Abstract
Background:
Some evidences show that immune infiltration is closely related to the clinical outcomes in cancers such as colorectal cancer. However, previous studies have not explained the diversity of cell types that make up the immune response. In particular, although some studies and reviews have shown that immunotherapy is important for cancer treatment, few studies have elucidated the relationship between prostate cancer (PCa) phenotype and immune infiltration.
Objectives:
In this study, we analyzed whether different types of tumor-infiltrating immune cells would affect the clinical phenotypes and survival of PCa based on a deconvolution algorithm and annotated gene expression profiles.
Materials and Methods:
The 22 subsets of immune cells inferred by CIBERSORT and the infiltration abundance of 6 immune cells calculated by TIMER were used to determine the associations between them and the PCa traits and survival response. In addition, the survival tree models were constructed to classify PCa patients into four subtypes, and the traits and prognosis were compared among these subtypes.
Results:
As a result, we found that some PCa patients with high death risk lacking immune infiltration were related to the poor prognosis. For the cell subsets studied and subtypes analysis, a low proportion of mast resting cells and T-cells follicular helper exhibited the obvious association with poor outcome.
Conclusions:
In summary, our study suggested the differences in the cellular composition of the immune infiltrate in PCa, and these differences might be important determinants for PCa traits and prognosis.
Keywords: Genomic signature, Immune infiltration, Prostate cancer
1. Background
Prostate cancer (PCa) is a common cancer threatening men’s health and quality of life. Over the past decades, the incidence of PCa has increased significantly ( 1 ). PCa displays obvious clinical heterogeneity, which makes PCa face great challenges in individualized clinical decision-making. More and more evidences of tumor cell genomic and biological changes show that it is important to identify PCa subtypes with different prognosis and therapeutic response for exploring potential molecular variation and drug targets ( 2 ). As far as we know, the malignant phenotypes of cancers are not only determined by the intrinsic activity of tumor cells, but also recruited and activated by immune cells in tumor related microenvironment ( 3 ).
At present, the role of immune cells in some malignant tumors and the concept of immunotherapy have been widely studied. Immune responders and immune non- responders consist of the immune cells ( 4 ). The degree of infiltration of immune cells may contribute to the stratification of patients. The infiltrated immune cells may improve the prediction of clinical prognosis and become the effective drug targets. For example, Li et al. analyzed tumor-infiltrating immune cells using over 10,000 RNA-seq samples for 23 cancer types from The Cancer Genome Atlas (TCGA). They calculated and inferred that immune infiltrates are associated with clinical features and cancer genetic changes of patients ( 5 ). By performing a review, Vitkin et al. speculated that the design of immunotherapy experiment of PCa should consider the cumulative influence of factors such as immune status before treatment, PTEN expression, and the influence of conventional therapy on anti-tumor immune response( 6 ). Similarly, Jansen et al. reviewed the evidences of the composition of infiltrative immune cells in tumor microenvironment. It is considered that the composition and tissue structure of tumor immune microenvironment is an important topic worthy of discussion in PCa research and an important field for future research( 7 ). Solinas et al. also suggested that a pre-existing immune infiltrate composition and activity may help to identify ideal candidates for immunotherapy, with the possible effects from early to late clinical management of tumor disease by a review of studies in the immune infiltrate in prostate, bladder and testicular tumors.
Most of previous studies investigated CD3+, CD4+, or CD8+ cells in prostate cancer patients undergoing prostatectomy or biopsy, and found that these cells are tumorigenic ( 8 ). It is reported that the higher numbers of regulatory T-cells (Treg) were associated with more advanced PCa stage ( 9 ). Some literatures show that tumor associated macrophages are related to the recurrence of PCa, and tumor-associated macrophages infiltration in prostate biopsies is a predictor of disease progression after PCa hormonal therapy ( 10 ). In addition, Nonomura et al. determined tumor-associated macrophage count is an significant prognostic factor of clinical outcome in patients with PCa( 11 ).
Interestingly, previous studies have shown that the response of B cells to tissue damage or death after androgen depletion promotes prostate recovery or abnormal proliferation( 12 ). We therefore consider it is necessary to investigate the association between the immune infiltration and clinical phenotypes and prognosis of PCa.
Recently, the systems biology tool CIBERSORT ( 3 ) uses deconvolution of bulk gene expression data and a complex algorithm to quantify various immune cell types in heterogeneous samples( 13 ). The relative proportion of 22 distinct functional subsets of immune cells was estimated by CIBERSORT. As another tool, TIMER (Tumor Immune Estimation Resource) detects immune cell infiltration in tumor tissue using RNA-Seq expression profile data ( 5 ). The infiltration abundance of six immune cells were detected and quantified in TIMER. TIMER removes highly expressed genes to eliminate bias effect and the co-linearity between immune cells to ensure the accuracy of inference ( 14 ).
2. Objectives
In this study, CIBERSORT and TIMER were used to quantitatively analyze the tumor-infiltrating immune cells subsets in PCa and to explore the relationship between immune response and PCa phenotype and survival. In particular, we applied immune cell infiltration and PCa-related phenotypes (such as PSA and gleason score) to construct the survival tree models to classify PCa patients into four subtypes, and the prognosis were compared among these subtypes. We found that some PCa patients with high death risk lacking immune infiltration were associated with the poor prognosis. For the cell subsets observed and subtypes analysis, a low proportion of mast resting cells and T-cells follicular helper displayed the strong association with poor outcome. This study may help to reveal the potential relationship between the intratumoral immune cells heterogeneity and disease progression in PCa.
3. Materials and Methods
3.1. Data Collection
The data of gene expression profiles and corresponding clinical information from PCa patients were downloaded from The Cancer Genome Atlas (TCGA) database (https://cancergenome.nih.gov/). Only patients diagnosed with PCa and patients with clinical and survival information available is included. For gene expression data of the TCGA dataset, reads were aligned to hg19 with Tophat2 ( 15 ), and FPKM (fragments per kilobase of transcript per million fragments mapped) values were generated and normalized. This process covert the count data to values which are more similar to microarray data and the microarray data format is suitable to CIBERSORT. Finally, 519 tumor samples were come into the analysis.
3.2. Calculation of Tumor Infiltration Abundance of Immune Cells
The relative proportions of 22 types of infiltrating immune cells were inferred using the CIBERSORT based on the normalized gene expression data ( 3 ). The 22 cell types inferred by CIBERSORT include B cells, T cells, natural killer cells, macrophages, dendritic cells, and other types of immune cells. CIBERSORT uses a set of reference gene expression values considered as a minimal representation of each cell type, and based on these values, support vector regression was used to infer the proportion of cell types ( 3 ). Here, we used LM22 gene file of CIBERSORT to analyze PCa data. We used CIBERSORT to implement this analysis, and the algorithm used the default signature matrix to run in 1000 permutations. In addition, TIMER (14) performed the estimation of infiltration abundance of six immune cells (B cells, CD4+ T cells, CD8+ T cells, Neutrphils, Macrophages and Dendritic cells) by constrained least- squares method, and this method is validated using pathological estimations. In this paper, CIBERSORT and TIMER simultaneously were applied to quantify the tumor-infiltrating immune cells subsets in PCa patients.
3.3. Statistical Analysis
Median of the fraction based on CIBERSORT or abundance based on TIMER of each cell type were compared between PCa survival patients and PCa death patients using the independent Manny-Whitney test. The potential relationship between immune cell infiltration and PCa related traits: prostate-specific antigen (PSA) and gleason score was investigated by Spearman correlation analysis. PSA level is suggested to be related with a high incidence of prostate tumors. The gleason score is directly related to clinical stage, survival, progression of metastatic disease, tumor size, margin status and pathologic stage. A high gleason score indicates that the tumor is more likely to exhibit aggressive behavior( 16 ).
The associations between inferred proportions (or abundances) of immune cell types based on CIBERSORT and TIMER and survival were performed. CIBERSORT proportions were divided into three levels (<33rd percentile, >33rd percentile and <66rd percentile, >66rd percentile) and these three levels for each of immune cells are taken as univariate to perform Cox regression analysis. The Hazard Ratios (HRs) and 95% confidence intervals are depicted, and p<0.1 was considered as significant. Also, the same analyses were implemented for the immune cells abundance inferred by TIMER. The relationship between immune cell infiltrate abundance and overall survival were evaluated and compared by Kaplan-Meier curves and log-rank test.
In addition, to investigate whether there are differences in immune cell infiltration and PCa related traits among different classes, which might be associated with different PCa outcome, we conducted the survival decision tree models to classify samples into difference classes and performed the Kaplan-Meier curves to evaluate the prognosis of these classes. The statistical analyses were performed using R software ( 17 ). P<0.05 was statistically significant, and statistical tests were all two-sided.
4. Results
4.1. Evaluation of Tumor-Infiltrating Immune Cells Based on CIBERSORT and TIMER
Based on CIBERSORT algorithm, we identified the difference of immune cells infiltration between PCa survival patients and PCa death patients in 22 types of immune cells.
In Figure 1, it is seen that the infiltration proportion of some immune cells are different between PCa survival patients and PCa death patients. PCa survival patients have a lower proportion of CD8+ T cells (P=0.007) and a higher proportion of Mast cells resting (P=0.018). These results indicated that PCa patients have aberrant immune infiltration and the potential heterogeneity. However, using TIMER tool, there was no significant difference between PCa death patients and PCa survival patients in infiltration abundance of six immune cells including B cells, CD4+ T cells, CD8+ T cells, Neutrphils, Macrophages and Dendritic cells.
Figure 1.
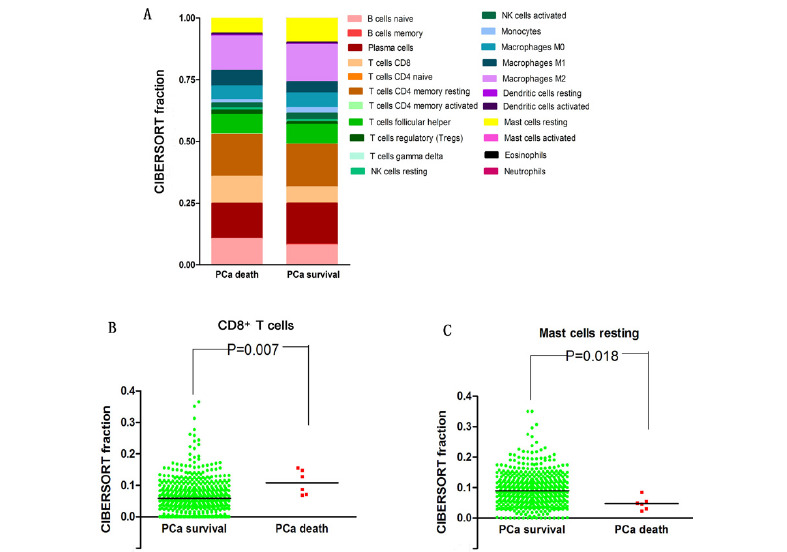
The comparison of CIBERSORT immune cells infiltration fractions between PCa survival patients and PCa death patients. (A) The distribution of 22 types of infiltrating immune cells based on the CIBERSORT for PCa survival patients and PCa death patients, respectively. (B) The comparison of CD8+ T cells infiltration fractions between PCa survival patients and PCa death patients. (C) The comparison of Mast cells resting infiltration fractions between PCa survival patients and PCa death patients.
4.2. The Correlation between Clinical Traits and Immune Cell Infiltration
We performed the Spearman correlation analysis ( 18 ) to investigate the potential relationship between immune cell infiltration and PCa-related traits: PSA and gleason score. The results showed that PSA is associated with Neutrophil, Plasma cells and Macrophages M1. Meanwhile, Macrophages M1 and Neutrophil display high infiltration in PCa patients with higher PSA (Fig. 2A, C) whereas the infiltration in Plasma cells is reduced as the PSA increased (Fig. 2B). Gleason score is associated with Macrophage, Plasma cells, T cells regulatory Tregs, Macrophages M1, Macrophages M2 and Mast cells resting. Meanwhile, Macrophages M1, Macrophages M2 and T cells regulatory Tregs display high infiltration in PCa patients with high gleason score (Fig. 2D, F, G and H) whereas the infiltration in Plasma cells and Mast cells resting are reduced as the gleason score increased (Fig. 2E, I).
Figure 2.
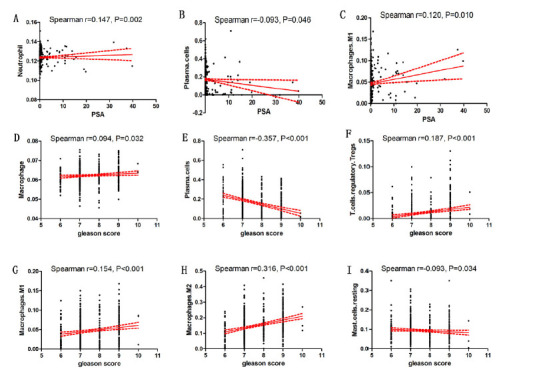
The correlation between PCa clinical traits and immune cell infiltration. (A) Neurophil (TIMER) and PSA; (B) Plasma cells (CIBERSORT) and PSA; (C) Macrophages M1 (CIBERSORT) and PSA; (D) Macrophages (TIMER) and gleason score; (E) Plasma cells (CIBERSORT) and gleason score; (F) T cells regulatory Tregs (CIBERSORT) and gleason score; (G) Macrophages M1 (CIBERSORT) and gleason score; (H) Macrophages M2 (CIBERSORT) and gleason score; (I) Mast cells resting (CIBERSORT) and gleason score.
4.3. The Association between PCa Prognostic and Tumor-Infiltrating Immune Cells
In order to further investigate whether there was significant correlation between immune subpopulation and PCa-related prognosis, we divided CIBERSORT immune infiltration proportions or TIMER immune infiltration abundances into three levels (<33rd percentile, >33rd percentile and <66rd percentile, >66rd percentile) and each of these three immune infiltration levels are taken as univariate to perform Cox regression analysis. For CIBERSORT immune infiltration proportions, the results showed that Mast cells resting, Dendritic cells activated and CD8+ T cells are significant (Fig. 3A). Mast cells resting (HR=0.299, 95% CI: 0.077-1.156) was associated with improved outcome, whereas Dendritic cells activated (HR=4.197, 95% CI: 0.768-22.928) and CD8+ T cells (HR=3.202, 95% CI: 0.974-14.673) were associated with poor outcome. Furthermore, for these three types of tumor- infiltrated immune cells, the median level is used to distinguish from low and high, and the Kaplan-Meier curve and log-rank tests showed that high-infiltrating Mast cells resting and low-infiltrating CD8+ T cells had a better prognosis (Fig. 3B and D). However, no significant results were found for the immune infiltration abundances predicted by TIMER.
Figure 3.
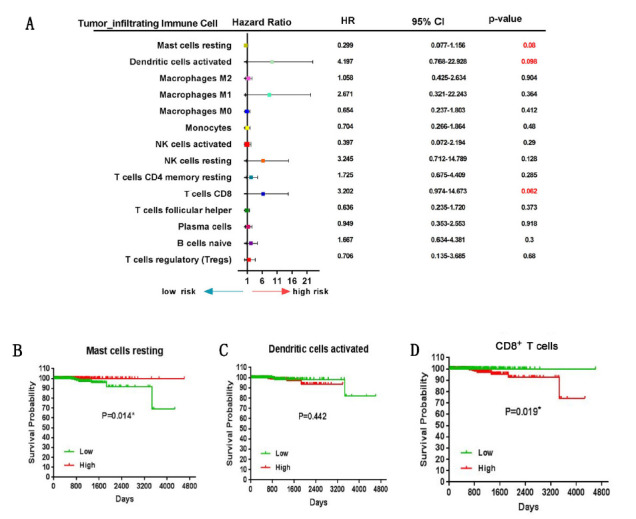
PCa Prognostic associations with tumor-infiltrating immune cells. (A) Hazard Ratio and 95% confidence intervals (horizontal lines). Number marked in red showing a P-value <0.1. (B) Kaplan-Meier curve of overall survival for Mast cells resting. (C) Kaplan-Meier curve of overall survival for Dendritic cells activated. (D) Kaplan-Meier curve of overall survival for CD8+ T cells. For (B), (C) and (D), the P-values are based on log- rank tests. Median is distinguishing from low and high of CIBERSORT immune infiltration proportions.
4.4. PCa Prognosis Related Distinct Classes Based on Survival Decision Tree Model
In particular, to investigate whether distinct classes of immune cell infiltration and PCa-related traits are associated with different PCa outcome, we conducted the survival decision tree models to classify samples and performed the Kaplan-Meier curves to evaluate the prognosis of these distinct classes. We used the immune infiltration fractions obtained by CIBERSORT and immune infiltration abundance obtained by TIMER to construct two survival decision trees respectively, and these tree models include age, PSA and gleason scores. The constructed tree based on CIBERFORT immune infiltration fractions revealed the identified four clusters, and the major split variable are Mast cells resting and T-cells follicular helper (Fig. 4A). It is seen that the lower Mast cells resting and T-cells follicular helper infiltration can cause the poor prognosis. The immune cells proportions of each cluster were shown in Figure 4B. Meanwhile, among these four clusters, B-cells naive (P<0.001), B-cells memory (P=0.001), Plasma cells (P<0.001), CD8+ T cells (P<0.001), CD4+ T cells memory activated (P=0.009), T-cells follicular helper (P<0.001), T-cells regulatory Tregs (P=0.018), NK cells resting (P<0.001), NK cells activated (P<0.001), Monocytes (P<0.001), Macrophages M1 (P<0.001), Mast cells resting (P<0.001) and Mast cells activated (P=0.002) are all significant. We found there were significant differences in gleason score among four clusters (P=0.042) (Fig. 4C). However, there was no significant difference in PSA (P=0.775). Moreover, we found that clusters were related to distinct patterns of survival. For instance, the cluster 4, defined by low levels of Mast cells resting and T-cells follicular helper, were associated with poor outcome compared with cluster 1, cluster 2 and cluster 3 (P<0.001) (Fig. 4D).
Figure 4.
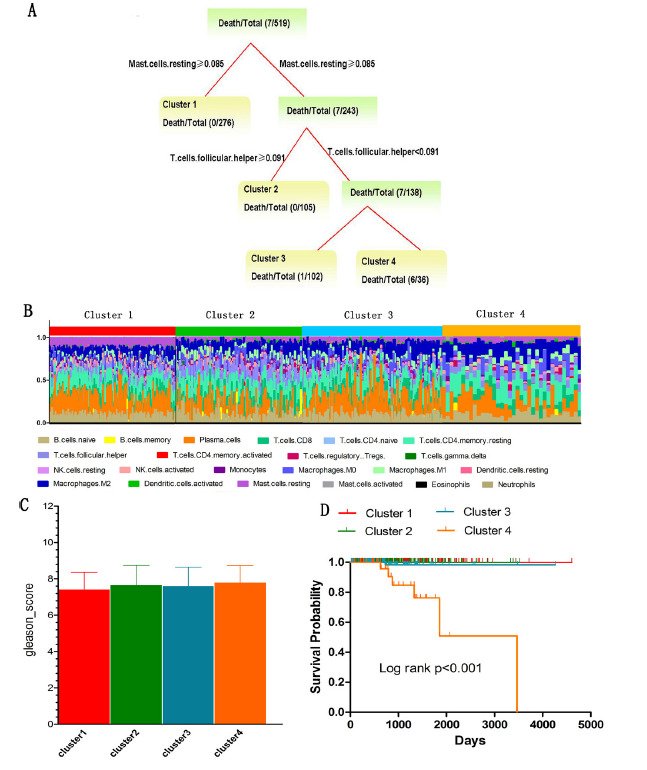
The distinct classes by combining immune cells infiltration and PCa-related traits. (A) The survival tree model based on CIBERSORT immune infiltration fractions. Each termination node in the tree represents the distinct class. (B) Stacked bar charts of immune cell proportions across samples is ordered according to cluster assignment. (C) Gleason score comparison for four distinct classes. (D) Kaplan-Meier survival analysis of patients among different clusters. P-values are based on log-rank tests.
The constructed tree based on TIMER immune infiltration abundance revealed the identified four clusters, and the major split variable is PSA, age and CD4+ T cells (Fig. 5A). We can see that the higher CD4+ T cells infiltration can cause the poor prognosis for those patients with age ≥ 64. We found that there were significant difference among four clusters in B-cells, CD4+ T cells, Neutrophil and Dendritic cell (DC) (P<0.01) (Fig. 5B). Also, there were significant differences among these four clusters in gleason score (P<0.001) and PSA (P<0.001), and the gleason score and PSA are special high levels in cluster 4 (Fig. 5C). Furthermore, we found that the clusters were related to distinct patterns of survival. We can see that the cluster 4, defined by high levels of PSA, were associated with poor outcome (Fig. 5D). In addition, the overall survival of cluster 1 and cluster 2 have better prognosis (compared with cluster 3 and cluster 4, P<0.05).
Figure 5.
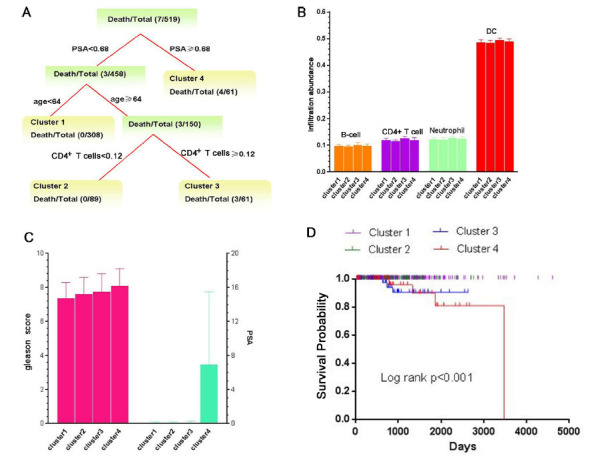
The distinct classes by combining immune cells infiltration and PCa-related traits. (A) The survival tree model based on TIMER immune infiltration abundances. Each termination node in the tree represents the distinct class. (B) The immune infiltration abundance comparison for four distinct classes. (C) Gleason score and PSA comparison for four distinct classes. (D) Kaplan-Meier survival analysis of patients among different clusters. P-values are based on from log-rank tests.
5. Discussion
In this study, CIBERSORT and TIMER were used to quantitatively detect tumor infiltrating immune cells and to explore the relationship between PCa phenotype and immune response and survival. We found that PCa survival patients have a low proportion of CD8+ T cells. By constructing decision tree model based on TIMER tool, among those older patients (age ≥ 64) with lower PSA, a slightly lower proportion of CD4+ T cells have a better prognosis. However, by constructing survival tree model based on CIBERSORT, we found that the higher infiltration levels of T-cells follicular helper can obtain the better prognosis obviously. In practice, some studies have found that the higher infiltration of CD4+ and CD8+ T cells is independently related to the improvement of survival after radical prostatectomy ( 19 ). Xiang et al. indicated that CD4+ T cells infiltration can promote chemotherapy resistance of PCa by regulating CCL5/ STAT3 signaling pathway( 20 ). A new study reported that CXCL 13-producing follicular helper CD4+ T cells infiltrating human breast cancer marks an organized immune response and predicts a good clinical outcome. This finding suggested follicular helper CD4+ T cells in cancer are an important immune factor in tumors, and their presence in tumor is a prognostic factor( 21 , 22 ). However, some other studies have not found that high levels of T cells infiltration contribute to the survival. For example, the spontaneous transgenic adenocarcinoma of the mouse prostate (TRAMP) model found that the transfer of naturally produced clonal amplification of PCa histone H4-specific population of CD8+ T cells to prostate tissue could reduce the tumor burden, but could not improve the survival rate( 23 ). Consider PCa has a relatively long expected survival, Ness et al. used Biochemical failure (BF) as endpoint to perform the survival analysis, and they found high T lymphocytes density in primary PCa tumors is associated with a short BF-free survival( 24 ). Furthermore, our study found that T cells regulatory Tregs is positive correlated with gleason score. This means that high level of Tregs is associated with aggressive behavior and poor prognosis. This result is consistent with some previous studies. For example, high levels of Tregs in the tumor microenvironment have been reported to be associated with poor prognosis in many cancers( 4 , 25 ). Many studies have provided the evidences that mast cells can prevent tumor growth ( 26 ), and found the negative regulation of mast cells on angiogenesis and tumor growth in patients with PCa ( 27 ). Our study found that the number of mast resting cells in PCa death patients was lower than that in PCa survival patients (Fig. 1C), which is consistent with previously reports. In addition, we found that the prognosis of mast cells at lower levels was poor in PCa patients, which is also validated by our constructed survival tree model. Similarly, the correlation analysis also showed that the immune infiltration levels of mast resting cells are negatively correlated with gleason scores, this means that a lower mast cell infiltration is associated with a higher gleason score, indicating that the tumor is more likely to exhibit aggressive behavior.
Interestingly, we found that Macrophages are positively correlated with PSA and gleason score. It is known that Macrophages play an important role in tumor growth and metastasis ( 28 ). Although many studies have detected the macrophage related markers in PCa samples, the results still remain controversial. For example, Hu et al. found tumor associated macrophages infiltration in prostate tumor in patients with metastasis is higher than that in patients without metastasis ( 29 ). Zhang et al. reported Macrophages M1 with high infiltration of PCa patients had a better prognosis(4). By applying a meta-analysis, Cao et al. suggested that the high density of tumor associated macrophages (TAMs) is related to the lower overall survival, but not to the biochemical recurrence or recurrence-free survival in PCa(30).
In addition, although Kaplan-Meier curve and log-rank test did not show that high infiltrated and low infiltrated DC had different prognosis, Dendritic cells activation is associated with poor prognosis based on the Cox regression analysis. In tumor environment, DC enters tumor tissue through blood circulation and interacts with tumor cells. Youlin et al. investigated whether Prostaglandin E2 (PGE2) can inhibit PCa progression in mice by antagonizing impairment of DC migration induced by tumor microenvironment, and they found that in RM-1-bearing mouse model, PGE2 treatment inhibited tumor growth and induced more tumor- infiltrating T cells and CD11c Dendritic cells at tumor sites( 31 ).
Specially, by applied TIMER tool, we performed a “Somatic Copy Number Alteration (SCNA)” analysis for the identified PCa-survival related genes in our previous study(2). “SCNA” module provides a comparison of tumor infiltration levels with changes in different somatic copy numbers for a given gene. The infiltration level of each SCNA category is compared with the normal subjects using two-sided Wilcoxon rank sum test ( 32 ). We found that CD8+ T cells of ARR3, KCNE1, PRKCA and SRC have significant differences (P<0.05) in PCa patients and normal subjects. The significant differences were seen from Macrophage of ARR3, PRKCA and SRC (P<0.05); Neutrophil of ARR3, MAPK15, RCOR2 and SRC (P<0.05); Dendritic cell of ARR3, PRKCA, RCOR2 and SRC (P<0.05) (Fig. 6). These findings suggested that the genes identified in our previous study exhibit different somatic copy number alterations in tumor- infiltrating immune cells.
Figure 6.
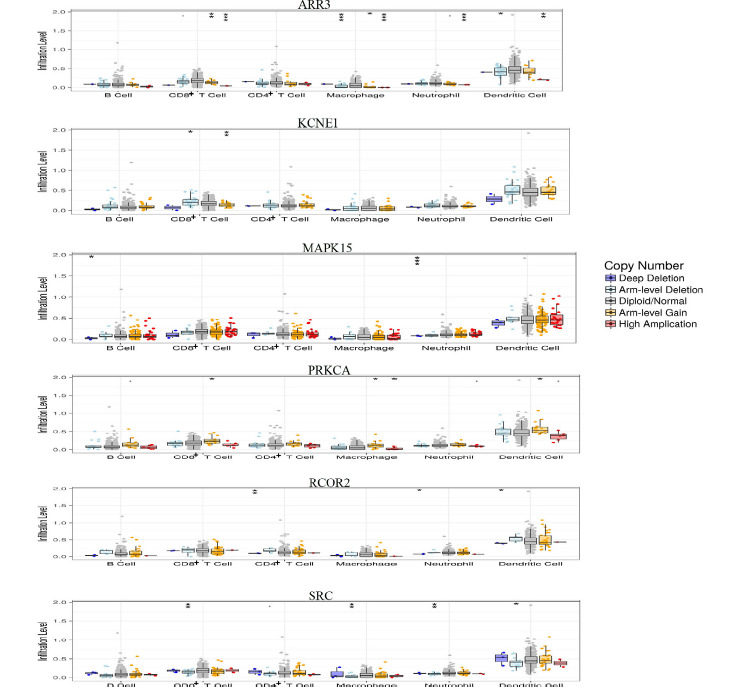
SCNA analysis for the identified PCa-survival related genes. Box plots show the distributions of each immune subset at each copy number status in given genes. The infiltration level of immune cells for each SCNA category is compared between PCa patients and the normal using two-sided Wilcoxon rank sum test. .P<0.1,*P<0.05, **P<0.01,***P<0.001.
Although our study provides some insights to understand the complex relationship between intratumoral immune cell heterogeneity and disease progression in PCa, the limitations should be pointed out. On one hand, although we selected two tools (CIBERSORT and TIMER) to obtain the infiltration levels of immune cells, these levels can only be obtained by algorithms, but not by experiments; therefore, in the future study, we will carry out the immunohistochemistry detection with experiments to validate these results. On the other hand, we only selected the limited datasets of PCa, and the sample size of death patients are too small, thus more available datasets will be used in future studies to address this issue. In addition, it is important to understand the relationships between tumor immune infiltration and RNA molecular regulation mechanism in the pathogenesis of PCa. For example, the hypothesis of endogenous competitive RNA provides a new way to study immune heterogeneity. Therefore, in future studies, we will integrate more molecular biomarkers, such as microRNA (miRNA) and long non-coding RNA (lncRNA) ( 33 ), to explore potential relationship between immune infiltration and PCa prognosis, and try to find out whether immune infiltration affects the survival of PCa patients.
6. Conclusion
In conclusion, our study found that PCa patients have different cellular composition of the immune infiltrate, and these differences might be important determinants of both traits and prognosis of PCa patients.
Acknowledgement
This work is supported by the Beijing Natural Science Foundation (Grant Nos. 7142015).
Conflict of Interest: The authors declare that they have no competing interests.
References
- 1.Zedan AH, Blavnsfeldt SG, Hansen TF, Nielsen BS, Marcussen N, Pleckaitis M, et al. Heterogeneity of miRNA expression in localized prostate cancer with clinicopathological correlations. PLoS One. 2017;12:e0179113. doi: 10.1371/journal.pone.0179113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin Hua, Hong Xia, Wenbin Xu, Ping Zhou. Risk stratification for prostate cancer via the integration of omics data of The Cancer Genome Atlas. Transl Cancer Res. 2018;7(3):706–719. doi: 10.21037/tcr.2018.06.01. [DOI] [Google Scholar]
- 3.Xiong Y. Profiles of immune infiltration in colorectal cancer and their clinical significant: A gene expression-based study. Cancer Med. 2018;7:4496–4508. doi: 10.1002/cam4.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang E, Dai F, Mao Y, He W, Liu F, Ma W, et al. Differences of the immune cell landscape between normal and tumor tissue in human prostate. Clin Transl Oncol. 2019 doi: 10.1007/s12094-019-02128-5. [DOI] [PubMed] [Google Scholar]
- 5.Li B, Severson E, Pignon J-C, Zhao H, Li T, Novak J, et al. Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biol. 2016;17(1):174. doi: 10.1186/s13059-016-1028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vitkin N. The Tummor Immune Contexture of Prostate Cancer. Front Immunol. 2019;10:603. doi: 10.3389/fimmu.2019.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jansen CS, Prokhnevska N, Kissick HT. The requirement for immune infiltration and organization in the tumor microenvironment for successful immunotherapy in prostate cancer. Urol Oncol. 2019;37(8):543–555. doi: 10.1016/j.urolonc.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strasner A, Karin M. Immune infiltration and prostate cancer. Front Oncol. 2015;5:128. doi: 10.3389/fonc.2015.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savage PA, Vosseller K, Kang C, Larimore K, Riedel E, Wojnoonski K, et al. Recognition of a ubiquitous self antigen by prostate cancer-infiltrating CD8+ T lymphocytes. Science. 2008;319(5860):215–220. doi: 10.1126/science. [DOI] [PubMed] [Google Scholar]
- 10.Gannon PO, Poisson AO, Delvoye N, Lapointe R, Mes-Masson AM, Saad F. Characterization of the intra-prostatic immune cell infiltration in androgen-deprived prostate cancer patients. J Immunol Methods. 2009;348(1-2):9–17. doi: 10.1016/j.jim.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Nonomura N, Takayama H, Nakayama M, Nakai Y, Kawashima A, Mukai M, et al. Infiltration of tumour-associated macrophages in prostate biopsy specimens is predictive of disease progression after hormonal therapy for prostate cancer. BJU Int. 2011;107(12):1918–1922. doi: 10.1111/j.1464-410X.2010.09804.x. [DOI] [PubMed] [Google Scholar]
- 12.Reddy KR, Guan Y, Qin G, Zhou Z, Jing N. Combined treatment targeting HIF-1α and Stat3 is a potent strategy for prostate cancer therapy. Prostate. 2011;71(16):1796–1809. doi: 10.1002/pros.21397. [DOI] [PubMed] [Google Scholar]
- 13.Chen B, Khodadoust MS, Liu CL, Newman AM, Alizadeh AA. Profiling Tumor Infiltrating Immune Cells with CIBERSORT. Methods Mol Biol. 2018;1711:243–259. doi: 10.1007/978-1-4939-7493-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei B, Kong W, Mou X, Wang S. Comprehensive analysis of tumor immune infiltration associated with endogenous competitive RNA networks in lung adenocarcinom. Pathol Res Pract. 2019;215(1):159–170. doi: 10.1016/j.prp.2018.10.032. [DOI] [PubMed] [Google Scholar]
- 15.Wang K, Singh D, Zeng Z, Coleman SJ, Huang Y, Savich GL, et al. MapSplice: Accurate mapping of RNA-seq reads for splice junction discovery. Nucleic Acids Res. 2010;38(18):e178. doi: 10.1093/nar/gkq622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jefferies MT, Pope CS, Kynaston HG, Clarke AR. Analysis of Fascin-1 in Relation to Gleason Risk Classification and Nuclear ETS-Related Gene Status of Human Prostate Carcinomas: An Immunohistochemical Study of Clinically Annotated Tumours From the Wales Cancer Bank. Biomark Cancer. 2017;9:1179299X17710944. doi: 10.1177/1179299X17710944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jalal H, Pechlivanoglou P, Krijkamp E. An Overview of R in Health Decision Sciences. Med Decis Making. 2017;37(7):735–746. doi: 10.1177/0272989X16686559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varn FS, Wang Y, Mullins DW, Fiering S, Cheng C. Systematic pan-cancer analysis reveals immune cell interactions in the tumor microenvironment. Cancer Res. 2017;77(6):1271–1282. doi: 10.1158/0008-5472.CAN-16-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, Attwood K, Versaggi C, Omilian A, Bshara W, Xu B, et al. Association of high CD8+ tumor infiltrating lymphocytes at prostatectomy with improved survival of prostate cancer patients. J Clin Oncol. 2018;36(15_suppl): 5068. doi: 10.1158/1078-0432.CCR-11-1899. [DOI] [Google Scholar]
- 20.Xiang P, Jin S, Yang Y, Sheng J, He Q, Song Y, et al. Infiltrating CD4+ T cells attenuate chemotherapy sensitivity in prostate cancer via CCL5 signaling. Prostate. 2019;79(9):1018–1031. doi: 10.1002/pros.23810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu-Trantien C, Willard-Gallo K. CXCL13-producing follicular helper CD4+ T cells infiltrating human breast cancer signal an organized immune response and predict a favorable clinical outcome. Cancer Res. 2013;73(1 Suppl) doi: 10.1158/1538-7445.TUMIMM2012-B87. [DOI] [Google Scholar]
- 22.Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, Wind Ad, et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. 2013;123(7):2873–2892. doi: 10.1172/JCI67428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savage PA, Vosseller K, Kang C, Larimore K, Riedel E, Wojnoonski K, et al. Recognition of a ubiquitous self antigen by prostate cancer-infiltrating CD8+ T lymphocytes. Science. 2008;319(5860):215–220. doi: 10.1126/science.1148886. [DOI] [PubMed] [Google Scholar]
- 24.Ness N, Andersen S, Valkov A, Nordby Y, Donnem T, Al-Saad S, et al. Infiltration of CD8þLymphocytes is an Independent Prognostic Factorof Biochemical Failure-Free Survival in Prostate Cancer. The Prostate. 2014;74:1452–1461. doi: 10.1002/pros.22862. [DOI] [PubMed] [Google Scholar]
- 25.Oleinika K, Nibbs RJ, Graham GJ, Fraser AR. Suppression, subversion and escape: the role of regulatory T cells in cancer progression. Clin Exp Immunol. 2013;171(1):36–45. doi: 10.1111/j.1365-2249.2012.04657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CLowman BR, Bissonnette E, Befus AD. Tumor necrosis factor- alpha dependent cytotoxicity of human skin mast cells is enhanced by anti-IgE antibodies. J Immunol. 1991;147(7):2253–2258. [PubMed] [Google Scholar]
- 27.Johansson A, Rudolfsson SH, Hammarsten P, Bergström SH, Pietras K, Jones J, et al. Mast cells are novel independent prognostic markers in prostate cancer and represent a target for therapy. Am J Pathol. 2010;177(2):1031–1041. doi: 10.2353/ajpath.2010.100070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Komohara Y, Fujiwara Y, Ohnishi K, Takeya M. Tumor- associated macrophages: Potential therapeutic targets for anti- cancer therapy. Adv Drug Deliv Rev. 2016;99(PtB):180–185. doi: 10.1016/j.addr.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Hu W, Qian Y, Yu F, Liu W, Wu Y, Fang X, et al. Alternatively activated macrophages are associated with metastasis and poor prognosis in prostate adenocarcinoma. Onco Lett. 2015;10(3):1390–1396. doi: 10.3892/ol.2015.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao J, Liu J, Xu R, Zhu X, Zhao X, Qian B-Z. Prognostic role of tumour-associated macrophages and macrophage scavenger receptor 1 in prostate cancer: a systematic review and meta- analysis. Oncotarget. 2017;8(47):83261–83269. doi: 10.18632/oncotarget.18743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Youlin K, Weiyang H, Simin L, Xin G. Prostaglandin E2 Inhibits Prostate Cancer Progression by Countervailing Tumor Microenvironment-Induced Impairment of Dendritic Cell Migration through LXRα/CCR7 Pathway. J Immunol Res. 2018;2018:5808962. doi: 10.1155/2018/5808962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shike M, Doane AS, Russo L, Cabal R, Reis-Filho JS, Gerald W, et al. The Effects of Soy Supplementation on Gene Expression in Breast Cancer: A Randomized Placebo-Controlled Study. J Natl Cancer Inst. 2014;106(9):pii dju189. doi: 10.1093/jnci/dju189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saghafi T, Taheri RA, Parkkila S, Emameh RZ. Phytochemicals as Modulators of Long Non-Coding RNAs and Inhibitors of Cancer-Related Carbonic Anhydrases. Int J Mol Sci. 2019;20(12):pii E2939. doi: 10.3390/ijms20122939. [DOI] [PMC free article] [PubMed] [Google Scholar]


