Abstract
BACKGROUND AND PURPOSE: Diffusion-weighted (DW) MR imaging is important in evaluating acute stroke, and knowledge of the signal intensity changes associated with acute stroke is valuable. Our purpose was to model the time course of the signal intensity of infarcts and to characterize the apparent diffusion coefficient (ADC) and T2 effects on total signal intensity.
METHODS: Ninety-two patients were included in this prospective cross-sectional study. Signal intensity in infarcts (4 hours to 417 days) and control regions were recorded on DW images (b = 0 and 1000 s/mm2), ADC maps, and ratio images (image with b = 1000 s/mm2 divided by image with b = 0 s/mm2). Cubic spline functions were used for polynomial fitting. The time courses of log signal intensity with log time were modeled. The independent contributions of T2 and ADC to the total signal intensity were retrospectively compared at 0–63 hours, 3–10 days, 11–57 days, and 57 days onward.
RESULTS: Mean signal intensity on DW images was maximal at 40 hours after infarction and normalized at 57 days. At 0–63 hours, the positive effect of ADC on signal intensity was greater than that of T2 (log value,13 ± 0.04 vs 0.11 ± 0.05; P = .04). At days 3–10, the positive T2 effect predominated (0.13 ± 0.08 vs 0.08 ± 0.04; P = .12). At 10–57 days, the positive T2 effect was greater than the negative ADC effect. After day 57, the negative ADC effect predominated.
CONCLUSION: The signal intensity of infarcts on DW images normalizes at 57 days, which is substantially later than previously suggested. T2 (shine-through) effect contributes largely to the total infarct signal intensity.
Diffusion-weighted (DW) MR imaging is a well-established clinical tool, most notably in the study of cerebral infarction (1–8). DW images are sensitive for detection of regions of acute cerebral ischemia, and they can provide clinically helpful information about infarct ages (3, 6, 9–11).
The signal intensity of brain infarcts on DW images is mainly influenced by T2 relaxation and the apparent diffusion coefficient (ADC) (12–13). Increases in T2, such as those typically seen with cerebral infarcts, cause increased signal intensity of lesions on DW images. The term shine-through has been used to refer to increases in lesion signal intensity on DW images that are due to increases in T2 effect (12, 14).
Investigators from several previously published studies have characterized the temporal evolution of water diffusibility (eg, ADC) within lesions after cerebral infarction (1, 3, 4, 7, 9, 15–17). As a result of these studies, knowledge now exists about the expected times at which ADC values are decreased, normal, and increased (1, 3, 7, 9). Because of the predictable time course of the ADC after infarction, ages of individual lesions can be estimated by using ADC values (1, 3, 7, 9). This fact may be of clinical value when the time of onset of ischemia is unclear or when multiple lesions are present.
In contrast to the substantial body of published work concerning the temporal evolution of ADC values after stroke, the temporal evolution of infarct signal intensity on DW images is less well documented in the literature. Specifically, while it is generally known that infarcts have increased signal intensity during the first days after cerebral infarction and that infarcts can have decreased signal intensity in the late, chronic stage, we are aware of only one report of the estimated time of normalization of the signal intensity on DW images after infarction (11). Although the signal intensity was suggested to normalize at approximately 14 days after symptom onset, data from more recent studies using a region-of-interest (ROI) assessment of the signal intensity suggest the possibility that the signal intensity remains elevated substantially later than 14 days (9, 16). However, these more recent studies provide limited information about the time of infarct normalization because they included few patients with infarcts older than 14 days. Additionally, they did not include any infarcts with normal or decreased signal intensity on DW images, and thus they could not provide an estimate of the time at which the signal intensity normalizes after infarction.
It has become clear that ADC maps provide valuable information in the setting of acute infarction. Nevertheless, we are aware of many radiologists in a variety of clinical settings who routinely rely on DW images alone (rather than ADC maps) for the assessment of patients with stroke. For such radiologists, the assumption (based on the available literature) that infarct signal intensity typically normalizes at about 14 days is a widely used rule. If a study showed that the infarct signal intensity typically normalizes at a time substantially later than 14 days, such information would likely help radiologists to be more accurate with their assessments of DW images.
The main purpose of this pilot study was to create a quantitative model of the time course of infarct signal intensity on DW images to determine the time at which the signal intensity returns to normal after its initial elevation after an infarction. Because prior studies have provided evidence that infarct signal intensity remains increased at least 30 days after infarction (9, 16), we determined to test the hypothesis that infarct signal intensity on clinical DW images normalizes at a time later than 30 days. The secondary purpose of this study was to characterize the temporal evolution of signal intensity on DW images with respect to the two major components of DW signal intensity: the diffusibility (ie, ADC) effect and the T2 (shine-through) effect.
Methods
Subjects
Over 14 months, 114 consecutive patients underwent clinical MR imaging at a university medical center after having stroke. A cross-sectional study design was used. The following patients were excluded: 1) those for whom the time of symptoms onset could not be accurately determined (eg, patients for whom the history was thought to be unreliable or for whom symptom onset was stepwise), 2) those who underwent treatment with either neuroprotective agents or thrombolytic agents, and 3) those with evidence of hemorrhage within the region of infarction. A total of 92 patients (48 women, 44 men) were included in the analysis. One image per patient was studied. Stroke etiology was determined according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification (18). The causes included large-artery atherosclerosis (n = 28), small-artery occlusion (n = 26), cardioembolism (n = 12), other determined etiologies (n = 4), and undetermined etiologies (n = 22). The patients’ ages ranged from 16 to 91 years (mean, 63.7 years ± 14.1). The time from symptom onset to imaging ranged from 4 hours to 417 days (median, 72 hours). This time was determined by interviewing all patients and available family members at the time of imaging. The human subjects review board at our institution reviewed and approved our protocol.
DW Imaging
All patients underwent clinical MR imaging with a 1.5-T MR imaging system (Signa; GE Medical Systems, Milwaukee, WI). DW imaging was performed by using a multisection, single-shot, spin-echo, echo-planar sequence. Diffusion gradients were applied sequentially in three orthogonal directions: anteroposterior, left-right, and superior-inferior, and imaging was performed by using the following parameters: TR/TEeff of 12,000/101ms and b values of 0 and 1000 s/mm2. An inversion recovery pulse (TI, 2200) designed to suppress CSF was used to try to limit the influence of partial-volume averaging effects on the accuracy of diffusion measurements (9, 19). The field of view was 40 × 20 cm and was displayed by using a matrix of 128 × 64. A section thickness of 5 mm and an intersection gap of 2.5 mm were used. The acquisition time for all DW imaging examinations was less than 2 minutes. The three orthogonal DW images were averaged to create an isotropic DW image. Finally, the image obtained with b = 0 s/mm2 and the resultant isotropic image with b = 1,000 s/mm2 were transferred to an imaging workstation (Advantage Windows workstation; GE Medical Systems). By using this workstation and commercial software (FuncTool; GE Medical Systems), two types of calculated images were created: The first type was a map of the isotropic ADC. The second type was the image proportional to the ratio of the image obtained with b = 1000 s/mm2 to that obtained with b = 0 s/mm2 (10, 14). This second calculated image, the ratio image, is essentially a DW image that is normalized to control for T2 effects and that contains image contrast due solely to diffusion effects (10, 14). Our software also applied a scaling factor of 1000 to facilitate display of the ratio image.
Analysis of Stroke Lesions
For each patient, one small (40-mm2) ROI was placed in the center of the infarct on the isotropic (ie, mean of three directions) image with b = 1000 s/mm2. For patients in whom infarcts were more conspicuous on the image with b = 0 s/mm2 than on the DW images (n = 17), the ROI was placed within the center of the lesion on the image with b = 0 s/mm2. The analysis software used in this study allowed simultaneous placement of matching ROIs on the image with b = 1000 s/mm2, the image with b = 0 s/mm2, the ADC map, and the ratio image.
As a control for signal intensity, a second ROI the same size as the first ROI was placed in the same (mirror image) location in the contralateral hemisphere. For each type of ROI (ie, the infarct ROI and the control ROI), four values were recorded: 1) the mean signal intensity on the image with b = 1000 s/mm2, 2) the mean signal intensity on the image with b = 0 s/mm2, 3) the mean ADC value, and 4) the mean signal intensity on the ratio image.
For each of the four types of image, a relative value of the signal intensity or ADC was computed by dividing the value in the lesion ROI by the value in the control ROI. Thus, for example, the relative signal intensity of the lesion on the DW image was defined as follows: SIDWI = SI DWI lesion/SIDWI control. We had previously determined that intraobserver variability for the determination of SIDWI was less than 1% (10). The relative signal intensity of the lesion on the non-DW image was defined as follows: SIb0 = SIb0 lesion/SIb0 control, where b0 indicates the image with b = 0 s/mm2. The relative ADC of the lesion was defined as rADC = ADClesion/ADCcontrol and the relative signal intensity on the ratio image was defined as SIRAT = SIRAT lesion/SIRAT control, where RAT indicates ratio.
Determination of the Time Course of Lesion Signal Intensity on DW Images
Using all patients, the log values of SIDWI were modeled as a function of the time interval (in log hours) that had elapsed between time of symptom onset and the time of imaging. The logarithmic scale was used to create more uniformly distributed points along the time axis so as to create more balance in the influence of the data points on the spline fit. Cubic spline functions with knots at log hour values of 1, 2, and 3 were used to derive the function (curve) of best fit. Spline functions were used to help control for the possibility of potentially different behavior (ie, linear vs nonlinear) in different regions of the curve. This curve then represented our model of the time course of relative signal intensity of infarcts. The time at which this curve achieved its maximal value was determined by setting the first derivative of the resultant polynomial equal to zero and solving for time. Additionally, the time at which the curve intersected zero (corresponding to the time of signal intensity normalization) was determined, along with 95% CI. The 95% CI for time points were computed by inverting the 95% confidence band for the fitted curves as functions of time; that is, the intersections of the edges of the confidence band determined the ends of the time CI.
Determination of the Time Course of ADC Changes
Lesion ADC values were compared with control ADC values for two groups: infarcts less than 7 days old and infarcts greater than 7 days old. Because we wished to compare the data from our study with those of previously published studies, we modeled the time course of the lesion ADC values. Values of rADC were compared with log hour times from symptom onset. Linear regression modeling of these data was performed. From this analysis, the time at which the fitted regression line intercepted the rADC value of 1 (ie, pseudonormalization of rADC) was determined.
Determination of the ADC Effect on DW Images
The values of log SIRAT and log hours from symptom onset were plotted together, and the function of best fit was determined in a manner analogous to that used to determine the time course for SIB0 and SIDWI. The resultant function represented our model of the time course of the component of infarct signal intensity due to changes in diffusibility (ADC). The times at which the function reached its maximal value and the time at which the function was equal to zero were determined.
Determination of the Time Course of the T2 Effect
T2 effect (ie, shine-through) was defined as SIb0, or the ratio of the signal intensity of the lesion to that of the control region on the image with b = 0 s/mm2 (9). The log values of SIb0 were modeled as a function of the time interval (in log hours) that had elapsed between symptom onset and imaging. A polynomial function that best fit the study data were determined in a manner analogous to that just described for the determination of the time course of lesion signal intensity on DW images. This polynomial function represented our model of the time course of the T2 effect on DW images. The time at which this function reached its maximal value was determined by using the first-derivative method described before.
Retrospective Comparison of Individual Contributions to Infarct Signal Intensity from ADC and T2 Effect
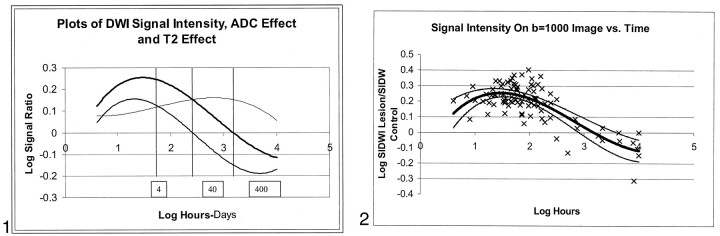
Graph plots of expected time courses of log SIb0, log SIDWI, and log SIRAT (plotted together) were visually inspected. This inspection showed that four distinct time periods were present; these were based on the magnitudes and signs (ie, positive or negative) of the relative contributions of SIb0 and SIRAT to SIDWI. The curves are shown plotted together in Figure 1. For each of the four time periods, the mean values of log SIb0 and log SIRAT were compared with each other.
Fig 1.
Simultaneous plots of signal intensity on DW images (thick line), signal intensity due to altered ADC (intermediate line), and signal intensity due to T2 effect (thin line). In this logarithmic plot, the effects of T2 and ADC add together to produce the total signal intensity seen on the DW image. The vertical lines separate four periods based on the magnitudes and signs (ie, positive or negative) of the relative contributions of SIb0 and SIRAT to SIDWI.
Statistical Methods
SIb0 and SIRAT were compared by taking their difference and comparing the value with 0 in each case by using two-tailed paired t tests. P values were determined for individual time periods by using Bonferroni adjustment. ADC values were compared by using two-tailed unpaired t tests. SAS statistical software (SAS, Cary, NC) was used.
Results
Determination of the Time Course of Infarct Signal Intensity on DW Images
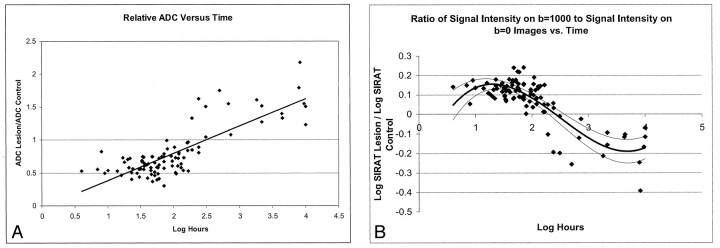
By using the fitted curve derived from our data, the log hour time after symptom onset for the expected maximal log SIDWI was 40.7 hours (95% CI: 8, 72 hours). At this time, the value of log SIDWI was found 0.25 (95% CI: 0.23, 0.27). The fitted curve intercepted the time axis at 57 days (95% CI: 34, 138 days). While nine of the 12 patients with an infarct older than 57 days had decreased SIDWI, two patients had normal signal intensity, and one patient with an infarct aged 90 days had slightly increased signal intensity (SIDWI = 1.07). The data and fitted curve are shown in Fig 2.
Fig 2.
Scatterplot of the log of infarct signal intensity on DW images versus the log of hours from symptom onset. The thick line represents the polynomial function of best fit for the study data. The thin lines represent the 95% confidence limits for this fitted curve. The expected signal intensity of lesions was greater than that of control regions during the first 57 days after symptom onset.
Determination of Time Course of Infarct ADC Values
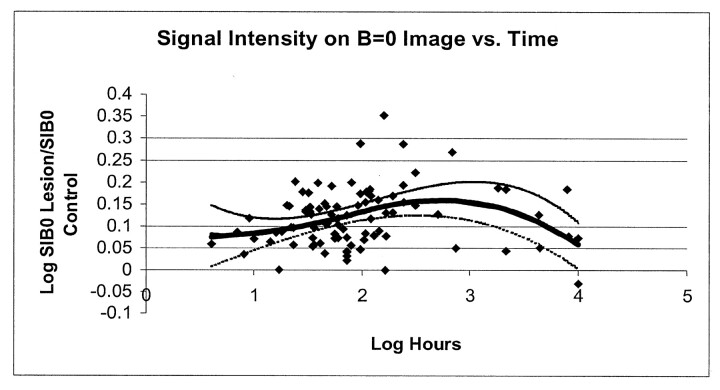
For patients with a symptom duration of less than 7 days, the mean lesion ADC value ± SD was (463 ± 144) × 10−6 mm2/s compared with mean control region ADC value of (742 ± 72) × 10−6 mm2/s (P < .0001), or 63% of normal values. For patients with a symptom duration of greater than 7 days, the mean lesion ADC value was (1027 ± 253) × 10−6 mm2/s compared with mean ADC value within control regions of (736 ± 70) × 10−6 mm2/s (P < .0001), or 140% of normal values. The regression analysis showed that the value of rADC equaled 1 or that it pseudonormalized at 9.8 days. The data for rADC versus time are plotted in Fig 3A.
Fig 3.
Comparison of infarct diffusibility with time.
A, Scatterplot of relative ADC versus log hours from symptom onset. The line represents the linear regression that fit the data. The line intercepts 1 (ie, infarcts had pseudonormal ADC values) at a time corresponding to 9.8 days.
B, Scatterplot of log of the ratio of lesion signal intensity on the image with b = 1000 s/mm2 to the lesion signal intensity on the image with b = 0 s/mm2. The component of infarct signal intensity due to the ADC effect (SIRAT) is the ratio of SIDWI lesion/SIb0 lesion to SIDWI control/SIb0 control. Decreased ADC contributes in a positive way to infarct signal intensity during the first 10 days after symptom onset. Increased lesion ADC contributes in a negative way to infarct signal intensity thereafter. The thick line represents the best fit for the study data. The thin lines represent the 95% confidence limits for this fitted curve.
Determination of the Time Course of the ADC Effect
The fitted curve derived from our data indicated that the time for maximal SIRAT was 28.2 hours after symptom onset (95% CI: 15.5, 37 hours). The value of log SIRAT at this time was 0.15 (95% CI: 0.12, 0.17). The fitted curve intercepted the time axis at 10.4 days after symptom onset (95% CI: 7.6, 16.6 days). The data and fitted curve are shown in Fig 3B.
Determination of the Time Course of the T2 Effect
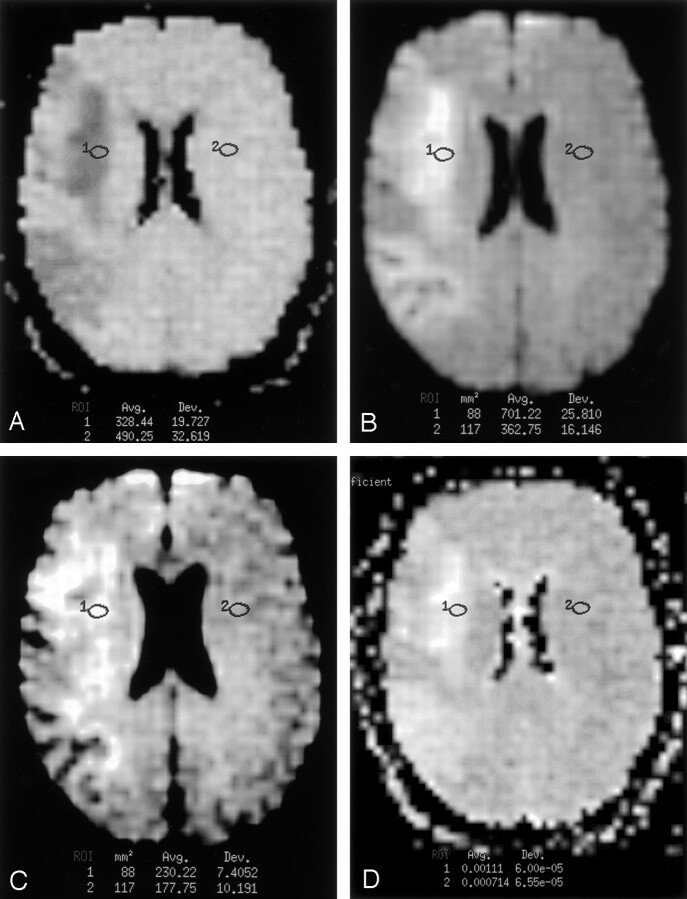
From the fitted curve derived from our data, the time after symptom onset for maximal SIb0 was 13.8 days (95% CI: 6.5, 36 days). At this time, the value of log SIb0 was 0.15 (95% CI: 0.12, 0.17). The data and fitted curve of SIb0 are plotted together in Figure 4.
Fig 4.
Scatterplot of the log of SIb0 versus the log of hours from symptom onset. The thick line represents the polynomial function of best fit. The thin lines represent the 95% confidence limits for this fitted curve. T2 shine-through was typically substantial and positive throughout the studied time course, and it was maximal at about 2 weeks.
Comparison of Individual Contributions of T2 Effect and ADC to Total Signal Intensity
The first two time periods (period 1 and period 2) were both characterized by positive values of log SIb0, log SIRAT, and log SIDWI. Period 1 extended from 4 hours to 63 hours (time at which curves for log SIb0 and log SIRAT intersected) and included the data of 45 patients. Period 2 thus extended from the end to period 1 to the time at which the curve describing log SIRAT intersected zero (normalization of ADC), and it lasted from 63 hours to 10 days. Period 2 included the data of 39 patients. The third time period (period 3) extended from the end of period 2 to the time at which the curve describing the time course of log SIDWI intersected zero (normalization of infarct signal intensity), and it lasted between 10 days and 57 days. Five patients were included in this time period. The fourth time period (period 4), was characterized by negative mean values of both log SIRAT and log SIDWI. This time period extended from the end of period 3 to the time of the images of the patients with the longest duration of symptoms (from 57 days onward), and included data of 12 patients. During period 1, the mean value (± SD) of the log SIRAT was 0.13 ± 0.04 and that of log SIb0 was 0.11 ± 0.05 (P = .04). During period 2, the mean value of log SIb0 was 0.13 ± 0.08, and the mean value of log SIRAT was 0.08 ± 0.04 (P = .12). During period 3, the mean value of log SIb0 was 0.16 ± 0.08, and the mean value of log SIRAT was −0.12 ± 0.08. During period 4, the mean value of log SIb0 was 0.10 ± 0.07, and the mean value of log SIRAT was −0.17 ± 0.10 (P = .001).
To summarize, before 10 days, DW signal intensity is increased as a result of positive contributions from both ADC and T2 effect, and the ADC contribution dominated in the first 3 days. After 10 days, DW signal intensity can either be increased (before 57 days) or decreased (after 57 days). Figure 1 graphically illustrates the duration of the four periods, along with their relative T2 and ADC components. Figures 5 and 6 illustrate typical findings in periods 3 and 4.
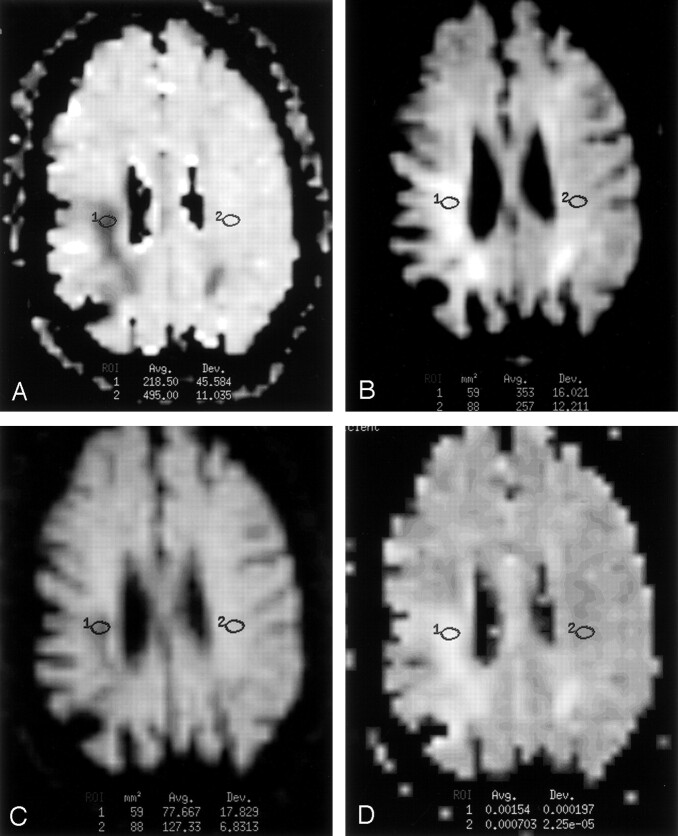
Fig 5.
Images in a 63-year-old woman with an onset of left-sided weakness 26 days before imaging. Regions 1 and 2 indicate the lesion ROI and control ROI, respectively.
A, Ratio DW image was computed as the 1000 times the ratio of the image with b = 1000 s/mm2 image divided by the image with b = 0 s/mm2. Image shows an infarct in the right frontal lobe with signal intensity (−33%) lower than that of the contralateral control tissue. This finding indicates increased tissue water motion in the infarct.
B, Fluid-suppressed image (b = 0 s/mm2, TEeff =101 milliseconds, TI = 2200 milliseconds) shows substantially increased signal intensity (+93%).
C, Standard DW image (b = 1000 s/mm2, TEeff = 101 milliseconds, TI = 2200 milliseconds) with both ADC and T2 effects shows increased signal intensity within the infarct (+30%). This is an example of a patient in period 3 (10–57 days). For patients imaged during this time period, the small negative effect of ADC on infarct signal intensity on DW images more than made up for by larger contribution of the increase of T2 to infarct signal intensity.
D, ADC map shows that infarct ADC is increased 55% over that of the control tissue.
Fig 6.
Images in a 74-year-old man with an onset of left-sided weakness 90 days before imaging.
A, Ratio DW image was computed as the 1000 times the ratio of the image with b = 1000 s/mm2 image divided by the image with b = 0 s/mm2. Image shows that the infarct has a signal intensity 56% less than that of the control tissue.
B, Fluid-suppressed image (b = 0 s/mm2, TEeff = 101 milliseconds, TI = 2200 milliseconds) shows infarct signal intensity 37% greater than that of the control tissue.
C, Standard DW image (b = 1000 s/mm2, TEeff = 101 milliseconds, TI = 2200 ms) with both ADC and T2 effects shows infarct signal intensity 39% less than that of the control tissue. This is an example of a patient in period 4 (later than 57 days). For patients imaged during this time period, the large negative effect of ADC on infarct signal intensity on DW images is greater than the smaller positive contribution of T2 effect.
D, ADC map shows that infarct ADC value is increased 119% compared with that of the contralateral tissue.
Discussion
Worldwide, stroke is the second leading cause of death and the leading cause of permanent disability (20). DW MR imaging is an important method of evaluating patients with acute stroke (1–9). Increased knowledge of the signal intensity changes seen in patients with stroke is likely to be of value.
Our finding that signal intensity of infarcts normalizes at an average of 57 days (with a lower 95% confidence limit of 34 days) after the onset of ischemia validated our pilot study hypothesis that normalization is seen later than 30 days. It is notable that our results substantially differ from those of Burdette et al (11), who found that the signal intensity of infarcts 14 days or older was normal. Several differences in the design of our study and that of Burdette et al may account for the different result. First, our study used an ROI analysis of the signal intensity of bright-appearing lesions rather than solely visual assessment. Second, our study included more patients with an infarct age greater than 14 days. The differences between our results and those of Burdette et al are unlikely to be due solely to technical differences. The DW sequences were similar in that both studies and involved b values of 1000 s/mm2, identical TR values, and similar TE values (101 milliseconds in our study and 97 milliseconds in the study by Burdette et al). The expected effects of varying b value and TE are important issues and discussed in more detail later.
Although our results were different from those reported by Burdette et al, the results of our study are, however, in basic agreement with the findings of Lansberg et al (9) and Huang et al (16), who reported increased signal intensity within infarcts as late as 72 days following symptom onset. Unlike the studies by Lansberg et al and Huang et al, our study included patients with infarcts of normal and decreased signal intensity on DW images, and thus we were able to provide an estimate of the time at which the signal intensity normalized.
In the second part of our study, the retrospective evaluation of the relative contributions of the ADC and T2 effects to the total signal intensity was intended to illustrate for the radiologist how the T2 and ADC effects act in combination to produce the signal intensity changes observed on DW images at various times. It is worth noting that our estimate of ADC effect (SIRAT) depended on a measurement of the signal intensity on the images with b = 0 s/mm2 and b = 1000 s/mm2. Thus, the estimate was not a completely independent measure. Nevertheless, we undertook this secondary assessment and comparison because we believed that a graphical and numerical comparison of the two effects over time would likely to increase our intuitive understanding of infarct signal intensity on DW images.
Our comparison of ADC effect with the T2 effect showed that, during the first 63 hours after symptom onset, the contribution to signal intensity provided by decrease in ADC was the major factor that determined signal intensity on clinical DW images. It is notable that, even during this initial period (period 1), shine-though still accounted for almost half (approximately 46%) of the increased signal intensity seen in infarcts. By contrast, during period 2 (days 3–10), the positive contribution from T2 was greater than the contribution of restricted diffusion, which was also substantial. During period 3 (days 10–57), ADC is typically increased; this increase has a negative effect on lesion signal intensity. However, because the magnitude of the positive contribution from T2 is typically greater than the magnitude of the negative contribution from increased ADC, the net effect of both contributions is slightly positive on DW images. During period 4 (after approximately 60 days), lesion ADC again becomes the major factor determining the signal intensity on DW images. Infarct signal intensity then is typically less than that of control regions. T2 effects are still present, but they are not enough to compensate for the decreased signal intensity produced by elevated ADC values.
According to our simple linear model of infarct ADC value, pseudonormalization of ADC values occurred on day 10 after the onset of symptoms. This finding is in accord with the findings of previously published studies that have shown that such pseudonormalization typically occurs between the seventh and 11th days after the onset of symptoms (3–4, 6, 12, 15). This agreement is important because it provides evidence that the infarct data of the present study are similar to those in previous reports. Therefore, our data concerning the normalization of infarct signal intensity are most likely generalizable to other groups of patients with stroke. The slight discrepancy between our estimate of the time at that ADC normalized (9.8 days) and the time that SIRAT normalized (10.4 days) is likely due to differences in the methods used to estimate these times (linear regression vs polynomial fitting).
Our use of an inversion pulse to suppress CSF was designed to limit the effect of volume-averaging artifacts on our diffusion measurements (9, 19). The use of inversion recovery pulses has been shown to result in measured ADC values lower than those found when inversion recovery is not applied (9, 19). Another effect of use of fluid-suppression inversion recovery is lesion-to-control signal-intensity ratios lower than those measured on T2-weighted images without fluid suppression (9). The T2 effect may possibly have been underestimated in our study However, because both the images obtained with b = 0 s/mm2 and those obtained with b = 1000 s/mm2 used the same pulse, the use of fluid suppression is unlikely to have substantially influenced our results.
While our data provide new information about the time of signal intensity normalization on DW images and about the individual contribution of T2 effect compared with diffusibility, we recognize a number of limitations that should be addressed in future studies. First, our cross-sectional study design could be improved upon in future studies with the use of a longitudinal study design. We used a cross-sectional study design because this was a pilot study without funding and a cross-sectional study design was most cost-effective. Future studies that provide for repeated imaging of patients (especially at time points between 30 and 120 days) will most likely help to decrease the variance in normalization time measured in this study. Second, although we tried to limit the effect of heterogeneity of the observed signal intensity and the ADC values in infarcts by using small ROIs and by studying the brightest areas, controlling for heterogeneity was beyond the scope of the present study. Future longitudinal studies would likely help in addressing this issue and also in determining the differences in the time courses in gray matter and white matter, if any. Third, the effects of patient age and infarct type (eg, lacunar infarcts and watershed infarcts) on time course of ADC (15, 16) could not be modeled separately in our study. These effects should be studied in future investigations with more patients. Finally, it is noteworthy that the signal intensity on DW images depends on the choice of user-defined parameters such as TE and b values. An important aim of this study was to provide quantitative information by using standard TE and b values so that our results would be generalizable. However, our results are expected to differ from those obtained by using substantially different TE and b values. For example, a TE substantially shorter than that used in our study would tend to decrease T2 weighting, and it would be expected to result in earlier normalization of the DW signal intensity. Larger b values (ie, greater diffusion weighting) would tend to increase lesion signal intensity before 10 days, and it would also tend to result in normalization earlier than seen in this study. It is our opinion that ADC values are preferable to DW signal intensity for differentiating infarcts less than 1 week of age from those more than 1 week of age. From the point of view of clinical practice, our results make plain that an infarct containing hyperintense regions on DW images could be acute, subacute, or chronic and that infarcts that are bright are not necessarily less than 2 weeks old, as previously thought.
Conclusion
Our study provides evidence that infarct signal intensity on DW images normalizes at a later time than that suggested in previous work. Our study also provides information about the relative contributions of the ADC and T2 effects for various infarct ages.
References
- 1.Warach S, Chien D, Li W, Ronthal M, Edelman RR. Fast magnetic resonance diffusion-weighted imaging of acute human stroke. Neurology 1992;42:1717–1723 [DOI] [PubMed] [Google Scholar]
- 2.Warach S, Gaa J, Siewert B, Wielopolski P, Edelman RR. Acute human stroke studied by whole brain echo planar diffusion-weighted magnetic resonance imaging. Ann Neurol 1995;37:231–241 [DOI] [PubMed] [Google Scholar]
- 3.Schlaug G, Siewert B, Benfield A, Edelman RR, Warach S. Time course of the apparent diffusion coefficient (ADC) abnormality in human stroke. Neurology 1997;49:113–119 [DOI] [PubMed] [Google Scholar]
- 4.Bastin ME, Rana AK, Wardlaw JM, Armitage PA, Keir SL. A study of the apparent diffusion coefficient of grey and white matter in human ischemic stroke. Neuroreport 2000;11:2867–2874 [DOI] [PubMed] [Google Scholar]
- 5.Desmond PM, Lovell AC, Rawlinson AA, et al. The value of apparent diffusion coefficient maps in early cerebral ischemia. AJNR Am J Neuroradiol 2001;22:1260–1267 [PMC free article] [PubMed] [Google Scholar]
- 6.Lutsep HL, Albers GW, DeCrespigny A, Kamat GN, Marks MP, Moseley ME. Clinical utility of diffusion-weighted magnetic resonance imaging in the assessment of ischemic stroke. Ann Neurol 1997;41:574–580 [DOI] [PubMed] [Google Scholar]
- 7.Marks MP, de Crespigny A, Lentz D, Enzmann DR, Albers GW, Moseley ME. Acute and chronic stroke: navigated spin-echo diffusion-weighted MR imaging [published correction appears in Radiology 1996;200:289]. Radiology 1996;199:403–408 [DOI] [PubMed] [Google Scholar]
- 8.Yang Q, Tress BM, Barber PA, et al. Serial study of apparent diffusion coefficient and anisotropy in patients with acute stroke. Stroke 1999;30:2382–2390 [DOI] [PubMed] [Google Scholar]
- 9.Lansberg MG, Thijs VN, O’Brien MW, et al. Evolution of apparent diffusion coefficient, diffusion-weighted, and T2-weighted signal intensity of acute stroke. AJNR Am J Neuroradiol 2001;22:637–644 [PMC free article] [PubMed] [Google Scholar]
- 10.Engelter ST, Provenzale JM, Petrella JR, Alberts MJ, DeLong DM, MacFall JR. Use of exponential diffusion imaging to determine the age of ischemic infarcts. J Neuroimaging 2001;11:141–147 [DOI] [PubMed] [Google Scholar]
- 11.Burdette JH, Ricci PE, Petitti N, Elster AD. Cerebral infarction: time course of signal intensity changes on diffusion weighted images. AJR Am J Roentgenol 1998;171:791–795 [DOI] [PubMed] [Google Scholar]
- 12.Burdette JH, Elster AD, Ricci PE. Acute cerebral infarction: quantification of spin-density and T2 shine-through phenomena on diffusion-weighted MR images. Radiology 1999;212:333–339 [DOI] [PubMed] [Google Scholar]
- 13.Knight RA, Dereski MO, Helpern JA, Ordidge RJ, Chopp M. Magnetic resonance imaging assessment of evolving focal cerebral ischemia: comparison with histopathology in rats. Stroke 1994;25:1252–1262 [DOI] [PubMed] [Google Scholar]
- 14.Provenzale JM, Engelter ST, Petrella JR, Smith JS, MacFall JR Use of MR exponential diffusion-weighted images to eradicate T2 “shine-through” effect. AJR Am J Roentgenol 1999;172:537–539 [DOI] [PubMed] [Google Scholar]
- 15.Copen WA, Schwamm LH, Gonzalez RG, et al. Ischemic stroke: effects of etiology and patient age on time course of the core apparent diffusion coefficient. Radiology 2001;221:27–34 [DOI] [PubMed] [Google Scholar]
- 16.Huang IJ, Chen CY, Chung HW, et al. Time course of cerebral infarction in the middle cerebral arterial territory: deep watershed versus territorial subtypes on diffusion-weighted MR images. Radiology 2001;221:35–42 [DOI] [PubMed] [Google Scholar]
- 17.Schwamm LH, Koroshetz WJ, Sorensen AG, et al. Time course of lesion development in patients with acute stroke: serial diffusion- and hemodynamic-weighted magnetic resonance imaging. Stroke 1998;29:2268–2276 [DOI] [PubMed] [Google Scholar]
- 18.Adams HPJ, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial. TOAST—Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41 [DOI] [PubMed] [Google Scholar]
- 19.Falconer JC, Narayana PA. Cerebrospinal fluid-suppressed high-resolution diffusion imaging of human brain. Magn Reson Med 1997;37:119–123 [DOI] [PubMed] [Google Scholar]
- 20.Hankey GJ. Stroke: how large a public problem and how can the neurologist help? Arch Neurol 1999;56:748–754 [DOI] [PubMed] [Google Scholar]