Abstract
Summary: A 5-year-old boy was evaluated for a left retrotympanic mass found at otoscopy. Subsequent petrous bone CT and MR angiographic examinations demonstrated bilateral aberrant internal carotid, bilateral stapedial artery persistence, and bilateral duplicated internal carotid arteries. Imaging findings and their clinical relevance are discussed. A second case of unilateral aberrant internal carotid artery with a persistent stapedial artery is included for comparison.
An aberrant internal carotid artery (ICA) in the middle ear is a rare congenital finding. If the diagnosis is not made before middle ear surgery is performed, hemorrhage, stroke, or death may occur as a result of injury to the vessel.
A persistent stapedial artery (PSA) is a more benign congenital diagnosis; however, symptoms of hearing loss and tinnitus may be present. Tinnitus was noted to resolve in one case after lysis and cauterization of the PSA (1). Stapes surgery is complicated by the presence of a PSA because the artery passes through the obturator foramen of the stapes. Stapes surgery may result in injury to the PSA; however, Govaerts et al (2) are not aware of cases in which damage to the PSA has led to unfavorable results.
A duplicated ICA is a rare congenital variant of little consequence other than possible associated findings such as an aberrant ICA. If neck or tonsil surgery is contemplated, knowledge of the presence of duplicated ICAs may be important.
Case Reports
Case 1
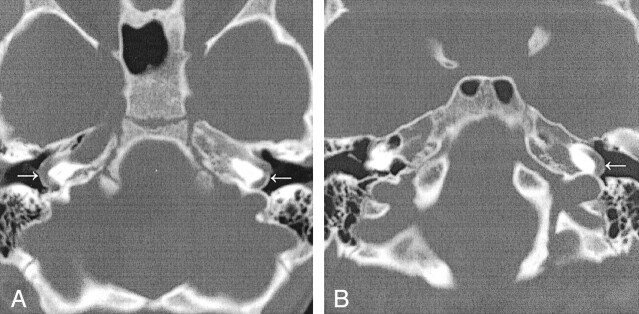
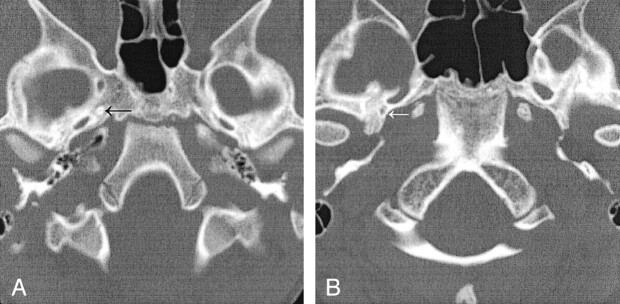
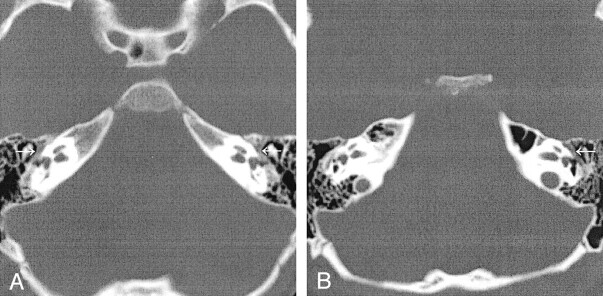
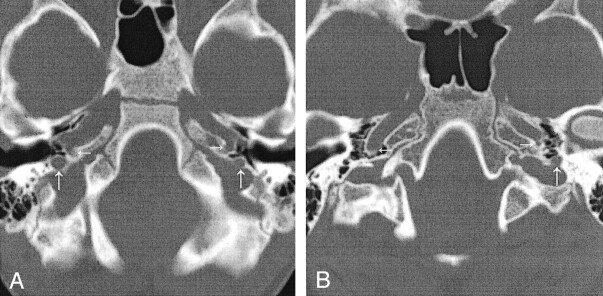
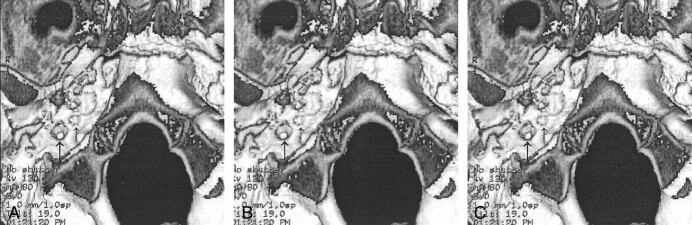
A 5-year-old boy presented to his pediatrician for a follow-up visit after treatment for otitis media of the left ear. A retrotympanic mass was noted during otoscopy of the left ear. Subsequent CT of the petrous bones demonstrated bilateral aberrant ICAs (Fig 1A), and an absent foramen spinosum bilaterally (Fig 2A). Bilateral PSAs were identified in the middle ear and facial nerve canal (Fig 3A). The exocranial orifice of the carotid canal was noted to be present but hypoplastic (Fig 4A,5).
Fig 1.
Aberrant ICAs (arrows) in the middle ear.
A, Case 1. CT scan demonstrates bilateral aberrant ICAs.
B, Case 2. CT scan demonstrates left aberrant ICA.
Fig 2.
Absent foramen spinosum.
A, Case 1. CT scan demonstrates bilateral absence of the foramen spinosum. A foramen of Vesalius is noted on this image on the right (arrow) and was present on the left on additional images (not shown).
B, Case 2. CT scan demonstrates a right foramen spinosum on the right (arrow) and absence on the left.
Fig 3.
Thickened contents of the horizontal facial canal.
A, Case 1. CT scan shows bilateral thickening from PSAs.
B, Case 2. CT scan shows left-sided thickening.
Fig 4.
Carotid canals and inferior tympanic canals.
A, Case 1. CT scan shows bilateral hypoplastic carotid canals (horizontal arrows) and enlarged bilateral inferior tympanic canals (vertical arrows) through which the aberrant ICA (inferior tympanic artery portion) passes.
B, Case 2. CT scan shows a normal right carotid canal (horizontal arrow on the patient’s right), a hypoplastic carotid canal (horizontal arrow on the patient’s left), and the inferior tympanic canal (vertical arrow) with the aberrant ICA.
Fig 5.
Case 1. 3D CT scans of the of the exocranial foramen with stereoscopic views. Images A and B are for cross-eyed viewing, and images B and C are for parallel-eye viewing. The right inferior tympanic canal (left arrow) shows a bony constriction. The hypoplastic right carotid canal is shown with the right arrow.
The patient had no symptoms related to these anatomic findings. Audiograms showed mild low-frequency hearing loss involving the left ear and normal hearing in the right ear. This has remained stable over the past 5 years.
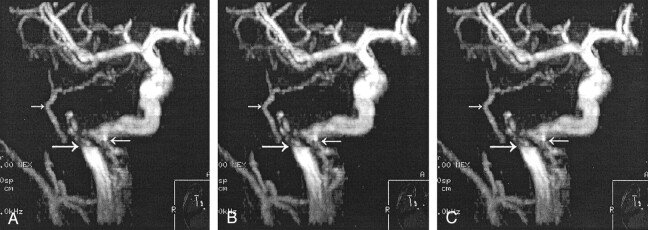
When the patient was aged 10 years, his parents noticed a left-ear bruit when they placed their ear next to the left ear of the patient. With exercise, this increased to a palpable thrill. A subtle right-ear bruit was audible with a stethoscope. However, the patient only reported hearing a mild bruit immediately after exertion. Repeat CT demonstrated no apparent change. MR angiography (MRA) demonstrated the known bilateral aberrant ICAs with the PSAs and confirmed flow in the native hypoplastic carotid canals (Fig 6). Also noted was the origin of right occipital artery from the larger of the duplicated right cervical ICA.
Fig 6.
Case 1. Stereoscopic MRA images of the right carotid. Images A and B are for cross-eyed viewing, and images B and C are for parallel-eyed viewing. The top left arrow shows the PSA supplying the middle meningeal distribution. The right arrow shows the duplicated ICA (carotid branch of the ascending pharyngeal artery). The bottom left arrow points to the turbulent blood flow at the inferior tympanic canal in the aberrant ICA. Views of the left ICA showed similar findings (not shown).
Case 2
An otolaryngologist evaluated a 10-year-old girl for otitis media. At otoscopy, a left retrotympanic pulsatile mass was noted. Subsequent CT scans of the petrous bone demonstrated a left aberrant ICA (Fig 1B), an absent left foramen spinosum (Fig 2B), a left PSA (Fig 3B), and a hypoplastic native exocranial carotid canal on the left side (Fig 4B). No further imaging studies were performed. Audiograms revealed a mild low-frequency hearing loss involving the left ear and normal hearing in the right ear. No clinical symptoms were referable to these anatomic findings.
Discussion
Many authors (1–6) have discussed the embryologic development of the cervical and cranial vasculature. Now, an aberrant ICA is generally accepted to be a collateral pathway that occurs as a result of agenesis of the first embryonic segment of the ICA. The inferior tympanic branch of the ascending pharyngeal artery anastomoses with the caroticotympanic artery (hyoid artery remnant) of the ICA. The aberrant ICA variant may therefore be more accurately referred to as “the inferior tympano-caroticotympanic variant,” as suggested by Lasjaunias (3). The inferior tympanic artery passes through the inferior tympanic canal (Jacobsen canal) at the skull base, with a resultant characteristic narrowing of the vessel.
Characteristic angiographic findings of aberrant ICA include lateral extension of the ICA well beyond the vestibular line of Lapayowker (7).
CT is considered one of the most reliable ways to diagnose an aberrant ICA (8–10). Findings include the following: A reduced-caliber aberrant ICA enters the middle ear through the enlarged inferior tympanic canal within the caroticojugular spine. The vessel takes a sharp turn anteriorly and crosses the promontory to continue on to the horizontal portion of the petrous carotid. Because the aberrant ICA is present in the middle ear, the normal bone covering the artery is absent. The vertical portion of the carotid canal may be absent or hypoplastic.
Dephasing of the spins due to turbulence, with loss of the signal intensity on the three-dimensional (3D) time-of-flight MRA image in case 1, is evident beyond the expected location of the inferior tympanic canal; this effect accounted for the patient’s bruits. MRA findings of aberrant ICA have been described (11); these include a lateral and superior location of the carotid genu in the middle ear on the anteroposterior projection and the posterior and superior location of this structure on the lateral projection.
The stapedial artery is persistent when the embryonic stapedial artery does not regress completely. This condition usually results in the stapedial artery supplying the middle meningeal artery territory. Congenital absence of the foramen spinosum is present in 3.2% of the population (12); therefore, additional findings demonstrating the course of the PSA are needed to confirm its presence. These findings include a small canaliculus exiting the carotid canal (in cases in which aberrant ICA is not present), the presence of a linear soft-tissue attenuation crossing the middle ear over the promontory, an enlarged facial nerve canal, or a separate and parallel canal (13).
In a case presented by Lasjaunias (3), courtesy of R. Willinsky, the duplicated ICAs had an appearance strikingly similar to that of case 1. The anatomic explanation of the two channels carrying the ICA flow is that one channel is the inferior tympanic artery branch of the ascending pharyngeal artery, and the other channel is the carotid branch of the superior pharyngeal branch of the pharyngeal trunk of the ascending pharyngeal artery. In case 1, we propose the same anatomic explanation for the bilateral duplicated channels of the ICAs. We hypothesize that the stenosis at the inferior tympanic canal bilaterally results in the need for an additional collateral supply from the carotid branch of the ascending pharyngeal artery.
The surgical incidence of PSA is quoted as 1:5,000 to 1:10,000 (0.02–0.01%) with stapes surgery (2). This incidence is different from that from a histopathologic study in which five PSAs were found in 1,045 temporal bones, for an incidence of 0.48% (14).
The incidence of aberrant ICA and the incidence of aberrant ICA with PSA is unknown; however, several case reports exist (1,6,15,16). Retrospective and prospective evaluation of cases of aberrant ICA may be helpful to determine the true incidence of this association. The incidence of a duplicated ICA is also unknown.
Six previous reports of bilateral aberrant ICAs (17–22) and 13 previous cases of bilateral PSAs (2,6,23) are known. No cases of bilateral duplicated ICAs or cases of bilateral aberrant carotid arteries with bilateral PSAs have been previously reported.
Conclusion
Recognizing the findings of an aberrant ICA is important to avoid potentially severe surgical complications. The identification of PSAs is also helpful in the evaluation of tinnitus and hearing loss.
We present what we believe to be the first reported case of bilateral aberrant ICAs with bilateral PSAs and bilateral duplicated ICAs.
Footnotes
Presented at the 40th Annual Meeting of the American Society of Neuroradiology, Vancouver, B.C., May 10–17, 2002.
References
- 1.Silbergleit R, Quint DJ, Mehta BA, Patel SC, Metes JJ, Noujaim SE. The persistent stapedial artery. AJNR Am J Neuroradiol 2000;21:572–577 [PMC free article] [PubMed] [Google Scholar]
- 2.Govaerts PJ, Marquet TF, Cremers CWRJ, Offeciers FE. Persistent stapedial artery: does it prevent successful surgery? Ann Otol Rhinol Laryngol 1993;102:724–728 [DOI] [PubMed] [Google Scholar]
- 3.Lasjaunias P, Berenstein A, Ter Brugge KG. Surgical Neuroangiography, Clinical Vascular Anatomy and Variations. 2nd ed. New York: Springer-Verlag;2001
- 4.Padget DH. The development of the cranial arteries in the human embryo. Contrib Embroyl 1948;32:205–261 [Google Scholar]
- 5.Congdod ED. Transformation of the aortic-arch system during the development of the human embryo. Contrib Embryol 1922;14:47–110 [Google Scholar]
- 6.Steffen TH. Vascular anomalies of the middle ear. Laryngoscope 1968;78:171–197 [DOI] [PubMed] [Google Scholar]
- 7.Lapayowker MS, Liebman EP, Ronis ML, Safer JN. Presentation of the internal carotid artery as a tumor of the middle ear. Radiology 1971;98:293–297 [DOI] [PubMed] [Google Scholar]
- 8.Swartz JD, Buzarnic ML, Naidich TP, Lowry LD, Doan HT. Aberrant internal carotid artery lying within the middle ear. Neuroradiology 1985;27:322–326 [DOI] [PubMed] [Google Scholar]
- 9.Lo WWM, Solti-Bohman LG, McElveen JT. Aberrant Carotid artery: radiologic diagnosis with emphasis on high-resolution computed tomography. Radiographics 1985;5:985–993 [DOI] [PubMed] [Google Scholar]
- 10.McElveen JT, Lo WWM, El Gabri TH, Nigri P. Aberrant internal carotid artery: classic findings on computed tomography. Otolaryngol Head Neck Surg 1986;94:616–621 [DOI] [PubMed] [Google Scholar]
- 11.Davis WL, Harnsberger HR. MR Angiography of an aberrant internal carotid artery. AJNR Am J Neuroradiol 1991;12:1225. [PMC free article] [PubMed] [Google Scholar]
- 12.Ginsberg LE, Pruett SW, Chen MYM, Elster AD. Skull-based foramina of the middle cranial fossa: reassessment of normal variation with high-resolution CT. AJNR Am J Neuroradiol 1994;15:283–291 [PMC free article] [PubMed] [Google Scholar]
- 13.Thiers FA, Sukai O, Poe DS, Curtin HD. Persistent stapedial artery: CT findings. AJNR Am J Neuroradiol 2000;21:1551–1554 [PMC free article] [PubMed] [Google Scholar]
- 14.Moreano EH, Puparella MM, Zelterman D, Goycoolea MV. Prevalence of facial canal dehiscence and of persistent stapedial artery in the human middle ear: a report of 1000 temporal bones. Laryngoscope 1994;104:309–320 [DOI] [PubMed] [Google Scholar]
- 15.Guinto FC, Garrabrant EC, Radcliffe WB. Radiology of the persistent stapedial artery. Radiology 1972;105:365–369 [DOI] [PubMed] [Google Scholar]
- 16.Lasjaunias P, Moret J, Manelfe C, Théron J, Hasso T, Seeger J Arterial anomalies at the base of the skull. Neuroradiology 1977;13:267–272 [DOI] [PubMed] [Google Scholar]
- 17.Goldman NC, Singleton GT, Holly EH. Aberrant internal carotid artery. Arch Otolaryngol 1971;94:269–273 [DOI] [PubMed] [Google Scholar]
- 18.Saito H, Chikamory Y, Yanagihara N. Aberrant carotid artery in the middle ear. Arch Oto Rhino Laryngol 1975;209:83–87 [DOI] [PubMed] [Google Scholar]
- 19.Campbell G, Renner G, Estrem SA. Bilateral aberrant internal carotid arteries. Otolaryng Head Neck Surg 1992;107:124–128 [DOI] [PubMed] [Google Scholar]
- 20.Glasscock ME, Seshul M, Seshul MB. Bilateral aberrant internal carotid artery case presentation. Arch Otolaryngol Head Neck Surg 1993;119:335–339 [DOI] [PubMed] [Google Scholar]
- 21.Ashikaga R, Araki Y, Ishida O. Bilateral aberrant internal carotid arteries. Neuroradiology 1995;37:655–657 [DOI] [PubMed] [Google Scholar]
- 22.Benton C, Bellet PS. Imaging of congenital anomalies of the temporal bone. Neuroimaging Clin N Am 2000;10:35–53 [PubMed] [Google Scholar]
- 23.Marion M, Hinojosa R, Kahn AA. Persistence of the stapedial artery: a histopathologic study. Otolaryngol Head Neck Surg 1985;93:298–312 [DOI] [PubMed] [Google Scholar]