Abstract
BACKGROUND AND PURPOSE: Diffusion tensor MR imaging has the potential to improve our ability to monitor several neurologic conditions. As a preliminary step to the assessment of the role of diffusion tensor MR imaging in the context of longitudinal and multicenter studies, we evaluated the effect of sequence-, imaging unit-, and imaging-reimaging-induced variations on diffusion tensor MR imaging quantities derived from histogram analysis of a large portion of the central brain of healthy volunteers.
METHODS: Each of eight healthy volunteers underwent imaging on two MR imaging units using three different pulsed gradient spin-echo single shot echo-planar pulse sequences (each of them having a different diffusion gradient scheme). Four additional healthy participants underwent imaging twice on the same imaging unit to assess imaging-reimaging variability.
RESULTS: For mean diffusivity histograms, the differences between inter-sequence and inter-imaging unit coefficients of variation were significant for all the considered quantities with P values ranging from .003 to <.001. Also, the inter-imaging unit coefficient of variation for average fractional anisotropy was significantly higher than the corresponding inter-sequence coefficient of variation (P = .002). In general, inter-sequence mean diffusivity histogram-derived metrics (coefficients of variation ranging from 1.72% to 5.56%) were more reproducible than were fractional anisotropy histogram-derived metrics (coefficients of variation ranging from 5.45% to 7.34%). Imaging-reimaging variability was found to fall in the range of inter-sequence coefficients of variation for all the considered quantities.
CONCLUSION: This study shows that inter-sequence, imaging-reimaging, and inter-imaging unit variabilities of diffusion tensor MR imaging-derived measurements are relatively low, suggesting that diffusion tensor MR imaging might provide additional measures of outcome with which to assess the evolution of brain structural damage in large scale studies of various neurologic conditions.
Diffusion-weighted MR imaging is a technique based on the measurement of the molecular translational motion of tissue water (1). In biologic tissues, because of the interactions between water molecules and cellular structures, diffusion is hindered by the presence of restricting barriers (2). As a consequence, diffusion-weighted MR imaging can provide valuable information regarding tissue structure at a microscopic level (3). In biologic tissue, especially in white matter, diffusion is an anisotropic phenomenon (ie, the diffusion coefficient is not the same in all directions). To fully describe the behavior of diffusing protons in such an anisotropic environment, diffusion tensor MR imaging has been introduced (4). From the tensor, it is possible to derive several scalar indexes, including the mean diffusivity, equal to one-third of the trace of the tensor, and the degree of diffusion anisotropy in every voxel (5).
Several pathologic conditions, such as multiple sclerosis, stroke, and Alzheimer disease, can alter the microstructural properties of the brain tissue and, as a consequence, affect diffusion tensor MR imaging-derived quantities (6–10). Diffusion tensor MR imaging abnormalities in these conditions have also been found to correlate significantly with clinical measures of outcome (6, 9, 11). Although these findings indicate the potential for diffusion tensor MR imaging to efficiently monitor the therapeutic effects of experimental treatments for numerous neurologic diseases, to date, no study has assessed the reproducibility of its measurement in vivo. Information regarding the impact of different MR images and imaging units on the stability of these measurements is a prerequisite for using diffusion tensor MR imaging-derived quantities in the context of longitudinal and multicenter studies. Diffusion characteristics can be analyzed on a region-of-interest basis or in a more global fashion by using histograms (12–14). Although the use of histogram analysis inevitably results in a loss of information regarding individual normal or diseased tissues, it allows the evaluation of all or large portions of the brain tissue in a fraction of the time that would be needed when using region-of-interest analysis. This is very attractive in the context of clinical trials, for which typically several hundreds of images have to be analyzed. Compared with a region of interest-based analysis, a histogram-based analysis has the additional advantage of being less affected by observer variability.
Against this background, we evaluated the effect of sequence-, imaging unit-, and imaging-reimaging-induced variations on measures derived from mean diffusivity and fractional anisotropy (5) histograms of the brain of healthy volunteers studied at different sites by using two different MR imaging units and three different diffusion tensor MR imaging sequences.
Methods
Participants
Twelve healthy volunteers (seven women and five men; mean age, 28.5 years; age range, 23–33 years) entered the study after providing written informed consent. None of the participants had a history of neurologic disorders, and they all had completely normal results of neurologic examination at the time the MR images were obtained.
MR Imaging Acquisition
Eight participants (six women and two men; mean age, 28.9 years; age range, 23–33 years) underwent two MR imaging examinations within a 3-month interval. The images were obtained by using two 1.5-T machines. The two imaging units will be referred to as imaging unit A (Siemens Magnetom Vision; Siemens, Erlangen, Germany) and imaging unit B (Philips Gyroscan ACS-NT; Philips Medical Systems, Eindhoven, The Netherlands). For imaging unit A, the maximum available gradient strength was 21 mT/m, with a maximum slew rate of 167 T/ms. For imaging unit B, the maximum available gradient strength was 21 mT/m, with a maximum slew rate of 105 T/ms. Two similar quadrature birdcage head coils were used on both occasions for RF signal intensity reception. The following sequences were obtained for all participants in each of the two imaging sessions: sequence 1) dual-echo turbo spin-echo (imaging unit A: 3300/16, 98 [TR/first TE, second TE]; echo train length, 5; matrix, 256 × 256; field of view, 250 × 250 mm; section orientation, axial; number of sections, 20; section thickness, 5 mm; intersection gap, 0 mm) (imaging unit B: 3300/11, 120; echo train length, 12; matrix, 256 × 256; field of view, 200 × 200 mm; section orientation, axial; number of sections, 16; section thickness, 5 mm; intersection gap, 1 mm); and sequence 2) three different pulsed gradient spin-echo single shot echo-planar pulse sequences with three different diffusion gradient schemes. In the first scheme, diffusion gradients were applied in six directions (x, y, z, xy, xz, yz, where x conventionally represents the read-out direction, y the phase-encoding direction, and z the section-selection direction). An additional set of images without diffusion weighting was obtained. This represents the simplest direction scheme, with the minimum number of encoding directions for diffusion tensor MR imaging measurements. In the second sequence, the diffusion gradients were also applied in six directions but with a slightly different scheme (xy, xz, yz, −xy, −xz, −yz). Again, an additional set of images without diffusion weighting was obtained. This scheme is a slightly modified version of the first, with a more symmetrical distribution of the direction of the diffusion gradients. In the third sequence, the diffusion encoding directions were eight (plus the set of images without diffusion weighting), chosen to cover the 3D space uniformly, according to the optimized scheme proposed by Jones et al (15). This scheme exploits a larger number of vectors than do the other two schemes, without any a priori knowledge of the degree of tissue anisotropy. As previously described (16), diffusion measurements were optimized by using only two b factors (b ≈ 0, and b = bMAX) for all sequences. The acquisition of each pulse sequence was repeated several times to improve the signal intensity-to-noise ratio, and the number of repetitions was chosen on a sequence-by-sequence basis to end up with a similar number of directions × number of repetitions per section for all the three sequences. This was done to obtain similar signal intensity-to-noise ratios. Additional details regarding diffusion gradients and numbers of acquisitions for each of the three pulsed gradient spin-echo single shot echo-planar pulse sequences are reported in Table 1. The following acquisition parameters were used for all the three pulsed gradient spin-echo single shot echo-planar pulse sequences: for imaging unit A: 4500/123 (TR/TE); maximum b factor, 1044 s/mm2; matrix, 128 × 128; field of view, 250 × 250 mm; number of sections, 16; section thickness, 5 mm; and for imaging unit B: 4200/128; maximum b factor, 1034 s/mm2; matrix, 128 × 128; field of view, 250 × 250 mm; number of sections, 16; section thickness, 5 mm. Imaging unit A used a sinusoidal ramp sampling, whereas imaging unit B used a trapezoidal ramp sampling method for echo-planar imaging read-out. The sections were always contiguous and acquired with axial orientation. The matrix size was always reconstructed to 256 × 256 by zero filling. Fat suppression was performed by using either a four-pulse binomial pulse train (imaging unit A) or a spectral selective inversion pulse (imaging unit B) to reduce chemical shift artifacts. Sections of all sequences were accurately positioned to run parallel to a line that joins the most inferoanterior and inferoposterior parts of the corpus callosum according to previously published guidelines (17). The most caudal section was positioned to traverse the inferior part of the pons. This brain portion was chosen because it is less sensitive to susceptibility artifacts.
TABLE 1:
Numbers of directions and repetitions for the three pulsed gradient spin-echo echo-planar imaging pulse sequences used in the study
| No. of Measurements | Total No. of Images per Section | No. of Diffusion Directions | |
|---|---|---|---|
| PGSE SS-EPI 1 | 10 | 7 | 6 |
| PGSE SS-EPI 2 | 10 | 7 | 6 |
| PGSE SS-EPI 3 | 8 | 9 | 8 |
Note.—PGSE SS-EPI indicates pulsed gradient spin-echo single shot echo-planar imaging. Additional information regarding the three diffusion sequences is presented in the Methods section.
To assess imaging-reimaging variability, the remaining four participants (one woman and three men; mean age, 29.0 years; age range, 29–32 years) underwent imaging twice on imaging unit A. The mean interval between the first and second examinations was 7 days. These MR imaging sessions included the dual-echo sequence and the first pulsed gradient spin-echo single shot echo-planar pulse sequence only. This diffusion gradient scheme was chosen because it is one of the most widely adopted for diffusion tensor MR imaging. Pulse sequence parameters and section positioning were the same as those reported for the other eight normal participants.
MR Imaging Postprocessing
All images were transferred to a workstation (Sun Microsystems, Mountain View, CA) for postprocessing. Pulsed gradient spin-echo single shot echo-planar pulse images were first averaged to improve the signal intensity-to-noise ratio and corrected for eddy current-induced distortions by using an algorithm that maximizes the mutual information between the diffusion-weighted and the diffusion-unweighted images (18). The diffusion-unweighted images served as template images for the registration. Assuming a mono-exponential relation between signal intensity and the product of the b matrix, the diffusion tensor was calculated for each pixel according to the following equation:
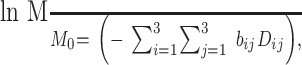 |
where M is the measured signal intensity when a particular diffusion-encoding gradient pair is played out, M0 is the T2-weighted signal intensity (ie, the diffusion-unweighted image), and bij are the elements of the b matrix. The elements of the diffusion tensor matrix (Dij) were estimated by linear regression (4), and the eigenvalues of the tensor (λ1, λ2, λ3) were derived after matrix diagonalization. The eigenvalues represent the effective diffusivities along the three orthogonal principal directions of the local coordinate system (ie, intrinsic to each voxel). The eigenvalues were sorted by their magnitude in descending order. Finally, the mean diffusivity and the fractional anisotropy were calculated and the corresponding maps were produced for all the pulse sequences. This means that for each healthy volunteer participating in the inter-imaging unit and inter-sequence variability assessment, six images of each of the eigenvalues and of mean diffusivity and fractional anisotropy were available at the end of this procedure, whereas only two images of each quantity were available for those participating in the imaging-reimaging variability assessment. Each of these sets of images was co-registered by using the long echo of the dual-echo sequence obtained on imaging unit A as the reference set of images and a rigid body technique based on mutual information (18). The quality of image registration was similar for the two sets of images obtained by using imaging units A and B. CSF and other extracerebral tissues were automatically removed from all images, and histograms of mean diffusivity, fractional anisotropy, and the eigenvalues were obtained, as previously described (14). For each histogram, the following quantities were derived: the relative peak height (after histogram normalization to correct for inter-participant differences in head size), the peak position, and the average value of the considered quantity.
Statistical Analysis
Inter-sequence variability was defined as the variability between diffusion histogram metrics obtained from different pulse sequences run on the same imaging unit. Inter-imaging unit variability was assessed as the variability between diffusion histogram metrics obtained for each of the three pulsed gradient spin-echo single shot echo-planar pulse sequences run on the two different imaging units. Imaging-reimaging variability was defined as the variability between diffusion histogram metrics obtained running the first sequence of pulsed gradient spin-echo single shot echo-planar pulse on the same imaging unit (imaging unit A) on two different occasions. Coefficients of variation were calculated to assess these variabilities. The coefficient of variation is defined as the SD of a random variable divided by its mean value. The standard errors of the mean coefficients of variation and the P values of the comparison of inter-sequence versus inter-imaging unit coefficients of variation were estimated by using a bootstrap resampling technique (19). Because of the small sample of patients studied for imaging-reimaging assessment, no formal statistical comparison was conducted for imaging-reimaging coefficients of variation versus inter-sequence or inter-imaging unit coefficients of variation.
Results
The mean diffusivity and fractional anisotropy histogram-derived metrics averaged over all eight volunteers (and the corresponding SDs) obtained for each of the three pulsed gradient spin-echo single shot echo-planar pulse sequences that had been used on each of the two imaging units are reported in Table 2.
TABLE 2:
Mean diffusivity and fractional anisotropy histogram-derived metrics, averaged over the eight participants, for each of the three pulsed gradient spin-echo single shot echo-planar imaging sequences run on two different imaging units
| Imaging Unit |
||
|---|---|---|
| A | B | |
| PGSE SS-EPI 1 | ||
| Average MD (SD) [×10−3 mm2/s] | 0.89 (0.01) | 0.85 (0.02) |
| Mean MD peak height (SD) | 98.0 (10.5) | 105.0 (7.7) |
| Mean MD peak position (SD) [×10−3 mm2/s] | 0.76 (0.02) | 0.74 (0.02) |
| Average FA (SD) | 0.24 (0.011) | 0.26 (0.003) |
| Mean FA peak height (SD) | 48.9 (4.5) | 49.8 (1.8) |
| Mean FA peak position (SD) | 0.1 (0.001) | 0.1 (0.001) |
| PGSE SS-EPI 2 | ||
| Average MD (SD) [×10−3 mm2/s] | 0.91 (0.02) | 0.84 (0.02) |
| Mean MD peak height (SD) | 98.8 (10.5) | 112.3 (10.0) |
| Mean MD peak position (SD) [×10−3 mm2/s] | 0.76 (0.03) | 0.72 (0.03) |
| Average FA (SD) | 0.22 (0.009) | 0.26 (0.006) |
| Mean FA peak height (SD) | 55.2 (4.5) | 45.7 (3.4) |
| Mean FA peak position (SD) | 0.1 (0.002) | 0.1 (0.001) |
| PGSE SS-EPI 3 | ||
| Average MD (SD) [×10−3 mm2/s] | 0.92 (0.02) | 0.85 (0.02) |
| Mean MD peak height (SD) | 98.1 (11.0) | 102.3 (10.6) |
| Mean MD peak position (SD) [×10−3 mm2/s] | 0.76 (0.02) | 0.73 (0.03) |
| Average FA (SD) | 0.22 (0.008) | 0.24 (0.010) |
| Mean FA peak height (SD) | 57.2 (5.2) | 50.0 (4.14) |
| Mean FA peak position (SD) | 0.1 (0.001) | 0.1 (0.001) |
Note.—PGSE SS-EPI indicates pulsed gradient spin-echo single shot echo-planar imaging; MD, mean diffusivity; FA, fractional anisotropy.
Tables 3 and 4 show the resulting inter-sequence, inter-imaging unit, and intra-imaging unit coefficients of variation calculated for histogram metrics derived from mean diffusivity, fractional anisotropy, and each of the diffusion tensor eigenvalues. The inter-imaging unit coefficients of variation were consistently higher than the inter-sequence coefficients of variation for all the diffusion histogram quantities. For mean diffusivity histograms, the differences between inter-sequence and inter-imaging unit coefficients of variation were significant for all the considered quantities with P values ranging from .003 to <.001. Also, the inter-imaging unit coefficient of variation for average fractional anisotropy was significantly higher than the corresponding inter-sequence coefficient of variation (P = .002). In general, inter-sequence mean diffusivity histogram-derived metrics (coefficients of variation ranging from 1.72% to 5.56%) were more reproducible than fractional anisotropy histogram-derived metrics (coefficients of variation ranging from 5.45% to 7.34%) (Table 3). Consistent with this, the reproducibility of the measurement decreased according to the sorting of the eigenvalues from λ1 to λ3 (Table 4). Mean imaging-reimaging coefficients of variation ranged from 1.81% to 14.3% (data not shown for each of the assessed quantities).
TABLE 3:
Inter-sequence and inter-imaging unit mean coefficients of variation (standard error of the means) for the mean diffusivity and fractional anisotropy histogram-derived metrics
| Inter-Sequence (%) | Inter-Imaging Unit (%) | P* | |
|---|---|---|---|
| Average MD | 1.72 (0.14) | 5.37 (0.7) | 0.003 |
| MD peak height | 5.56 (0.6) | 10.19 (1.0) | <0.001 |
| MD peak position | 1.90 (0.2) | 4.20 (0.43) | <0.001 |
| Average FA | 5.45 (0.4) | 7.71 (0.6) | 0.002 |
| FA peak height | 7.34 (0.9) | 6.07 (0.8) | n.s. |
| FA peak location | 7.30 (0.6) | 8.74 (0.9) | n.s. |
Note.—MD indicates mean diffusivity; FA, fractional anisotropy; n.s., not significant.
Statistical analysis: comparisons were conducted by using a bootstrap resampling technique.
TABLE 4:
Inter-sequence and inter-imaging unit mean coefficients of variation (standard error of the means) for the λ1, λ2, and λ3 histogram-derived metrics
| Inter-Sequence (%) | Inter-Imaging Unit (%) | P* | |
|---|---|---|---|
| Average λ1 | 1.63 (0.1) | 4.13 (0.4) | <0.001 |
| λ1 peak height | 5.18 (0.6) | 6.93 (0.8) | n.s. |
| λ1 peak position | 1.78 (0.2) | 4.76 (0.6) | <0.001 |
| Average λ2 | 2.02 (0.3) | 5.39 (0.6) | <0.001 |
| λ2 peak height | 3.30 (0.3) | 4.37 (0.5) | n.s. |
| λ2 peak position | 1.50 (0.1) | 5.33 (0.6) | <0.001 |
| Average λ3 | 4.71 (0.5) | 6.80 (0.7) | 0.009 |
| λ3 peak height | 5.49 (0.6) | 6.89 (0.8) | n.s. |
| λ3 peak position | 6.06 (0.7) | 12.19 (1.1) | <0.001 |
Note.—λ1–λ3 = eigenvalues of the diffusion tensor; n.s., not significant.
Statistical analysis: comparisons were conducted by using a bootstrap resampling technique.
Discussion
Diffusion tensor MR imaging holds significant promise for the monitoring of the evolution of several neurologic conditions, either natural or modified by treatment. Diffusion tensor MR imaging provides in vivo quantitative information regarding the size, shape, orientation, and geometry of the investigated tissues (3), and as a consequence, it enables us to obtain reliable estimates of the structural integrity of the overall brain tissue or of large portions of it (12–14). Consistent with this notion, several studies have shown moderate to strong correlations between diffusion tensor MR imaging findings and the clinical status of patients with several neurologic diseases, including multiple sclerosis (6, 7), stroke (11), and Alzheimer disease (9). In addition, in the last few years, the increased availability of echo-planar imaging on most of the commercial imaging units has made it possible, at least in principle, to conduct large scale multicenter studies (as it is the case for many phase II and virtually all phase III treatment trials) by using diffusion tensor MR imaging.
Previous studies have shown that several factors, which include biologic activity, observer reproducibility, pulse sequences, accuracy in repositioning and the use of different imaging units, influence the measurements of quantities derived from conventional and magnetization transfer MR imaging (20, 21). Clearly, some of these potentially confounding factors can be easily controlled when planning multicenter studies (22). Conversely, the use of different MR imaging units is hard to avoid in this context. This is not only because individual participating centers typically have different MR imaging units but also because an upgrade of at least some of the MR machines involved in such studies is likely to occur, considering the duration of multicenter trials (treatment trials in clinical neurology are typically of 2- to 3-year duration) and the lifetime of an imaging unit (typically 5–10 years). In case of diffusion tensor MR imaging, it might also be challenging to standardize across centers the acquisition schemes of the pulse sequences used. This is because of hardware constraints or limited ability to modify the diffusion tensor MR imaging pulse sequence without collaboration with imaging unit manufacturers. For instance, typical factors that can vary among different MR imaging units (even among different models made by the same manufacturer) are fat suppression techniques, form of echo-planar image read-out, eddy current compensation measures, shim techniques, timing and orientation of diffusion-weighting gradients, and strategies to remove background gradients.
This has prompted us to investigate the impact of the use of different imaging units and pulse sequences on diffusion tensor MR imaging histogram-derived quantities. We studied healthy volunteers to assess inter-sequence, inter-imaging unit, and intra-imaging unit variabilities without the confounding factor of disease-induced biologic variation. Such an investigation is important because it indicates the range of technique-related “noise” which may mimic or mask “true” pathologic variations. We chose to assess histogram-derived metrics rather than region of interest-derived metrics, because in the context of large scale studies, and, in particular, in the case of multifocal diseases, it might be unrealistic to try to obtain diffusion tensor MR imaging measures from different brain regions and tissues. Another major advantage of the histogram approach is that it is less operator-dependent than region-of-interest analysis. This can be important in the case of measures, such as eigenvalues and fractional anisotropy, which are characterized by inherently large spatial fluctuations within the human brain and, as a consequence, could be significantly influenced even by slight variations in region-of-interest placement. Although obtaining diffusion tensor MR imaging data regarding white and gray matter separately might be rewarding to improve our understanding of pathobiology of various neurologic conditions, we decided not to segment parenchymal pixels into gray and white matter because, as discussed earlier for region-of-interest analysis, it might be unfeasible to apply such an approach in a reliable way in the context of multicenter studies. Also, it is likely that data derived from white and gray matter in isolation would behave similarly to those obtained from pooled parenchymal pixels in terms of sensitivity to MR imaging and sequence variations.
Perhaps not unexpectedly, this study indicates that both the use of different pulse sequences and the use of different MR imaging units introduce variability in the measurements of diffusion tensor MR imaging histogram-derived quantities. However, it also shows that inter-imaging unit variability is significantly higher than inter-sequence variability, whereas the latter yielded reproducibility measures similar to those obtained for imaging-reimaging. This is not unexpected, because one of the factors that might influence inter-sequence variability is the difference in gradient strength that may occur on different axes because of an imperfect gradient calibration. As a consequence, slightly different patient repositioning within the imaging unit or a change in section orientation might impact diffusion tensor MR imaging quantities in a similar way. All of this has major implications for real-life studies because both the sequences and the imaging units used in the present study are representative of those that might be used in multicenter studies. Nevertheless, the variabilities found in the present study for diffusion tensor MR imaging metrics were lower than those reported for magnetization transfer ratio histograms (22). Although we cannot assume that inter-imaging unit variability of diffusion tensor MR imaging measurements would be the same for all potentially available imaging units and although comparisons among different studies in this context have always to be exercised with caution, one reason for this finding might be that diffusion tensor MR imaging is potentially less affected by “environmental” conditions than other MR imaging-derived measures. Water molecular diffusion is a physical property of tissues and not an MR property and, as a consequence, it is expected to be more stable than other MR quantities across different imaging units.
In this study, we tried to use widely available, similar, but not fully identical pulse sequences to match as closely as possible the situation that might be faced when planning real-life multicenter studies. The pulse sequences we used were designed to have either the same, or at least as similar as possible, TEs and b factors. Note that the b factor determines the strength of the diffusion weighting and is tied to the TE. Therefore, these two parameters affect the amount of signal intensity decay and the signal intensity-to-noise ratio. The b factor was optimized by estimating the TE that would produce the smallest measurement error (23), to minimize the variability of the measured diffusivity (15). Consistent with these considerations, we observed a relatively low variability of mean diffusivity histogram-derived metrics within different diffusion-weighting gradient schemes that had been run on the same imaging units.
We also found that the variability of mean diffusivity measurement is generally lower than that of fractional anisotropy histogram-derived metrics. This agrees with the observation that λ1 histogram-derived metrics have lower coefficients of variation than those of λ2, which in turn are lower than those of λ3. Because mean diffusivity is obtained by averaging the three eigenvalues, its value is dominated by that of the largest eigenvalue. Conversely, fractional anisotropy reflects the difference among the three eigenvalues and as a consequence is more sensitive to the variability among them and to background noise (5, 24, 25). Moreover, fractional anisotropy varies throughout the different white matter regions and, even more markedly, between white and gray matter (26, 27) whereas mean diffusivity is relatively constant in gray and white matter of healthy participants (6, 7). Thus, fractional anisotropy histograms are likely to be strongly influenced by section positioning, especially when only a portion of the brain, although large, is analyzed (28). In this situation, even a slight inaccuracy in image repositioning can constitute an additional source of variability for fractional anisotropy histogram-derived metrics. Nevertheless, considering that participants were not moved from the imaging units during the same imaging session and considering that they were carefully repositioned by using a standardized procedure when undergoing reimaging, we think that most of the variability in fractional anisotropy measurements is likely to be secondary to background noise.
Conclusion
We have shown that diffusion tensor MR imaging-derived quantities are affected by the use of different imaging units and, less markedly, by the use of different pulse sequences and by imaging-reimaging. This confirms conventional wisdom that individual patients included in multicenter studies should undergo imaging, if at all possible, with the same MR machine for the whole duration of the study. Nevertheless, our results also show that inter-sequence and inter-imaging unit variabilities of diffusion tensor MR imaging-derived measurements are relatively low and at least are not higher than those found for other MR metrics already applied in multicenter studies (21, 22). This indicates the potential of adding diffusion tensor MR imaging-derived quantities to the assessment of brain structural changes in multicenter and longitudinal studies. The reliability of diffusion tensor MR imaging measurements might be improved further by the collection of data from healthy volunteers, to obtain a center-by-center normalization of data from patients. In this context, mean diffusivity seems to be very promising, considering that it provides quantitative information regarding tissue integrity and that mean diffusivity histogram-derived measurements are relatively stable across imaging units and sequences. Also, mean diffusivity data can be obtained with relatively simple diffusion-weighted MR images, which are available on most of the clinical imaging units, whereas measures of anisotropy require more demanding and specifically designed diffusion tensor MR imaging sequences and more advanced postprocessing to be implemented.
Acknowledgments
We are grateful to the people who volunteered for this study.
Footnotes
This study was supported by grants from the Fondazione Italiana Sclerosi Multipla, the Italian Ministry of Health (contract number ICS 030.5/RF0079), the Hertie Foundation (to F.F.; GHS-319/94), and the National Institutes of Health (to R.B.; NIH-1R01NS35959).
References
- 1.Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 1986;161:401–407 [DOI] [PubMed] [Google Scholar]
- 2.Nicholson C, Phillips JM. Ion diffusion modified by tortuosity and volume fraction in the extracellular microenvironment of the rat cerebellum. J Physiol 1981;321:225–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le Bihan D, Turner R, Pekar J, Moonen CT. Diffusion and perfusion imaging by gradient sensitization: design, strategy and significance. J Magn Reson Imaging 1991;1:7–8 [DOI] [PubMed] [Google Scholar]
- 4.Basser PJ, Mattiello J, Le Bihan D. Estimation of the effective self-diffusion tensor from the NMR spin-echo. J Magn Reson B 1994;103:247–254 [DOI] [PubMed] [Google Scholar]
- 5.Pierpaoli C, Basser PJ. Towards a quantitative assessment of diffusion anisotropy. Magn Reson Med 1996;36:893–906 [DOI] [PubMed] [Google Scholar]
- 6.Filippi M, Cercignani M, Inglese M, Horsfield MA, Comi G. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology 2001;56:304–311 [DOI] [PubMed] [Google Scholar]
- 7.Bammer R, Augustin M, Strasser-Fuchs S, et al. Magnetic resonance diffusion tensor imaging for characterizing diffuse and focal white matter abnormalities in multiple sclerosis. Magn Reson Med 2000;44:583–591 [DOI] [PubMed] [Google Scholar]
- 8.Warach S, Chien D, Li W, Ronthal M, Edelman RR. Fast magnetic resonance diffusion-weighted imaging of acute human stroke. Neurology 1992;42:1717–1723 [DOI] [PubMed] [Google Scholar]
- 9.Bozzali M, Franceschi M, Falini A, et al. Quantification of tissue damage in AD using diffusion tensor and magnetization transfer MRI. Neurology 2001;157:1135–1137 [DOI] [PubMed] [Google Scholar]
- 10.Werring DJ, Brassat D, Droogan AG, et al. The pathogenesis of lesions and normal-appearing white matter changes in multiple sclerosis. Brain 2000;123:1667–1676 [DOI] [PubMed] [Google Scholar]
- 11.Warach S, Dashe JF, Edelman RR. Clinical outcome in ischemic lesion predicted by early diffusion-weighted and perfusion magnetic resonance imaging: a preliminary analysis. J Cereb Blood Flow Metab 1996;16:53–59 [DOI] [PubMed] [Google Scholar]
- 12.Cercignani M, Iannucci G, Rocca MA, Comi G, Horsfield MA, Filippi M. Pathologic damage in MS assessed by diffusion-weighted and magnetization transfer MRI. Neurology 2000;54:1139–1144 [DOI] [PubMed] [Google Scholar]
- 13.Nusbaum AO, Tang CY, Wei TC, Buchsbaum MS, Atlas SW. Whole-brain diffusion MR histogram differ between MS subtypes. Neurology 2000;54:1421–1427 [DOI] [PubMed] [Google Scholar]
- 14.Cercignani M, Inglese M, Pagani E, Comi G, Filippi M. Mean diffusivity and fractional anisotropy histograms of patients with multiple sclerosis. AJNR Am J Neuroradiol 2001;22:952–958 [PMC free article] [PubMed] [Google Scholar]
- 15.Jones DK, Horsfield MA, Simmons A. Optimal strategies for measuring diffusion anisotropic systems by magnetic resonance imaging. Magn Reson Med 1999;42:512–525 [PubMed] [Google Scholar]
- 16.Bito Y, Hirata S, Yamamoto E. Optimal gradient factors for ADC measurements. Proceedings of the 3rd Annual Meeting of ISMRM, Nice, France,1995. , p.1344
- 17.Miller DH, Barkhof F, Berry I, Kappos L, Scotti G, Thomson AJ. Magnetic resonance imaging in monitoring the treatment of multiple sclerosis: concerted action guidelines. J Neurol Neurosurg Psychiatry 1991;54:683–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Studholme C, Hill DL, Hawkes DJ. Automated three-dimensional registration of magnetic resonance and positron emission tomography brain images by multiresolution optimization of voxel similarity measures. Med Phys 1996;24:25–35 [DOI] [PubMed] [Google Scholar]
- 19.Efron B, Tibshirani R. An Introduction to the Bootstrap. New York: Chapman & Hall;1993
- 20.Filippi M, van Waesberghe JH, Horsfield MA, et al. Interscanner variation in brain MRI lesion load measurements in MS: implications for clinical trials. Neurology 1997;49:371–377 [DOI] [PubMed] [Google Scholar]
- 21.Sormani MP, Iannucci G, Rocca MA, et al. Reproducibility of magnetization transfer ratio histogram-derived measures of the brain in healthy volunteers. AJNR Am J Neuroradiol 2000;21:133–136 [PMC free article] [PubMed] [Google Scholar]
- 22.Filippi M, Horsfield MA, Ader HJ, et al. Guidelines for using quantitative measures of brain magnetic resonance imaging abnormalities in monitoring the treatment of multiple sclerosis. Ann Neurol 1998;43:499–506 [DOI] [PubMed] [Google Scholar]
- 23.Chien D, Buxton R, Kwong K, Brady T, Rosen B. MR diffusion imaging of the human brain. J Comput Assist Tomogr 1990;14:514–520 [DOI] [PubMed] [Google Scholar]
- 24.Bastin ME, Armitage PA, Marshall I. A theoretical study of the effect of experimental noise on the measurement of anisotropy in diffusion imaging. Magn Reson Imaging 1998;16:773–785 [DOI] [PubMed] [Google Scholar]
- 25.Basser PJ Pajevic S. Statistical artifacts in diffusion tensor MRI (DT-MRI) caused by background noise. Magn Reson Med 2000;44:41–50 [DOI] [PubMed] [Google Scholar]
- 26.Chenevert TL, Brunberg JA, Pipe JG. Ansiotropic diffusion within human white matter: demonstration with NMR techniques in vivo. Radiology 1990;177:401–405 [DOI] [PubMed] [Google Scholar]
- 27.Hajnal JV, Doran M, Hall AS. MR imaging of anisotropically restricted diffusion of water in the nervous system: technical, anatomic, and pathologic considerations. J Comput Assisted Tomogr 1991;15:1–18 [DOI] [PubMed] [Google Scholar]
- 28.Bammer R, Abbehusem C, Rao A, et al. On the changes in diffusion anisotropy histograms. Mult Scler 2001;7[suppl 1]:S87 [Google Scholar]


