Abstract
BACKGROUND AND PURPOSE: Dural arteriovenous fistulas (DAVFs) with disturbed regional cerebral blood flow (rCBF) include retrograde leptomeningeal venous drainage (RLVD). We examined rCBF disturbances in patients with DAVFs by studying MR imaging and single photon emission CT (SPECT) changes before and after treatment.
METHODS: In 22 patients with DAVFs and RLVD, we studied their symptoms, pre- and post-treatment MR imaging and SPECT findings, and treatment results. Patients were assigned to two groups: Type 1 included those with RLVD into more than one venous sinus, and type 2, those with RLVD into a single venous sinus.
RESULTS: Eleven patients had type 1 RLVD. In these patients, preoperative T2-weighted MR images showed no hyperintense areas, and angiographic evidence showed flow into more than one venous sinus. The other 11 patients had type 2 RLVD. In these patients, preoperative SPECT demonstrated hypoperfused areas that coincided with hyperintense areas on T2-weighted MR images. After treatment, the hyperintense areas disappeared, and symptoms improved in seven of these patients (type 2a). Their preoperative SPECT studies demonstrated preservation of vasoreactivity after an acetazolamide challenge. In the other four patients (Type 2b), the hyperintense areas and symptoms persisted after treatment. Their preoperative SPECT studies revealed a marked disturbance of vasoreactivity.
CONCLUSION: In patients with drainage into a single venous sinus, we consistently observed areas of hyperintensity on MR images. These results and findings of hypoperfusion on SPECT scans apparently reflect venous congestion, whereas unpreserved vasoreactivity after an acetazolamide challenge on SPECT scans reflects venous infarction. The preservation of vasoreactivity after the challenge appears to be a good prognostic indicator.
Patients with dural arteriovenous fistulas (DAVFs) and cerebral ischemia and/or cerebral hemorrhage manifest regional cerebral blood flow (rCBF) abnormalities due to disturbance of venous perfusion (1–3). The angiographic characteristics of DAVFs with disturbed rCBF include retrograde leptomeningeal venous drainage (RLVD) in the draining system (4). While single photon emission CT (SPECT) results, positron emission tomography (PET) findings (5–7), and signal intensity abnormalities on MR images are useful for their identification (8), the degree of rCBF disturbance in DAVFs with RLVD remains to be elucidated. We examined rCBF disturbances in patients with DAVFs by studying the changes on MR images and SPECT scans before and after treatment. We also assessed symptomatic improvements achieved with surgical and/or interventional therapy.
Methods
Between April 1994 and December 2001, we treated 22 patients with DAVFs and RLVD. They included nine men and 13 women ranging in age from 31 to 71 years. In all cases, continuous-mode angiography provided detailed information about the location of the fistula, the arterial supply, and the venous drainage pattern. Patients’ symptoms, angiographic findings, and areas with abnormal signal intensity on T2-weighted MR imaging before treatment are listed in Table 1. Of the 22 patients, two had an intracerebral hemorrhage, and one had a subarachnoid hemorrhage. Progressive mental disturbance was recognized in seven patients, and exophthalmos was observed in six. Two patients each reported double vision and headache, and one patient each experienced dysesthesia of the lower limbs and a visual field disturbance.
TABLE 1:
Clinical features in 20 patients with RVLD in DAVFs
| Patient/Age, y/Sex | Clinical Presentation | Angiographic Findings |
|||
|---|---|---|---|---|---|
| Location* | Varices | Drainage Pattern | Accessory Route | ||
| A: Type 1, Non-hyperintensity on T2-Weighted MR Images | |||||
| 1/66/M | Mental disturbance | SSS | Multiple | RLVD only | Yes |
| 2/66/F | Exophthalmos | CS | None | RLVD only | Yes |
| 3/62/F | Exophthalmos | CS | None | RLVD only | Yes |
| 4/63/M | Subarachnoid hemorrhage | T-SS | Single | RLVD only | Yes |
| 5/68/F | Double vision | CS | None | RLVD only | Yes |
| 6/49/F | Cerebral hematoma | T-SS | Single | Sinus, RLVD | Yes |
| 7/67/M | Cerebral hematoma | Sig S | Multiple | Sinus, RLVD | Yes |
| 8/59/M | Mental disturbance | CS | Single | Sinus, RLVD | Yes |
| 9/52/F | Mental disturbance | CS | Single | Sinus, RLVD | Yes |
| 10/60/F | Mental disturbance | CS | None | Sinus, RLVD | Yes |
| 11/53/F | Double vision | CS | Single | Sinus, RLVD | Yes |
| B: Type 2, Hyperintensity on T2-Weighted MR Images | |||||
| 12/51/F | Mental disturbance | T-SS | Single | Sinus, RLVD | No |
| 13/58/M | Lower-limb hypesthesia | SSS | Single | RLVD only | No |
| 14/66/F | Exophthalmos | CS | None | RLVD only | No |
| 15/31/F | Exophthalmos | CS | None | Sinus, RLVD | No |
| 16/50/M | Exophthalmos | CS | None | Sinus, RLVD | No |
| 17/71/M | Headache | T-SS | None | Sinus, RLVD | No |
| 18/63/F | Headache | CS | None | Sinus, RLVD | No |
| 19/47/M | Visual field disturbance | T-SS | None | RLVD only | No |
| 20/50/F | Mental disturbance | T-SS | None | RLVD only | No |
| 21/63/M | Mental disturbance | T-SS | None | RLVD only | No |
| 22/70/F | Exophthalmos | CS | None | RLVD only | No |
CS indicates cavernous sinus; SSS, superior sagittal sinus; T-SS, transverse-sigmoid sinus; and SigS, sigmoid sinus.
On the basis of detailed continuous-mode angiographic study (15 frames per second), we assigned the 22 patients into two groups according to the RLVD pattern. Type 1 (n = 11) comprised those with angiographic evidence of RLVD into more than one venous sinus, and type 2 (n = 11) included those with RLVD into a single venous sinus. The feeding arteries were the external carotid and/or internal carotid arteries. Nine patients had one (n = 7) or more (n = 2) varices in the venous drainage.
All patients underwent SPECT with technetium-99m hexamethyl-propylene amine oxime (99mTc-HMPAO) at a dose of 740 MBq. Patients in whom preoperative T2-weighted MR images showed lesions with abnormal intensity lesions or in whom SPECT scans showed areas of abnormal perfusion were re-examined by using the same studies after their DAVFs were treated with open and/or interventional surgery.
Results
Of the 22 patients, 11 had type 1 RLVD. These patients had drainage into more than one venous sinus, and their preoperative T2-weighted MR images showed no hyperintense areas. The other 11 patients had type 2 RLVD with drainage into a single venous sinus. On T1-weighted images, the lesion was surrounded by an area of hypointensity; on T2-weighted images, an area of hyperintensity was seen (Table 1). Of the 11 type 1 patients, three experienced hemorrhagic episodes; no type 2 patients had evidence of hemorrhage. Single or multiple varices were noted in seven type 1 patients, and two type 2 patients had single varices in the RLVD route. Although SPECT images did not demonstrate areas of hypoperfusion in any of the 11 type 1 patients, all such images did in the 11 type 2 patients. These areas coincided with the areas of hyperintensity on T2-weighted MR images (Table 2).
TABLE 2:
MR Imaging and SPECT findings in nine patients with DAVF hyperintensity on T2-Weighted MR Images
| Patient | Before Treatmen Findings |
After Treatment Findings |
Outcome | ||||
|---|---|---|---|---|---|---|---|
| T2-Weighted MR Imaging | SPECT |
T2-Weighted MR Imaging | SPECT |
||||
| At Rest | With Acetazolamide | At Rest | With Acetazolamide | ||||
| Type 2a | |||||||
| 12 | Hyperintensity | Hypoperfusion | Preserved | T2-normalization at 1 mo | Normal | Not done | Good |
| 13 | Hyperintensity | Hypoperfusion | Preserved | T2-normalization at 2 wk | Normal | Not done | Good |
| 14 | Hyperintensity | Hypoperfusion | Preserved | T2-normalization at 2 wk | Normal | Not done | Good |
| 15 | Hyperintensity | Hypoperfusion | Preserved | T2-normalization at 1 wk | Normal | Not done | Good |
| 16 | Hyperintensity | Hypoperfusion | Preserved | T2-normalization at 3 wk | Normal | Not done | Good |
| 17 | Hyperintensity | Hypoperfusion | Preserved | T2-normalization at 2 wk | Normal | Not done | Good |
| 18 | Hyperintensity | Hypoperfusion | Preserved | T2-normalization at 3 wk | Normal | Not done | Good |
| Type 2b | |||||||
| 19 | Hyperintensity | Hypoperfusion | Non-preserved | T2-hyperintensity continued, subcortical hemorrhage | Hypoperfusion, peripheral hyperperfusion | Non-preserved | Poor |
| 20 | Hyperintensity | Hypoperfusion | Non-preserved | T2-hyperintensity continued | Hypoperfusion | Non-preserved | Poor |
| 21 | Hyperintensity | Hypoperfusion | Non-preserved | T2-hyperintensity continued | Hypoperfusion | Non-preserved | Poor |
| 22 | Hyperintensity | Hypoperfusion | Non-preserved | T2-hyperintensity continued | Hypoperfusion | Non-preserved | Poor |
In all type 1 patients, surgery improved their preoperative symptoms of progressive mental disturbance, exophthalmos, and double vision. However, two patients who had had an intracerebral hematoma continued to experience mild hemiplegia. None of the type 1 patients were in worse condition after treatment.
Surgical intervention resulted in the disappearance of the hyperintense areas and normalization of the hypoperfusion in seven of 11 type 2 patients. Their symptoms of progressive mental disturbance, dysesthesia of the lower limbs, and headache were improved at 2–4 weeks after treatment. After an acetazolamide challenge, images in these seven patients demonstrated preservation of vasoreactivity (type 2a). In the remaining four type 2 patients, we noted the persistence of the areas of hyperintensity and hypoperfusion despite treatment. Vasoreactivity had not been preserved after an acetazolamide challenge (type 2b) in these patients, and their symptoms of visual field disturbances and progressive mental disturbances were not completely resolved after treatment.
Illustrative Cases
Type 1RLVD into More Than One Venous Sinus and No Hyperintensity on Preoperative T2-Weighted MR Images. Case 7.—
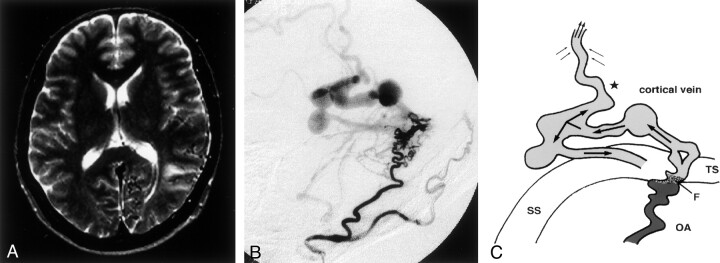
This 67-year-old man had experienced a cerebral hemorrhage in the left temporal lobe. Angiograms demonstrated DAVFs in the left transverse sigmoid sinus. T1-weighted MR imaging showed a small hypointense area in the left temporal lobe. It originated at the site of the hematoma. Multiple signal-void lesions were observed near the transverse sinus. MR imaging revealed no area with abnormal intensity on the cortical surface (Fig 1A), and SPECT demonstrated no areas of hypoperfusion. Angiographic study showed that the lesion was fed by the left occipital and middle meningeal arteries. On continuous-mode angiograms, these feeders flowed directly into the dilated abnormal vein through the fistula point. Retrograde venous flow was recognized in the cortical vein, and final drainage was into the left transverse-sigmoid sinus. Additional drainage into the superior sagittal sinus was observed. Multiple varices were recognized in the path of the cortical venous drainage (Fig 1B and C).
Fig 1.
Type 1. Case 7 in a 67-year-old man. with cerebral hemorrhage.
A, T2-weighted MR image reveals no hyperintense lesion in the left temporo-parietal lobe.
B, Left external carotid angiogram, lateral projection, shows DAVFs adjacent to the left transverse sinus. Venous drainage is retrograde into the left transverse sinus. An accessory drainage route into the superior sagittal sinus is recognized. Multiple varices are seen in the venous drainage path.
C, Schematic diagram of a DAVF with an accessory route (star) in the retrograde venous drainage (single arrows). The accessory route with retrograde flow (top double arrows) and the surrounding venous flow (left and right double arrows) drain into another sinus through this accessory route. F indicates the fistula point; OA, occipital artery; SS, sigmoid sinus; and TS, transverse sinus.
Embolization with permanent particles (200–400-μm cellulose porous beads [CPB]) (9, 10) resulted in occlusion of the fistulas via the occipital and middle meningeal arteries. However, because the DAVFs were not completely obstructed after embolization followed by interruption of the RLVD, the patient underwent direct surgery. Hemostatic clips were applied to the draining vein at the exit site of the fistulas. His postoperative course was uneventful. However, right mild hemiplegia resulting from his intracerebral hematoma persisted. Angiographic study performed 6 months after surgery revealed no residual DAVFs.
Type 2a RLVD into a Single Venous Sinus and Hyperintensity with Post-Treatment Disappearance of the Lesion on T2-Weighted MR Images. Case 13.—
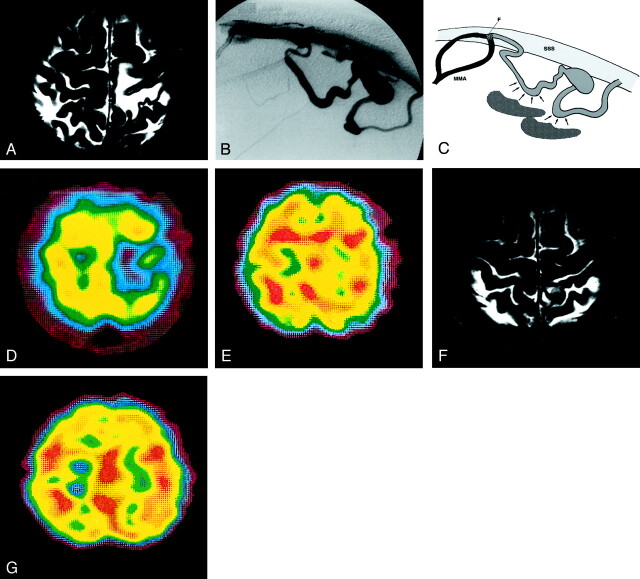
At the time of admission, this 58-year-old man had a 1-year history of progressively worsening dysesthesia of the right lower limb. MR imaging revealed a signal-void lesion with irregular enhancement adjacent to the superior sagittal sinus. Areas of hypointensity were observed on T1-weighted MR images, and hyperintense areas were seen on T2-weighted MR images in the left parietal lobe (Fig 2A).
Fig 2.
Type 2a. Case 13 in a 58-year-old man.
A, T2-weighted MR image reveals a hyperintense lesion in the left parietal lobe.
B, External carotid angiogram, lateral projection, of the left middle meningeal artery, reveals DAVFs at the superior sagittal sinus. The feeder middle meningeal arteries drain directly into the cortical vein. Final venous drainage is into the superior sagittal sinus via a varix. No accessory route is recognized.
C, Schematic drawing of a DAVF without an accessory route in the retrograde venous drainage. The surrounding flow (arrows) cannot drain into another area, resulting in severe venous congestion (shadow). F indicates fistula point; MMA, middle meningeal artery; and SSS, superior sagittal sinus
D, 99mTc-HMPAO SPECT scan shows an area of hypoperfusion at the site of the lesion.
E, After an acetazolamide challenge, the area of hypoperfusion is increased.
F, Post-treatment T2-weighted MR image shows the disappearance of the hyperintense area.
G, Post-treatment SPECT image reveals normal perfusion of the left parietal lobe.
Angiographic study demonstrated DAVFs in the superior sagittal sinus. We closely examined the route of the vessels on continuous-mode angiograms. The feeding arteries were the bilateral middle meningeal arteries. They drained directly into the cortical vein via two fistula points. A single large varix was recognized in the cortical venous drainage. The retrograde venous route was separated into two parts and finally drained into the superior sagittal sinus without flow into another sinus (Fig 2B and C). The area of hypoperfusion coincided with the hyperintense area on T2-weighted MR images and was increased after the acetazolamide challenge (Fig 2D and E). Endovascular embolization via the transarterial approach with 2-cm straight coils (Target Scientific Corp., Boston, MA) resulted in occlusion of the fistulas. Subsequent angiograms demonstrated complete disappearance of the DAVFs. Images demonstrated pooling of the contrast medium in a venous lake. However, an angiogram obtained 1 month later revealed slight refilling of the DAVFs, and direct surgery was performed. The draining vein was permanently interrupted with hemostatic clips at the exit of the fistula. After 2 weeks, the patient’s dysesthesia of the right lower limb improved, and T2-weighted MR images showed the disappearance of the hyperintense area (Fig 2F). Post-treatment SPECT revealed normal perfusion of the left parietal lobe (Fig 2G). Angiographic study performed 6 months after surgery revealed no residual DAVFs.
Type 2b RLVD into a Single Venous Sinus and Persistent Hyperintensity on Post-Treatment T2-Weighted MR Images. Case 21.—
This 63-year-old man had a 6-month history of mental disturbances. T2-weighted MR images showed areas of hyperintensity at the left temporo-occipital lobe (Fig 3A). On continuous-mode angiography, the feeders were the left occipital arteries. A DAVF was recognized in the left transverse-sigmoid sinus with RLVD into a single venous sinus (Fig 3B). The hypoperfusion area on SPECT images coincided with the area of hyperintensity on T2-weighted MR images; it did not increased after the acetazolamide challenge (Fig 3C and D).
Fig 3.
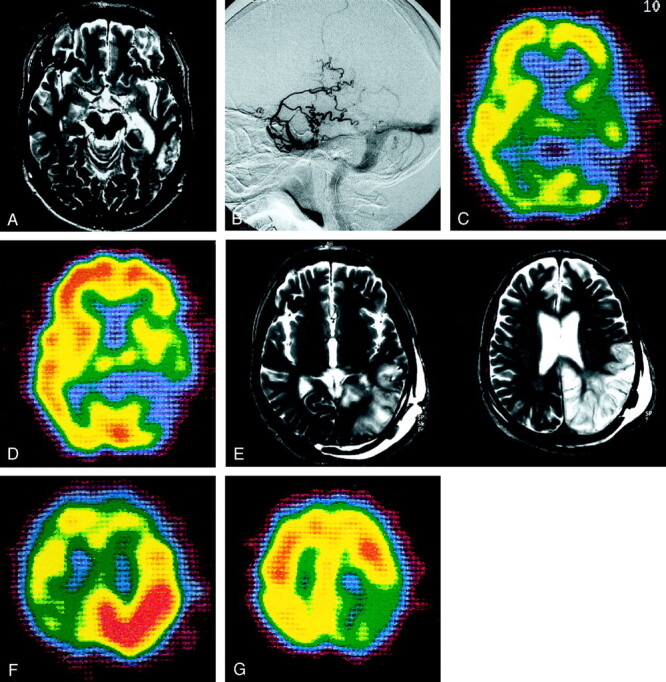
Type 2b. Case 21 in a 63-year-old man.
A, T2-weighted MR image reveals a hyperintense lesion in the left temporo-occipital lobe.
B, External carotid angiogram, lateral projection, of the left occipital artery shows DAVFs adjacent to the left transverse sinus. No accessory route is recognized.
C, 99mTc-HMPAO SPECT scan shows a hypoperfused area at the site of the lesion.
D, The hypoperfused area is not increased after the acetazolamide challenge.
E, After treatment, the hyperintense area seen on the T2-weighted MR image persists and expands to the left parietal lobe.
F, SPECT image obtained immediately after treatment reveals hyperperfusion in the left parietal lobe.
G, SPECT image obtained 6 months after treatment demonstrates hypoperfusion in the left parietal lobe.
The left occipital arteries were embolized with CPB. However, because a subsequent angiogram revealed slight refilling of the DAVF, direct surgery was performed. Permanent clips were applied to the draining vein at the site where the fistula exited without disturbing the normal venous return. The patient’s postoperative course was uneventful. After treatment, the hyperintense area on T2-weighted MR image not only persisted but also expanded to the left parietal lobe (Fig 3E). SPECT images obtained immediately after treatment revealed hyperperfusion in the left parietal lobe (Fig 3F). After 6 months, SPECT demonstrated hypoperfusion at the same site (Fig 3G). Angiographic study performed 6 months after surgery revealed no residual DAVFs; however, the patient continued to be slightly disoriented.
Discussion
Some DAVFs produce only tinnitus, whereas others are associated with neurologic deficits or intracranial hemorrhage (1,11–13). The type of symptoms and focal neurologic deficits are determined by the characteristics of the venous drainage (3, 12,14, 15). DAVFs with RLVD tended to be associated with variceal or aneurysmal venous dilatation (14, 15), and patients tended to develop venous hypertension, which resulted in neurologic deficits due to hemorrhage, brain edema, and/or ischemia (14).
Willinsky et al (8) reported that, in DAVFs with cortical venous drainage, T2-weighted MR images depicted areas of hyperintensity in the venous territory. They proposed that this finding is direct evidence of venous hypertension and congestive encephalopathy. We already reported that the pattern of venous drainage and MR imaging findings are correlated in DAVF patients with RLVD (16).
In our series, 11 of 22 patients with DAVF had RLVD into more than one venous sinus (type 1). In the others, type 2 RLVD was into a single venous sinus. Hyperintensity on T2-weighted images was noted in all 11 type 2 patients; none of the type 1 patients had these areas of hyperintensity. Moreover, seven type 1 patients had one or more varices in the RLVD route. Only two of the 11 type 2 patients had varices; in both cases, they were single varices. Three type 1 patients had hemorrhagic episodes, whereas no type 2 RLVD patients experienced hemorrhage.
We speculate that, in patients with drainage into more than one venous sinus, the retrograde venous flow increased within a short time, resulting in increased hemodynamic stress, varix formation, and the development of hemorrhage or neurologic deficits shortly after the appearance of this type DAVF. Our previous histopathologic study (17) showed that, in type 1 patients, the thickness of the vessel wall was extremely irregular. This finding suggests that they have a tendency to develop varices in the RLVD route and for hemorrhagic episodes. On the other hand, in type 2 patients with drainage into a single venous sinus, the volume of retrograde venous drainage was diminished due to the absence of an accessory route. Therefore, their retrograde venous flow was not markedly increased, and venous perfusion was slowly impaired over a long period, without bleeding. The resulting venous congestion was visualized as areas of hyperintensity on T2-weighted MR images. Pathologically, the entire media was thickened with prominent local intimal thickening (17). Type 2 patients had a weaker tendency to develop varices and hemorrhagic episodes.
In patients with DAVFs, SPECT is useful for evaluating the degree of rCBF disturbance (5). In all of our type 1 patients without hyperintense areas on T2-weighted MR images, SPECT scans did not show hypoperfusion. On the other hand, in all type 2 patients, the areas of hyperintensity on T2-weighted MR images coincided with areas of hypoperfusion on SPECT scans. We posit that these differences represent different degrees of rCBF disturbance in DAVFs with or without accessory drainage. The areas of hyperintensity on T2-weighted MR images that coincide with the areas of hypoperfusion on SPECT scans may reflect the presence of venous congestion due to rCBF disturbance, and the degree of this disturbance may be more severe in patients without accessory drainage.
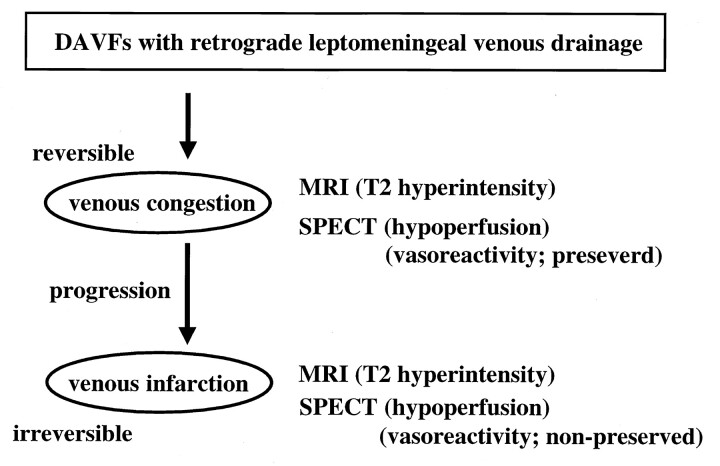
In type 1 patients, the DAVFs tended to progress rapidly, and hemorrhagic episodes occurred in the early stage of progression. Therefore, these patients experienced severe clinical episodes before the manifestation of hypoperfusion on SPECT scans. In seven type 2a patients, all of whom had preserved vasoreactivity after the acetazolamide challenge, the hyperintensity on T2-weighted MR images disappeared after treatment. We posit that, together, the preoperative findings reflected the presence of venous congestion that was reversible by surgical intervention. We further suggest that unpreserved vasoreactivity after an acetazolamide challenge reflects venous infarction that cannot be reversed with surgery and that the degree of rCBF disturbance is more severe in these patients (type 2b) than in the others. The examination of vasoreactivity after an acetazolamide challenge was important for evaluating the degree of rCBF disturbance in patients with DAVF and RLVD (Fig 4). Kuroda et al (18) reported that the acetazolamide test is valuable for assessing the cerebral perfusion reserve and for predicting the long-term prognosis of patients with internal carotid artery occlusion.
Fig 4.
Diagram summarizes our MR imaging and SPECT findings in DAVFs with RLVD.
In patients with DAVF, RLVD, and preserved vasoreactivity, surgery improved their symptoms of mental disturbance, dysesthesia of the right lower limb, and headache at 2–4 weeks after treatment. In seven type 2a patients with venous congestion, the interval between symptom onset and successful treatment was 2–4 months (mean, 3.58 months). In four type 2b patients with venous infarction, the interval between symptom onset and surgery was 5–12 months (mean, 7.5 months). The duration of rCBF disturbance attributable to RLVD is also an important factor in evaluating the degree of rCBF disturbance (19, 20).
We ascribe the presence of RLVD with drainage into more than one venous sinus in DAVF patients to different pathologies and a complex interplay of yet unknown factors that lead to the development of these accessory routes.
Conclusion
In patients with DAVF and RLVD, the pattern of venous drainage is correlated with the MR imaging findings. In patients with drainage into more than one venous sinus, preoperative T2-weighted images showed no hyperintense areas. Patients with drainage into a single venous sinus presented with venous congestion, which was reflected as areas of hyperintensity that coincided with areas of hypoperfusion on SPECT scans. In patients with preserved vasoreactivity after the acetazolamide challenge, surgery tended to alleviate their preoperative symptoms. Their rCBF disturbance was attributable to venous congestion. On the other hand, in patients with unpreserved vasoreactivity after the acetazolamide challenge, surgery tended not to alleviate their symptoms. Their rCBF disturbance was due to venous infarction. Therefore, we conclude that the preservation of vasoreactivity after an acetazolamide challenge can be interpreted as a good prognostic indicator.
References
- 1.Awad IA, Little JR, Akarawi WP. Intracranial dural arteriovenous malformations: factors predisposing to an aggressive neurological course. J Neurosurg 1990;72:839–850 [DOI] [PubMed] [Google Scholar]
- 2.Hurst RW, Bagley LJ, Galetta S. Dementia resulting from dural arteriovenous fistulas: the pathologic findings of venous hypertensive encephalopathy. AJNR Am J Neuroradiol 1998;19:1267–1273 [PMC free article] [PubMed] [Google Scholar]
- 3.Lasjaunias P, Chiu M, ter Brugge K. Neurological manifestations of intracranial dural arteriovenous malformations. J Neurosurg 1986;64:724–730 [DOI] [PubMed] [Google Scholar]
- 4.Goto K, Sidipratomo P, Ogata N. Combining endovascular and neurosurgical treatments of high-risk dural arteriovenous fistulas in the lateral sinus and the confluence of the sinuses. J Neurosurg 1999;90:289–299 [DOI] [PubMed] [Google Scholar]
- 5.Iwama T, Hashimoto N, Takagi Y. Hemodynamic and metabolic disturbances in patients with intracranial dural arteriovenous fistulas: positron emission tomography evaluation before and after treatment. J Neurosurg 1997;86:806–811 [DOI] [PubMed] [Google Scholar]
- 6.Kawaguchi T, Fujita S, Yamada H. Hemodynamics before and after the total removal of a dural arteriovenous malformation of the posterior fossa: case report. Surg Neurol 1988;30:457–461 [DOI] [PubMed] [Google Scholar]
- 7.Kuroda S, Ushikoshi S, Houkin K. Postoperative hyperperfusion in dural arteriovenous fistula associated with venous ischemia: case report. Surg Neurol 1998;49:406–411 [DOI] [PubMed] [Google Scholar]
- 8.Willinsky R, Terbrugge K, Montanera W. Venous congestion: an MR finding in dural arteriovenous malformations with cortical venous drainage. AJNR Am J Neuroradiol 1994;15:1501–1507 [PMC free article] [PubMed] [Google Scholar]
- 9.Hamada J, Kai Y, Nagahiro S, Hashimoto N, Iwata H, Ushio Y. Embolization with cellulose porous beads, II: Clinical trial. AJNR Am J Neuroradiol 1996;17:1901–1906 [PMC free article] [PubMed] [Google Scholar]
- 10.Hamada J, Ushio Y, Kazekawa K, Tsukahara T, Hashimoto N, Iwata H. Embolization with cellulose porous beads, I: an experimental study. AJNR Am J Neuroradiol 1996;17:1895–1899 [PMC free article] [PubMed] [Google Scholar]
- 11.Castaigne P, Bories J, Brunet P, Merland JJ, Meininger V. Meningeal arterio-venous fistulas with cortical venous drainage. Revue Neurologique 1976;132:169–181 [PubMed] [Google Scholar]
- 12.Djindjian R, Merland JJ, Theron J. Superselective arteriography of the external carotid artery. New York: Springer-Verlag;1977;606628
- 13.Houser OW, Baker H Jr., Rhoton A Jr, Okazaki H. Intracranial dural arteriovenous malformations. Radiology 1972;105:55–64 [DOI] [PubMed] [Google Scholar]
- 14.Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg 1995;82:166–179 [DOI] [PubMed] [Google Scholar]
- 15.Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology 1995;194:671–680 [DOI] [PubMed] [Google Scholar]
- 16.Kai Y, Hamada J, Morioka M. Correlation between magnetic resonance images and draining patterns in dural arteriovenous fistulas with leptomeningeal venous drainage. Acta Neurochir 2000;142:413–418 [DOI] [PubMed] [Google Scholar]
- 17.Hamada J, Yano S, Kai Y. Histopathological study of venous aneurysms in patients with dural arteriovenous fistulas. J Neurosurg 2000;92:1023–1027 [DOI] [PubMed] [Google Scholar]
- 18.Kuroda S, Kamiyama H, Abe H, Houkin K, Isobe M, Mitsumori K. Acetazolamide test in detecting reduced cerebral perfusion reserve and predicting long-term prognosis in patients with internal carotid artery occlusion. Neurosurgery 1993;32:912–918 [DOI] [PubMed] [Google Scholar]
- 19.Collice M, D’Aliberti G, Talamonti G, et al. Surgical interruption of leptomeningeal drainage as treatment for intracranial dural arteriovenous fistulas without dural sinus drainage. J Neurosurg 1996;84:810–817 [DOI] [PubMed] [Google Scholar]
- 20.Thompson BG, Doppman JL, Oldfield EH. Treatment of cranial dural arteriovenous fistulae by interruption of leptomeningeal venous drainage. J Neurosurg 1994;80:617–623 [DOI] [PubMed] [Google Scholar]