Abstract
BACKGROUND AND PURPOSE: A novel plastic embedding approach was used to histologically evaluate inflammatory changes and scar formation over time and to better visualize the mesh attenuation within the aneurysm lumen of acutely ruptured aneurysms after treatment with GDCs.
METHODS: Autopsies were performed on six patients with acute subarachnoid hemorrhage who had died between 5 and 272 days after GDC treatment. The aneurysms containing the platinum coils were embedded in plastic along with the intact parent vessels, were sliced, and were ground to a thickness of 5 to 10 μm. In addition, 250-μm-thick sections were prepared. Histologic examinations were performed. Three exemplary cases representing the time lapse from treatment to death are discussed in detail.
RESULTS: At the three exemplary time periods (5, 13, and 272 days) after coiling, a continuing healing process could be observed. At 5 days after placement of GDCs, a blood clot consisting of erythrocytes and fibrin was found throughout the cavity. Thirteen days after the procedure, large foamy macrophages were observed near the coils in the aneurysm cavity. Two hundred seventy-two days after the intervention, scar formation within the aneurysm appeared to be completed, with vascularized connective tissue filling the cavity and embedding the coils. Large foreign body giant cells were found adjacent to the coils. A layer of long slender cells, resembling endothelium, sealed the aneurysm neck.
CONCLUSION: We postulate that within days after GDC treatment, blood clotting and thrombus formation prevent rebleeding and that solid scar formation covered by a layer of long slender cells, resembling endothelium, seals the aneurysm over time.
The implementation of GDCs or detachable balloons offers neuroradiologists treatment options (1–4) similar to those available to neurosurgeons (5–8) in certain cases. Assuming that endovascular therapy results are comparable with those achieved by surgery for aneurysms localized in the posterior circulation (1, 9, 10), a large comparative study is currently being conducted (International Subarachnoid Aneurysm Trial, Oxford, England). To date, only few histologic studies have shown how time affects the coils embedded in the aneurysm, its neck, or its walls (11–14) because of the methodologic difficulty that coil removal destroys the aneurysm sac. The purpose of our study was to histologically evaluate the inflammatory changes and scar formation that occur over time in acutely ruptured aneurysms after treatment with GDCs.
Methods
Preparation of the Aneurysms
Between November 1992 and February 1999, 247 patients with intracerebral aneurysms were treated with GDC placement in our department of neuroradiology. Autopsies were rarely possible because of lack of consent from near relatives or because patients were transferred to other institutions or to their homes before they died. Autopsies were also considered of limited value, because a meaningful dissection of the treated aneurysms was hardly possible; the aneurysm structures and the position of the coils within were destroyed by the incising scalpel. To attempt a new preparation by which a coiled aneurysm can be sliced without destroying the tissue surrounding the embedded coils, a preparation method such as that used for thin bone sections was applied (15). Autopsies were thus performed on six consecutive patients with acute subarachnoid hemorrhage who had died between 5 and 272 days after GDC treatment. In no case studied was aneurysm rebleeding considered to be the cause of death (Table 1). Near relatives of the deceased agreed to autopsy in all cases. The brains were dissected after fixation in 10% formalin for 2 to 3 weeks. Basal leptomeninges were removed and the cerebral arterial circulus carefully isolated (by C.H.) together with the aneurysm. A plastification process was performed (by G.D.) in all cases. After being cleansed, the aneurysms were dehydrated in a mixture of petroleum and gasoline and were then submerged in a plastic medium (Technovit 7200 VLC, Kulzer) (15). After polymerization of the plastic under blue light, the specimens were cut to a thickness of 2 mm at an orientation perpendicular to the plane of the orifice of the aneurysm. The sections cutting through the center of the aneurysm were further processed by grinding one surface flat (plane) with a grinding machine (Exact, Norderstedt, Germany). The plastic sections were then mounted on glass slides with the ground side down by using Eukitt (Kindler, Germany), and the top surface was ground to a thickness of 5 to 10 μm. In addition, 250-μm-thick sections were prepared to allow visualization of the number and orientation of platinum coils within the aneurysm (Fig 1). The samples were stained with toluidine blue and embedded in Eukitt.
Patients, aneurysms, and clinical characteristics in six autopsied cases
| Case No. | Sex | Age at Time of Death | H&H | Aneurysm Size | Time Lapse (days) | Aneurysm Location | Cause of Death | Survival (days) |
|---|---|---|---|---|---|---|---|---|
| 1* | M | 43 | III | l | 1 | Medial cerebral artery | Brain swelling | 5 |
| 2 | F | 41 | III | m | 1 | Superior cerebellar artery | Brain swelling | 12 |
| 3* | F | 48 | IV | m | 2 | Anterior communicating artery | Brain swelling | 13 |
| 4 | F | 58 | III | s | 1 | Anterior communicating artery | Vasospasm | 20 |
| 5 | F | 43 | IV | m | 1 | Basilary tip | Brain swelling | 26 |
| 6* | F | 72 | V | m | 1 | Basilary tip | Frontal cerebral bleeding | 272 |
Note.—H&H indicates Hunt and Hess Scale grade at admission;
, case discussed in present text; m, male; f, female; l, large (>15 mm); m, medium (6–15 mm); s, small (<6 mm). Time lapse is number of days between first bleeding and treatment; survival is number of days survived after treatment.
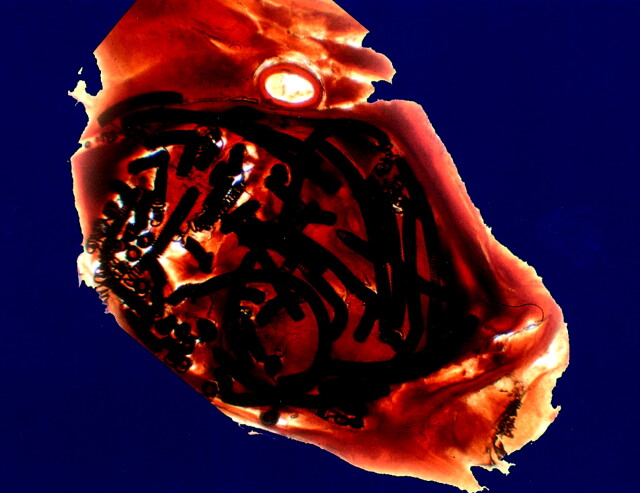
Fig 1.
Distribution of platinum coils in a plasticized medial cerebral artery aneurysm is shown by this arbitrarily chosen 250-μm-thick section from case 1 (see Table 1). The scale can be extrapolated from the coil diameter of 0.010 in.
Case Selection
To examine the time course regarding treatment effects, three exemplary cases were selected as illustrative: one in which death occurred 5 days after treatment, one in which death occurred 13 days after treatment, and one in which death occurred 272 days after treatment (Table 1). An examination of the clinical course of any of the patients was not the subject of the present study; rather, the effect of coiling at different points of time was studied.
Results
For a summary of the clinical data, see Table 1.
Autopsy and Histologic Findings
Case 1.—
Autopsy disclosed a deep venous thrombosis in the right leg and a fulminate thromboembolism in the pulmonary arteries. Neuropathologic examination of the brain revealed a massive brain swelling with cerebellar herniation and central pontine hemorrhage as the cause of death. Fresh hemorrhagic cortical infarcts were present in the territories of both posterior cerebral arteries, the left medial cerebral artery, and the right anterior cerebral artery. Gross subarachnoid hemorrhage, ≤5 mm thick, covered both frontal lobes. At the trifurcation of the left medial cerebral artery, an aneurysm measuring 10 × 5 × 5 mm was found. Coils could be observed through the thin translucent wall of the aneurysm.
Histologic examination showed the infarcts to be <1 day old, presenting as edematous tissue with ischemic neurons and granulocyte extravasation. The aneurysm orifice measured 2.2 mm at the plane of the histologic slide. The wall of the aneurysm was composed of connective tissue and measured between 140 and 600 μm in thickness. The aneurysm was loosely filled with coils (Fig 2C). All vessels were patent, and no coil protruded from the neck of the aneurysm into the lumen of the artery. The cavity of the aneurysm was occluded with a thrombus consisting of erythrocytes and fibrin (Fig 2D). At the border between blood clot and arterial wall, small groups of macrophages or fibroblasts or both could be seen (Fig 2E).
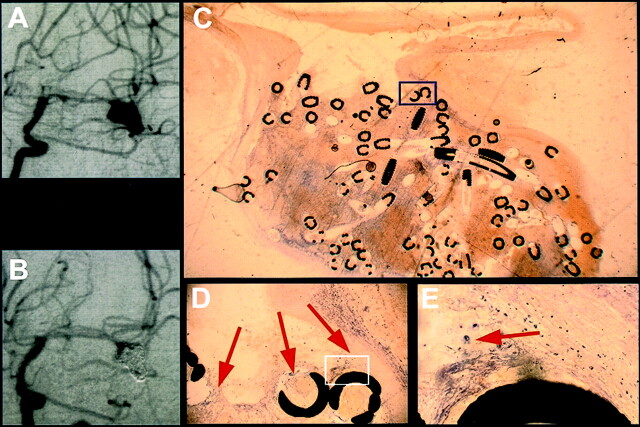
Fig 2.
Exemplary short-term results from case 1, in which death occurred 5 days after treatment (see Table 1).
A, Angiogram obtained before treatment of a medial cerebral artery aneurysm.
B, Angiogram obtained after GDC treatment of a medial cerebral artery aneurysm.
C, Histologic 5- to 10-μm-thick section of plasticized aneurysm 5 days after treatment depicts a thrombus consisting of fibrin and erythrocytes. The scale can be extrapolated from the coil diameter of 0.01 in.
D, ×10 magnification of the inset shown in C depicts fibrin clotting and erythrocytes (arrows).
E, ×40 magnification of the inset shown in D depicts single macrophages (arrow).
Case 2.—
The cause of death for this 42-year-old woman was a massive edema due to infarction of the left basal ganglia (territory of the medial cerebral artery) and the upper cerebellar hemispheres and vermis (territories of the superior cerebellar arteries). Histologic examination showed the necrotic tissue to be invaded by macrophages and the neurons to have eosinophilic condensation of the cytoplasm and nuclear pyknosis. In addition, there were foci in the cerebellar infarct, which were approximately 2 weeks old and corresponded to an infarct that had developed after coiling of the aneurysm of the left superior cerebellar artery.
A slight degeneration of the upper vermis with focal cerebellar siderosis was noticed, and in the leptomeninges, signs of recurrent hemorrhage were observed, which were most prominent over the base of the brain and the brain stem. The cerebral arteries showed severe atherosclerotic changes, which consisted of intimal proliferation, reduplication of the elastic lamina, intramural cholesterol clefts, and nests of macrophages. At the branching of the basilar artery and the posterior cerebral arteries, an aneurysm was found, which had a diameter of 8 mm. No signs of a perforation of the vascular wall by the coils were observed. Serial sections with four successive planes showed the aneurysm to be filled with only few coils nestling against the wall of the sac of the aneurysm. The lumen of the sac was only partially filled by a fresh blood clot composed of erythrocytes and fibrin. No signs of organization within the clot were observed. The orifice of the aneurysm was free and measured 1.3 mm in diameter. The internal elastic lamina ended at the neck of the aneurysm. No coils protruded through the wall of the aneurysm, which was 220 to 640 μm thick and consisted mainly of collagenous tissue. Focal intimal proliferation with nests of foamy macrophages was noticed.
Case 3.—
The main macroscopic findings at autopsy consisted of massive brain edema with cerebellar herniation and compression of the ventricles, gross subarachnoid hemorrhage located predominantly at the base of the brain, and a spherical aneurysm of the anterior communicating artery measuring 5 mm in diameter. Platinum coils were visible through the thin translucent wall of the aneurysm.
At the microscopic level, fresh ischemic changes presenting as scattered eosinophilic neurons with pyknotic nuclei were observed in the cortex. The cavity of the aneurysm was only partially filled with coils, some of which were orientated along the wall of the aneurysm forming a basket shape (Fig 3C). At the plane of the histologic slide, the aneurysm orifice measured 1.2 mm. The thickness of the saccular wall varied between 250 and 600 μm. The cavity of the aneurysm was completely occluded by a thrombus that extended into the distal parent artery (see arrows in Fig 3C). The thrombus protruded into the arterial lumen and was completely covered by a layer of long slender cells, resembling endothelium (Fig 3D). Large foamy macrophages could be observed within the thrombus, near the core of the aneurysm and near the coils (Fig 3E).
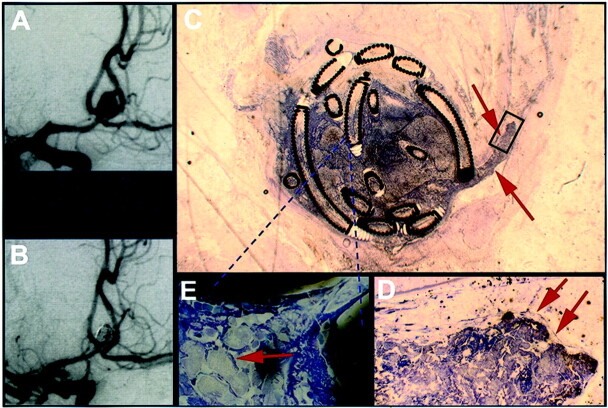
Fig 3.
Exemplary mid-term results from case 3, in which death occurred 13 days after treatment (see Table 1).
A, Angiogram obtained before treatment of an anterior communicating artery aneurysm.
B, Angiogram obtained after GDC treatment of an anterior communicating artery aneurysm.
C, Histologic 5- to 10-μm-thick section of plasticized aneurysm 13 days after treatment shows thrombus extending into the parent vessel (arrows). The scale can be extrapolated from the coil diameter of 0.010 in.
D, ×20 magnification of the inset in shown in C depicts that the thrombus projecting into the feeding vessel is partially coated with endothelium (arrows).
E, ×100 magnification of the area in C indicated by the dashed lines depicts foamy giant cells between the platinum coils (arrow).
Case 4.—
As in cases 2 and 3, a massive brain edema was found, which in case 4 led to compression of the ventricles, temporal and tonsillar herniation, and a central pontine hemorrhage. Bilateral cerebral infarcts, which clinically had developed after recurrent vasospasms, were found in the territories of the anterior and medial cerebral arteries. The lesions in the anterior cerebral arteries were only a few days old. The necroses in the medial cerebral artery were of different age, some being only a few days old with eosinophilic neurons with pyknotic nuclei and an edematous border surrounding the infarct, others showing capillary proliferation and macrophage invasion. In the oldest infarcts, a prominent siderosis and tissue disintegration were found.
Further, fresh ischemic necroses were found bilaterally in the cerebellum and vermis. At the base of the brain, recurrent subarachnoid hemorrhage was observed in the interhemispheric fissure with siderosis in the leptomeninges and parenchyma. An aneurysm measuring 4 mm in diameter was found in the anterior communicating artery. No other aneurysms were noticed.
Histologically, the wall of the aneurysm was 50 to 275 μm thick and was composed of connective tissue. Only few coils were observed to be nested against the wall of the sac of the aneurysm. None of the four sections cut through the plane of the orifice of the aneurysm. Within the lumen of the sac, a fresh coagulum consisting of fibrin and erythrocytes was found. The anterior cerebral arteries near the aneurysm showed excentric intimal proliferations composed of collagen, fibrocytes, and some macrophages occluding >50% of the vascular lumen.
Case 5.—
The cause of death in case 5 was a massive brain edema with severe herniation and central pontine hemorrhage. In the territory of the left posterior cerebral artery, recurrent hemorrhagic infarction was found. Histologic investigation showed the infarction to be between 1 day and approximately 3 weeks old. In addition, fresh, approximately 2-week-old necroses were observed in the right upper cerebellar hemisphere. One of the leptomeningeal arteries cranial to the cerebellar infarction showed an old thrombosis.
In both cerebral hemispheres, including the hippocampi, the thalamus, and the mamillary bodies, patchy ischemic neuronal necroses were found. A large subarachnoid hemorrhage was observed over both cerebral hemispheres, the base of the brain, and the brain stem. At the top of the basilar artery, a coiled aneurysm measuring 5 mm in diameter was found. Histologic examination showed that the aneurysm had a thin wall and measured only 7 to 80 μm in diameter. None of the three preparations cut through the orifice of the sac of the aneurysm. Only a few coils were found in the aneurysm. Most of the lumen of the sac was filled by a fresh clot. No degenerative changes or thromboses were found in the basilar artery.
Case 6.—
The cause of death and main finding at autopsy was massive recurrent intracerebral hypertensive hemorrhage, which was located in the right frontal white matter, the putamen, and the thalamus and caudally extended to the splenium of the corpus callosum, reaching a maximum diameter of 40 × 60 mm in the plane of the chiasm. The maximal displacement of midline structures was 20 mm.
Old subarachnoid hemorrhage was observed over the ventral part of the brain stem and the cerebellar tonsils, showing as an orange discoloration. At the junction between the basilar artery and the posterior cerebral arteries, a spherical aneurysm measuring 8 mm in diameter was seen. In the wall of the aneurysm, a small atherosclerotic plaque was seen.
Histologic examination showed the hemorrhagic tissue to have changes of different ages, with masses of fresh blood and groups of erythrophages and foamy macrophages, a partially obliterated capillary network, and old thromboses. The vessels passing through the hemorrhagic parenchyma showed marked arteriosclerotic alterations.
The wall of the aneurysm was very thin, measuring only between 60 and 400 μm, and in some parts consisted of only a few collagenous lamellae. Adjacent to the aneurysm wall, some small veins and arteries were observed (Fig 4C). In the plane of the histologic slide, the orifice of the aneurysm measured 4 mm. No coil protruded from the neck of the aneurysm into the artery. Vascularized connective tissue completely filled the aneurysms cavity and embedded the coils (Fig 4D). Some large multinucleated giant cells were found adjacent to the coils (Fig 4E). The orifice of the aneurysm was completely covered by a layer of long slender cells, resembling endothelium (Fig 4F).
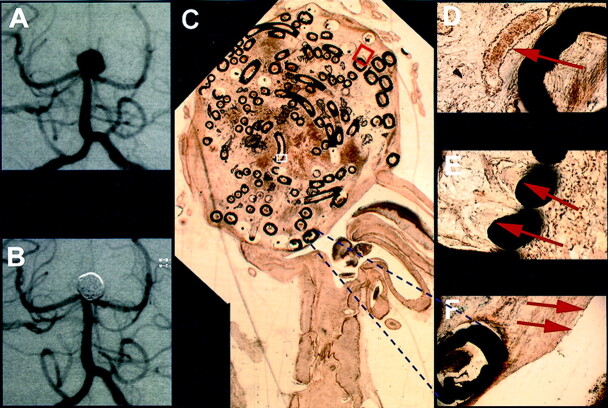
Fig 4.
Exemplary long-term results from case 6, in which death occurred 272 days after treatment (Table 1).
A, Angiogram obtained before treatment of a basilar tip aneurysm.
B, Angiogram obtained after treatment of a basilar tip aneurysm.
C, Histologic 5- to 10-μm-thick section of plasticized aneurysm depicts scar-like connective tissue in the former aneurysm lumen 272 days after treatment. The scale can be extrapolated from the two coil diameters of 0.010 and 0.015 in.
D, ×20 magnification of the red inset shown in C depicts vascularized connecting tissue surrounding platinum coils (arrow).
E, ×40 magnification of the white inset shown in C depicts foreign body giant cells with multiple nuclei adjacent to coils (arrows).
F, ×10 magnification of the area indicated by the dashed lines depicts scar tissue covered by a layer of long slender cells, resembling endothelium, demarcating the aneurysm orifice against the parent vessel (arrows).
Discussion
An in vitro study by Gobin et al (16) showed that although no pressure gradient changes between parent vessels and the aneurysm lumen could be observed after insertion of a single platinum coil, the blood flow pulsatility into the aneurysms could be clearly and significantly reduced. By stopping blood flow into the aneurysm, a coagulative cascade can be evoked, which leads to thrombosis and eventually to aneurysm clotting. Guglielmi’s favored therapeutically induced electrothrombosis at the moment of coil release (17) may trigger thromboembolization, but others have observed that the mere introduction of coils seems adequate to almost immediately initiate clotting (18).
It is presumed that such an endovascular treatment prevents spontaneous hemorrhages, which otherwise often occur in untreated aneurysms. Such mechanisms have been described in the literature as shown by histologic examinations in animals after coil occlusion (12) and in a human autopsy study conducted 36 hours after coil insertion, in which a thromboembolized clot consisting of fibrin and thrombocytes was found (19). Our autopsy results in the case in which death occurred 5 days after aneurysm treatment similarly revealed a thromboembolized clot consisting of erythrocytes and fibrin between the inserted coils within the aneurysm lumen. Similar findings have been reported (14, 20).
The inserted platinum coil immediately and decisively relieves the influx of pulsating blood (16), allowing initial clotting. The hemodynamic pressure imposed on the inserted platinum coil mesh is dissipated over the wall of the aneurysm cavity. This, combined with the reaction of the aneurysm wall to the foreign body (coil), initiates scar formation in the initially unorganized blood clot, resembling a normal wound healing process (14, 20). As would be expected in normal wound healing, foamy macrophages were found between the coils in case three (after 13 days) in the present study (Fig 3E). In an incompletely occluded aneurysm, others similarly found scar formation after 42 days, stemming from the periphery and leading toward the core of the aneurysm as indicated by capillary proliferation, with a remaining nonreactive central blood clot (14). Favorable conditions can lead to formation of vascularized connective tissue within the aneurysm cavity after coil insertion (13, 21). This was seen in the present case 6 (in which death occurred 9 months after endovascular treatment) (Fig 4). The neck was also sealed by a layer of cells, resembling endothelium, as was also observed by others in a case in which death occurred 33 months after therapy (13).
The wider the aneurysm neck in proportion to its volume, the less likely it is that a sufficient number of platinum coils can be placed to form an adequate mesh (22). Several authors describe that the diameter of the aneurysm neck relative to total aneurysm diameter are simple geometrical predictors for the success of aneurysm treatment (23–28). Platinum coils can also protrude further into the parent vessel lumen than desired, thus contributing to flow conditions detrimental to scar formation. The unfortunate relationship of thrombus-coated coils to those coils protruding into the blood stream of the parent vessel (29) also illustrates an explanation for coil migration: it is easily imaginable that coils in a liquid or semi-liquid mass can move. A higher leverage effect of blood pulsation can especially be imagined in cases of a “wide open” area between platinum coils as part of a low attenuation mesh. It is thus not surprising that regular angiographic follow-up examinations performed after 1 year showed that coils or coil meshes had changed their positions in ≤15% of the cases (4).
In cases of coil migration, different effects have been described around or between coils. The observations include aneurysm recanalization (24–28), nonreactive thrombus cores without macrophage immigration or scar formation even after 2 to 6 months (11), and thrombus organization emanating from the aneurysm periphery after 6 weeks (14).
If the coil mesh attenuation is high enough at the portal (neck) level, a continuous water-hammer effect (30) at the early stage of scar formation may cause a “settling” rearrangement or movement of the platinum coils but it may not dislodge the coil mesh to such an extent that single coil segments can be exposed to the blood flow or that bordering thrombotic material can be lysed. After continuous scar formation with capillary proliferation (Fig 4D), a normal scar can form, on which endothelium can develop (13).
Conclusion
In conclusion, the presented findings are in accordance with studies on experimentally induced aneurysms in animals. They confirm that scar and endothelium formation may occur after treatment with GDCs, leading to elimination of the aneurysm.
Acknowledgments
The authors thank Jarold Knispel, PhD, for language advice.
References
- 1.McDougall CG, Halbach VV, Dowd CF, Higashida RT, Larse DW, Hieshima GB. Endovascular treatment of basilar tip aneurysms using electrolytically detachable coils. J Neurosurg 1996;84:393–399 [DOI] [PubMed] [Google Scholar]
- 2.Nichols DA, Brown RD Jr, Thielen KR, Meyer FB, Atkinson JL, Piepgras DG. Endovascular treatment of ruptured posterior circulation aneurysms using electrolytically detachable coils. J Neurosurg 1997;87:374–380 [DOI] [PubMed] [Google Scholar]
- 3.Raymond J, Roy D, Bojanowski M, Moumdjian R. Endovascular treatment of acutely ruptured and unruptured aneurysms of the basilar bifurcation. J Neurosurg 1997;86:211–219 [DOI] [PubMed] [Google Scholar]
- 4.Byrne JV, Sohn MJ, Molyneux AJ, Chir B. Five-year experience in using coil embolization for ruptured intracranial aneurysms: outcomes and incidence of late rebleeding. J Neurosurg 1999;90:656–663 [DOI] [PubMed] [Google Scholar]
- 5.Drake CG. Surgical treatment of ruptured aneurysms of the basilar artery: experience with 14 cases. J Neurosurg 1965;23:457–473 [DOI] [PubMed] [Google Scholar]
- 6.Hernesniemi J, Vapalahti M, Niskanen M, Kari A. Management outcome for vertebrobasilar artery aneurysms by early surgery. Neurosurgery 1992;31:857–862 [DOI] [PubMed] [Google Scholar]
- 7.Kassell NF, Torner JC, Haley EC Jr, Jane JA, Adams HP, Kongable GL. The International Cooperative Study on the Timing of Aneurysm Surgery: part 1. overall management results. J Neurosurg 1990;73:18–36 [DOI] [PubMed] [Google Scholar]
- 8.Kassell NF, Torner JC, Jane JA, Haley EC Jr, Adams HP. The International Cooperative Study on the Timing of Aneurysm Surgery: part 2. surgical results. J Neurosurg 1990;73:37–47 [DOI] [PubMed] [Google Scholar]
- 9.Groden C, Grzyska U, Eckert B, Freckmann N, Herrmann HD, Zeumer H. Comparison of open surgery and interventions with endovascular controlled detachable coils in treatment of acutely ruptured basilar tip aneurysms. J Neurovasc Dis 1998;3:131–139 [Google Scholar]
- 10.Pierot L, Boulin A, Castaings L, Rey A, Moret J. Selective occlusion of basilar artery aneurysms using controlled detachable coils: report of 35 cases. Neurosurgery 1996;38:948–953 [DOI] [PubMed] [Google Scholar]
- 11.Molyneux AJ, Ellison DW, Morris J, Byrne JV. Histological findings in giant aneurysms treated with Guglielmi detachable coils: report of two cases with autopsy correlation. J Neurosurg 1995;83:129–132 [DOI] [PubMed] [Google Scholar]
- 12.Tenjin H, Fushiki S, Nakahara Y, et al. Effect of Guglielmi detachable coils on experimental carotid artery aneurysms in primates. Stroke 1995;26:2075–2080 [DOI] [PubMed] [Google Scholar]
- 13.Castro E, Fortea F, Villoria F, Lacruz C, Ferreras B, Carrillo R. Long-term histopathologic findings in two cerebral aneurysms embolized with Guglielmi detachable coils. AJNR Am J Neuroradiol 1999;20:549–552 [PMC free article] [PubMed] [Google Scholar]
- 14.Shimizu S, Kurata A, Takano M, et al. Tissue response of a small saccular aneurysm after incomplete occlusion with a Guglielmi detachable coil. AJNR Am J Neuroradiol 1999;20:546–548 [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn M, Vogel M, Delling G. Undecalcified preparation of bone tissue: report of technical experience and development of new methods. Virchows Arch A Pathol Anat Histopathol 1991;418:1–7 [DOI] [PubMed] [Google Scholar]
- 16.Gobin YP, Counord JL, Flaud P, Duffaux J. In vitro study of haemodynamics in a giant saccular aneurysm model: influence of flow dynamics in the parent vessel and effects of coil embolisation. Neuroradiology 1994;36:530–536 [DOI] [PubMed] [Google Scholar]
- 17.Guglielmi G, Viñuela F, Sepetka I, Macellari V. Electrothrombosis of saccular aneurysms via endovascular approach: part 1. electrochemical basis, technique, and experimental results. J Neurosurg 1991;75:1–7 [DOI] [PubMed] [Google Scholar]
- 18.Koizumi T, Kawano T, Kazekawa K, et al. Histological findings in aneurysm treated with IDC: scanning electron microscopical study [in Japanese]. No Shinkei Geka 1997;25:1027–1031 [PubMed] [Google Scholar]
- 19.Stiver SI, Porter PJ, Willinsky RA, Wallace MC. Acute human histopathology of an intracranial aneurysm treated using Guglielmi detachable coils: case report and review of the literature. Neurosurgery 1998;43:1203–1208 [DOI] [PubMed] [Google Scholar]
- 20.Horowitz MB, Purdy PD, Burns D, Bellotto D. Scanning electron microscopic findings in a basilar tip aneurysm embolized with Guglielmi detachable coils. AJNR Am J Neuroradiol 1997;18:688–690 [PMC free article] [PubMed] [Google Scholar]
- 21.Bavinzski G, Talazoglu V, Killer M, et al. Gross and microscopic histopathological findings in aneurysms of the human brain treated with Guglielmi detachable coils. J Neurosurg 1999;91:284–293 [DOI] [PubMed] [Google Scholar]
- 22.Malisch TW, Guglielmi G, Viñuela F, et al. Intracranial aneurysms treated with the Guglielmi detachable coil: midterm clinical results in a consecutive series of 100 patients. J Neurosurg 1997;87:176–183 [DOI] [PubMed] [Google Scholar]
- 23.Satoh K, Satomi J, Matsubara S, Nagahiro S. Measurement of volume ratio to predict coil compaction, on aneurysmal embolization. Intervent Neuroradiol 1998;4:179–182 [DOI] [PubMed] [Google Scholar]
- 24.Debrun GM, Aletich VA, Kehrli P, et al. Aneurysm geometry: an important criterion in selecting patients for Guglielmi detachable coiling. Neurol Med Chir (Tokyo) 1998;[suppl 38]:1–20 [DOI] [PubMed] [Google Scholar]
- 25.Debrun GM, Aletich VA, Kehrli P, Misra M, Ausman JI, Charbel F. Selection of cerebral aneurysms for treatment using Guglielmi detachable coils: the preliminary University of Illinois at Chicago experience. Neurosurgery 1998;43:1281–1295 [DOI] [PubMed] [Google Scholar]
- 26.Gruber A, Killer M, Bavinzski G, Richling B. Clinical and angiographic results of endosaccular coiling treatment of giant and very large intracranial aneurysms: a 7-year, single-center experience. Neurosurgery 1999;45:793–803 [DOI] [PubMed] [Google Scholar]
- 27.Viñuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 1997;86:475–482 [DOI] [PubMed] [Google Scholar]
- 28.Turjman F, Massoud TF, Sayre J, Viñuela F. Predictors of aneurysmal occlusion in the period immediately after endovascular treatment with detachable coils: a multivariate analysis. AJNR Am J Neuroradiol 1998;19:1645–1651 [PMC free article] [PubMed] [Google Scholar]
- 29.Macdonald RL, Mojtahedi S, Johns L, Kowalczuk A. Randomized comparison of Guglielmi detachable coils and cellulose acetate polymer for treatment of aneurysms in dogs. Stroke 1998;29:478–485 [DOI] [PubMed] [Google Scholar]
- 30.Kwan ES, Heilman CB, Shucart WA, Klucznik RP. Enlargement of basilar artery aneurysms following balloon occlusion: “water-hammer effect”: report of two cases. J Neurosurg 1991;75:963–968 [DOI] [PubMed] [Google Scholar]