Abstract
BACKGROUND AND PURPOSE: Patients in poor clinical condition (Hunt and Hess grade 4 or 5) after subarachnoid hemorrhage (SAH) have historically fared poorly and many often were excluded from aggressive treatment. Early aggressive surgical treatment of SAH can produce good-quality survival for a higher percentage of patients than previously reported. We assessed the outcome of patients with Hunt and Hess grade 4 or 5 who were treated with Guglielmi detachable coil (GDC) embolization.
METHODS: We retrospectively evaluated the records of 27 consecutive grade 4 and 5 patients with 29 aneurysms treated within 72 hours of SAH by using GDCs. Percentage aneurysm occlusion after embolization, perioperative complications, and symptoms of vasospasm were evaluated. Outcome was assessed with the Glasgow Outcome Scale.
RESULTS: Sixteen patients (59%) were grade 4, and 11 (41%) were grade 5. Eighteen (67%) had one aneurysm, six (22%) had two aneurysms, and three (11%) had three aneurysms. Twenty-nine aneurysms were treated. Fourteen (48%) were completely occluded, and four (14%) were nearly completely occluded (≥95% occlusion) at embolization. Eleven aneurysms (38%) had partial coiling (<95% occlusion). In the 27 patients, one technical (4%) and one clinical (4%) complication occurred at embolization. No rehemorrhage occurred in any patients (follow-up, 6–44 months; mean, 23 months). Twenty-five (92%) had vasospasm, and seven required endovascular treatment because of worsening clinical status. Sixteen patients (59%) died within 30 days of SAH. Eight patients (30%) had a good clinical outcome at a mean follow-up of 23 months.
CONCLUSION: Patients with Hunt and Hess grade 4 or 5 after SAH can undergo successful coil embolization of the aneurysms despite their poor medical condition and a high frequency of vasospasm at the time of treatment. Morbidity and mortality rates with this disease are still high. These findings compare favorably with those published in surgical series for aggressively treated patients with Hunt and Hess grade 4 or 5.
Results of community- and population-based studies indicate that fewer than one-third of people with a ruptured cerebral aneurysm return to their premorbid state. The clinical status of the patient at admission is probably the single most important predictor of outcome. The subgroup of patients who present with a depressed level of consciousness generally do poorly. In the International Cooperative Study on the Timing of Aneurysm Surgery (1), only 11.1% of patients who were comatose at admission (n = 315) made a good recovery and 72.1% died. Two grading systems—the Hunt and Hess system (2) and the World Federation of Neurologic Surgeons Scale (3)—are the most frequently used by physicians to evaluate the clinical severity of subarachnoid hemorrhage (SAH). These systems are comparable as outcome predictors. Patients who are poor clinical grade (Hunt and Hess grades 4 and 5) generally compose about 20–30% of those admitted to the hospital with aneurysmal SAH (1, 2, 4).
Historically, patients with poor grades according to this clinical grading scale have fared poorly. Early published series stressed treating patients with poor grades (grades 4 and 5) by delaying surgery until they showed clinical improvement (grades 1–3). This treatment paradigm resulted in a favorable outcome of 3.8–18%, with a mortality rate of 68–87.4% (5–9). Later, in the early and mid-1990s, several published reports suggested that early aggressive medical and surgical therapy brought about an improvement in patient outcome. Favorable outcome in these series ranged from 7% to 42.6% (10–16).
The use of the Guglielmi detachable coil (GDC) for the endovascular treatment of intracranial aneurysms was first reported in 1991 (17). Several series have since described the successful coil embolization of both ruptured and unruptured aneurysms (18–21). The GDC has been shown to be effective in preventing early rebleeding (22). Only two studies have reported outcome data following GDC treatment of patients with a poor grade (Hunt and Hess grade 4 or 5) (23, 24). The objective of this study was to assess the technical success and clinical outcome in patients with Hunt and Hess grade 4 or 5 after SAH who were treated with the GDC system.
Methods
We performed a retrospective review of the records of 27 consecutive patients who were Hunt and Hess grade 4 or 5 at presentation after SAH between June 1995 and January 2001 and who were treated with GDC embolization at our institution. These patients were all treated within 72 hours of the onset of SAH. The grade assigned to the patient was the immediate pretreatment grade and not an earlier grade (after resuscitation in the emergency department or immediately after placement of an external ventricular drain). All patients treated at our institution were evaluated by a combined neurosurgery and interventional neuroradiology team. These patients were usually judged to be candidates for endovascular therapy because their aneurysm morphology was thought to be conducive for either complete occlusion or near-complete occlusion. Our intent was to completely thrombose the aneurysm whenever possible. In some cases, coil embolization was performed to partially occlude the aneurysm to protect the dome from acute rebleeding. During this time period, some patients who were grade 4 or 5 did undergo surgery at our institution, and some patients were considered candidates for another endovascular procedure (ie, parent-vessel occlusion). In general, those patients receiving these other therapies were not thought to be candidates for intraaneurysmal coil embolization. A total of 19 additional patients with grades 4 or 5 were seen during this time but not treated with coil embolization alone. Thirteen underwent surgery. In the remaining six patients, three had no treatment and three had permanent vessel occlusion.
All procedures were performed with the patient under general anesthesia and with neurophysiologic monitoring by using somatosensory evoked potentials and electroencephalographic monitoring. Patients were monitored by a dedicated neurophysiologist. In cases with multiple aneurysms, we attempted to identify the most likely aneurysm to have bled (based on CT and angiographic images). If it was not thought possible to identify the most likely bleeding aneurysm, more than one aneurysm underwent coil embolization. SAH was aggressively managed by using daily transcranial Doppler sonography, calcium antagonist, and triple-H (hypertension, hypervolemia, hemodilution) therapy when appropriate. In those patients with continued symptoms thought to be due to vasospasm despite aggressive medical management, endovascular therapy with angioplasty and/or papaverine was used. Patients were evaluated for percentage occlusion of the aneurysm at coil embolization, perioperative complications, and angiographic and clinical vasospasm. Surviving patients (those alive after 30 days) were followed up for an average of 23 months (range, 6–44 months). Patient outcome was assessed by using the modified Rankin scale.
Results
The patient population consisted of 18 women and nine men with an average age of 64 years (range, 37–87 years). Eighteen (67%) of the 27 patients were older than 60 years. Sixteen (59%) were Hunt and Hess grade 4 and 11 (41%) grade 5. Eighteen patients (67%) had one aneurysm, six (22%) had two aneurysms, and three (11%) had three. Thus, there were a total of 39 aneurysms. Twenty-nine of these were treated by using the GDC system. Tables 1 and 2 show the results of treatment for the patients with grades 4 and 5, respectively. Eighteen (62%) of the 29 treated aneurysms were located in the anterior circulation and 11 (38%) in the posterior circulation. Specifically, eight aneurysms were located on the anterior communicating artery, two on the middle cerebral artery, four on the internal carotid artery, four on the posterior communicating artery, five on the basilar artery, two on the posterior inferior cerebellar artery, two on the superior cerebellar artery, and one each on the vertebral artery and posterior cerebral artery. Twenty-three aneurysms (79%) were smaller than 10 mm in diameter, four (14%) were 11–25 mm, and two (7%) were larger than 25 mm.
TABLE 1:
Patients with Hunt and Hess grade 4
| Patient No. | No. of Aneurysms | CT Fisher Grade | Aneurysm Location | Percentage Occlusion | Rankin Outcome Score | Vasospasm | Vasospasm Treatment |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 3 | R PCOM | 90 | 2 | Y | HHH, PA |
| 2 | 3 | 4 | L ICA | 80 | 6 | Y | HHH |
| 3 | 3 | 3 | Basilar | 100 | 4 | Y | HHH |
| R P1–P2 | 95 | ||||||
| 4 | 3 | 4 | R ICA | 100 | 6 | Y | HHH |
| 5 | 1 | 3 | L ICA | 80 | 1 | N | — |
| 6 | 1 | 4 | ACOM | 100 | 0 | Y | HHH |
| 7 | 1 | 4 | L SCA | 95 | 1 | N | — |
| 8 | 1 | 3 | Basilar | 100 | 1 | Y | HHH |
| 9 | 1 | 4 | ACOM | 100 | 6 | Y | HHH, PA |
| 10 | 1 | 4 | L ICA | 90 | 6 | Y | HHH |
| 11 | 1 | 4 | R SCA | 100 | 6 | Y | HHH |
| 12 | 2 | 3 | L ICA | 100 | 6 | Y | HHH, PA |
| 13 | 1 | 4 | L VERT | 100 | 6 | Y | HHH |
| 14 | 1 | 3 | R PICA | 100 | 6 | Y | HHH, PA |
| 15 | 2 | 3 | R MCA | 95 | 6 | Y | HHH |
| 16 | 1 | 4 | R ACOM | 100 | 6 | Y | HHH, PA |
Note.—ACOM indicates anterior communicating artery; HHH = hypertension, hypervolemia, hemodilution therapy; ICA = internal carotid artery; L = left; MCA = middle cerebral artery; PA = papavarine; PCOM = posterior communicating artery; PICA = posterior inferior cerebellar artery; R = right; SCA = superior cerebellar artery; VERT = vertebral artery.
TABLE 2:
Patients with Hunt and Hess grade 5
| Patient No. | No. of Aneurysms | CT Fisher Grade | Aneurysm Location | Percentage Occlusion | Rankin Outcome Score | Vasospasm | Vasospasm Treatment |
|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | ACOM | 60 | 4 | Y | HHH |
| 2 | 2 | 4 | PCOM | 90 | 6 | Y | HHH |
| 3 | 1 | 4 | Basilar | 90 | 6 | Y | HHH |
| 4 | 1 | 4 | L ACOM | 100 | 6 | Y | HHH |
| 5 | 1 | 4 | R ACOM | 90 | 0 | Y | HHH, PA |
| 6 | 1 | 3 | Basilar | 90 | 6 | Y | HHH, PA, PTA |
| 7 | 2 | 4 | ACOM | 50 | 4 | Y | HHH |
| R PCOM | 75 | ||||||
| 8 | 2 | 3 | L MCA | 100 | 2 | Y | HHH |
| 9 | 1 | 3 | R PCOM | 100 | 6 | Y | HHH |
| 10 | 1 | 4 | ACOM | 100 | 6 | Y | HHH |
| 11 | 1 | 3 | Basilar | 95 | 2 | Y | HHH |
Note.—ACOM indicates anterior communicating artery; HHH = hypertension, hypervolemia, hemodilution therapy; L = left; MCA = middle cerebral artery; PA = papavarine; PCOM = posterior communicating artery; PTA = angioplasty; R = right.
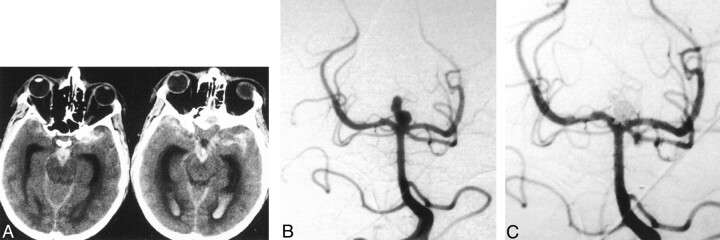
Fourteen aneurysms (48%) were completely occluded (Fig 1), and four (14%) were judged to be nearly completely occluded with only a small remnant remaining (≥ 95% occlusion). These accounted for 62% of the treated aneurysms. In 11 cases (38%), the aneurysm morphology was such that it was decided to partially coil the aneurysm (<95% occlusion) to protect the dome from rebleeding in the time interval immediately following the hemorrhage.
Fig 1.
45-year-old man with sudden severe headache and loss of consciousness was classified as Hunt and Hess grade 5.
A, Axial nonenhanced CT images demonstrate extensive subarachnoid and intraventricular blood.
B, Diagnostic angiogram shows a basilar tip aneurysm. The superior lobulations may represent an associated pseudoaneurysm.
C, Angiogram obtained after coil embolization shows complete occlusion of the aneurysm. There is minimal protrusion of coil loops into the basilar tip, but the patent vessel is widely patent. The patient had a good recovery and at 6-month follow-up was able to resume normal daily life.
At the time of treatment, 25 (92%) of the 27 patients had radiographic evidence of vasospasm. Eleven patients had Fisher grade three CT scans, and 16 had Fisher grade 4 scans. All patients were treated with triple-H therapy and nimodipine. Seven patients had angiographic (and clinical) vasospasm that required endovascular treatment with angioplasty and papavarine
No rebleeding occurred in the patient population during the follow-up period. In the 27 patients, one technical complication (4%) and one clinical complication (4%) occurred at the time of the procedure. In the patient with a technical complication, the left internal carotid artery was dissected during access, and a stent was then placed. The vessel remained patent with no change in the patient’s clinical status. In a second patient, at the time of coil embolization acute thrombosis of the basilar artery occurred. Despite immediate thrombolytic (tissue plasminogen activator) treatment, this patient had a major cerebral infarction and later died.
Sixteen patients (59%) died within 30 days of the SAH. Ten of the 16 patients were grade 4 and six were grade 5. Twelve of the 16 patients died within 1 week of SAH. Nine patients in the series were judged to have died after loss of brain stem function either secondary to the acute bleed or from subsequent infarction. Five other patients experienced cardiopulmonary arrest, one had a pulmonary embolus, and one had multisystem organ failure.
Eleven patients (41%) survived beyond 1 month. Eight patients (30%) had a favorable outcome (modified Rankin score ≤2). Three of the eleven patients remained in a vegetative (n = 2) or severely disabled (n = 1) condition, and these three patients died within 1 year of SAH.
Of the 16 grade 4 patients, five (31%) had a good outcome. Of the 11 grade 5 patients, three (27%) had a good outcome. Five (29%) of the 17 patients with anterior circulation aneurysms had a good outcome, three (30%) of the 10 patients with posterior circulation aneurysms had a good outcome.
In the 19 patients not treated with intraaneurysmal coil embolization alone, three (16%) had a good outcome (modified Rankin score ≤2). Eight patients (42%) died, four were severely disabled or in a vegetative state, and four were returned to the referring hospital after surgical therapy and were lost to follow-up.
Discussion
In 1968, Hunt and Hess (2) published their results of treating 275 consecutive cases of intracranial aneurysm at Ohio State University. All grades 1 and 2 patients were taken to surgery as soon as possible. The grades 3–5 patients were treated conservatively until they improved to a grade 1 or 2. Exceptions were made for patients with multiple, repeated bleeding episodes and those with significant mass effect from a hematoma. In their experience, the survival for patients admitted at grade 1, 2, or 3 was 70%. However, fewer than 20% of the grade 4 or 5 patients survived.
More recent data suggest that urgent preoperative resuscitation, early surgery, and aggressive treatment of vasospasm have improved the outcome in patients with good grades. Le Roux et al (25) demonstrated that 86% of patients with Hunt and Hess grades 1–3 return to independent function. This rate was 96% for the grade 1 patients.
Patients with poor grades (Hunt and Hess grades 4 and 5) were historically excluded from early surgical treatment. Bailes et al (10) followed up 19 patients with aneurysms who were poor grade and treated conservatively; they noted a mean survival of 31.8 hours and 100% mortality. Hijdra et al (7) evaluated 42 patients in whom treatment was delayed until clinical improvement was observed before therapy was started. Their mortality rate was 71%, with a favorable outcome of 5%. Ohno et al (9) also reported on delayed treatment until clinical improvement. In that study, 32 patients (15%) had a favorable outcome. Outcome from other studies revealed that more than 90% of untreated poor-grade patients die (4–6).
These results for poor-grade patients led to consideration of an aggressive approach, including rapid resuscitation, control of intracranial pressure, early surgery, and ischemia prophylaxis. Use of these techniques in poor-grade patients has improved outcomes. Hutchinson et al (26) resuscitated all the poor-grade patients in their study for the first 24 hours and selected only the patients who demonstrated a purposeful response to painful stimuli for angiography and potential aneurysm surgery. In the overall population of 102 patients, there was a favorable outcome of 25% and a mortality rate of 67%. Nowak et al (13) evaluated 69 of 102 poor-grade patients who were treated surgically. In the surgical group, 33% had a favorable outcome (GOS score 1 or 2). The patients treated surgically included 27 patients treated after placement of an external ventricular drain and subsequent clinical improvement, 17 operated on despite no clinical improvement after placement of an external ventricular drain, and 25 patients who were operated on early secondary to a large hematoma. Several authors have reported on placement of external ventricular drains with selective aggressive approach to patients with grade 4 or 5 after SAH. They had favorable outcomes ranging from 20% to 42% (14, 15, 27, 28).
The GDC system was first made available for approved use in 1995. This device has since gained widespread acceptance as an alternative treatment technique for intracranial aneurysms. Series reporting results with mid and longer term follow-up have been quite limited, however. In addition, the use of GDCs for ruptured aneurysms in patients with a poorer clinical grade have generally not been reported as separate series, and it is difficult to determine outcome data for this separate group of patients. Two prior reports have concentrated on the endovascular treatment of patients with poor clinical grades who were treated by using the GDC (23, 24). Kremer et al (23) compared endovascular treatment in anterior versus posterior circulation aneurysms in patients who were grade 4 or 5. In this series, 18 patients had anterior and 22 had posterior circulation aneurysms. The reported results do have some confounding variables, however. First, four patients had balloon occlusions. Second, a larger number of patients were treated late following the SAH. Fourteen (35%) were treated after 3 days and 10 (25%) were treated after 1 week. Outcome data in a series such as this may be biased because later treatment may select patients that do better by virtue of the fact that they have survived longer without treatment. Nevertheless, 16 (40%) of 40 patients had good outcomes (GOS score 4 or 5) in that study (23). Of interest, eight (50%) of the 16 patients with good outcomes in that series were treated after more than 3 days following SAH.
Groden et al (24) evaluated patients with grade 4 or 5 by comparing endovascular therapy to surgery for anterior circulation aneurysms. In this series, 20 patients were treated endovascularly. This report also includes patients treated with balloon occlusion (one patient) and a significant number of patients who were treated late, with five (25%) of 20 patients treated 1 week after ictus. In this study group, six (30%) of 20 patients had a good outcome (GOS score 4 or 5)
A recent series evaluated overall outcome with endovascular treatment in 80 patients with aneurysm who were grade 4 and 5 and were treated during a 6-year period (29). In this series, 42 patients (52.5%) had favorable GOS scores; 30 patients (37.5%) died. However, 30 patients (37.5%) were treated 3 days or more following SAH, with 12 patients (15%) treated beyond 2 weeks. Some of the bias with selection of patients receiving late treatment may apply here as well. For example, 21 (42%) of 50 patients treated in the first 3 days died, whereas five (27%) of 18 patients treated after 1 week died.
In our series, the 27 patients with 29 aneurysms were all Hunt and Hess grade 4 or 5 (poor grade) and were treated by using the GDC system within 72 hours after ictus. Sixteen patients (59%) died within 30 days of SAH, whereas 11 (41%) survived. Eight patients (30%) had a favorable outcome (modified Rankin ≤2). Therefore, our results are similar to what is seen in aggressively treated surgical patients (ie, those treated within 72 hours after ictus with early surgery).
Our series also included a large number of elderly patients, which may well reduce survival. The mean age in our population was 64 years, with 12 patients (44%) older than 65 years. Advanced age may well affect outcome. For example, Hutchinson et al (26) reported good outcomes in 25% of 102 patients with grade 4 or 5 who were treated surgically. In this patient population, however, those older than 65 years (33 [32%] of 102 patients) generally had poor outcomes, with only two (65) of 33 having a GOS score of 4 or 5.
Conclusion
Patients who are acutely ill with high Hunt and Hess grades after SAH can undergo successful coil embolization despite their poor medical condition and a high frequency of vasospasm. Many of these patients have a good clinical outcome. There still remain high rates of morbidity and mortality with this disease. These findings compare favorably with those published in the surgical literature.
References
- 1.Kassell NF, Torner JC, Jane JA, Haley EC Jr, Adams HP. The international cooperative study on the timing of aneurysm surgery. II: surgical results. J Neurosurg 1990;73:37–47 [DOI] [PubMed] [Google Scholar]
- 2.Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 1968;28:14–20 [DOI] [PubMed] [Google Scholar]
- 3.Drake CG. Report of World Federation of Neurological Surgeons committee on universal subarachnoid hemorrhage grading scale. J Neurosurg 1988;86:985–986 [DOI] [PubMed] [Google Scholar]
- 4.Longstreth WT, Nelson LM, Koepsell TD, van Belle G. Clinical course of spontaneous subarachnoid hemorrhage: a popula-tion based study in King County. Washington Neurol 1993;43:712–718 [DOI] [PubMed] [Google Scholar]
- 5.Adams HP, Kassell NF, Torner JC, Nibbelink DW, Sahs AL. Early management of aneurysmal SAH: a report to the cooperative aneurysm study. J Neurosurg 1981;54:141–145 [DOI] [PubMed] [Google Scholar]
- 6.Testa C, Andreoli A, Arista A, Limoni P, Tognetti F. Overall results in 304 consecutive patients with acute spontaneous subarachnoid hemorrhage. Surg Neurol 1985;24:377–385 [DOI] [PubMed] [Google Scholar]
- 7.Hijdra A, van Gijn J, Nagelkerke NJD, Vermeulen M, van Crevel H. Prediction of delayed cerebral ischemia, rebleeding, and outcome after aneurysmal subarachnoid hemorrhage. Stroke 1988;19:1250–1256 [DOI] [PubMed] [Google Scholar]
- 8.Freckmann N, Noll M, Winkler D, Nowak G, Rehn H, Neuss M, Hermann HD. Does the timing of aneurysm surgery neglect the real problems of the subarachnoid haemorrhage? Acta Neurochir (Wien) 1987;89:91–99 [DOI] [PubMed] [Google Scholar]
- 9.Ohno K, Suzuki R, Masaoka H, Monma S, Matsushima Y, Inaba Y. A review of 102 consecutive patients with intracranial aneurysms in a community hospital in Japan. Acta Neurochir (Wien) 1988;94:23–27 [DOI] [PubMed] [Google Scholar]
- 10.Bailes JE, Spetzler RF, Hadley MN, Baldwin HZ. Management morbidity and mortality of poor grade aneurysm patients. J Neurosurg 1990;72:559–566 [DOI] [PubMed] [Google Scholar]
- 11.Medlock MD, Dulebohn SC, Elwood PW. Prophylactic hypervolemia without calcium channel blockers in early aneurysm surgery. Neurosurgery 1992;30:12–16 [DOI] [PubMed] [Google Scholar]
- 12.Miyaoka M, Sato K, Ishii S. A clinical study of the relationship of timing to outcome of surgery for ruptured cerebral aneurysms: a retrospective analysis of 1622 cases. J Neurosurg 1993;79:373–378 [DOI] [PubMed] [Google Scholar]
- 13.Nowak G, Schwachenwald R, Arnold H. Early management in poor grade aneurysm patients. Acta Neurochir 1994;126:33–37 [DOI] [PubMed] [Google Scholar]
- 14.Seifert V, Trost HA, Stolke D. Management of morbidity and mortality in grade IV and V patient with aneurysmal subarachnoid hemorrhage. Acta Neurochir 1990;103:5–10 [DOI] [PubMed] [Google Scholar]
- 15.Steudel WI, Reif J, Voges M. Modulated surgery in the management of ruptured intracranial aneurysm in poor grade patients. Neurol Res 1994;16:49–53 [DOI] [PubMed] [Google Scholar]
- 16.LeRoux PD, Winn HR. Intracranial aneurysms and subarachnoid hemorrhage management of the poor grade patient. Acta Neurochir Suppl 1999;72:7–26 [DOI] [PubMed] [Google Scholar]
- 17.Guglielmi G, Vinuela F, Dion J, Duckwiler G. Electrolysis of saccular aneurysms via endovascular approach. II: preliminary clinical experience. J Neurosurg 1991;75:8–14 [DOI] [PubMed] [Google Scholar]
- 18.Vinuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 1997;86:475–482 [DOI] [PubMed] [Google Scholar]
- 19.Steiger HJ, Medele R, Bruckmann H, Schroth G, Reulen HJ. Interdisciplinary management results in 100 patients with ruptured and unruptured posterior circulation aneurysms. Acta Neurochir (Wein) 1999;141:359–367 [DOI] [PubMed] [Google Scholar]
- 20.Koivisto T, Vanninen R, Hurskainen H, Saari T, Hernesniemi J, Vapalahti M. Outcome of early endovascular versus surgical treatment of ruptured cerebral aneurysms. Stroke 2000;10:2369–2377 [DOI] [PubMed] [Google Scholar]
- 21.Kuether T, Nesbit GM, Barnwell SL. Clinical and angiographic outcomes, with treatment data for patients with cerebral aneurysms treated with Guglielmi detachable coils: a single center experience. Neurosurgery 1998;43:1016–1023 [DOI] [PubMed] [Google Scholar]
- 22.Graves VB, Strother CM, Duff TA, Perl J. Early treatment of ruptured aneurysms with detachable coils: effect on subsequent bleeding. Neurosurgery 1995;37:640–647 [DOI] [PubMed] [Google Scholar]
- 23.Kremer C, Groden C, Hansen HC, Gryska U, Zeumer H. Outcome after endovascular treatment of Hunt and Hess Grade IV or V aneurysms: comparison of anterior versus posterior circulation. Stroke 1999;30:2617–2622 [DOI] [PubMed] [Google Scholar]
- 24.Groden C, Kremer C, Regelsberger J, Hansen HC, Zeumer H. Comparison of operative and endovascular treatment of anterior circulation aneurysms in patients in poor grades. Neuroradiology 2001;43:778–783 [DOI] [PubMed] [Google Scholar]
- 25.Le Roux PD, Elliott JP, Downey L, et al. Improved outcome after rupture of anterior circulation aneurysms: a retrospective 10 year review of 224 good grade patients. J Neurosurg 1995;83:394–402 [DOI] [PubMed] [Google Scholar]
- 26.Hutchinson PJ, Power DM, Tripathi P, Kirkpatrick PJ. Outcome from poor grade aneurysmal subarachnoid haemorrhage: which poor grade subarachnoid haemorrhage patients benefit from aneurysm clipping? Br J Neurosurg 2000;14:105–109 [DOI] [PubMed] [Google Scholar]
- 27.Ungersbock K, Bocher-Schwarz H, Ulrich P, Wild A, Perneezky D. Aneurysm of patients in poor grade condition: indication and experience. Neurol Res 1994;16:31–34 [DOI] [PubMed] [Google Scholar]
- 28.Le Roux PD, Elliott JP, Newell DW, Grady MS, Winn HR. Predicting outcome in poor-grade patients with subarachnoid hemorrhage: a retrospective review of 159 aggressively managed cases. J Neurosurg 1996;85:39–49 [DOI] [PubMed] [Google Scholar]
- 29.Bracard S, Lebdinsky A, Anxionnat R, et al. Endovascuolar treatment of Hunt and Hess Grade IV and V aneurysms. AJNR Am J Neuroadiol 2002;23:953–957 [PMC free article] [PubMed] [Google Scholar]