Abstract
BACKGROUND AND PURPOSE: In the treatment of cerebral aneurysms, platinum coils often fail to elicit a fibrotic response. We tested the hypothesis that a new, collagen-based endovascular coil would improve angiographic and histologic outcomes as compared with those achieved with platinum coils in a rabbit model of saccular aneurysms.
METHODS: Elastase-induced aneurysms were created in 12 New Zealand White rabbits (body weight, 3–4 kg). Embolization was performed either by use of collagen-based coils (n = 6) or platinum coils (n = 6). In both coil groups, subjects were kept alive for either 2 weeks (n = 3 [collagen], n = 3 [platinum]) or 10 weeks (n = 3 [collagen], n = 3 [platinum]) after embolization and then were sacrificed. Digital subtraction angiography (DSA) was performed immediately after embolization and immediately before sacrifice. Postmortem histologic analysis of all coils was performed.
RESULTS: Collagen-based coils were loosely packed in all cases because of limitations in size of coils available for embolization. In all six aneurysms packed with collagen-based coils, progressive thrombosis was noted at follow-up (DSA). Platinum coil samples were densely packed in all six cases. Progressive thrombosis was seen in one case, and interval regrowth was present in one case. Two weeks after embolization, collagen-based coil samples showed a marked peri-coil cellular response. Ten weeks after embolization, collagen-based samples had dense connective tissue matrix deposition in two of three cases. Platinum coils had unorganized thrombus at 2 weeks; loose-matrix deposition was only seen in the 10-week samples. Smooth muscle actin-positive cells were seen across the neck of the aneurysm in four of six collagen-based coil cases.
CONCLUSION: Collagen-based coils show a marked cellular response in animal-model aneurysms, with resultant high rates of progressive occlusion after embolization. Dense matrix deposition is commonly seen with collagen-based coils. This contrasts with low rates of progressive thrombosis and high rates of loose matrix deposition seen with platinum coils.
Since the development of the Guglielmi detachable coil (GDC; Target Therapeutics, Fremont, CA), platinum coils have become widely accepted as a valuable alternative to open surgery for the treatment of cerebral aneurysms; however, low rates of initial complete aneurysm obliteration, as well as high rates of late aneurysm recanalization, have been noted in wide-necked and large or giant aneurysms treated with platinum microcoils (1, 2). The mechanisms responsible for aneurysm recanalization remain unclear, but limited human histologic data suggest that the thrombus induced by coil placement remains unorganized; this unorganized, loose thrombus may be unable to withstand continued arterial pulsation (3). Conversely, it remains possible that the thrombus, because of persistent flow, cannot organize and thus undergoes thrombolysis.
Previous investigators have suggested that the inert nature of the platinum used to construct the GDC may explain the lack of thrombus organization (4). On the basis of this theory, many investigators have proposed modifications aimed at increasing the coil’s “biologic activity,” defined as improvement in thrombus organization, collagen formation, or endothelialization within and adjacent to the aneurysm cavity (4–11). The addition of collagen to platinum coils has received substantial attention (4, 6, 8, 11).
The purpose of the current study was to compare angiographic and histologic outcomes of a new, collagen-based endovascular coil as compared with those of platinum endovascular coils in a rabbit model of saccular aneurysms.
Methods
Aneurysm Creation
Twelve New Zealand White rabbits (body weight, 3–4 kg) underwent a protocol approved by the animal research committee of our institution for creation of elastase-induced aneurysm formation followed by embolization. Detailed procedures for aneurysm creation have been described elsewhere (12). In brief, anesthesia was induced with an intramuscular injection of a mixture of ketamine hydrochloride (Ketaved; Vedco, St. Joseph, MO [30 mg/kg body weight]) and xylazine hydrochloride (Tranquived; Vedco [6 mg/kg body weight]). Maintenance anesthetic was administered with an intramuscular injection of one-third the original anesthetic dose. Using a sterile technique, the right common carotid artery (CCA) was exposed and ligated distally. A 5F sheath (Cordis Endovascular, Miami Lakes, FL) was advanced retrograde in the CCA to a point approximately 3 cm cephalad to the CCA origin. Through this indwelling sheath, a 3F Fogarty balloon (Baxter Healthcare Corporation, Irvine, CA) was advanced to the origin of the right CCA at its junction with the subclavian artery. The balloon was inflated with just enough iodinated contrast medium (Omnipaque 300, Nycomed, Princeton, NJ) to achieve flow arrest in the CCA. Porcine elastase (5.23 U/mg, 40.1 mg/mL; Worthington Biochemical Corporation, Lakewood, NJ) mixed with saline and iodinated contrast medium was incubated in the dead space of the CCA, above the inflated balloon, for 20 minutes. After incubation of the elastase solution, the balloon and sheath were removed, and the CCA was ligated below the sheath entry site.
Collagen-Based Coil Device
The collagen-based coils were constructed from highly purified bovine type I collagen, which was incorporated with tantalum particles (<10 μm; Atlantic Equipment Engineers, Bergenfield, NJ). They were cross-linked with 1.0% glutaraldehyde in phosphate-buffered saline solution (PBS; pH, 7.4) for 20 hours at room temperature for in vivo stability. The cross-linked collagen-based coils were extensively rinsed in PBS (pH, 7.4) for 48 hours to eliminate the glutaraldehyde residuals. Although quantitative glutaraldehyde testing was not performed, we did perform cytotoxicity testing to ensure that glutaraldehyde residuals were below the level that would cause cytotoxicity (author’s [S.T.L.] unpublished data). The coils were constructed as single filaments of approximately 0.15–0.20 mm in diameter. Coils of 6 mm and 4 mm secondary shape with a length of 4–10 cm were prepared.
Embolization Procedures
Aneurysms were allowed to mature for at least 3 weeks after creation to allow for maturation of the aneurysm cavity (13). For the embolization procedure, anesthesia was induced and maintained as during aneurysm creating. Using a sterile technique, surgical exposure of the right common femoral artery was performed and a 5F vascular sheath was placed. Heparin (100 U/kg) was administered intravenously. A 5F catheter (Envoy; Cordis Endovascular) was advanced into the brachiocephalic artery. Using coaxial technique, an Excel microcatheter (Target Therapeutics, for platinum coils) or a Jetstream 18 (MicroInterventional Systems, [Mountainview, CA] for collagen-based coils) was advanced into the aneurysm. The size of the aneurysm was assessed by direct comparison with radiopaque sizing devices.
Embolization was performed in aneurysms either by use of collagen-based coils (n = 6 subjects) or detachable platinum coils (GDC; Target Therapeutics; n = 6 subjects). Platinum coils were advanced and detached as done clinically. Collagen-based coils were pushed through the microcatheter with a TruPush coil pusher (Cordis). When necessary, collagen-based coils were pushed out of the microcatheter by saline injection. Subjects were either kept alive for 2 weeks (n = 3, collagen-based coils; n = 3, platinum coils) or for 10 weeks (n = 3, collagen-based coils; n = 3, platinum coils) after embolization and then sacrificed. After coil embolization, the catheters were removed. The vascular sheath was removed, and the proximal aspect of the femoral artery was ligated with a 3–0 silk suture.
Follow-up Angiography
Subjects underwent intraarterial digital subtraction angiography (DSA) at the time of sacrifice with a 5F diagnostic catheter placed into the brachiocephalic artery from a femoral approach. All follow-up angiograms were compared with the immediate postembolization angiograms to assess for interval change in coil configuration or aneurysm filling.
Histologic Preparation
At the time of sacrifice, the subjects were deeply anesthetized and then euthanized by an overdose injection of pentobarbital. The mediastinum was dissected, and the aortic arch and proximal great vessels, including the coil-embolized segment of the artery, were exposed and dissected, free from surrounding tissues. The tissue was immediately placed in 2% paraformaldehyde. After fixation for at least 24 hours, samples were embedded in paraffin. Platinum coil samples embedded in paraffin were subsequently sectioned at 1000-μm intervals with a slow-speed saw (Isomet, Beuhler, IN). These thick sections were then viewed under a dissecting microscope, and, using gentle traction, the platinum coil winds were removed. After removal of all platinum, the samples were re-embedded in paraffin and sectioned at 7-μm intervals. Collagen-based coil samples embedded in paraffin were sectioned at 7-μm increments without the need for presectioning manipulation. Sections were stained with hematoxylin and eosin, Masson trichrome, and Movat pentachrome. Immunohistochemical staining was performed on collagen-based coil samples, using antibodies against alpha-smooth muscle actin and rabbit anti-macrophage-11.
Results
Angiographic Findings at Embolization
Results of angiographic evaluation are summarized in Table. Collagen-based coils were successfully placed in six subjects (Figs 1, 2). When collagen-based coils were placed within the aneurysm cavities, the coils generally conformed in excellent fashion to the shape of the aneurysm. Collagen coils were loosely packed in all cases because of limitations in the size of coils available for embolization as well as some difficulty in pushing coils into aneurysms. Difficulty in advancing the collagen coils into the aneurysm cavities was probably related to friction at the coil-coil pusher interface. In two collagen-based coil subjects, saline injection was required to push the coils into the aneurysm cavity. Such saline injection was effective in advancing the collagen-based coil but resulted in relatively uncontrolled deployment and untoward herniation of coils into the parent artery in two cases (Fig 2). Because of the relatively small number of different coil diameters, we were unable to pack the collagen-based coil subjects as densely as we were the platinum coil subjects. Immediately after embolization, percentage of occlusion ranged from 30% to 90% (mean, 62%).
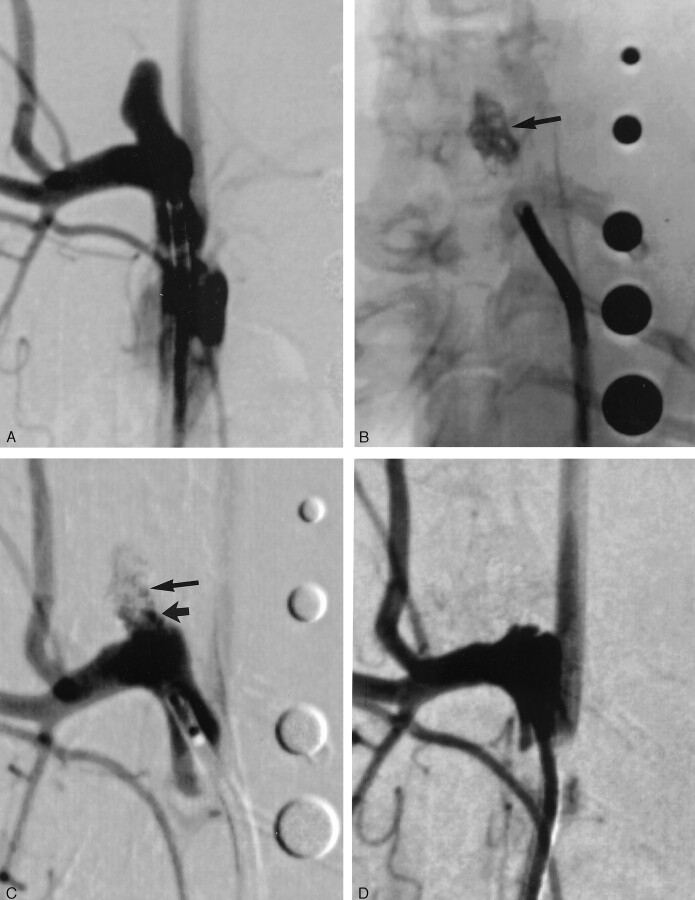
Fig 1.
Subject 1, aneurysm packed with collagen-based coil (2-week sample).
A, DSA image, showing aneurysm cavity.
B, Radiograph, showing collagen coils after implantation (arrow). Radiopaque sizing markers range from 2 mm to 6 mm in diameter.
C, DSA image, immediately after coil implantation, demonstrating residual flow at the neck (short arrow) and in the body (long arrow) of the aneurysm cavity.
D, DSA image, 2 weeks after implantation, showing interval progressive aneurysm occlusion, with no residual flow in the aneurysm cavity.
Fig 2.
Subject 8, aneurysm packed with collagen coil (10-week sample).
A, DSA image, showing aneurysm cavity.
B, Radiograph, showing collagen coils after implantation. Loose packing was present in the aneurysm cavity (long arrow) with a loop of coil present in the parent artery distal to the aneurysm cavity (short arrow).
C, DSA image, immediately after coil implantation, demonstrating large amount of residual flow in the body of the aneurysm cavity. No compromise of flow existed in the parent artery distal to the aneurysm cavity.
D, DSA image, 10 weeks after implantation, showing interval progressive aneurysm occlusion, with no residual flow in the aneurysm cavity and a triangular neck remnant (long arrow). Flow remained unimpeded in the region of the coils in the distal parent artery (short arrow).
Platinum coils were placed in six subjects. All of these aneurysms were densely packed with no adverse events. Percentage of occlusion immediately after embolization ranged from 70% to 95% (mean, 86%).
Angiographic Findings at Follow-up
Collagen-based coils.
At follow-up, all six aneurysms packed with collagen-based coils demonstrated progressive thrombosis. Five of these six aneurysms showed 100% occlusion at follow-up (Fig 1). One aneurysm in the 10-week sample showed marked progressive occlusion with a persistent triangular neck remnant (Fig 2). No evidence of untoward thrombus formation existed along the neck or in the parent artery in any of these cases. In one of three cases followed for 2 weeks, moderate parent artery compromise occurred because of coil segment protrusion from inadvertent herniation of coils into the parent artery that occurred during embolization.
Platinum coils.
DSA follow-up in the six subjects that underwent embolization with platinum coils demonstrated minimal progressive aneurysm occlusion in one subject, no change in four subjects, and minimal residual aneurysm filling in one subject.
Histologic Findings
Collagen-based coils.
All three aneurysms harvested at 2 weeks after embolization demonstrated diffuse intraaneurysmal cellular proliferation and thick tissue layers covering the neck (Fig 3). Cellular infiltration consisted of spindle cells, mononuclear cells, and some leukocytes. Spindle cells in the aneurysm cavity and across the aneurysm neck stained positive for SMA, indicating smooth muscle or myofibroblastic infiltration.
Fig 3.
Subject 3, aneurysm packed with collagen coil (2-week sample).
A, Histologic specimen showing the neck of the aneurysm covered by a thick layer of tissue consisting of nucleated cells (arrows). (Hematoxylin and eosin stain, original magnification ×40.)
B, Histologic speciment showing dense blue staining indicates collagen deposition within the aneurysm cavity. Vascular channels are also present. (Trichrome stain, original magnification ×100.).
Ten weeks after embolization, no residual unorganized thrombus was seen in any subject (Fig 4). Both dense and loose connective tissue infiltration was seen. Spindle cells were present in all subjects. Chronic inflammatory changes in the immediate vicinity of coil loops were present in one subject.
Fig 4.
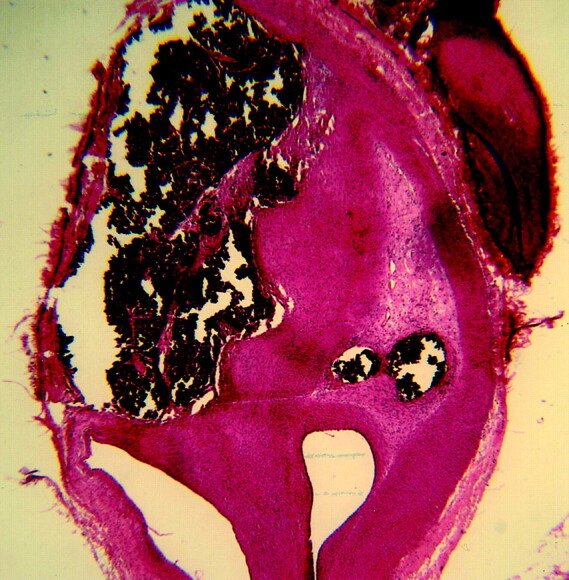
Subject 7, aneurysms packed with collagen-based coil (10-week sample). Histologic specimen showing the aneurysm cavity is filled with dense matrix and spindle cells. (Hematoxylin and eosin stain, original magnification ×20.).
Platinum coils.
Among the cases treated by platinum-coil embolization, all aneurysms followed for 2 weeks demonstrated unorganized thrombus filling the entire dome of the aneurysm with minimal inflammatory response (Fig 5). Thin fibrin was present at the neck in each of the three samples, and no evidence of spindle cell infiltration was present in any of the three samples. Dominant findings in aneurysms followed for 10 weeks after platinum coil embolization were unorganized thrombus in one case and loose connective tissue present in the other two cases (Fig 6). Unorganized thrombus was present in at least a portion of all these samples.
Fig 5.
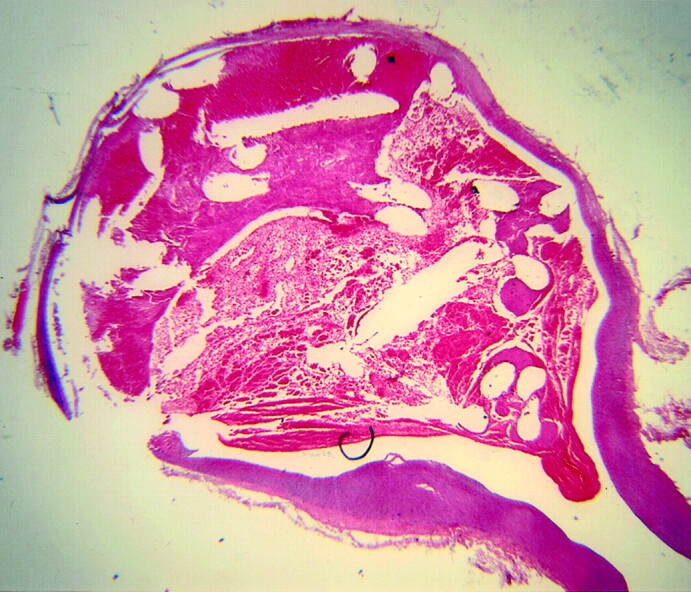
Subject 5, aneurysms packed with platinum coil (2-week sample). Histologic specimen showing the aneurysm cavity is filled with unorganized thrombus. The thrombus in the dome is laminated, suggesting ongoing turnover of unorganized thrombus. (Hematoxylin and eosin stain, original magnification ×20.).
Fig 6.
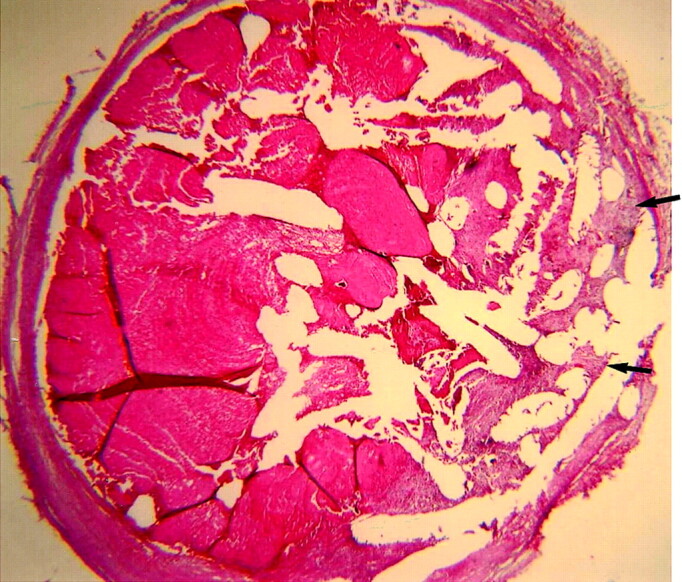
Subject 12, aneurysm packed with platinum coil (10-week sample). Histologic specimen showing the aneurysm cavity remains nearly completely filled with unorganized thrombus, with a small amount of loose matrix along the periphery (arrows). (Hematoxylin and eosin stain, original magnification ×20.).
Discussion
The hypothesis of the present investigation was based on numerous studies that have asserted that a collagen surface enhances cell adhesion, proliferation, and extracellular matrix synthesis, all of which contribute to fibrogenesis (14–16). Therefore, the objective of the current study was to test the hypothesis of collagen-induced fibrogenesis as a means to facilitate the occlusion of cerebral aneurysms. To facilitate the accurate placement of the collagen-based coil, we incorporated heavy-metal tantalum particles into the coils for radiopacity. The coil size and shape of the collagen filament was preformed and chemically cross-linked with glutaraldehyde to provide coil shape memory and also to improve the biomechanical characteristics for coil delivery. Because glutaraldehyde residuals are known to cause tissue reactions in terms of cytotoxicity and inflammation (17), cross-linked coils were rinsed extensively in PBS solution to remove the free glutaraldehyde residuals.
The incorporation of tantalum particles into the collagen filament at a weight ratio greater than 50% of tantalum provided sufficient radiopacity for coil placement. Similar to platinum coils, the collagen-based coils were of low thrombogenicity, even when segments of collagen-based coil protruded into the parent artery during delivery. We interpret this low thrombogenicity to the cross-linking effect of glutaraldehyde; cross-linking renders the collagen surface to be negatively charged by eliminating the e-amino groups of lysines and hydroxylysines from the surface of the collagen coils. It is known that a negatively charged surface is more hemocompatible (18).
The collagen-based prototypes used in the current study were not retrievable. Therefore, when problems occurred during coil delivery, we could not retrieve the coil from the aneurysm or parent artery. This design limitation resulted in partial protrusion of the coil into the parent artery in two of six subjects. Because the coil is hemocompatible, this did not result in arterial occlusion during the course of the current study. It is anticipated that a detachable form of the collagen-based coil could be developed in the future.
Unlike platinum coils, the collagen-based coils showed marked progressive occlusion angiographically. The angiographic observation was corroborated with the histologic findings that showed substantially more cellular activity, intraaneurysmal proliferation of SMA-positive cells, and fibrous tissue deposition as compared with that seen with platinum coils; however, the types of cells observed in the collagen-based coils also suggested some degree of inflammatory reaction. It is possible that the collagen-bound glutaraldehyde molecules or its polymeric moieties not directly involved in cross-linking formation induced the inflammatory response or that the inflammatory response might be partially caused by the tantalum particles.
Previous authors have proposed the use of collagen as an adjunct to platinum coils for aneurysm embolization. A swine model was used to evaluate collagen-coated and collagen filament-bearing microcoils in two separate studies (6, 11). Both studies showed complete obliteration of aneurysm cavities with the modified platinum coil devices; however, swine aneurysms in general tend to heal rapidly, even with standard platinum coils, so the relevance of these previous data to human aneurysms remains unclear. Szikora et al (8) used a canine model to evaluate a collagen-bearing microcoil. These authors noted that the collagen induced a local proliferation of fibroblasts but did not improve the angiographic outcomes as compared with standard coils. The collagen filament-bearing coils generally do not provide large surface areas for cell adhesion. The angiographic outcomes observed in the current study are consistent with the hypothesis that maximizing collagen surface exposure to cells will enhance aneurysm occlusion.
The current study demonstrates the feasibility of performing embolization in saccular aneurysms by use of coils constructed from purified type I collagen. The present study, although promising, has several limitations. Thus, a number of improvements must be made before the collagen-based coil can be used clinically. The coil must be able to be delivered, detached, visualized in situ, and retrieved. We believe that each of these objectives can be achieved by applying current microcatheter technology and the knowledge and expertise accumulated in engineering collagen-based materials for endovascular applications. This work is currently in progress.
Conclusion
Collagen-based coils demonstrate an active cellular response in model aneurysms and resultant high rates of progressive occlusion after embolization. Dense matrix deposition is commonly seen with collagen-based coils. This contrasts with low rates of progressive thrombosis and high rates of loose matrix deposition seen with platinum coils.
Acknowledgments
We thank Cynthia Dodson for assistance in animal care and Thomas D. Young for assistance in histologic processing.
Footnotes
Supported by National Institutes of Health grant 1R43NS40630 (to S.-T.L.)
Presented at the 40th annual meeting of the American Society of Neuroradiology, Vancouver, B.C., May 11–17, 2002.
Most of this work was performed in the Department of Radiology, University of Virginia, Charlottesville, VA (D.F.K., N.H.F.).
References
- 1.Hayakawa M, Murayama Y, Duckwiler GR, et al. Natural history of the neck remnant of a cerebral aneurysm treated with the Guglielmi detachable coil system. J Neurosurg 2000;93:561–568 [DOI] [PubMed] [Google Scholar]
- 2.Gruber A, Killer M, Bavinzski G, Richling B. Clinical and angiographic results of endosaccular coiling treatment of giant and very large intracranial aneurysms: a 7-year, single-center experience. Neurosurgery 1999;45:793–803; discussion 803–804 [DOI] [PubMed] [Google Scholar]
- 3.Molyneux AJ, Ellison DW, Morris J, Byrne JV. Histological findings in giant aneurysms treated with Guglielmi detachable coils: report of two cases with autopsy correlation. J Neurosurg 1995;83:129–132 [DOI] [PubMed] [Google Scholar]
- 4.Murayama Y, Suzuki Y, Vinuela F, et al. Development of a biologically active Guglielmi detachable coil for the treatment of cerebral aneurysms. Part I. In vitro study. AJNR Am J Neuroradiol 1999;20:1986–1991 [PMC free article] [PubMed] [Google Scholar]
- 5.Murayama Y, Vinuela F, Suzuki Y, et al. Development of the biologically active Guglielmi detachable coil for the treatment of cerebral aneurysms. Part II. An experimental study in a swine aneurysm model. AJNR Am J Neuroradiol 1999;20:1992–1999 [PMC free article] [PubMed] [Google Scholar]
- 6.Dawson RC, 3d, Shengelaia GG, Krisht AF, Bonner GD. Histologic effects of collagen-filled interlocking detachable coils in the ablation of experimental aneurysms in swine. AJNR Am J Neuroradiol 1996;17:853–858 [PMC free article] [PubMed] [Google Scholar]
- 7.Kallmes DF, Borland MK, Cloft HJ, et al. In vitro proliferation and adhesion of basic fibroblast growth factor-producing fibroblasts on platinum coils. Radiology 1998;206:237–243 [DOI] [PubMed] [Google Scholar]
- 8.Szikora I, Wakhloo AK, Guterman LR, et al. Initial experience with collagen-filled Guglielmi detachable coils for endovascular treatment of experimental aneurysms. AJNR Am J Neuroradiol 1997;18:667–672 [PMC free article] [PubMed] [Google Scholar]
- 9.Kallmes DF, Williams AD, Cloft HJ, et al. Platinum coil-mediated implantation of growth factor-secreting endovascular tissue grafts: an in vivo study. Radiology 1998;207:519–523 [DOI] [PubMed] [Google Scholar]
- 10.de Gast AN, Altes TA, Marx WF, et al. Transforming growth factor beta-coated platinum coils for endovascular treatment of aneurysms: an animal study. Neurosurgery 2001;49:690–694; discussion 694–696 [DOI] [PubMed] [Google Scholar]
- 11.Dawson RC, Krisht AF, Barrow DL, et al. Treatment of experimental aneurysms using collagen-coated microcoils. Neurosurgery 1995;36:133–139; discussion 139–140 [DOI] [PubMed] [Google Scholar]
- 12.Altes TA, Cloft HJ, Short JG, et al. 1999 ARRS Executive Council Award. Creation of saccular aneurysms in the rabbit: a model suitable for testing endovascular devices. AJR Am J Roentgenol 2000;174:349–354 [DOI] [PubMed] [Google Scholar]
- 13.Fujiwara NH, Cloft HJ, Marx WF, et al. Serial angiography in an elastase-induced aneurysm model in rabbits: evidence for progressive aneurysm enlargement after creation. AJNR Am J Neuroradiol 2001;22:698–703 [PMC free article] [PubMed] [Google Scholar]
- 14.Kleinman HK, Klebe RJ, Martin GR. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol 1981;88:473–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klebe RJ, Rosenberger PG, Naylor SL, et al. Cell attachment to collagen: isolation of a cell attachment mutant. Exp Cell Res 1977;104:119–125 [DOI] [PubMed] [Google Scholar]
- 16.Macarak EJ, Howard PS. Adhesion of endothelial cells to extracellular matrix proteins. J Cell Physiol 1983;116:76–86 [DOI] [PubMed] [Google Scholar]
- 17.Speer DP, Chvapil M, Eskelson CD, Ulreich J. Biological effects of residual glutaraldehyde in glutaraldehyde-tanned collagen biomaterials. J Biomed Mater Res 1980;14:753–764 [DOI] [PubMed] [Google Scholar]
- 18.Sawyer PN, Stanczewski B, Kirschenbaum D. The development of polymeric cardiovascular collagen prostheses. Artif Organs 1977;1:83–91 [DOI] [PubMed] [Google Scholar]