1 (89.3–96.8) | Baden (2021) | |
| Pfizer | 95.0 (90.3–97.6) | Polack (2020) | |
| AstraZeneca | 70.4 (54.8–80.6) | Voysey (2021) | |
| For asymptomatic cases | |||
| Moderna | 61.8 (30.7–78.9) | Baden et al. (2021) | |
| Pfizer | 52.4 (29.5–68.4) | Polack et al. (2020) | |
| AstraZeneca (UK arm) | 27.3 (−17.2–54.9) | Voysey (2021) | |
| Adverse effects of vaccine (%) | |||
| Moderna | 27.4 | Beta(2281,12396) | Baden et al. (2021) |
| Pfizer | 27.0 | Beta(2619, 16241) | Polack et al. (2020) |
| AstraZeneca | 33.6 | Beta(4039,7982) | |
| Utility | |||
| Isolation at home | 0.81 | Kohli et al. (2021) | |
| Hospitalization | |||
| Low risk | 0.70 | ||
| Medium risk | 0.50 | ||
| High risk | 0.40 | ||
| Direct cost, U.S. $ | |||
| Confirmatory diagnosis | 50 | ||
| Vaccine price (per dose) | |||
| Moderna | 31 | ||
| Pfizer | 14 | ||
| AstraZeneca | 5 | ||
| Vaccine administration (per dose) | 10 | 0–10 | |
| Hospitalization (per day) | National Health Insurance Administration | ||
| Negative pressure isolation ward | 146.43 | Triangular (73.2, 146.43, 219.6) | |
| Intensive care unit | 243.23 | Triangular (121.6, 243.23, 364.8) | |
| Non-invasive positive pressure ventilation | 30 | Triangular (15, 30, 45) | |
| Computer Tomography | 152.0 | Triangular (76, 152, 228) | |
| Indirect cost, U.S. $ | |||
| Hospitalization (per day) | 84.6 | ||
| Vaccine jab (half-day) | 42.3 | ||
| Adverse effect due to vaccination (2-day) | 169.2 | ||
∗Dirichlet distributions were applied for the daily transition probabilities for events of low risk, medium risk, high risk, recovery, and death.
∗The expected GDP per capital in Taiwan in 2020 was $30,981.
Note that the high risk state corresponds to the medical needs for intensive care unit (ICU) management and the low and medium risk states require ward care equipped with negative pressure facility. As the disease progresses to medium risk state the use of non-invasive ventilator is required.
To take into account the uncertainty inherited from the evolution of COVID-19 in terms of the proportions of asymptomatic cases and hospitalization needs and the daily probabilities of disease progression after being admitted to hospital, a probabilistic approach was adopted. For the asymptomatic proportion, the Beta distribution of Beta(111, 552) was used (Table 1). Regarding the daily transition rates of hospitalized COVID-19 patients across five disease states, a Dirichlet distribution with the marginal summation of 1000 was adopted.
Vaccine efficacy
The parameters on the effectiveness of vaccination in preventing asymptomatic and symptomatic COVID-19 cases were derived from the published literatures of phase 3 clinical trials including the BNT162b2 (Pfizer-BioNTech), mRNA-1273 (Moderna), and AZD1222 (Oxford-AstraZeneca).16, 17, 18 Information on the point and interval estimates of vaccine efficacy of symptomatic and asymptomatic cases was abstracted. The prevalence of adverse effects of fever or more severe was borrowed from the findings in the phase 4 post-market reports and was incorporated into the cost-effectiveness analysis by using Beta distributions. In the current analysis, we assumed no vaccine jab would be required once 70% of population was vaccinated or infected with COVID-19.
Cost
In the current cost-effectiveness analysis, both healthcare payer and societal perspectives were adopted. The direct cost associated with the prevention and treatment for COVID-19 cases was considered. It includes the cost for testing using RT-PCR for the identification of infected cases. For the prevention strategy with vaccination, the cost for vaccination and its administration were considered.19 The aggregated cost for hospitalized COVID-19 patients including caring in the facility of isolation ward, supportive care, and oxygenation for the low, medium, and high risk patients was used. For patients in medium risk state, the cost for using non-invasive positive pressure ventilation was included. Regarding the patients in high risk state, the aggregated cost for the management of patients in the facility of ICU with the precaution for infection control and the use of invasive ventilator or ECMO was used. For the high risk patients, the cost for using computed tomography to evaluate the severity of pulmonary lesions was also applied. Information on the cost was collected from the National Health Insurance Administration, Taiwan.20 Triangular distributions were used for costs to account for the uncertainties of relevant costs.
We considered the indirect cost pertaining to productivity loss due to COVID-19 related hospitalization, half-day course for vaccine jab, and two-day sick leave if there was adverse effect from vaccination. The unit of one-day productivity loss was calculated based on the expected GDP per capital in Taiwan 2020 (USD $30,981).
As far as the global economic and educational losses attributed to COVID-19 is concerned, the global value of vaccine capacity was projected based on Castillo et al. study (2021) where 3 billion annual vaccine course was associated with the global benefit of USD$17.4 trillion.8 Reaching 70% vaccine coverage would be projected to gain a benefit of $840.955 per person. This was further discounted by the efficacy of each vaccine.
Cost-utility analysis
For the economic evaluation, we borrowed the scenario in Israel for our simulation. The cohort size was 8,362,864. The initial condition of 11,016 COVID-19 cases on Nov 1, 2020, the date when the second surge of epidemic was about to rebound after a well-controlled period, was applied. Vaccine of SARS-CoV-2 was administered since day 51. In the initial 15 days, the daily vaccine jabs were 40,000. It increased to 80,000 afterwards.
A constellation of COVID-19 related outcomes were collected, including numbers of asymptomatic and symptomatic infectives, days of hospitalization, number of death, and the quality adjusted life days (QALDs) gained in the simulated cohort with and without vaccination administered. The QALDs of low, medium, and high risk COVID-19 was set as a previous study did.21 We used one-day for a Markov cycle. The time horizon in this analysis was 180 days. As this is a short period, neither cost nor utility was discounted in the current analysis. The incremental cost-utility ratio (ICUR) for cost per QALD gained was calculated as the difference of cost for different vaccines versus no vaccine strategy divided by the QALD gained from the vaccination program.
Cost-benefit analysis
We performed the cost-benefit analysis for the COVID-19 vaccines with benefit-cost ratio (BCR) of four approaches. The first one (BCR1) was from the payer's perspective with BC ratio calculated as saving on COVID-19 related medical cost divided by the direct cost of vaccine. The second one (BCR2) was from societal perspective with BC ratio calculated as saving on the direct cost of medical expenditure and the indirect cost divided by the direct cost of vaccine. The latter two are from macro viewpoint to consider the economic impacts due to productivity and education loss and the value of life. Therefore, we obtained the third BC ratio (BCR3) with cost saving on the abovementioned medical cost plus indirect cost together with the economic impacts in terms of productivity and education loss divided (see Supplementary Materials) by the investment on vaccine. Note that the investment cost was measured with the vaccine price and administration fee (Moderna $82, Pfizer $48, and AstraZeneca $30 for two doses) regardless the coverage of vaccination. Finally, the contingent valuation method was applied for the value of life to value the benefit in terms of the value of statistical life (VSL).22 , 23 , 24 The fourth BC ratio (BCR4) with the product of the reduced number of death (life saved from vaccination) and VSL ($2.7 million)25 divided by the investment on vaccine.
Parameter uncertainty
The one-way sensitivity analyses were applied to examining the robustness of the cost-utility analysis of COVID-19 vaccine with varying values of parameters, including vaccine efficacy, the progress of vaccine administration, the reproductive number, proportion of asymptomatic cases, vaccine price, the fee for administration, and cost of hospitalization.
For the simultaneous consideration of parameter uncertainties, we conducted the Monte Carlo simulation with 500 second-order parameter samples in light of distributions of parameters (Table 1). For each parameter sample, the microsimulation was conducted with 10,000 first-order simulation trials. An incremental cost-utility scatter plot was depicted to determine the spread of the ICURs.
Results
Simulated effectiveness of vaccination
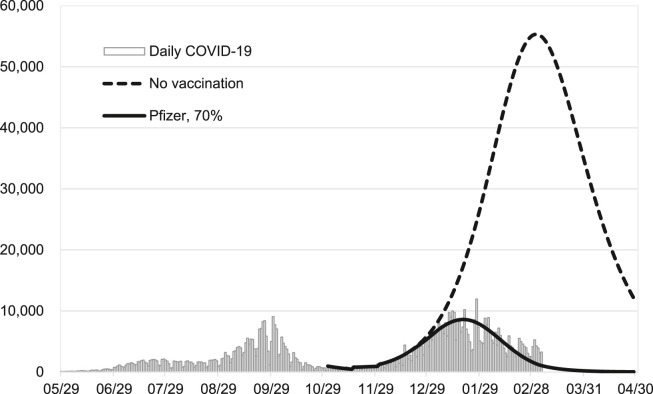
Fig. 2 shows the empirical confirmed COVID-19 cases in Israel between March 2020 and February 2021. The predicted daily count with 70% coverage of Pfizer vaccine fits well with the observed epidemic curve. This suggests a good validity of our model. We also predicted the expected daily confirmed cases from November 2020, the starting time of the second wave in Israel, till the end of April 2021 in the scenarios without being administered by vaccine.
Figure 2.
A validation plot with the empirical and expected daily count of confirmed COVID-19 cases with and without vaccination until April 30 2021 in Israel.
Table 2 shows a series of COVID-19 related outcomes, including confirmed asymptomatic and symptomatic COVID-19 cases, accumulated days of hospitalization, and death with and without vaccines. The administration of COVID-19 vaccine to the simulated cohort led to a substantial reduction of each outcome, including cases, hospitalization, and deaths. Take Moderna vaccine as an example, the jabs were associated with the reductions of 2.9 million symptomatic cases, 0.58 million asymptomatic cases, 4.66 million hospitalization days, and 44508 deaths. These were commensurate with the effectiveness of Moderna vaccine in reducing asymptomatic and symptomatic cases by 85.78% (95% CI: 85.69, 85.88%) and 87.37% (95% CI: 87.33–87.41%), less hospitalization days by 85.09% (95% CI: 84.97, 85.04%), and fewer deaths by 84.17% (95% CI: 83.81, 84.54%). The effectiveness of the Pfizer vaccine was only slightly different (less than 1%) from that of the Moderna vaccine with respect to outcomes. The administration of AstraZeneca vaccine led to a less extent of effectiveness in reducing asymptomatic cases, symptomatic cases, hospitalization, and death as 76.86% (95% CI: 76.73, 76.99%), 80.99% (95% CI: 80.94, 81.04%), 78.59% (95% CI: 78.55, 78.63%), and 77.55% (95% CI: 77.10, 77.99%), respectively.
Table 2.
Numbers of a cascade of COVID-19 related events with and without vaccination.
| Strategy | Asymptomatic COVID-19 |
Symptomatic COVID-19 |
Hospitalization (days) |
Death |
||||
|---|---|---|---|---|---|---|---|---|
| No. | 1-RR | No. | 1-RR | No. | 1-RR | No. | 1-RR | |
| Vaccination | ||||||||
| Moderna | 96,325 | 0.8578 (0.8569, 0.8588) | 417,891 | 0.8737 (0.8733, 0.8741) | 816,780 | 0.8509 (0.8497, 0.8504) | 8368 | 0.8417 (0.8381, 0.8454) |
| Pfizer | 100,096 | 0.8522 (0.8513, 0.8532) | 418,654 | 0.8734 (0.8730, 0.8738) | 818,076 | 0.8506 (0.8503, 0.8510) | 8381 | 0.8415 (0.8378, 0.8451) |
| AstraZeneca | 156,754 | 0.7686 (0.7673, 0.7699) | 628,858 | 0.8099 (0.8094, 0.8104) | 1,172,640 | 0.7859 (0.7855, 0.7863) | 11,872 | 0.7755 (0.7710, 0.7799) |
| No vaccination | 677,467 | 3,307,633 | 5,476,842 | 52,876 | ||||
RR: relative risk; 1-RR refers to the effectiveness in terms of the reductions of asymptomatic and symptomatic cases, duration of hospitalization, and deaths.
Cost-utility analysis
Taken together, the Moderna COVID-19 vaccine yielded an average 0.8284 quality-adjusted life days (QALDs) gained per person with less cost incurred, which indicated that Moderna vaccine was a dominate strategy against no vaccine (ICUR = −321.1441) (Table 3 ). The incremental QALDs gained of Pfizer vaccine was close to that of Moderna, but a larger saving in cost was observed due to the cheaper price of Pfizer (USD $14 per dose) compared to Moderna (USD $31 per dose). The ICUR for Pfizer was −356.7512. As far as AstraZeneca is concerned, the incremental QALD was smaller (0.7456) than the other two. Although the vaccine price was the cheapest (USD $5 per dose) among the three, the incremental cost saving was also the least owing to the higher demand for medical needs. The ICUR for AstraZeneca was −341.4381. Nonetheless, all three vaccines with a coverage rate of 70% were dominant against no vaccination.
Table 3.
Base case results of the cost-effectiveness analysis for three COVID-19 vaccines.
| Strategy | Effectiveness (QALD) | Incremental QALD | Cost (USD) | Incremental cost | ICUR |
|---|---|---|---|---|---|
| Vaccination | |||||
| Moderna | 179.8286 | 0.8284 | 155.4759 | −266.0500 | −321.1441 |
| Pfizer | 179.8120 | 0.8119 | 131.8955 | −289.6303 | −356.7512 |
| AstraZeneca | 179.7458 | 0.7456 | 166.9397 | −254.5862 | −341.4381 |
| No vaccination | 179.0002 | 421.5258 | – | ||
ICUR: incremental cost-utility ratio; QALD: quality-adjusted life day.
Cost-benefit analysis
Table 4 shows the results of the CBA. We itemized the direct cost of vaccine and the associated medical expenditure, the indirect cost related to vaccine jabs and hospitalization by four strategies. The BCR1 suggested that, from the payer's perspective, one dollar of vaccine investment would lead to a return of USD $2.79, USD $4.77, and USD $7.21 for Moderna, Pfizer, and AstraZeneca, respectively. The BCR2 from the societal perspectives (including the cost saving of the direct cost on medical expenditure and the indirect cost) suggested that one dollar of investment would lead to a return of USD $6.05, USD $10.39, and USD $14.46 for Moderna, Pfizer, and AstraZeneca, respectively. Furthermore, when the global economic and education losses were considered, the BCR3 for the three vaccines were inflated to USD $13.54, USD $23.32, and USD $28.85. The BCR4 considering the value of life further brought the corresponding figures to USD $175.84, USD $300.30, and USD $442.79.
Table 4.
The results of the cost-benefit analysis for three COVID-19 vaccines.
| Costsa | No vaccination | Vaccination |
Net cost (saving) of no vaccination versus vaccination |
||||
|---|---|---|---|---|---|---|---|
| Moderna | Pfizer | AstraZeneca | Moderna | Pfizer | AstraZeneca | ||
| Direct cost | |||||||
| Vaccine | 0.0000 | 52.7244 | 30.8519 | 18.9079 | 52.7244 | 30.8519 | 18.9079 |
| COVID-19 medical cost | 172.9218 | 25.7249 | 25.7741 | 36.5047 | (147.1968) | (147.1477) | (136.4170) |
| Indirect cost related to activities for vaccine jab and medical needs | 248.6040 | 77.0265 | 75.2695 | 111.5270 | (171.5775) | (173.3345) | (137.0771) |
| Benefit-cost ratio | |||||||
| Payer's perspective (BCR1) | – | – | – | – | 2.79 | 4.77 | 7.21 |
| Societal perspective (BCR2) | – | – | – | – | 6.05 | 10.39 | 14.46 |
| Economic impacts | |||||||
| Due to productivity and education loss | – | – | – | – | (791.34) | (798.91) | (592.03) |
| Value of statistical life | – | – | – | – | (14418.71) | (14414.57) | (13283.61) |
| Benefit-cost ratio | |||||||
| In terms of productivity and education loss (BCR3) | – | – | – | – | 13.54 | 23.32 | 28.85 |
| In terms of value of statistical life (BCR4) | – | – | – | – | 175.84 | 300.30 | 442.79 |
Data presented in individual average.
Parameter uncertainty
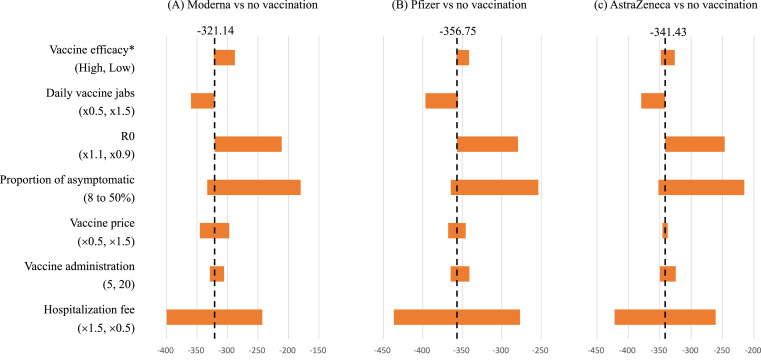
The results of the one-way sensitivity analysis of key parameters are presented in the tornado diagram (Fig. 3 ). It shows that higher vaccine efficacy, high daily volume of vaccination, higher contagiousness, lower proportion of asymptomatic cases, lower vaccine price, lower administration fee for vaccination, and higher level of medical cost led to lower ICURs.
Figure 3.
Tornado plots for the one-way sensitivity analyses of three COVID-19 vaccines. The Y-axis shows variables and its range for the one-way sensitivity analyses. The two numbers included in the parentheses corresponding to the left and right ends of the Tornado diagram for each variable. The axis shows the value of incremental cost-utility ratio. The dash line for the three comparison indicates the base-case estimate. ∗ Vaccine efficacy: High—98% and 80% for reducing symptomatic and asymptomatic cases; Low: 50% and 0% for reducing symptomatic and asymptomatic cases.
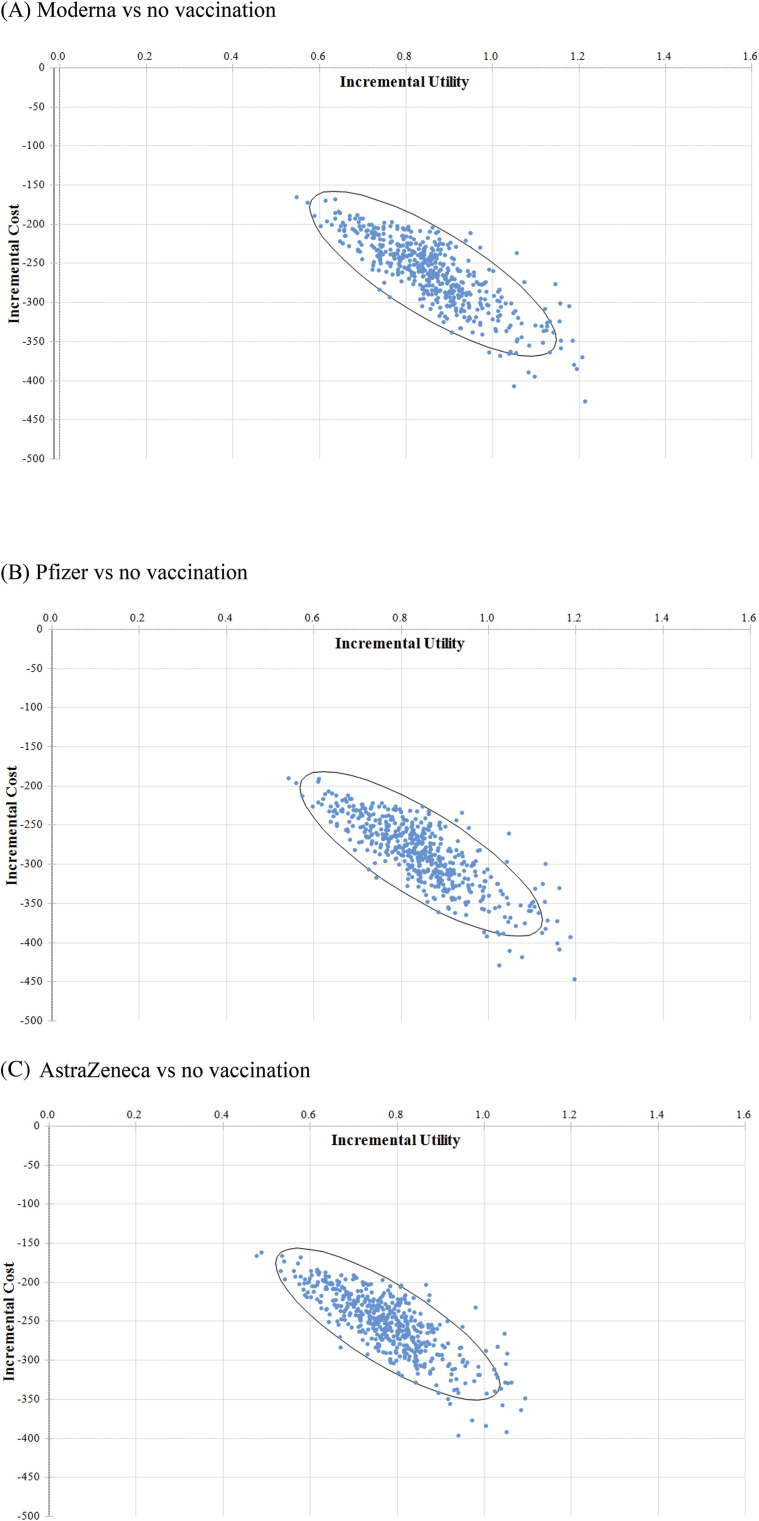
Considering the joint uncertainty of these parameters, the incremental cost-effectiveness scatter plot shows mass vaccination against COVID-19 was almost 100% cost-saving for all three vaccines with 70% coverage rate (Fig. 4 ).
Figure 4.
The incremental cost-effectiveness scatter plots.
Discussion
On the basis of the joint information on SARS-CoV-2 transmission, COVID-19 progression, and vaccination distribution the effectiveness of the mass vaccination strategy can alleviate asymptomatic and symptomatic COVID-19 by about 85–88% given a coverage rate of 70% with Moderna and Pfizer vaccines and by about 75–81% for AstraZeneca, in the context of a country with several surges of community-acquired COVID-19 outbreak such as Israel. Following such a efficacy in the containment of COVID-19 outbreak in community, the effectiveness in reducing the days of hospitalization was estimated as 85% for Moderna and Pfizer vaccines and 78% for AstraZeneca, showing the remarkable effectiveness of mass vaccination in bringing down the medical needs for countries confronted with COVID-19 outbreak. This benefit further results in averting COVID-19 death by 84% for Moderna and Pfizer vaccines and 77% for AstraZeneca. While translating the effectiveness related to all the tracks from infectious process to recovery or death into the framework of economical appraisal, all the three vaccines are cost-saving against no vaccination program. The ICURs per QALD gained for mass vaccination with Moderna, Pfizer, and AstraZeneca ranged between USD $ −357 and USD $ −321. When the global economic and education losses were considered, the BC ratio for the three vaccines were inflated to USD $13.54, USD $23.32, and USD $28.85.
Spurred by the rampage of COVID-19 pandemic in 2020, the schedule on the development of vaccines have been accelerated with at least six vaccines completing phase 3 clinical trial in the beginning of 2021. Following the gradual filling of the gap between demand and supply for vaccination distribution, it is pressing for economical appraisals to guide an informed decision making for health decision maker.8 , 26 , 27
In line with current consensus on scaling up the supply of vaccination for global distribution for the containment of COVID-19 pandemic, our results support the strategy of mass vaccination with rapid distribution. Our findings on the cost-effectiveness on mass vaccination strategy are also supported by recent literatures. Padula et al. reported the cost-effectiveness of a series of vaccination strategies targeting at the hospitalized COVID-19 patients with the focus on vaccination priorities in America.28 The authors reported the uniformly cost-effectiveness of vaccination strategies for hospitalized COVID-19 patients using the willingness-to-pay threshold of USD 50,000. They found that the effectiveness of mass vaccination strategy in more than 50% reduction for hospitalization days and mortality with the reduction of health cost by 90%. The probability of being cost-effective for mass vaccination strategy was around 70% given the wiliness-to-pay threshold of USD $50,000.
In addition to strengthen the evidence of cost-effectiveness in vaccination distribution, our analysis is based on a comprehensive framework covering both the transmission of SARS-CoV-2 in community and the progression of hospitalized COVID-19 patients. Facilitated by such a hinged approach, our analysis is capable of covering the benefit of vaccination strategy form reducing the asymptomatic and symptomatic COVID-19 cases to the clinical outcomes of hospitalized patients resulting from the active immunization. The developed model can be applied to other potentially used vaccines, such as MVC-COV1901 vaccine by Medigen Corp, Taiwan. This can also provide the basis of incorporating strategies combining prevention and treatment measures that are expected to evolve with the elucidation of pathophysiological mechanisms of COVID-19.28
There are several limitations in this study. Firstly, in the current analysis, we only focus on the discussion of vaccine strategies. A comprehensive economic evaluation incorporating different level of containment measures, such as social distancing, border control, and other non-pharmaceutical interventions would be needed. Secondly, the serious adverse events of vaccination such as thrombosis and anaphylactic reactions were not included in the current evaluation due to the rarity of these events and the uncertainty in the evaluation.29 , 30 With the wide-spread rolling out of vaccination and the continuous monitoring of adverse events at global scale, this impact can be incorporated in the further study. Thirdly, considering the rapid evolution on the pandemic, the time frame in the current analysis was set at 180 days. This is also the durability of the immunity conferred by the available vaccines. However, the uncertainty about the duration of immunity needs more researches to support. The fourth limitation is that our current CUA or CBA analysis have not taken into account vaccine hesitancy, which may disfavor the results of CEA and CBA. This would become the subject of an ongoing research. Finally, we have not considered whether and to what extent the effectiveness of vaccine would be affected by emerging viral variants like the UK variant and the Africa variant. These have relied on more researches on the quantification of cross-protection from the current vaccines.
In conclusion, mass vaccination against COVID-19 with three current available vaccines is cost-saving for gaining more lives and less costs incurred. These findings provide the evidence for informed decision making and all stakeholders for the discovery, production, and delivery of COVID-19 vaccine.
Funding
This study was supported by Ministry of Science and Technology, Taiwan (MOST 109-2327-B-002-009).
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jfma.2021.05.020.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.WHO . 2020. COVID-19 situation reports.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed 31st March, 2021) [Google Scholar]
- 2.Cutler D.M., Summers L.H. The COVID-19 pandemic and the $16 trillion virus. JAMA. 2020;324(15):1495–1496. doi: 10.1001/jama.2020.19759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inoue H., Todo Y. The propagation of economic impacts through supply chains: the case of a mega-city lockdown to prevent the spread of COVID-19. PloS One. 2020;15(9) doi: 10.1371/journal.pone.0239251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Riel D., de Wit E. Next-generation vaccine platforms for COVID-19. Nat Mater. 2020;19(8):810–812. doi: 10.1038/s41563-020-0746-0. [DOI] [PubMed] [Google Scholar]
- 5.Deb B., Shah H., Goel S. Current global vaccine and drug efforts against COVID-19: pros and cons of bypassing ani-mal trials. J Biosci. 2020;45:82. doi: 10.1007/s12038-020-00053-2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le T.T., Andreadakis Z., Kumar A., Román R.G., Tollefsen S., Saville M. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19:305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 7.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A. BNT162b2 mRNA covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021 doi: 10.1056/NEJMoa2101765. NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castillo J.C., Ahuja A., Athey S., Baker A., Budish E., Chipty T. Market design to accelerate COVID-19 vaccine supply. Science. 2021;371(6534):1107–1109. doi: 10.1126/science.abg0889. [DOI] [PubMed] [Google Scholar]
- 9.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai C.C., Hsu C.Y., Jen H.H., Yen A.M., Chan C.C., Chen H.H. The bayesian susceptible-exposed-infected-recovered model for the outbreak of COVID-19 on the diamond princess cruise ship. Stoch Environ Res Risk Assess. 2021 Jan 26:1–15. doi: 10.1007/s00477-020-01968-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byambasuren O., Cardona M., Bell K., Clark J., McLaws M.L., Glasziou P. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. JAMMI. 2020;5(4):223–234. doi: 10.3138/jammi-2020-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jen H.H., Chen S.Y., Chang W.J., Chen C.N., Yen A.M.F., Chang R.E. Evaluating medical capacity for hospitalization and intensive care unit of COVID-19: a Queue model approach. J Formos Med Assoc. 2021 May 9 doi: 10.1016/j.jfma.2021.05.002. S0929-6646(21)00187-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jen H.H., Chang W.J., Lin T.Y., Hsu C.Y., Yen A.M., Lai C.C. Evaluating clinical efficacy of antiviral therapy for COVID-19: a surrogate endpoint approach. Infect Dis Ther. 2021 Mar 18:1–11. doi: 10.1007/s40121-021-00431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C. Remdesivir for the treatment of covid-19—preliminary report. N Engl J Med. 2020;383(10):993–994. doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 15.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C. Remdesivir for the treatment of covid-19 - final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S. C4591001 clinical trial group. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020 Dec 31;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., COVE Study Group Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021 Feb 4;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Oxford COVID Vaccine Trial Group Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021 Jan 9;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wouters O.J., Shadlen K.C., Salcher-Konrad M., Pollard A.J., Larson H.J., Teerawattananon Y. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet. 2021 Mar 13;397(10278):1023–1034. doi: 10.1016/S0140-6736(21)00306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National health insurance administration. https://www.nhi.gov.tw/Content_List.aspx?n=58ED9C8D8417D00B
- 21.Kohli M., Maschio M., Becker D., Weinstein M.C. The potential public health and economic value of a hypothetical COVID-19 vaccine in the United States: use of cost-effectiveness modeling to inform vaccination prioritization. Vaccine. 2021;39(7):1157–1164. doi: 10.1016/j.vaccine.2020.12.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J.T., Hammitt J.K., Wang J.D., Tsou M.W. Valuation of the risk of SARS in taiwan. Health Econ. 2005;14(1):83–91. doi: 10.1002/hec.911. [DOI] [PubMed] [Google Scholar]
- 23.Hammitt J.K. Valuing mortality risk in the time of COVID-19. J Risk Uncertain. 2020;61:129–154. doi: 10.1007/s11166-020-09338-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellavance F., Dionne G., Lebeau M. The value of a statistical life: a meta-analysis with a mixed effects regression model. J Health Econ. 2009;28:444–464. doi: 10.1016/j.jhealeco.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Mallow P.J. Estimates of the value of life lost from COVID-19 in Ohio. J Comp Eff Res. 2021;10(4):281–284. doi: 10.2217/cer-2020-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suwantika A.A., Boersma C., Postma M.J. The potential impact of COVID-19 pandemic on the immunization performance in Indonesia. Expert Rev Vaccines. 2020 Aug;19(8):687–690. doi: 10.1080/14760584.2020.1800461. [DOI] [PubMed] [Google Scholar]
- 27.Appleby J. Will covid-19 vaccines be cost effective-and does it matter? BMJ. 2020 Nov 26:371. doi: 10.1136/bmj.m4491. m4491. [DOI] [PubMed] [Google Scholar]
- 28.Padula W.V., Malaviya S., Reid N.M., Tierce J., Alexander G.C. 2020. Economic value of treatment and vaccine to address the COVID-19 pandemic: A U.S. Cost-effectiveness and budget impact analysis.https://ssrn.com/abstract=3586694 Available at SSRN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahase E. AstraZeneca vaccine: blood clots are "extremely rare" and benefits outweigh risks, regulators conclude. BMJ. 2021;373:n931. doi: 10.1136/bmj.n931. [DOI] [PubMed] [Google Scholar]
- 30.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021 doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.