Abstract
Summary: The imaging characteristics of spontaneous intracranial hypotension have been well described in the clinical and imaging literature. We present a case of spontaneous intracranial hypotension with typical clinical and laboratory features that were thought to be suspicious for a ruptured aneurysm. Blood in the CSF in conjunction with headaches led to cerebral angiography that showed diffuse enlargement of cortical and medullary veins. The angiographic findings were diagnostic of spontaneous intracranial hypotension and consistent with the Monro-Kellie hypothesis.
Spontaneous intracranial hypotension (SIH) was initially described by Schaltenbrand (1) as a spontaneous positional headache, neck stiffness, nausea, tinnitus, and vertigo in patients with low CSF pressure. The diagnosis is based on clinical presentation, CSF evaluation, and diffuse pachymeningeal enhancement on MR images (2, 3). We present a case of spontaneous intracranial hypotension with typical clinical and MR imaging findings and with pertinent findings shown by cerebral angiography.
Case Report
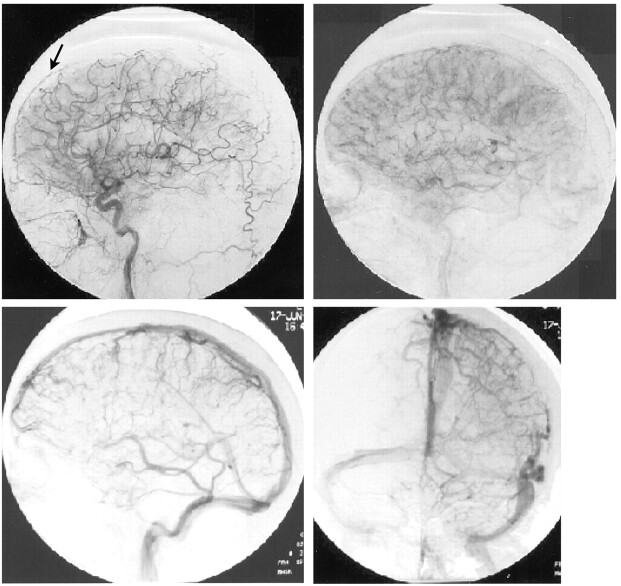
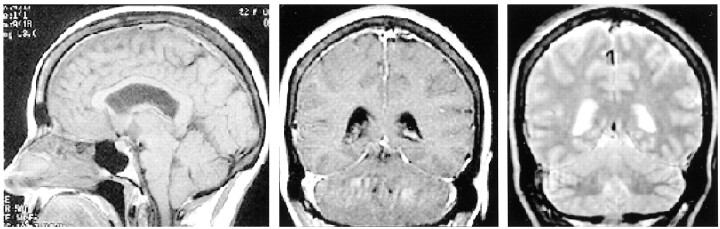
A 50-year-old woman presented with severe headaches that she experienced while in the upright position but that were relieved when she was supine. No abnormal neurologic findings were revealed by physical examination. A lumbar puncture was performed with an undetectable opening pressure. CSF studies revealed 629 red blood cells without evidence of a traumatic tap, elevated protein of 257 mg/dL, and glucose of 89 mg/dL and was positive for lymphocytes and neutrophils. Initial CT showed decreased basal cistern visualization interpreted at an outside institution as possible subacute subarachnoid hemorrhage. Cerebral angiography was requested by the neurosurgery service to identify a possible source of subarachnoid hemorrhage. The angiogram excluded an intracranial aneurysm but showed an enlarged anterior falx artery and enlargement of cortical and medullary veins (Fig 1). MR imaging showed diffuse meningeal enhancement, increased T2 signal intensity within the pachymeninges, decreased size of the prepontine and superior cerebellar cisterns, and downward displacement of hypothalamic structures and the cerebellar tonsils (Fig 2). Myelography and radionuclide cisternography did not reveal a CSF leak. The angiographic and MR imaging findings were indicative of spontaneous intracranial hypotension.
Fig 1.
Arteriograms of the left carotid artery. Arterial and venous phases show an enlarged anterior falx artery (arrow) and multiple enlarged cortical and medullary veins, associated with SIH.
Fig 2.
MR images of SIH. Sagittal view T1-weighted image (left) shows depressed cerebellar tonsils, hypothalamic structures, and effacement of the prepontine cistern. Coronal view T1-weighted image (center) shows diffuse dural thickening and enhancement, and coronal view T2-weighted image (right) shows dural high T2 signal intensity. These findings resolved with the symptoms.
The patient received supportive treatment, and her symptoms improved. Follow-up MR imaging performed 1 month later showed near complete resolution of the meningeal enhancement, improved patency of the basal cisterns, and less hypothalamic/tonsilar displacement.
Discussion
Diagnostic imaging of patients with suspected SIH has led to a number of characteristic descriptions. Findings on MR imaging include subdural fluid collections, downward descent of the midbrain and cerebellar tonsils, and a reduction in the size of the prepontine cistern. However, the most characteristic finding on MR imaging is diffuse pachymeningeal enhancement (2).
Many hypothesize that the dural enhancement of SIH is due to venous engorgement as opposed to inflammatory changes (3, 4). This is primarily based on the Monro-Kellie hypothesis, which states that with an intact skull, the sum of the brain volume plus CSF volume plus intracranial blood volume is constant (5). Therefore, a reduction in the CSF volume should result in an increase in the intracranial blood volume. With an increase in venous blood, there is presumably accumulation of contrast material in the pachymeninges due to the lack of a blood-brain barrier. Furthermore, Chung et al (2) have suggested that the increase in dural signal intensity on long TR weighted images may discriminate SIH from pachymeningitis. Meningeal biopsies in cases of SIH lack evidence of inflammation, infection, or neoplasia, further supporting this hypothesis (6).
Visualization of venous engorgement in association with SIH has infrequently been described. The MR imaging literature has described dilated cervical epidural veins in cases of orthostatic headaches (7, 8). Although there are numerous differential considerations, generally, no secondary signs support another cause for cervical epidural venous dilation. These findings will spontaneously resolve with the patient’s symptoms and are thought to be the sequelae of CSF hypotension. A paucity of angiographic descriptions of SIH is available in the literature. The only published data of which we are aware are presented by Christoforidis et al (4), who described an attenuated meningeal blush and an enlarged anterior falx artery. Cerebral angiography in our case of SIH showed an enlarged anterior falx artery and markedly enlarged cortical and medullary veins. The venous engorgement shown is in accordance with the Monro-Kellie rule. This further supports the hypothesis that diffuse pachymeningeal enhancement seen on MR images in cases of SIH is a hydrostatic phenomena due to increased intracranial blood volume.
The presence of >600 red cells in the CSF was the driving force behind the arteriogram obtained in this case. Yet, abnormal amounts of blood and protein in the CSF is a common feature of SIH and is thought to be a result of meningeal hyperemia and red blood cell diapedesis (9, 10). Up to 1100 cells have been seen with nontraumatic lumbar punctures in cases of SIH (2, 4). Furthermore, a traumatic lumbar puncture is more likely with low CSF pressure. Xanthochromia being the hallmark of chronic subarachnoid hemorrhage is a common observation in association with SIH. It has been suggested that SIH may have clinical and diagnostic features that are similar to those of subarachnoid hemorrhage caused by other factors (10). However, the presence of subarachnoid hemorrhage in conjunction with characteristic clinical features of SIH should lead first to MR imaging. Had MR imaging been available in our case, the patient likely would not have undergone arteriography to exclude another source of subarachnoid hemorrhage. Should angiography be required in atypical clinical scenarios, the findings can be diagnostic of SIH and are explained by the Monro-Kellie hypothesis.
References
- 1.Schaltenbrand G. Normal and pathological physiology of cerebrospinal fluid circulation. Lancet 1953;I:805–809 [DOI] [PubMed] [Google Scholar]
- 2.Chung SJ, Kim JS, Lee MC. Syndrome of cerebral spinal fluid hypovolemia: clinical and imaging features and outcome. Neurology 2000;55:1321–1327 [DOI] [PubMed] [Google Scholar]
- 3.Fishman RA, Dillon WP. Dural enhancement and cerebral displacement secondary to intracranial hypotension. Neurology 1993;43:609–611 [DOI] [PubMed] [Google Scholar]
- 4.Christoforidis GA, Mehta BA, Landi JL, Czarnecki EJ, Piaskowski RA. Spontaneous intracranial hypotension: report of four cases and review of the literature. Neuroradiology 1998;40:636–643 [DOI] [PubMed] [Google Scholar]
- 5.Mokri B. The Monro-Kellie hypothesis: applications in CSF volume depletion. Neurology 2001;56:1746–1748 [DOI] [PubMed] [Google Scholar]
- 6.Mokri B, Parisi JE, Scheithauer BW, Piepgras DG, Miller GM. Meningeal biopsy in intracranial hypotension: meningeal enhancement on MRI. Neurology 1995;45:1801–1807 [DOI] [PubMed] [Google Scholar]
- 7.Clarot F, Callonnec F, Douvrin F. Giant cervical epidural veins after lumbar puncture in a case of intracranial hypotension. AJNR Am J Neuroradiol 2000;21:787–789 [PMC free article] [PubMed] [Google Scholar]
- 8.Forderreuther S, Yousry I, Empl M, Straube A. Dilated cervical epidural veins and extra arachnoid fluid collection in orthostatic headaches. Neurology 2001;57:527–529 [DOI] [PubMed] [Google Scholar]
- 9.Rando TA, Fishman RA. Spontaneous intracranial hypotension: report of 2 cases and review of the literature. Neurology 1992;42:481–487 [DOI] [PubMed] [Google Scholar]
- 10.Schievink WI, Wijdicks EF, Meyer FB, Sonntag VK. Spontaneous intracranial hypotension mimicking aneurysmal subarachnoid hemorrhage. Neurosurgery 2001;48:513–517 [DOI] [PubMed] [Google Scholar]