Abstract
Summary: We report serial MR findings in a 54-year-old woman with eosinophilic meningoencephalitis due to Toxocara canis infection, a parasitic disease contracted through exposure with soil contaminated by the eggs of the roundworm. MR imaging revealed several enhancing subcortical and white matter lesions in both lobes. Antihelminthic chemotherapy yielded marked improvement of the neurologic deficits and cerebral lesions. The specific MR findings—low signal intensity on T1-weighted images, high signal intensity on T2-weighted images, and contrast enhancement—and the clinical and epidemiologic features of CNS involvement are herein reviewed.
Many parasites can cause CNS infections, with toxoplasmosis, cysticercosis, and schistosomiasis being the most common. CNS involvement by other parasites, such as Toxocara canis, is extremely rare (1).
T canis, a roundworm common in dogs and other canids, may lead to three main forms of the disease, depending on the number of larvae ingested: occult, ocular, and visceral larva migrans. The last is characterized by generalized illness, abdominal symptoms, a skin rash, and symptoms arising from larval invasion of different organs (2). Among these organs, the clinically most important are the liver, lungs, eyes, and CNS (3). As a manifestation of visceral larva migrans, CNS involvement has been described on only a few occasions; descriptions include eosinophilic meningitis, encephalitis, or a combination of these entities. Our review of the medical literature produced only four cases of T canis encephalitis (4–7) and four cases of myelitis (8–11) that were investigated with MR imaging.
We describe a case of toxocaral encephalitis assessed by using CT and MR imaging. This case is notable for its neuroradiologic findings, which are presented herein to highlight the clinical entity of cerebral toxocaral disease.
Case Report
A 54-year-old woman presented with left-arm clumsiness, a gait disturbance, and behavioral disorders. These symptoms had appeared 4 days before her admission to the hospital. Laboratory findings showed eosinophilia of 36% in 15,800 white blood cells (WBCs), thrombocytosis of 42,0000/mm3, hypergammaglobulinemia, and an elevated erythrocyte sedimentation rate (ESR) of 91. Results of all other routine blood tests were normal. Findings from a buffy coat test confirmed this isolated hypereosinophilia. EEG showed a diffusely, mildly slowed background and focal slow activity at 2.5–4 Hz, with interspersed sharp waves in and from the left hemisphere. CSF examination demonstrated a clear, colorless fluid with five to six cells per microliter (normal value, less than five cells per microliter), a glucose level of 52 mg/dL, a protein level of 15 mmol/L, and an elevated LDH of 113. Plain cranial CT performed at admission showed hypoattenuating areas in the white matter in both hemispheres, with a hyperattenuating center in one of these areas (Fig 1). Eight days later, cranial MR imaging was performed by using a 1.5-T superconducting unit with conventional T1-weighted spin-echo sequences (TE/TR, 600/20) and fluid-attenuated inversion recovery (FLAIR) sequences (TE/TR/TI, 8000/160/2200) before and after the injection of contrast material, as well as T2-weighted fast spin-echo sequences (2000/20–80). Axial and coronal images were obtained.
Fig 1.
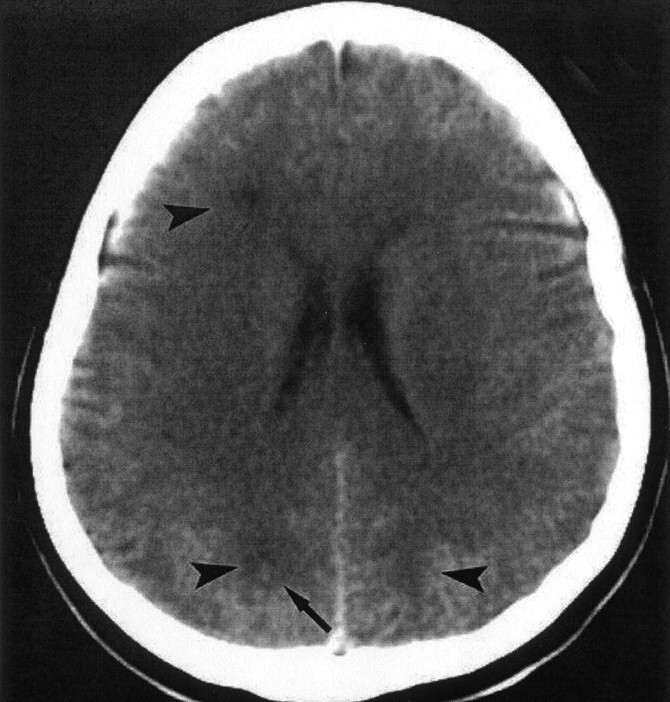
Nonenhanced cranial CT scan obtained at admission shows multiple hypoattenuating lesions in the right frontal lobe and in both occipital lobes (arrowheads). Note the hyperattenuating center of the lesion in the right occipital lobe (arrow).
MR imaging revealed several subcortical lesions in the right frontal and occipital lobes, and a chain of sharply demarcated white matter lesions were present in both centra semiovale. On the T1-weighted images, most of the lesions were hypointense, but one lesion in the right occipital lobe showed high signal intensity (Fig 2A). On T2-weighted and FLAIR images, all lesions were hyperintense (Fig 2B). After the intravenous administration of gadopentetate dimeglumine, all lesions showed intense contrast enhancement in their centers (Fig 2C). Meningeal enhancement was observed close to the occipital subcortical lesion on the right side (Fig 2D). Results of abdominal and cardiac sonography, Doppler sonography of the intracranial and extracranial arteries, and ophthalmologic examination were normal. The search for parasitic organisms in CSF and feces yielded negative findings. Serologic examination of the CSF and blood revealed negative findings for toxoplasmosis, schistosomiasis, ascariasis, echinococcosis, and cysticercosis. Results of enzyme-linked immunosorbent assay (ELISA) for T canis antibodies were positive in serum (immunoglobin G [IgG] 2+, immunoglobulin M [IgM] 2+) and negative in the CSF. Titers of this level were indicative of a current infection with T canis larvae.
Fig 2.
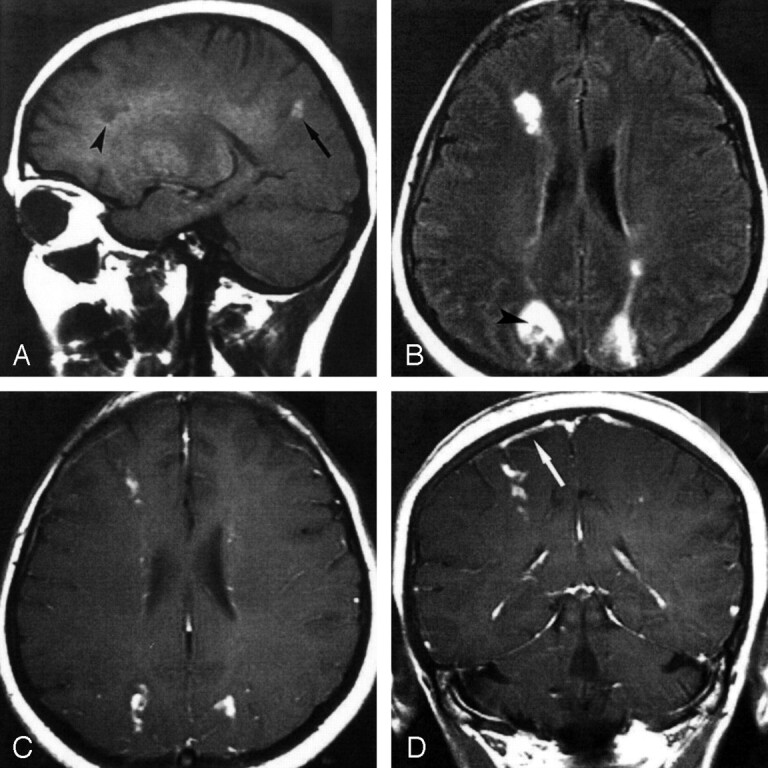
MR images before treatment.
A, On this nonenhanced sagittal T1-weighted MR image, the occipital lesion appears hyperintense (arrowhead), whereas the frontal lesion is hypointense (arrow).
B, Axial FLAIR MR image shows multiple hyperintense lesions. The center of the right occipital lesion appears hypointense (arrowhead).
C and D, Axial (C) and coronal (D) contrast-enhanced T1-weighted MR images show marked contrast enhancement of the lesions. Note the area of parasagittal meningeal enhancement (arrow) on the right side, close to the frontal-lobe lesion.
A treatment scheme combining albendazole (S: 1 × 2) and methylprednisolone 32 mg daily was initiated, and the patient’s condition rapidly improved. She was discharged 1 month after admission, with left-arm weakness and clumsiness. At that time, blood results revealed a reduction of the eosinophil count (7.6% in 7390 WBC) and the ESR (37). Anti-helminthic therapy was continued for 1 week, with progressive improvement in the neurologic status (with residual minor left-arm clumsiness).
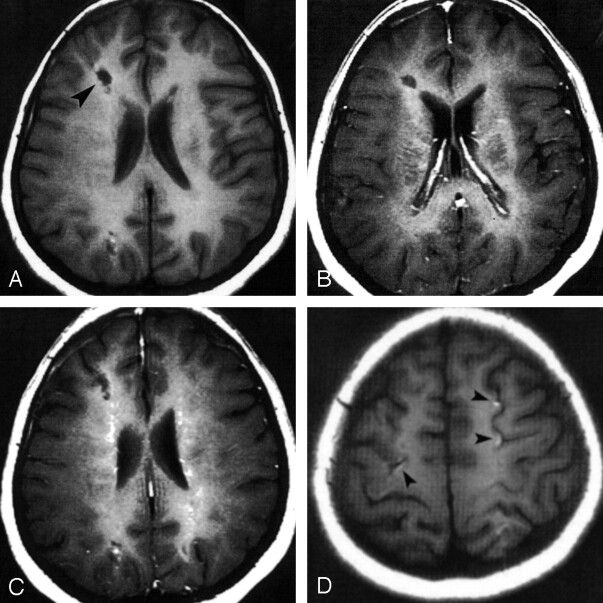
Follow-up T1-weighted MR images obtained 8 months after the initiation of treatment revealed hypointensity in the lesions that was more pronounced than that of MR images obtained at admission. No enhancement was noted on the postcontrast images. Additional findings were hyperintense cortical lesions in both T1- and T2-weighted images. These were in close proximity to the subcortical lesions (Fig 3).
Fig 3.
Follow-up axial MR images obtained 8 months after the initiation of treatment.
A, Nonenhanced T1-weighted image the right frontal lesion (arrowhead) shows signal intensity almost identical to that of the CSF. This finding indicates chronic gliotic changes.
B and C, Consequent contrast-enhanced T1-weighted images reveal the absence of enhancement.
D, Nonenhanced T1-weighted image at the convexity shows multiple, small, hyperintense cortical lesions due to cortical necrosis (arrowheads).
The patient continued to have residual left-arm clumsiness, and her blood tests revealed further reduction of eosinophils (0.8% in 7340 WBCs). This finding indicated that the migration of the larvae was completed and that the tissues were no longer exposed to the parasite. However, titers for T canis antibodies were still positive in serum (IgG 2+, IgM 2+). The patient refused to undergo a repeat lumbar puncture.
Discussion
Infection with T canis is a common worldwide human helminthiasis that rarely affects the brain or the spinal cord. Seroprevalence is high in developed countries, especially in rural areas, and also in some tropical islands (12). An area is considered to pose a high risk for Toxocara infection when the infestation rate among dogs is over 7%. In Thessaloniki, Greece, the reported data indicate that infestation rates among dogs and cats is 29% and 66%, respectively, and the rate of soil contamination is 35% (13). For this reason, infection is possible in Thessaloniki even among those without close contact with dogs.
Humans become infected after ingesting embryonated eggs from soil (geophagia, pica) or after exposure to dirty hands or raw vegetables or to larvae from undercooked giblets. People then serve as transport hosts of T canis. After the infective eggs are ingested, they hatch. The larvae penetrate the intestinal wall and are carried by the circulation to a wide variety of tissues (liver, heart, lungs, muscle, eyes, CNS), where each worm becomes encapsulated by a collagenous capsule in a granulomatous reaction. Although the larvae do not grow or undergo any further development in these sites, they are metabolically active, and therefore, they can cause severe local reactions that are the bases of toxocariasis. They give off an array of enzymes, waste products, and cuticular components that cause tissue damage, necrosis, and a marked inflammatory reaction, in which eosinophils are a major component. Some have suggested that toxic eosinophil proteins released into the brain and other tissues contribute to the pathologic changes and clinical signs seen with this infection (2).
The site of T canis invasion depends on multiple factors, including the number of ingested larvae, genetic factors of the host, and whether previous exposure has occurred (2). T canis larvae are known to invade the CNS of animals; however, in contrast to Baylisascaris procyonis, Toxocara organisms are not often associated with clinical CNS disease. Similarly, clinically overt brain disease in humans is less common with Toxocara than with other organisms, although a few cases of overwhelming infection are known (14). Clinical CNS disease is related to the number of larvae entering the brain and to the severity of CNS damage and inflammation (15).
In 1951, Beautymann and Woolf (16) were the first to publish evidence of toxocaral cerebral infection. They found second-stage larva in the left thalamus of an English child whose death was attributed to poliomyelitis. Since then, many reports have described eosinophilic granulomas and vasculitic lesions in the brains of children and adults (17,18). These lesions are predominantly in the cerebral and cerebellar white matter, with or without the presence of larvae (2). The latter observation is due to the fact that larvae enmeshed in a granulomatous reaction are not permanently imprisoned by this host reaction, as they can apparently burrow out of the reaction, migrate elsewhere, and elicit the same reaction anew. For this reason, the cellular composition of the granulomas cannot be used to indicate the length of infection (19).
Toxocariasis is not the only helminthic parasitic infection that causes eosinophilic meningoencephalitis. Many other parasites and fungi are associated with eosinophilic meningoencephalitis. These include B procyonis, Coccidioides immitis, and Angiostrongylus cantonensis, and they vary in terms of geographic locale and patterns of CNS involvement (15).
The diagnosis of neurotoxocariasis is based on several findings: high serum titers of T canis antibodies (measured with sensitive immunologic methods, ELISA or Western blotting that use Toxocara excretory-secretory antigens) (12), eosinophilia in the blood and/or CSF, the demonstration of an intrathecal synthesis of anti-T canis antibodies, and close contact with dogs. The clinical and radiologic improvement, as well as the normalization of the CSF parameters during antihelminthic therapy, supports the diagnosis (8).
Despite the normal CSF findings (eg, five to six cells per milliliter, negative antibody titer) in our patient, our diagnosis was completely documented. Several reports indicate that serum or even CSF Toxocara titers are of limited value for diagnosis because the results are often negative or borderline, especially in patients with a pure cerebral infestation (5,6). Therefore, the diagnosis was based on the positive Toxocara titer in the serum, the marked reduction of the activity (contrast enhancement) of the lesions on MR imaging, and the improvement of the patient’s neurologic status during antihelminthic therapy. The striking recovery was attributed to the treatment rather than the spontaneous course of the disease.
Recent reports (5,20,21) suggest the simultaneous administration of immunosuppressants (eg, corticosteroids) with albendazole, although we are aware of no results confirming the superiority of this combined treatment to single therapy (8).
Neurotoxocariasis is mainly manifested by a granulomatous process, as in other parasitic CNS infestations (2). MR images show multifocal, circumscribed lesions in the brain with strong contrast enhancement or a combination of circumscribed and diffuse changes in chronic infections; these are nonspecific findings (5,6).
In the English-language literature, four reports of cerebral toxocaral disease investigated with MR imaging are available. They all describe multiple subcortical, cortical, or white matter lesions that were hypoattenuating on CT scans, hyperintense on T2-weighted MR images, and homogeneously enhancing (4–7). Only Ruttinger and Hadidi (6) have described a case in which a marked decrease in the size and number of the lesions was present after antihelminthic treatment.
Four other reports describe spinal cord involvement with solitary or multiple lesions that were hyperintense on T2-weighted images and that enhanced strongly after the administration of contrast material (8–11). In all of the cases mentioned as well as in our case, involvement of only the CNS occurred with normal findings in the skin, lungs, and liver.
In our case, the lesions were located in both hemispheres, mostly subcortically. At admission, they were all hypoattenuating on CT scans, hypointense on T1-weighted images, and hyperintense on T2-weighted images. An exception was the center of the right occipital-lobe lesion, which was hyperattenuating on CT scans and hyperintense on both T1- and T2-weighted MR images. This finding was suggestive of microhemorrhages or cortical necrosis due to infarction. The granulomas had strong homogeneous enhancement on the first MR image, and this was thought to result from a focal disruption of the blood-brain barrier due to a reactive inflammatory process; this was suggestive of an active infection (8).
The focal meningeal enhancement observed near the right occipital subcortical lesion is probably due to the extension of the inflammation in the subarachnoid space. This observation has been described once before in the radiologic literature (22). The CT and MR imaging findings on admission, in combination with the clinical presentation, were more suggestive of an inflammatory process than of brain infarcts.
At follow-up, the more pronounced hypointensity of the lesions on T1-weighted images and their hyperintensity on T2-weighted images were obviously the result of chronic gliotic changes, and the absence of enhancement on postcontrast images was suggestive of inactive infection. MR imaging also revealed hyperintense cortical areas (on both T1- and T2-weighted images) near the granulomas. These were a result of cortical necrosis presumably due to multiple brain infarcts caused by immune vasculitis.
Immune vasculitis is also a well-known complication of other parasitic diseases (eg, neurocysticercosis) in which the parasite compromises primarily the small cerebral vessels (5,23). In an angiographic study of 28 patients with cerebral subarachnoid cysticercosis, Barinagarrementeria and Del Brutto (24) demonstrated that 53% of all patients with subarachnoid cysticercosis had angiographically documented cerebral arteritis and that most of them were symptomatic (80%).
To our knowledge, only one angiographically documented report describes cerebral vasculitis in neurotoxocariasis (5). This case involved the occlusion of multiple small branches of the middle cerebral artery that resulted in multiple brain infarcts. On the other hand, in a few reported cases, cerebral infarction developed during anti-helminthic treatment (Herxheimer reaction) (24).
In our case, cerebral infarctions were close to the granulomas and developed during antihelminthic treatment; therefore, their cause was difficult to define. Therefore, whether cerebral infarctions are due to an acute inflammatory reaction to the Toxocara antigen, to a delayed-type hypersensitivity to antihelminthic drugs, or both, is unclear.
This case report confirms that CT and MR findings of cerebral toxocariasis are nonspecific and that serologic studies of blood and CSF are necessary to establish the diagnosis. On the other hand, serial MR imaging during antihelminthic chemotherapy for neurotoxocariasis is a valuable tool for monitoring the course of the disease, whereas serum tests for T canis antibodies may be less useful because results can remain positive for months or years after clinical improvement occurs, and T canis antibodies in the CSF of patients with pure cerebral toxocariasis may be negative (8,10).
Conclusion
In a patient with hypereosinophilia of unknown origin and cerebral granulomatous disease, the differential diagnosis of T canis infection must not be overlooked (5).
References
- 1.Bowen BC, Post MJD. Intracranial infection. In: Atlas SW, ed. Magnetic Resonance Imaging of the Brain and Spine. New York: Raven Press;1991. :501–537
- 2.Kayes SG. Human toxocariasis and the visceral larva migrans syndrome: correlative immunopathology. In: Freedman DO, ed. Immunopathogenetic Aspects of Disease Induced by Helminth Parasites: Chemical Immunology. Vol 66. Basel: Karger;1997. :99–124 [DOI] [PubMed]
- 3.Glickman LT, Schantz PM. Epidemiology and pathogenesis of zoonotic Toxocariasis. Epidemiol Rev 1981;3:230–250 [DOI] [PubMed] [Google Scholar]
- 4.Komiyama A, Hasegawa O, Nakamura S, Ohno S, Kons K. Optic neuritis in cerebral toxocariasis. J Neurol Neurosurg Psychiatry 1995;59:197–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sommer C, Ringelstein EB, Biniek R, Glockner WN. Adult Toxocara canis encephalitis. J Neurol Neurosurg Psychiatry 1994;57:229–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruttinger P, Hadidi H. MRI in cerebral toxocaral disease. J Neurol Neurosurg Psychiatry 1991;54:361–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zachariah S, Zachariah B, Varghese R. Neuroimaging studies of cerebral “visceral larva migrans” syndrome. J Neuroimaging 1994;4:39–40 [DOI] [PubMed] [Google Scholar]
- 8.Goffette S, Jeanjean AP, Duprez TPJ, Bigaignon G, Sindic CJM. Eosinophilic pleocytosis and myelitis related to Toxocara canis infection. Eur J Neurol 2000;7:703–706 [DOI] [PubMed] [Google Scholar]
- 9.Strupp M, Pfister HW, Eichenlaub S, Arbusow V. Meningomyelitis in a case of toxocariasis with markedly isolated CSF eosinophilia and an MRI-documented related thoracic cord lesion. J Neurol 1999;246:741–744 [DOI] [PubMed] [Google Scholar]
- 10.Duprez TPJ, Bigaignon G, Delgrange E, et al. MRI of cervical cord lesions and their resolution in Toxocara canis myelopathy. Neuroradiology 1996;38:792–795 [DOI] [PubMed] [Google Scholar]
- 11.Kumar J, Kimm J MR in Toxocara canis myelopathy. AJNR Am J Neuroradiol 1994;15:1918–1920 [PMC free article] [PubMed] [Google Scholar]
- 12.Magnaval JF, Glickman LT, Dorchies P, Morassin B. Highlights of human toxocariasis. Korean J Parasitol 2001;39:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haralambidis ST. Toxocarosis. In: Haralambidis ST, ed. Issues of Parasitology that Concern Public Health in Thessaloniki. Thessaloniki, Greece: University Studio Press;1993. :41–45
- 14.Kazacos K. Visceral, ocular and neural larva migrans. In: Connor D, Chandler F, Schwartz D, Manz H, Lack E, eds. Pathology of Infectious Diseases. Stamford: Appleton & Lange;1997. :1459–1473
- 15.Rowley HA, Uht RM, Kazacos KR, Sakamari J, Wheaton WV, et al. Radiologic-pathologic findings in Raccoon roundworm (Bayliscaris procyonis) encephalitis. AJNR Am J Neuroradiol 2000;21:415–420 [PMC free article] [PubMed] [Google Scholar]
- 16.Beautymann W, Woolf A. An Ascaris larva in the brain in association with acute anterior poliomyelitis. J Pathol Bacteriol 1951;63:635–647 [DOI] [PubMed] [Google Scholar]
- 17.Dent JH, Nichols RL, Beaver PC, Carrera GM, Staggers RJ. Visceral larva migrans with a case report. Am J Pathol 1956;32:777–803 [PMC free article] [PubMed] [Google Scholar]
- 18.Hill IR, Denham DA, Scholtz CL. Toxocara canis larvae in the brain of a British child. Trans R Soc Trop Med Hyg 1985;79:352–354 [DOI] [PubMed] [Google Scholar]
- 19.Kayes SG, Oaks JA. Development of the granulomatous response in murine Toxocariasis: initial events. Am J Pathol 1978;93:277–294 [PMC free article] [PubMed] [Google Scholar]
- 20.Del Brutto OH. Cysticercosis and cerebrovascular disease: a review. J Neurol Neurosurg Psychiatry 1992;55:252–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Brutto OH, Sotelo J, Roman GC. Therapy for neurocysticercosis: a reappraisal. Clin Infect Dis 1993;17:730–735 [DOI] [PubMed] [Google Scholar]
- 22.Villano M, Cerillo A, Narciso N, Vizioli L, Del Basso de Caro M. A rare case of Toxocara canis arachnoidea. J Neurosurg Sci 1992;36:67–69 [PubMed] [Google Scholar]
- 23.Bang OY, Heo JH, Choi SA, Kim DI. Large cerebral infarction during Praziquantel therapy in neurocysticercosis. Stroke 1997;28:211–213 [DOI] [PubMed] [Google Scholar]
- 24.Barinagarrementeria F, Del Brutto OH. Lacunar syndrome due to cysticercosis. Arch Neurol 1989;46:415–417 [DOI] [PubMed] [Google Scholar]