Abstract
BACKGROUND AND PURPOSE: We developed an experimental canine subarachnoid hemorrhage (SAH) model in which one-time blood injection by means of a microcatheter into the ventral cisterna magna (CM) is performed without direct CM puncture. We assessed the severity and duration of the vasospasm produced in this model.
METHODS: Fresh autologous blood (0.25 or 0.5 ml/kg) or saline (0.5 ml/kg) was injected into the ventral CM of dogs through a microcatheter inserted at the lumbar region. Serial angiograms were obtained on days 3, 7, 10, and 14, and chronologic changes in the mean diameter of the basilar artery (BA) were recorded. The BA was examined histologically on day 7 after injection.
RESULTS: A remarkable amount of clot was present in front of the brain stem at 24 hours after SAH induction. The clot was smaller in the 0.25 ml/kg SAH than in the 0.5 ml/kg SAH group. On day 3, narrowing of the BA was apparent in both SAH groups compared with the control (P < .05). The BA gradually returned to nearly normal on day 14 in the 0.25 ml/kg SAH group. Arterial narrowing was more severe and persistent in the 0.5 ml/kg SAH than in the 0.25 ml/kg SAH group (P < .05). Histologic examination of the BA on the 7th postinjection day confirmed narrowing of the lumen, indicative of arterial spasm, in both SAH groups.
CONCLUSION: Our method of SAH induction by means of a single injection of blood directly into the ventral CM through a microcatheter induced severe, prolonged spasms in the canine BA. Because our model facilitates the induction of different-sized clots, we could control of the severity and duration of the induced vasospasms.
The treatment of subarachnoid hemorrhage (SAH) remains one of the major challenges in neurosurgery. A considerable proportion of the neurologic morbidity and mortality of SAH results from delayed cerebral ischemia due to cerebral vasospasm in response to residual blood clots in the subarachnoid space after SAH. Because in vivo experiments using human vessels are not possible, animal SAH models have been developed to study the pathogenesis and treatment of cerebral vasospasm (1). In 1972, Kuwayama et al (2) described the only currently available animal model of one-time injection of autologous blood into the cisterna magna (CM). Using beagles and a small-gauge needle, they succeeded in producing angiographic vasospasms. However, the severity of the vasospasms was variable on the 7th post-SAH day. In 1983, Varsos et al (3) reported a significant modification of this model. Also using a small-gauge needle, they performed two injections of autologous blood into the canine CM at 48-hour intervals and demonstrated enhancement in the severity and duration of the vasospasms. They documented significant angiographic vasospasms refractory to all tested pharmacologic vasodilators. This “two-hemorrhage” model has since been used extensively in the study of SAH and vasospasm.
We developed a method for SAH induction in which we advance a microcatheter, introduced into the subarachnoid space at the lumbar region, into the canine CM for blood injection. In this maneuver, the position of the microcatheter tip in the CSF space is under the control of the operator. Therefore, we were able to inject blood directly into the “ventral side” of the brain stem for effective clot production around the basilar artery (BA). Using our method, we delivered a single injection of autologous blood into the ventral CM by means of the microcatheter and found that severe chronic BA vasospasm persisted for about 14 days.
Ours is the first, to our knowledge, successful attempt of subarachnoid blood injection through a microcatheter introduced into the ventral CM. We compared the severity and duration of the vasospasm induced by our model with that by the two-hemorrhage model.
Methods
All experiments were performed with the prior approval of the Animal Experimentation Ethics Committee of Kumamoto University and according to its Animal Care Guidelines.
Animal Groups
Adult mongrel dogs (n = 20) weighing between 10 and 15 kg were randomly divided into three groups and injection into the ventral CM was performed. Group 1 (control, n = 6) received 0.5 ml/kg physiologic saline; group 2 (n = 7) and group 3 (n = 7) were injected with 0.25 ml/kg and 0.5 ml/kg blood, respectively, (SAH groups). One dog from each SAH group was killed at 24 hours after SAH induction to examine subarachnoid clot formation. On the 7th postinjection day, one dog from each of the three groups was killed and the BA was submitted for histologic examination. The remaining five dogs in each group were followed up with angiography for 14 days to evaluate the chronologic change of the BA diameter.
Animal Preparation before SAH Induction
All 20 dogs were anesthetized with intravenous pentobarbital (25 mg/kg) and mechanically ventilated with room air delivered by a respirator. The ventilation rate and tidal volume were adjusted to maintain arterial blood gas and pH levels within physiologic limits. The body temperature was maintained at 37°C by means of a heating pad placed beneath the animal. Mean arterial blood pressure and blood gases were continuously monitored with a 4F catheter inserted into the femoral artery. Another 4F catheter was inserted into the contralateral femoral artery and advanced under fluoroscopic guidance into the left vertebral artery up to the C4 spinal level for subsequent angiographic procedures. All angiograms were obtained after mechanical injection of 5 ml of Iopamidol 300 (Iopamiron 300; Schering, Osaka, Japan), corresponding to 300 mg of iodine per ml (Iopamidol 300; Schering, Osaka, Japan), with use of an autoinjector (M-800C; Nemoto Kyorindo Co., Ltd., Tokyo, Japan). Identical injection-exposure parameters, timing, and magnification were used throughout (5 ml, 2 ml/s; exposure delay, 1.5 seconds; 74 kV; 100 mAs); magnification and exposure factors were kept constant throughout the experiments.
Microcatheter Introduction and Blood Injection into the Ventral CM
After obtaining baseline cerebral angiograms, the dogs were placed in the prone position. A puncture was made with a 17-gauge Tuohy needle at the L2–3 interface, and a guidewire was introduced through the needle into the subarachnoid space. The needle was withdrawn over the guidewire and replaced by a 4F catheter introducer (Supersheath; Medikit Co., Ltd., Tokyo, Japan) to prevent CSF leakage during microcatheter introduction. After confirming that the catheter introducer had entered the subarachnoid space, a Tracker-18 microcatheter (Boston Scientific Corp., Boston, MA) and a Terumo-GT microguidewire (Terumo, Natick, MA) were introduced into the subarachnoid space through the catheter introducer and advanced to the ventral CM. During advancement to the ventral CM, the microcatheter and microguidewire were continuously monitored under fluoroscopic visualization and advanced slowly and carefully by using an over-the-wire technique to prevent injury to the spinal cord, nerve roots, or vascular structures. When the microcatheter reached the ventral CM, 0.25 ml/kg (group 2) or 0.5 ml/kg (group 3) of clear CSF was withdrawn, and an equivalent amount of fresh autologous arterial blood from the femoral artery was immediately injected (2 ml/min) through the microcatheter. In the control group, the same method was used to deliver 0.5 ml/kg physiologic saline into the ventral CM. Then, the table was tilted 30° to lower the head of the animal for 30 minutes to permit pooling and clotting of blood around the BA. At 24 hours after SAH induction, one dog from each group was killed with an intravenous bolus injection (50 mg/kg) of sodium pentobarbital, for gross observation of the clot around the BA.
Angiographic Estimation of the Diameter of the BA
Repeat angiograms were obtained on days 3, 7, 10, and 14 after SAH induction and used to evaluate chronologically the changes in the diameter of the BA from the pre-SAH induction baseline. Measurements were taken at three sites along the vessel: at 1 cm distal to its origin, midway between the proximal and distal ends, and at 1 cm proximal to its termination (4). The baseline diameter at each site was recorded as values a, b, and c. The diameters measured on different post-SAH days at the same site were recorded as values d, e, and f. The degree of vasospasm at the different post-SAH time points was calculated by using the formula [(d/a + e/b + f/c)/3] × 100. The measurements were repeated three times by three researchers (J.H., Y.K., T.T.) blinded to the animal groups.
Histologic Examination
On the 7th post-SAH day, one dog from each SAH group and the control group was killed with an intravenous bolus injection (50 mg/kg) of sodium pentobarbital. The BA was removed with the brain stem and embedded in paraffin. Then, 4.5-μm-thick sections were cut, stained with hematoxylin-eosin and elastica van Gieson, and inspected under a microscope.
Statistical Analysis
Data are expressed as the mean ± standard error of the mean. Differences among the three groups were determined by using the Student’s t test. Differences of P less than .05 were considered to be statistically significant.
Results
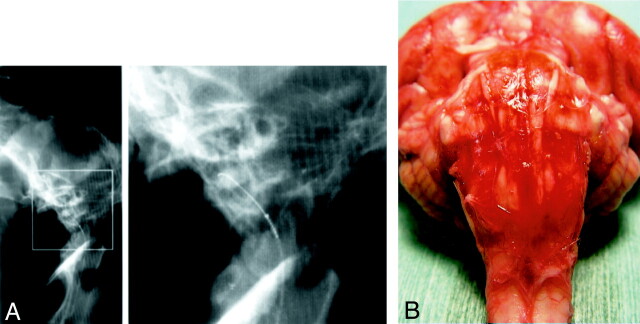
None of the dogs subjected to experimental SAH died from SAH, and none exhibited hemiparesis. The maneuver used to introduce the microcatheter into the ventral CM went smoothly. The average time required to advance the microcatheter into the ventral CM from the lumbar region was approximately 5 minutes. As shown in Fig 1A, use of the microguidewire facilitated visualization of the microcatheter under fluoroscopic guidance and its advancement through the subarachnoid space into the ventral CM.
Fig 1.
A, Lateral (left) conventional radiograph of the occipital cranium and cervical spine of a dog demonstrate the microcatheter and microguidewire inserted into the ventral CM. Right part of A is an enlargement of the rectangular area on the left.
B, Photograph of a gross specimen shows the ventral surface of the brain stem after injection of 0.5 ml/kg autologous fresh blood. The BA is completely embedded in clotted subarachnoid blood.
Inspection of the Clot Around the BA
Gross examination at 24 hours after the infusion of 0.5 ml/kg autologous fresh blood revealed a remarkable amount of clotted blood at the ventral surface of the brain stem (Fig 1B). The clot was smaller in the 0.25 ml/kg than in the 0.5 ml/kg SAH group. Autopsy of the animals demonstrated no spinal cord or nerve injury by the insertion of the microguidwire and/or microcatheter.
Angiographic Estimation of the Diameter of the BA
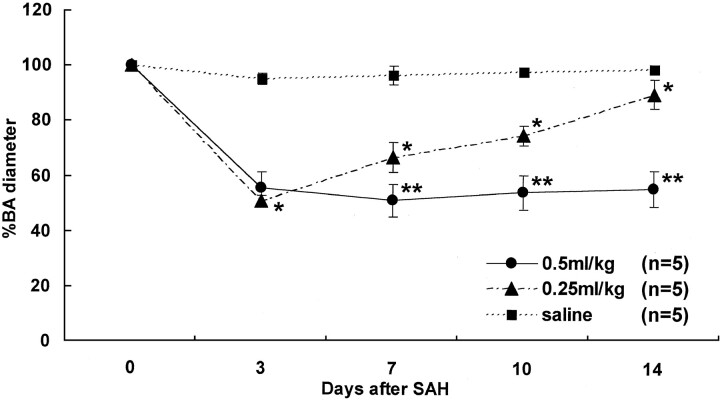
Representative serial angiograms of the BA in the control group and each SAH group are shown in Fig 2. No arterial constriction was noted during the 14-day observation in the control animals injected with 0.5 ml/kg physiologic saline (Fig 2A). In the 0.25 ml/kg SAH group, severe constriction was present on day 3, but the diameter of the BA gradually returned to normal over the course of 14 days (Fig 2B). Arterial constriction was severe on days 3 and 7 in the 0.5 ml/kg SAH group, and severe vasoconstriction persisted on days 10 and 14 (Fig 2C). Figure 3 is a graphic demonstration of the chronologic changes in the mean diameter of the BA in each group. The BA of the controls (n = 5) exhibited no significant angiographic changes. In the 0.25 ml/kg SAH group (n = 5), the mean diameter of the BA was severely constricted on day 3 to 51.0 ± 2.0% of the baseline value; it gradually returned to 66.8 ± 5.4% by day 7, 75.0 ± 3.5% by day 10, and 90.1 ± 5.3% by day 14. Significant arterial narrowing was observed in the 0.25 ml/kg SAH group on days 3, 7, 10, and 14 compared with the control group (P < .05). In the 0.5 ml/kg SAH group (n = 5), the mean diameter of the BA showed a reduction to 55.6 ± 5.7% of the baseline on day 3 and to 51.3 ± 6.0% on day 7. It was 54.3 ± 6.1% on day 10 and 55.9 ± 6.4% on day 14. On day 3, both SAH groups manifested almost identical arterial narrowing. However, on days 7, 10, and 14, arterial narrowing was significantly more severe and persistent in the 0.5 ml/kg than in the 0.25 ml/kg SAH group (P < .05).
Fig 2.
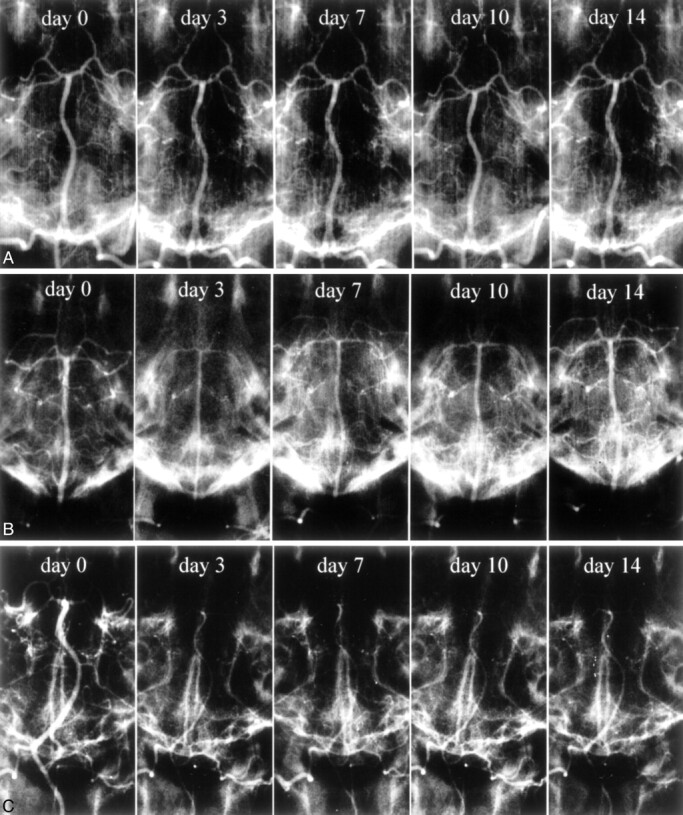
Representative serial angiograms of the canine BA.
A, Physiologic saline (0.5 ml/kg) was injected into the ventral CM. No constriction is noted during the 14-day observation period.
B, Autologous blood (0.25 ml/kg) was injected into the ventral CM. Severe constriction is present on day 3. Over time, the diameter of the BA gradually returns to normal.
C, Autologous blood (0.5 ml/kg) was injected into the ventral CM. There are sites with severe arterial constriction on days 3 and 7 after SAH induction; severe vasoconstriction persists on days 10 and 14.
Fig 3.
Graphic presentation of the percent changes in the diameter of the BA on days 0 (before SAH), 3, 7, 10, and 14. Autologous fresh blood (0.5 or 0.25 ml/kg) or physiologic saline was injected into the ventral CM. Apparent arterial narrowing was observed in the 0.25 ml/kg SAH group on days 3, 7, 10, and 14 compared with the control group (* indicates P < .05). Arterial narrowing was more severe and persistent in the 0.5 ml/kg SAH group on days 7, 10, and 14 than in the the 0.25 ml/kg SAH group (** indicates P < .05). No arterial narrowing was apparent in the saline-injected control group.
Histologic Examination
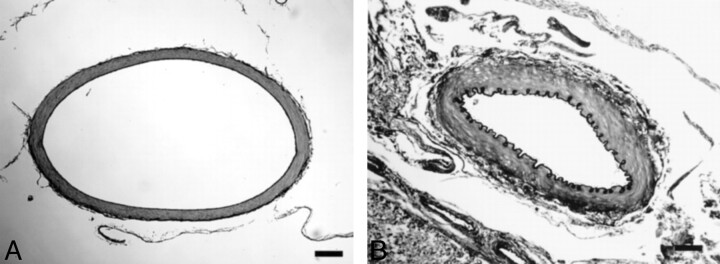
At light microscopy on day 7, the BA of the controls exhibited no significant histopathologic changes (Fig 4A). The BA harvested 7 days after the induction of 0.5 ml/kg SAH showed narrowing of the lumen, corrugation of the internal elastic lamina, and subendothelial thickening and proliferation (Fig 4B). The BA of the 0.25 ml/kg SAH model revealed slight attenuation of the histopathologic changes noted in the 0.5 ml/kg SAH model.
Fig 4.
Light-microscopic appearance of the BA removed at autopsy on the 7th post-SAH day (elastica van Gieson stain; original magnification, x100). Black bars in A and B indicate 100 μm.
A, Physiologic saline (0.5 ml/kg) was injected into the ventral CM. The diameter of the BA is normal.
B, Autologous blood (0.5 ml/kg) was injected into the ventral CM. There is severe narrowing of the vessel diameter, with folding and corrugation of the internal elastic lamina.
Discussion
In earlier models (2, 3), SAH was induced by dorsal CM puncture and the dogs were tilted head down to facilitate gravity-induced contact of blood with the BA. In the two-hemorrhage model (3), blood was injected twice at 48-hour intervals to render more severe and prolonged arterial constriction. To increase the efficacy of clot-production around the BA, we devised a method to inject blood directly into the ventral side of the brain stem. Our introduction of a microcatheter into the CM by lumbar tap allows us to advance the microcatheter to the ventral side of the brain stem and makes it possible to inject fresh autologous blood directly into the ventral CM. We confirmed the presence of a remarkable amount of clotted blood at the base of the brain stem at 24 hours after the single injection of 0.5 ml/kg blood. Serial angiograms obtained during a 2-week period after SAH induction documented that the BA was severely and chronically constricted. Our pathologic findings on the BA coincide with those of others (3, 5) in terms of corrugation of the internal elastic lamina, hypertrophy of the intima, and constriction of the BA diameter.
Sako et al (6) who used the two-hemorrhage SAH model delivered two injections of 0.8 ml/kg arterial blood into the dorsal CM by puncture with a small-gauge needle. They reported arterial narrowing of about 60% of the baseline on the 7th day after the first injection; it was about 65% on day 14. In our 0.5 ml/kg SAH model, maximum arterial narrowing was also noted on day 7: the diameter of the BA was 51.3% of the baseline diameter, and arterial constriction persisted during our 2-week observation period; it was about 55.9% on day 14. Although our model employs a single injection and a smaller volume of injected autologous blood, the degree and duration of vasospasms were comparable to those of the two-hemorrhage model (3, 6). Our direct single injection of blood into the ventral CM led to effective clot formation around the BA and enhanced the severity of the vasospasm. Using our model, we can control the volume of the induced clot and thus the severity and duration of the vasospasms: in dogs injected with 0.25 ml/kg autologous blood, the spasms were milder and of shorter duration than those in dogs treated with 0.5 ml/kg. Serial angiograms in the 0.25 ml/kg SAH model demonstrated that the BA was severely constricted to 51% of the baseline diameter on day 3; subsequently it gradually returned to the pre-SAH baseline.
To avoid spinal cord or nerve root injury and the induction of mechanical vasospasm in the BA upon introduction of the microcatheter into the ventral CM, our model requires great care during microcatheter and microguidewire manipulation in the subarachnoid space. We found that by using continuous fluoroscopic visualization we were able to avoid any iatrogenic injury.
Conclusion
We developed a reliable model of SAH and vasospasm in dogs, in which a single injection of selected amounts of autologous blood is delivered through a microcatheter introduced into the ventral CM. Our model produces BA vasospasms the severity and duration of which can be controlled by altering the volume of injected blood.
Acknowledgments
We thank Mrs. Masayo Obata for technical assistance.
References
- 1.Megyesi JF, Vollrath B, Cook DA, Findlay JM. In vivo animal models of cerebral vasospasm: a review. Neurosurgery 2000;46:448–460; discussion 460–461 [PubMed] [Google Scholar]
- 2.Kuwayama A, Zervas NT, Belson R, Shintani A, Pickren K. A model for experimental cerebral arterial spasm. Stroke 1972;3:49–56 [DOI] [PubMed] [Google Scholar]
- 3.Varsos VG, Liszczak TM, Han DH, et al. Delayed cerebral vasospasm is not reversible by aminophylline, nifedipine, or papaverine in a “two-hemorrhage” canine model. J Neurosurg 1983;58:11–17 [DOI] [PubMed] [Google Scholar]
- 4.Ohkuma H, Parney I, Megyesi J, Ghahary A, Findlay JM. Antisense preproendothelin-oligo DNA therapy for vasospasm in a canine model of subarachnoid hemorrhage. J Neurosurg 1999;90:1105–1114 [DOI] [PubMed] [Google Scholar]
- 5.Seifert V, Eisert WG, Stolke D, Goetz C. Efficacy of single intracisternal bolus injection of recombinant tissue plasminogen activator to prevent delayed cerebral vasospasm after experimental subarachnoid hemorrhage. Neurosurgery 1989;25:590–598 [DOI] [PubMed] [Google Scholar]
- 6.Sako M, Nishihara J, Ohta S, Wang J, Sakaki S. Role of protein kinase C in the pathogenesis of cerebral vasospasm after subarachnoid hemorrhage. J Cereb Blood Flow Metab 1993;13:247–254 [DOI] [PubMed] [Google Scholar]