Abstract
BACKGROUND AND PURPOSE: Treatment-induced white matter (WM) injury in medulloblastoma survivors, as manifested by deterioration of cognitive function, is prevalent. However, no reliable imaging method exists for early detection and quantification. Our goal was to determine whether anisotropy of WM is reduced in medulloblastoma survivors and whether fractional anisotropy (FA) can be used as an index for evaluation of treatment-induced WM injury.
METHODS: We evaluated nine medulloblastoma survivors treated with surgery, cranial irradiation, and chemotherapy by use of diffusion-tensor (DT) imaging and compared FA findings in selected WM sites (cerebellar hemispheres, pons, medulla oblongata, frontal periventricular WM, parietal periventricular WM, and corona radiata) with those of healthy age-matched control subjects. FA maps were compared with conventional T2-weighted images. FA was also compared with age at treatment, time interval since treatment, and deterioration of school performance. The two-tailed paired t test was used to determine statistical significance (P < .05).
RESULTS: Significant reduction of FA (P < .05) was seen in all anatomic sites in the patient group compared with FA in control subjects, except in the frontal periventricular WM, even in areas with normal appearance on T2-weighted images. FA reduction ranged from 12.4–19% (mean, 16.5%). Compared with control subjects, posterior fossa and supratentorial WM FA in patients were reduced by 14.6% (SD 1.9%) and 18.4% (SD 0.55%), respectively (P = .029). Reduction of supratentorial WM FA correlated with younger age at treatment (< 5 years), longer interval since treatment (> 5 years), and deterioration of school performance.
CONCLUSION: DT imaging and use of the index FA is potentially useful for early detection and monitoring of treatment-induced WM injury in children with medulloblastoma.
Treatment for medulloblastoma, the most common malignant brain tumor of childhood, consists of combination surgery, craniospinal radiation therapy, and chemotherapy. Although multitechnique treatment has led to improved long-term survival, currently reported to be around 70–80% (1, 2), it is associated with significant morbidity from treatment-related complications, including neurotoxicity from cranial irradiation and chemotherapy. Symptoms usually occur several months after radiation therapy and chemotherapy and involve diverse aspects of cognition, including memory, intelligence, attention, personality dysfunction, and other high-level cognitive functions (3–6). This is especially profound in younger children because of the effect of therapy on normal brain growth and development. In a study of young survivors of medulloblastoma, significant neuropsychological deficits were found in all long-term survivors (4).
WM is recognized as the most vulnerable element in the brain to radiation injury (7, 8). On conventional MR images, post-treatment leukomalacia often begins as hyperintense foci in the deep WM adjacent to the anterior and posterior horns of the lateral ventricles, progressing to more peripheral regions in the centrum semiovale and then coalescing to involve the entire WM (7–11). Correlation between MR imaging findings and neurodevelopmental abnormalities, including cognitive function, has been poor (8–11). Most studies have shown that conventional MR imaging is of limited benefit, because it lacks the sensitivity to depict early injury, with several authors reporting deterioration of patient intellectual function without associated MR abnormality (11–13).
To date, no reliable method exists for detection and quantification of treatment-induced WM injury. DT imaging, an MR technique, is able to reveal and quantify the direction of molecular mobility in tissues, which in WM is normally anisotropic (ie, it is directional) because of axonal fibers running in parallel (14). Recent DT studies of cerebral ischemia and infarction (15, 16) and demyelinating (17, 18) and dysmyelinating diseases (19, 20) have shown reduction in anisotropy in the diseased WM (15, 16). As far as we are aware, no report yet exists of the application of DT imaging in the evaluation of treatment-induced WM injury.
We hypothesize that in treatment-induced WM injury, loss of anisotropy would occur as a result of ischemia, demyelination, and gliosis. In a cohort of medulloblastoma survivors who previously underwent treatment by surgery, cranial irradiation, and chemotherapy, our goal was to determine whether 1) anisotropy of WM is reduced, 2) DT imaging can depict changes in the WM not visible on conventional MR images, and 3) quantification of anisotropy by use of the index FA correlates with clinical parameters including age at treatment, time interval since treatment, and school performance.
Methods
Patient Data
Nine medulloblastoma survivors, who were previously healthy and developmentally normal before tumor diagnosis, were enrolled into the study after giving informed consent (Table 1). The children were between 3 and 19 years of age (mean, 10.8 years) at the time of MR imaging and were between 3 and 14 years of age (mean, 7.8 years) at treatment. The time interval between treatment and MR imaging ranged between 1 and 6 years (mean, 3.6 years). All children underwent tumor resection followed by craniospinal irradiation. The whole cranium was irradiated with lateral opposing fields to 30.6–40 Gy in 1.8–2-Gy daily fractions. After whole cranial irradiation, the lateral opposing fields were reduced to the posterior cranial fossa, and an additional boost was given to bring the posterior fossa dose to total 50.4–54 Gy. Radiation therapy was given before chemotherapy for those patients older than 3 years at diagnosis. For patients younger than 3 years, in an effort to reduce radiation damage in the very young brain, adjuvant chemotherapy was given after tumor resection, and the start of radiation therapy was delayed until the child was 3 years old or when there was evidence of disease progression. The chemotherapy regimen was either vincristine, cyclophosphamide, cisplatin, and VP16 (baby Pediatric Oncology Group [POG] protocol) (21) or CCNU (17), cisplatin, and vincristine (CCV protocol) (1).
TABLE 1:
Clinical, MR, and fractional anisotropy data in nine medulloblastoma survivors
| Patient (No.) | Age (y) | Age (y) at Treatment | Interval (y) | Total CRT Dose (Gy) | Chemo | WM | VI | FA ▵ (%) | Postoperative Complications | Deterioration in School Performance |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 19 | 13 | 6 | 54 | babyPOG | 0 | 0.31 | 19.2 | None | Moderate |
| 2 | 16 | 14 | 2 | 54.6 | CCV | 0 | 0.22 | 21.9 | None | Moderate |
| 3 | 13 | 12 | 1 | 50.4 | CCV | 0 | 0.27 | 9.9 | None | Mild |
| 4 | 13 | 7 | 6 | 54 | babyPOG | 0 | 0.24 | 11.6 | Cerebellar mutism | Moderate |
| 5 | 10 | 4 | 6 | 54 | babyPOG | 3 | 0.33 | 46.2 | Hydrocephalus, shunt infection | Severe |
| 6 | 9 | 3 | 6 | 54 | babyPOG | 1 | 0.31 | 15.8 | None | Moderate |
| 7 | 7 | 5 | 2 | 50.4 | babyPOG | 0 | 0.25 | 21.1 | None | Moderate |
| 8 | 7 | 5 | 2 | 54 | CCV | 0 | 0.24 | 4 | Bleeding during surgery | Mild |
| 9 | 3 | 3 | 1 | 50.4 | babyPOG | 2 | 0.30 | 18.2 | Cerebellar mutism | Moderate* |
Note.—CRT signifies cranial radiation therapy; Chemo, chemotherapy regimen; WM, white matter grade determined by MR imaging (4-point scale [see Methods]); VI, ventricular index (maximum width of the frontal horns divided by the maximum internal skull diameter); FA ▵ (%), percentage of reduction in the supratentorial white matter fractional anisotropy of patients versus controls.
Delayed developmental milestones.
Control Subjects
Nine healthy age-matched children were selected as control subjects after informed consent was obtained. These patients underwent MR imaging of the brain or pituitary gland for clinical indications such as headache, precocious puberty, and sensorineural hearing loss and were confirmed to have normal MR images and no neurologic deficit.
MR Imaging
MR imaging was performed with a Signa 1.5-T imager (General Electric [GE] Medical Systems, Milwaukee, WI) with a standard head coil. The following sequences were performed in all patients: axial spin-echo T1-weighted imaging (500/10 [TR/TE]; section thickness, 5 mm with 1.5-mm gap; field of view [FOV], 23 cm; acquisition matrix, 256 × 224), fast spin-echo proton density–weighted imaging (2400/10 [TR/TE]), T2-weighted imaging (4000/100 [TR/TE]; section thickness, 5 mm with 1.5-mm gap; FOV, 23 cm; acquisition matrix, 320 × 224), coronal fluid-attenuated inversion recovery imaging (9000/140/2250 [TR/TE/TI]; section thickness, 5 mm with 1.5-mm gap; FOV, 23 cm).
DT imaging was performed by single-shot spin-echo echo-planar imaging (10,000/100 [TR/TE]; FOV, 28 cm; acquisition matrix, 128 × 128). By use of a section thickness of 5 mm with 1.5-mm gap, images were acquired through the entire brain (approximately 18 images). Diffusion-sensitizing gradient encoding was applied in 25 directions by use of a diffusion-weighted factor (b = 1200 s/mm2), and one image was acquired without use of a diffusion gradient (ie, b = 0 s/mm2). The gradient directions were chosen by using the technique described by Basser (22, 23). Twenty-six images were obtained at each section, yielding an approximate total of 486 images. DT imaging time was approximately 5 minutes. The diffusion-weighted MR images were transferred to an Advantage Workstation (GE Medical Systems) for data processing by using a commercial software program (FUNCTOOL; GE Medical Systems). Maps of mean diffusivity (MD) and FA were generated (22).
Conventional MR images were evaluated for the severity of WM changes and atrophy. WM changes detected on T2-weighted images were graded on a scale of 0 to 3 (0, normal or no change; 1, mild or high signal-intensity changes involving only the deep periventricular frontal lobe or temporal lobe or both that did not extend to the gray matter (GM)-WM junction; 2, moderate nonconfluent changes involving the WM of the frontal and temporal lobe that extended to the GM-WM junction and into the centrum semiovale; 3, severe confluent changes from anterior to posterior that involved the WM of the frontal, temporal, parietal, and occipital lobe and was continuous throughout the centrum semiovale.
The ventricular index, obtained by measuring the maximal width of the frontal horns divided by the maximal internal skull diameter, was also recorded.
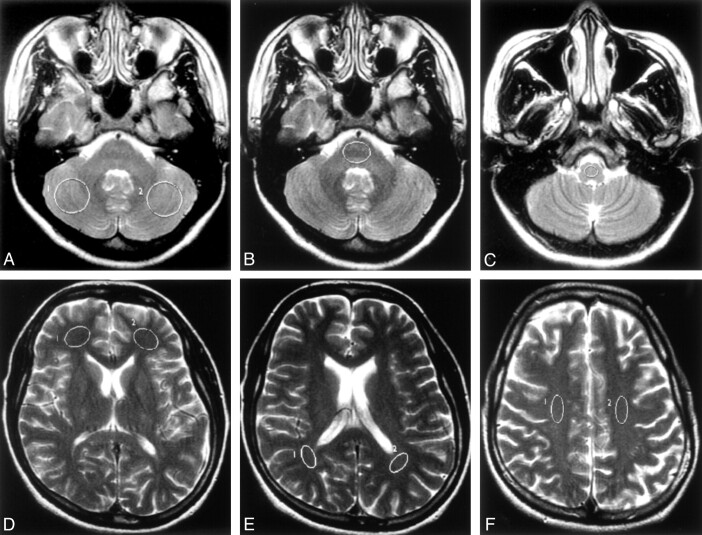
Region-of-Interest Analysis (Fig. 1)
Fig 1.
Axial T2-weighted images (100/4000/2 [TE/TR/NEX]) showing the locations of typical ROIs used in the study.
A, Bilateral cerebellar hemispheres.
B, Pons.
C, Medulla oblongata.
D, Bilateral frontal periventricular WM.
E, Bilateral parietal periventricular WM.
F, Bilateral corona radiata.
Quantitative values of MD and FA were measured by manually placing round or oval-shaped regions of interest (ROIs) onto the MD and FA maps (Fig 2b, 3b), respectively (performed by the same radiologist [P.-L.K.]). The images from the patient and the corresponding age-matched control subject were placed adjacent to each other on the workstation, and the ROIs were placed on identical sites and were of similar size in both subjects, as far as possible. The following anatomic sites were selected for evaluation: 1) bilateral cerebellar hemispheres, 2) pons, 3) medulla oblongata, 4) bilateral frontal periventricular WM, (5) bilateral parietal periventricular WM, and (6) bilateral corona radiata. The regions were first defined on the conventional T2-weighted images, and the ROIs were transferred onto the FA and MD maps at the identical level. The ROIs were placed such that they encompassed as much WM as possible, and care was taken to avoid GM and CSF (except in the cerebellar hemispheres).
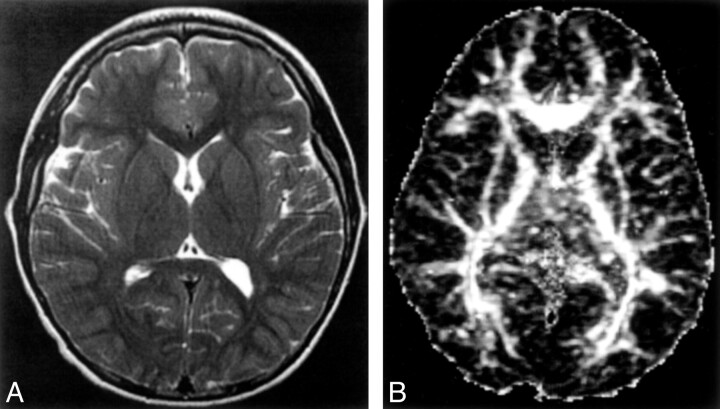
Fig 2.
Healthy 10-year-old control showing MR images at the level of the basal ganglia
A, Axial T2-weighted images (100/4000/2 [TE/TR/NEX]).
B, Axial echo-planar spin-echo DT imaging-derived FA maps (minimum/10000/1200/1 [TE/TR/b factor/NEX]; b = 1200 s/mm2 × 25 directions and b = 0).
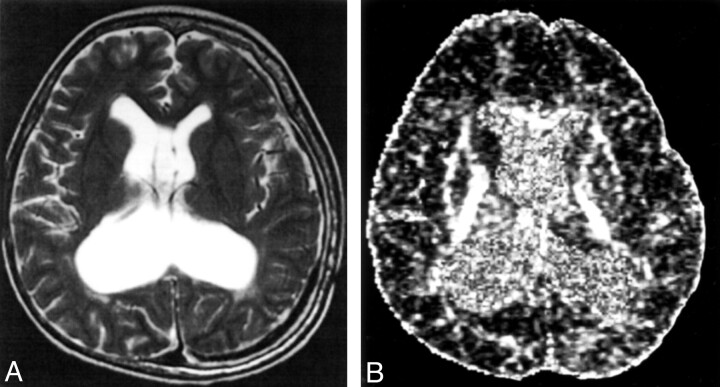
Fig 3.
A 10-year-old medulloblastoma survivor with treatment-induced WM injury and postsurgical complications of hydrocephalus and shunt infection.
A, Axial T2-weighted images (100/4000/2 [TE/TR/NEX]).
B, Axial echo-planar spin-echo DT imaging-derived FA maps (minimum/10000/1200/1 [TE/TR/b factor/NEX]; b = 1200 s/mm2 × 25 directions and b = 0) showing (a) grade 3 white matter changes and (b) reduced signal intensity in the WM compared with the healthy age-matched control, in keeping with reduced FA.
Clinical Parameters
The following clinical parameters were obtained: 1) age at treatment, 2) time interval since treatment, 3) the presence of postoperative complications, and 4) global intellectual outcome as reflected by school performance and the need for special school placement. The severity of deterioration in learning capacity was graded as mild, moderate, or severe according to a patient’s actual learning performance in school. Patients with mild deterioration had subtle changes and were still doing well in regular school. Patients with moderate deterioration have done significantly poorer in school and were either in the remedial class in regular school or admitted to a special school. Patients with severe deterioration had developmental delay and were unable to attend school. One patient (case 9) was too young to be graded by school performance and was instead graded by her achievement of developmental milestones.
Statistical Analysis
Mean FAs and mean MDs of the ROIs were analyzed and compared between the medulloblastoma group and the control group for each of the corresponding anatomic sites, by use of the two-tailed paired t test to detect any significant differences between the two groups. For all bilateral regions, the values were averaged for statistical analyses.
We compared the mean reductions in FA between the posterior fossa (cerebellar hemispheres, pons, and medulla) and supratentorial WM (frontal and parietal periventricular WM and corona radiata) in the medulloblastoma group. We also evaluated differences in supratentorial WM FA reduction between 1) children less than 5 years of age (n = 3) and those 5 years of age or older (n = 6) at treatment and 2) children with intervals since treatment of less than 5 years (n = 5) and 5 years or more (n = 4). The two-tailed paired t test was used to detect significant differences between the above groups. A P value of <.05 was considered to indicate statistical significance.
Results
MR Imaging Findings (Table 1)
Post-treatment leukomalacia was detected on T2-weighted images in three patients, one each of grade 1, 2, and 3 (Fig. 3a). Ventricular index ranged from 0.22 to 0.33 (mean 0.27). No other WM lesions were present in the posterior fossa or supratentorial region.
Fractional Anisotropy Measurements (Table 2)
TABLE 2:
Fractional anisotropy and mean diffusivity at different anatomic sites in nine medulloblastoma survivors (patients) and age-matched controls
| Fractional anisotropy (FA) |
Mean Diffusivity (MD) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Patient (SD) | Control (SD) | Δ (%) | P Value | Patient (SD) | Control (SD) | Δ (%) | P Value | |
| Pons | 0.30 (0.04) | 0.35 (0.02) | −12.4 | 0.004* | 1.11 (0.14) | 1.14 (0.05) | −2.8 | 0.422 |
| Cereb | 0.15 (0.03) | 0.17 (0.04) | −15.7 | 0.042† | 1.37 (0.25) | 1.09 (0.04) | 25.9 | 0.010† |
| Medulla | 0.31 (0.06) | 0.36 (0.06) | −15.7 | 0.030† | 1.28 (0.21) | 1.17 (0.13) | 9.3 | 0.270 |
| FWM | 0.24 (0.08) | 0.30 (0.06) | −18.4 | 0.079 | 1.30 (0.15) | 1.22 (0.06) | 6.7 | 0.066 |
| PWM | 0.33 (0.07) | 0.41 (0.05) | −19 | 0.028† | 1.24 (0.10) | 1.17 (0.08) | 5.7 | 0.090 |
| CR | 0.38 (0.10) | 0.47 (0.10) | −17.9 | 0.008* | 0.95 (0.12) | 0.92 (0.12) | 7.1 | 0.300 |
Note.—Cereb indicates cerebellar hemisphere; FWM, frontal white matter; PWM, parietal white matter; and CR, corona radiata.
Δ (%) = Percentage of reduction in FA of patients compared with controls.
P < .01
P < .05.
There was a decrease in FA in all anatomic sites of the patient group compared with measures in the healthy age-matched control group (ie, cerebellar hemispheres, pons, medulla oblongata, periventricular WM in the frontal and parietal lobes, and the corona radiata). The percentage of reduction ranged between 12.4% and 19%, with a mean of 16.5%, and this reduction was statistically significant (P < .05) in all anatomic sites except the frontal periventricular WM.
Compared with the findings in the control group, the mean reductions in the posterior fossa FA and the supratentorial FA in the patient group were 14.6% (SD 1.9%) and 18.4% (SD 0.55%), respectively, and the difference was statistically significant (P = .029).
Within the patient group (Table 1), the reduction in supratentorial FA of the group less than 5 years of age at treatment was 26.7% (SD 16.9%) and for the group 5 years of age or older at treatment was 14.6% (SD 7.2%). The mean reduction in supratentorial FA in the group with an interval since treatment of less than 5 years was 15% (SD 7.8%) and in the group with an interval since treatment of 5 years or more was 23.2% (SD 15.6%). These differences were, however, not statistically significant by t tests (P = .16 and .336, respectively).
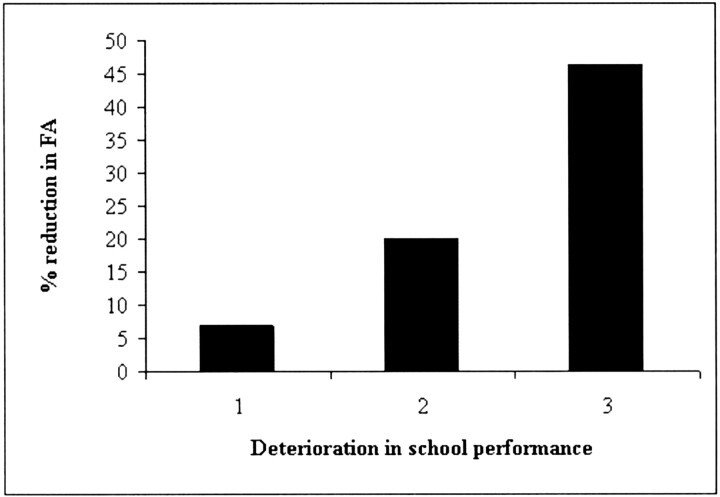
With regard to school performance (Table 1), all patients had deterioration, with two having mild deterioration, six having moderate deterioration, and one having severe deterioration. The reductions in supratentorial FA of the mild, moderate, and severe groups were 7%, 20%, and 46.2%, respectively (Fig 4).
Fig 4.
Graph showing relationship between severity of deterioration of school performance of the nine medulloblastoma survivors, and percentage reduction in supratentorial FA of the medulloblastoma survivors compared with healthy age-matched controls. 1, mild deterioration; 2, moderate deterioration; 3, severe deterioration.
The patient with the highest percentage of reduction in supratentorial FA (patient 5; 46.2%) was treated at 4 years of age and subsequently had postoperative complications of hydrocephalus, ventriculo-peritoneal shunt insertion complicated by infection (Fig. 3). He presently has marked ventriculomegaly, daily seizures that are not well controlled with antiepileptics, developmental delay, and learning and speech problems. Other postoperative complications were cerebellar mutism in two patients and intraoperative bleeding in one patient, leading to termination of the procedure.
Mean Diffusivity Measurements (Table 2)
In the patient group, MD was increased in all sites except the pons. The amount of increase ranged from 5.7% to 25.9%. In none of the sites was there statistical significance compared with findings in the control group, except in the cerebellar hemispheres (P = .01).
Discussion
To our knowledge, this is the first study to evaluate the application of DT imaging in treatment-related WM injury. We have shown that FA is significantly reduced in multiple anatomic sites in medulloblastoma survivors who have undergone surgery, cranial irradiation, and chemotherapy compared with healthy age-matched control subjects, even in areas with normal-appearing WM on conventional MR images. In all the anatomic sites except the pons, MD was increased, but this increase did not reach statistical significance, except for the cerebellar hemispheres, which suggests that MD is less sensitive than FA for the detection of abnormalities. Because of the presence of cerebellar atrophy in most patients, the increase in MD in the cerebellar hemispheres is most likely the result of volume averaging with the adjacent CSF.
We have also found that, among the medulloblastoma group, reduction in supratentorial WM FA paralleled the severity of deterioration in school performance. We have therefore shown that DT imaging, by use of the quantitative index FA, is sensitive and potentially useful for the early detection and subsequent monitoring of treatment-related neurotoxicity. Although we recognize the limitations of small patient numbers in this pilot study, our results suggest that supratentorial WM FA correlates with intellectual outcome in treatment-induced neurotoxicity. The association between WM and cognitive function has been highlighted in a study showing correlation between WM volume and IQ in medulloblastoma survivors (24). Only one other study has evaluated the relationship between FA and cognitive function, and it was in patients with multiple sclerosis (25). In that study, FA of brain tissue was lower for the cognitively impaired than for the unimpaired patients, although the difference was not found to be statistically significant (25). Early recognition and accurate quantification of treatment-induced neurotoxicity are important for successful neuropsychological intervention or modification of a drug regimen or both and, in the future, to assess the effectiveness of drugs that may prevent injury to normal brain tissue. Because this is a pilot study, we intend to follow up with full cognitive-function tests, increase patient numbers, and perform longitudinal studies to evaluate the sequential changes of FA after treatment and also to determine whether FA reduction precedes deterioration of cognitive function.
Histopathologic findings of radiation injury in animal models include vascular endothelial injury, infarction, WM necrosis, and demyelination (7, 8). The proposed pathophysiologies for irradiation- or chemotherapy-related neurotoxic injury are vascular, leading to obstruction, ischemia, and necrosis and toxicity to glial cells, leading to demyelination (7, 8). Our results are consistent with the findings of previous studies that have shown reduction in anisotropy in disease processes that affect myelin or axonal integrity in ischemic diseases, demyelinating and dysmyelinating WM diseases such as leukoaraiosis (15) and chronic brain ischemia (26), multiple sclerosis (17, 18), Krabbe disease (20), and X-linked adrenoleukodystrophy (19). It was proposed that, in chronic ischemia, the loss of ordered axonal tracts and accompanying nondirectionally orientated gliosis result in loss of FA (15).
More severe reduction in FA was found with younger age at treatment and longer interval since treatment. On the assumption that reduction in FA is associated with impaired cognitive function, these findings are in agreement with the recognized risk factors of poor neuropsychological outcome in post-treatment neurotoxicity of patients with medulloblastoma, which include young age at treatment (5, 6,27–29), higher dose intensity and volume (28, 29), and the presence of surgical complications (29, 30). Regarding interval since treatment, the association with increasingly poor cognitive function is less well established; however, a few series have found this to be true in patients with acute lymphoblastic leukemia (31). In an MR spectroscopy study of treatment-induced leukoencephalopathy in patients with acute lymphoblastic leukemia, Chan et al (32) found significant correlations between reduction in N-acetylaspartate (NAA), a neuronal marker, and increased interval since diagnosis. The study, however, did not find a significant association between NAA-creatine ratios and academic performance. Other studies have suggested that MR spectroscopy may be more sensitive in the detection of radiation-induced brain injury compared with conventional T2-weighted MR imaging (33), although this has not been a consistent finding (34). In a study of treatment-induced neurotoxicity in children, there was neither a significant difference in NAA between patients and healthy control subjects nor a relationship between NAA and intellectual impairment, which suggests that MR spectroscopy is not sensitive enough for the evaluation of treatment-induced neurotoxicity (34).
Despite receiving a lower irradiation dose than that in the posterior fossa, the supratentorial WM had more severe reduction in FA. There are a few factors that could explain this anomaly. Measurement of the anatomic structures in the posterior fossa (ie, pons, medulla, and cerebellar hemispheres) is subject to higher chance of partial volume effect from CSF because of the small size of the structures (pons and medulla) and CSF between the folia of the atrophied cerebellar hemispheres. By contrast, it may also be possible that the supratentorial WM is inherently more susceptible to irradiation. Although there is no histopathologic study to support this, in a study comparing intellectual outcome in children with posterior fossa tumors, those who underwent irradiation to the whole brain (medulloblastoma patients) were found to have more severe neurocognitive deficits than were those who had irradiation to the posterior fossa only (ependymoma patients). The study concluded that neurocognitive deficits were related to irradiation to the cerebral hemispheres and not the posterior fossa (30).
Conclusion
Significant reduction in anisotropy is found in post-treatment WM of medulloblastoma survivors compared with that in healthy age-matched control subjects, even in normal-appearing areas on T2-weighted images. We have also found a trend of FA reduction with young age at treatment, increased interval since treatment, and poor intellectual outcome. Our results suggest that DT imaging, by use of the anisotropy index, FA, is potentially useful for early detection and monitoring of treatment-induced WM injury in children with medulloblastoma.
Acknowledgments
We thank Dr. Guang Cao, GE Medical Systems Asia, and Dr. Paul K. M. Au-Yeung and Mr. Lawrence Yip, Department of Radiology, Queen Mary Hospital, Hong Kong, for their support and technical assistance.
References
- 1.Packer RJ, Goldwein J, Nicholson HS, et al. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: a Children’s Cancer Group Study. J Clin Oncol 1999;17:2127–2136 [DOI] [PubMed] [Google Scholar]
- 2.Chan GCF, Li CK, Luk CW, et al. Treatment of childhood medulloblastoma with combined chemotherapy and craniospinal irradiation: the Hong Kong Experience. Neurooncology 2002. (abstract, in press)
- 3.Johnson DL, McCabe MA, Nicholson HS, et al. Quality of long-term survival in young children with medulloblastoma. J Neurosurg 1994;80:1004–1010 [DOI] [PubMed] [Google Scholar]
- 4.Walter AW, Mulhern RK, Gajjar A, et al. Survival and neurodevelopmental outcome of young children with medulloblastoma at St. Jude Children’s Research Hospital. J Clin Oncol 1999;17:3720–3728 [DOI] [PubMed] [Google Scholar]
- 5.Packer RJ, Sposto R, Atkins TE, et al. Quality of life in children with primitive neuroectodermal tumors (medulloblastoma) of the posterior fossa. Pediatr Neurosci 1987;13:169–175 [DOI] [PubMed] [Google Scholar]
- 6.Silverman CL, Palkes H, Talent B, Kovnar E, Clouse JW, Thomas PRM. Late effects of radiotherapy on patients with cerebellar medulloblastoma. Cancer 1984;54:825–829 [DOI] [PubMed] [Google Scholar]
- 7.Valk PE, Dillon WP. Radiation injury of the brain. AJNR Am J Neuroradiol 1991;12:45–62 [PMC free article] [PubMed] [Google Scholar]
- 8.Ball WS, Prenger EC, Ballard ET. Neurotoxicity of radio/chemotherapy in children: pathologic and MR correlation. AJNR Am J Neuroradiol 1992;13:761–776 [PMC free article] [PubMed] [Google Scholar]
- 9.Curran WJ, Hecht-Leavitt C, Schut L, Zimmerman RA, Nelson DF. Magnetic resonance imaging of cranial radiation lesions. Int J Radiat Oncol Biol Phys 1987;13:1093–1098 [DOI] [PubMed] [Google Scholar]
- 10.Tsuruda JS, Kortman KE, Bradley WG, Wheeler DC, Van Dalsem W, Bradley TP. Radiation effects on cerebral white matter: MR evaluation. AJR Am J Roentgenol 1987;149:165–171 [DOI] [PubMed] [Google Scholar]
- 11.Constine LS, Konski A, Ekholm S, McDonald S, Rubin P. Adverse effects of brain irradiation correlated with MR and CT imaging. Int J Radiat Oncol Biol Phys 1988;15:319–330 [DOI] [PubMed] [Google Scholar]
- 12.Harila-Saari AH, Pääkkö EL, Vainionpää LK, Pyhtinen J, Lanning BM. A longitudinal magnetic resonance imaging study of the brain in survivors of childhood acute lymphoblastic leukemia. Cancer 1998;83:2608–2617 [PubMed] [Google Scholar]
- 13.Wilson DA, Nitschke R, Bowman ME, Chaffin MJ, Sexauer CL, Prince JR. Transient white matter changes on MRI images in children undergoing chemotherapy for acute lymphocytic leukemia: correlation with neuropsychologic deficiencies. Radiology 1991;180:205–209 [DOI] [PubMed] [Google Scholar]
- 14.Bihan DL, Mangin JF, Poupon C, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 2001;13:534–546 [DOI] [PubMed] [Google Scholar]
- 15.Jones DK, Lythgoe D, Horsfield MA, Simmons A, Williams SCR, Markus HS. Characterization of white matter damage in ischemic leukoaraiosis with diffusion tensor MRI. Stroke 1999;30:393–397 [DOI] [PubMed] [Google Scholar]
- 16.Sorensen AG, Wu O, Copen WA, et al. Human acute cerebral ischemia: detection of changes in water diffusion anisotropy by using MR imaging. Radiology 1999;212:785–792 [DOI] [PubMed] [Google Scholar]
- 17.Guo AC, MacFall JR, Provenzale JM. Multiple sclerosis: diffusion tensor MR imaging for evaluation of normal-appearing white matter. Radiology 2002;222:729–736 [DOI] [PubMed] [Google Scholar]
- 18.Ciccarelli O, Werring DJ, Wheeler-Kingshott CAM. Investigation of MS normal-appearing brain using diffusion tensor MRI with clinical correlations. Neurology 2001;56:926–933 [DOI] [PubMed] [Google Scholar]
- 19.Ito R, Melhem ER, Mori S, Eichler FS, Raymond GV, Moser HW. Diffusion tensor brain MR imaging in X-linked cerebral adrenoleukodystrophy. Neurology 2001;56:544–547 [DOI] [PubMed] [Google Scholar]
- 20.Guo AC, Petrella JR, Kurtzberg J, Provenzale JM. Evaluation of white matter anisotropy in Krabbe disease with diffusion tensor MR imaging: initial experience. Radiology 2001;218:809–815 [DOI] [PubMed] [Google Scholar]
- 21.Duffner PK, Horowitz ME, Krischer JP, et al. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med 1993;328:1725–1731 [DOI] [PubMed] [Google Scholar]
- 22.Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed 1995;8:333–344 [DOI] [PubMed] [Google Scholar]
- 23.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J 1994;66:259–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mulhern RK, Reddick WE, Palmer SL, et al. Neurocognitive deficits in medulloblastoma survivors and white matter loss. Ann Neurol 1999;46:834–841 [DOI] [PubMed] [Google Scholar]
- 25.Rovaris M, Iannucci G, Falautano M, et al. Cognitive dysfunction in patients with mildly disabling relapsing-remitting multiple sclerosis: an exploratory study with diffusion tensor MR imaging. J Neurol Sci 2002;195:103–109 [DOI] [PubMed] [Google Scholar]
- 26.Inoue T, Ogasawara K, Konno H, Ogawa A, Kabasawa H. Diffusion tensor imaging in patients with chronic brain ishcemia. Proc Intl Soc Magn Reson Med 2002;10
- 27.Chin HW, Maruyama Y. Age at treatment and long-term performance results in medulloblastoma. Cancer 1984;53:1952–1958 [DOI] [PubMed] [Google Scholar]
- 28.Mulhern RK, Kepner JL, Thomas PR, Armstrong FD, Friedman HS, Kun LE. Neuropsychologic functioning of survivors of childhood medulloblastoma randomized to receive conventional or reduced-dose craniospinal irradiation: a pediatric oncology group study. J Clin Oncol 1998;16:1723–1728 [DOI] [PubMed] [Google Scholar]
- 29.Kimmings E, Kleinlugtebeld ATH. Medulloblastoma: factors influencing the educational potential of surviving children. Br J Neurosurg 1995;9:611–617 [DOI] [PubMed] [Google Scholar]
- 30.Hoppe-Hirsch E, Brunet L, Laroussinie F, et al. Intellectual outcome in children with malignant tumors of the posterior fossa: influence of the field of irradiation and quality of surgery. Childs Nerv Syst 1995;11:340–346 [DOI] [PubMed] [Google Scholar]
- 31.Jankovic M, Brouwers P, Valsecci MG, et al. Association of 1800cGy cranial irradiation with intellectual function in children with acute lymphoblastic leukaemia. Lancet 1994;344:224–227 [DOI] [PubMed] [Google Scholar]
- 32.Chan YL, Roebuck DJ, Yuen MP, et al. Long-term cerebral metabolite changes on proton magnetic resonance spectroscopy in patients cured of acute lymphoblastic leukemia with previous intrathecal methotrexate and cranial irradiation prophylaxis. Int J Radiat Oncol Biol Phys 2001;50:759–763 [DOI] [PubMed] [Google Scholar]
- 33.Chan YL, Yeung DKW, Leung SF, Cao G. Proton magnetic resonance spectroscopy of late delayed radiation-induced injury of the brain. J Magn Reson Imaging 1999;10:130–137 [DOI] [PubMed] [Google Scholar]
- 34.Davidson A, Tait DM, Payne GS, et al. Magnetic resonance spectroscopy in the evaluation of neurotoxicity following cranial irradiation for childhood cancer. Br J Radiol 2000;73:421–424 [DOI] [PubMed] [Google Scholar]