Abstract
Summary: Since Kojewnikoff’s first description of epilepsia partialis continua, isolated case reports and small case series have elucidated the disease. However, few imaging studies have been performed. We report a case in which serial diffusion and perfusion MR imaging allowed monitoring of the changes in hemodynamic and cell membrane permeability until the resolution of clinical and electrical epileptic activity.
Epilepsia partialis continua (EPC) can be defined as a form of partial status epilepticus with simple motor manifestations that are maintained for over 1 hour. In EPC, clonic activity is restricted to one body part and recurs at fairly regular intervals (1, 2).
In patients with status epilepticus, brain imaging studies have documented an array of accompanying alterations. Such studies have also allowed follow-up of the abnormal electrical activity, which is, at least in part, topographically correlated with the EEG epileptiform anomalies. Hemodynamic changes in focal seizures are characterized by ictal hyperperfusion and postictal hypoperfusion (3, 4). Transient ultrastructural changes have been recently demonstrated with the use of diffusion MR imaging. These changes consist of the redistribution of intracellular and extracellular water, presumably due to an alteration in cell membrane permeability or cytotoxic edema or both (4–7). Hemodynamic and ultrastructural changes in areas remote from the site of the primary epileptogenic activity have also been reported in focal epilepsy. These changes consisting of areas with high blood flow or decreased apparent diffusion coefficients (ADCs) that are located in the cerebellar hemisphere contralateral to the cerebral areas involved (6, 8, 9).
We report a case in which serial EEG and MR imaging studies allowed monitoring of the whole spectrum of hemodynamic, ultrastructural, and electrical changes in a woman with EPC until the clinical and electrical conditions resolved. Perturbation of the blood flow and the abnormal cellular water distribution were demonstrated, both in cortical areas and in the contralateral cerebellar hemisphere during the ictal phase. This change was followed by the reestablishment of near-normal perfusion and membrane-ion homeostasis.
Case Report
An 83-year-old woman was bedridden as a result of a 2-year history of osteoporosis. This patient was admitted at our hospital after a sudden onset of involuntary mouth movements on the left side that spread to the ipsilateral arm and leg. After unrevealing brain CT and EEG, which demonstrated abnormal electric activity, therapy with phenytoin was started.
After the first event, the woman presented with seizures occurring every 5–10 minutes. The frequency of her seizures gradually decreased in the following days, until they completely resolved after 5 days. EEG performed during an ictal period revealed a focus for the seizures in the right temporal region, with subsequent spread to the adjacent cortex. EEG showed complete disappearance of the abnormal electrical activity at day 14 after admission, and the electrical activity remained within normal in the following days.
The patient underwent MR imaging at admission and at days 2, 7, and 15 days after the onset of her seizures. Images were obtained with a 1.5-T magnet (Gyroscan NT-2000; Philips, Best, the Netherlands). Fluid-attenuated inversion recovery (FLAIR) imaging, diffusion-weighted (DW) imaging, and perfusion-weighted (PW) imaging were used, and sections were selected to include the cerebral and cerebellar hemispheres. Maps of the ADC and relative cerebral blood flow (rCBF) were generated.
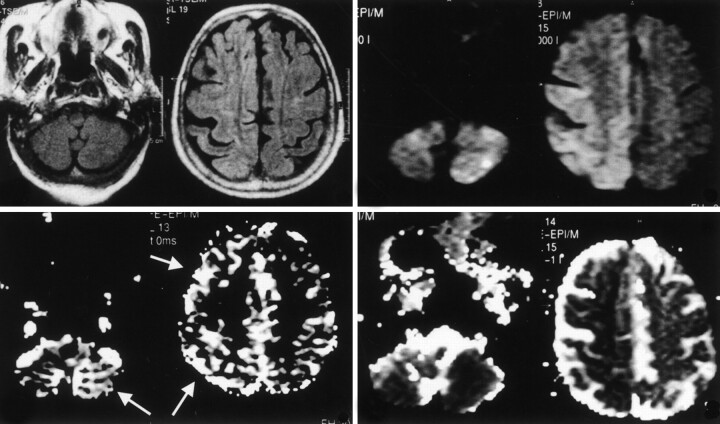
PW imaging was performed by using a multishot echo-planar gradient-echo sequence in 12 axial sections. Parameters for this were as follows: TR/TE/NEX, 440/30/1; flip angle, 45°; FOV, 240; matrix, 80 × 128; section thickness, 7 mm; gap, 0 mm; and total imaging time, 1 minute 30 seconds. Forty dynamic images were acquired before, during, and after the intravenous injection of a bolus of 20 mL of gadopentetate dimeglumine (Magnevist; Schering, Berlin, Germany). The 40 sequential images were analyzed at the MR workstation by using dedicated software (Packman; Philips). For each pixel, the measured time course of the signal intensity was fitted to a modified gamma function. The fit yielded the mean transit time (MTT), which was calculated as the first moment of the curve and the area under the curve (which is proportional to the cerebral blood volume [CBV]). From these two quantities, an index of rCBF was calculated by using the following equation: rCBF=CBV/MTT. The resulting rCBF image was displayed as a gray-scale map (Fig 1B, 2B). FLAIR MR images obtained at admission showed slight and diffuse cortical hyperintensity in the right hemisphere due to edema and scattered neuronal necrosis. DW images showed marked hyperintensity in the cerebral cortex on the side of the epileptic focus; this corresponded to a 35% decrease in the ADC. Regions of interest drawn in the same cortical areas on the rCBF maps showed marked hyperemia (35% increase compared with the contralateral side). Apparently, perturbation of the rCBF extended beyond the visible alterations in ADC. Interestingly, both parameters, ADC and rCBF, were also altered in the cerebellar hemisphere contralateral to the epileptogenic focus (30% decrease in the ADC and 45% increase in the rCBF) (Fig 1).
Fig 1.
Images obtained during the ictal phase on day 1 in an 83-year-old woman with EPC. Top left, T2-weighted FLAIR MR images show diffuse and slight hyperintensity in the right frontotemporal cortex. Bottom left, rCBF maps show hyperperfusion of the right cerebral hemisphere and the contralateral cerebellum (arrows). Top right, On DW images, the same regions show marked hyperintensity. Bottom right, Corresponding decreased ADC is shown.
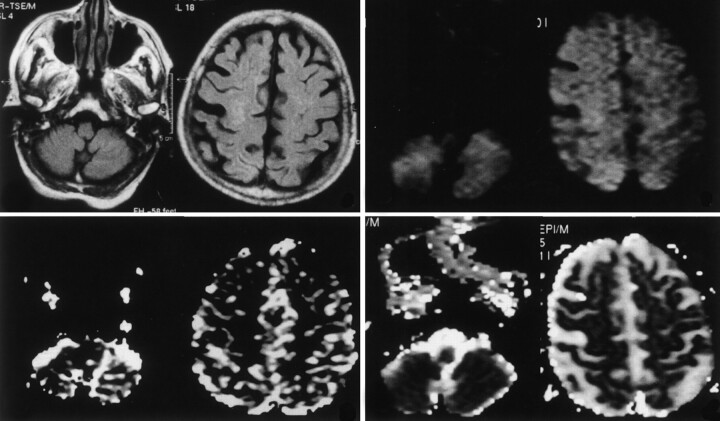
Fig 2.
Postictal studies obtained on day 15 after the onset of seizures. Top left, FLAIR MR images demonstrate near-complete resolution of the cortical hyperintensity. rCBF maps (bottom left), DW images (top right), and ADC maps (bottom right) show near-complete resolution of the previous perturbations, both in the cerebral cortex and in the contralateral cerebellar hemisphere.
FLAIR images obtained on days 2, 7, and 15 days after admission demonstrated near-complete resolution of the cortical hyperintensity. Both the ADC maps and rCBF maps demonstrated a gradual return to normal values (Fig 2). The normalization of the hemodynamic, ultrastructural, and electrical parameters was correlated with an improvement in the patient’s clinical status and with a decreased frequency of seizures.
Discussion
This report illustrates the spectrum of electrical, hemodynamic, and ultrastructural changes that occur in the brain during evolving status epilepticus. The main perturbation in EPC consists of abnormal electrical activity, which produces a marked increase in cerebral blood flow (1). The EEG changes are also associated with perturbation of membrane-ion homeostasis, with subsequent intracellular edema. If such changes are prolonged or severe enough, neuronal necrosis inevitably follows (4, 8, 9). Changes were demonstrated in our case, both in the areas primarily involved in the seizure activity and in the areas remote from but functionally connected to the epileptogenic cortex.
Such a phenomenon was previously demonstrated in epilepsy, with tracer studies or with diffusion MR imaging (4, 6–11, 13–15), and it has been interpreted as a remote effect mediated by the corticopontocerebellar pathway (16). This phenomenon is similar to that of crossed cerebellar diaschisis, which was first demonstrated in patients with stroke. This observation implies a decrease in neuronal activity due to an interruption of the afferent axonal supply. Changes in cerebral blood flow in the hemisphere opposite a cerebral infarction have been explained by using a different hypothesis involving neurogenic, vasogenic, and chemical mechanisms (10, 17). In epilepsy, crossed cerebellar changes can be explained by the same mechanism, namely, increased neuronal input from the seizure-induced hyperactivity of the contralateral limbs. It is also worth mentioning that the cerebellum is thought to have an inhibitory function on epilepsy by means of the release of the inhibitory transmitter gamma amino butyric acid from the Purkinje cells. Therefore, an alternative hypothesis could be that cerebellar changes in epilepsy are due to an increased demand for inhibition, which results in the depletion of amino acids and subsequent influx of calcium into the neurons until toxic levels are reached (5, 18).
In our case, the prompt choice of an appropriate therapy led to the gradual normalization of diffusion, perfusion, and electrical activity that corresponded to a marked improvement in the patient’s clinical status.
Transient decreases in ADC have been described in several complicated clinical settings other than epilepsy; these include venous sinus thrombosis, hemiplegic migraine, transient ischemic attacks, and hyperacute arterial infarction (19). In these conditions, normalization of ADC values has been explained as being the result of the full restoration of energy metabolism. This restoration occurs after a transitory phase in which an array of phenomena occur; these include an increase in calcium and glutamate levels, an inhibition of protein synthesis, a disturbance of metabolites, depolarization, and mitochondrial dysfunction.
Reversible decreases in ADC and transitory hyperperfusion have been described, particularly in patients with migraines associated with an aura. Conceivably, the mechanisms underlying the DW imaging and PW imaging findings in migraine with aura and in status epilepticus are similar; these findings include vasodilatation and the abnormal release of excitatory amino acids (20).
MR diffusion and perfusion techniques allow the direct and noninvasive visualization of evolving status epilepticus. Identification of the primary site of abnormal electrical activity and the cascade of phenomena accompanying and following the epileptiform discharge may assist clinicians in initiating a timely therapeutic intervention. These techniques may also provide immediate and continuous feedback about the evolution of the disease.
Conclusion
Previous reports have described the clinical evolution of status epilepticus with the use of either DW imaging or PW imaging techniques. As illustrated in this article, PW imaging and DW imaging, respectively, allow the evaluation of increased cerebral blood flow and increased intracellular edema that occurs in the brain during EPC. The combined use of these two MR imaging techniques allows a more complete depiction of the spectrum of alterations that characterize evolving status epilepticus.
Acknowledgments
The authors would like to thank Mr. Maurizio Carmellini for his technical support.
References
- 1.Schomer DL. Focal status epilepticus and epilepsia partialis continua in adults and children. Epilepsia 1993;34:29–36 [DOI] [PubMed] [Google Scholar]
- 2.Biraben A, Chauvel P. Epilepsia partialis continua. In: Engel J, Pedley TA, eds. Epilepsy: A Comprehensive Textbook. Philadelphia: Lippincott-Raven;1998. :2447–2453
- 3.Duncan R. Epilepsy, cerebral blood flow, and cerebral metabolic rate. Cerebrovasc Brain Metab Rev 1992;4:105–121 [PubMed] [Google Scholar]
- 4.Wall CJ, Kendall EJ, Obenaus A. Rapid alterations in diffusion-weighted images with anatomic correlates in a rodent model of status epilepticus. AJNR Am J Neuroradiol 2000;21:1841–1852 [PMC free article] [PubMed] [Google Scholar]
- 5.Men S, Lee DH, Barron JR, Muñoz DG. Selective neuronal necrosis associated with status epilepticus: MR findings. AJNR Am J Neuroradiol 2000;21:1837–1840 [PMC free article] [PubMed] [Google Scholar]
- 6.Wieshmann UC, Symms MR, Shorvon SD. Diffusion changes in status epilepticus. Lancet 1997;16:493–494 [DOI] [PubMed] [Google Scholar]
- 7.Nakasu Y, Nakasu S, Morikawa S, Uemura S, Inubushi T, Handa J. Diffusion-weighted MR in experimental sustained seizures elicited with kainic acid. AJNR Am J Neuroradiol 1995;16:1185–1192 [PMC free article] [PubMed] [Google Scholar]
- 8.Duncan R, Patterson J, Bone I, Wyper DJ. Reversible cerebellar diaschisis in focal epilepsy. Lancet 1987;12:625–626 [DOI] [PubMed] [Google Scholar]
- 9.Wasterlain CG, Fujikawa DG, Penix L, Sankar R. Pathophysiological mechanisms of brain damage from status epilepticus. Epilepsia 1993;34:37–53 [DOI] [PubMed] [Google Scholar]
- 10.Reivich M. Crossed cerebellar diaschisis. AJNR Am J Neuroradiol 1992;13:62–64 [PMC free article] [PubMed] [Google Scholar]
- 11.Biersack HJ, Grunwald F, Linke DB. Transient cerebellar diaschisis. Lancet 1988;9:825. [DOI] [PubMed] [Google Scholar]
- 12.SPECT and PET in epilepsy. Lancet 1989;21:135–137 [PubMed] [Google Scholar]
- 13.Duncan R, Patterson J, Hadley DM, Bone I. Unilateral cerebellar damage in focal epilepsy. J Neurol Neurosurg Psychiatry 1990;53:436–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeong-Ah Kim, Jin Il Chung, Pyeong Ho Yoon, et al. Transient MR signal changes in patients with generalized tonicoclonic seizure or status epilepticus: periictal diffusion-weighted imaging. AJNR Am J Neuroradiol 2001;22:1149–1160 [PMC free article] [PubMed] [Google Scholar]
- 15.Chan S, Chin SS, Kartha K, et al. Reversible signal abnormalities in the hippocampus and neocortex after prolonged seizures. AJNR Am J Neuroradiol 1996;17:1725–1731 [PMC free article] [PubMed] [Google Scholar]
- 16.Sagiuchi T, Ishii K, Asano Y, et al. Interictal crossed cerebellar hyperperfusion on Tc-99m ECD SPECT. Ann Nucl Med 2001;15:369–372 [DOI] [PubMed] [Google Scholar]
- 17.Lavy S, Eldad M, Portnoy Z. Effect of cerebral infarction on the regional cerebral blood flow of the contralateral hemisphere. Stroke 1975;6:160–163 [DOI] [PubMed] [Google Scholar]
- 18.Dam AM, Dam M. Neuropathology. In: Dam M, Gram L, eds. Comprehensive Epileptology. New York: Raven Press;1991. :43–55
- 19.Grant PE, He J, Halpern EF, et al. Frequency and clinical context of decreased apparent diffusion coefficient reversal in the human brain. Radiology 2001;221:43–50 [DOI] [PubMed] [Google Scholar]
- 20.Chabriat H, Vahedi K, Clark CA, et al. Decreased hemispheric water mobility in hemiplegic migraine related to mutation of CACNA1A gene. Neurology 2000;25:510–512 [DOI] [PubMed] [Google Scholar]