Abstract
Thromboembolic events are potential complications of neurointerventional procedures. The mainstay of therapy for these complications is both supportive therapy and the use of fibrinolytic agents. In some cases, the occluding clot seems to be resistant to fibrinolytic or anti-platelet agents. We herein report our successful attempt at clot removal by using a microsnare to mechanically capture and remove a small resistant organized clot fragment that was occluding one of the post-trifurcation M2 divisions of a middle cerebral artery. This complication occurred during coil embolization of an ipsilateral posterior communicating aneurysm.
Thromboembolic events during neurointerventional procedures are recognized complications (1). When they occur, they are generally treated with systemic support therapy, including oxygen, IV administered fluids, plasma volume expanders, and heparin (2), together with the use of fibrinolytic drugs (3) or, more recently, anti-platelet agents (4, 5). Despite these methods, there are cases in which the occluding material is resistant to pharmacologic dissolution and the decision must then be made either to do nothing further in the immediate setting or to attempt removing the occlusive material, thereby reestablishing flow distal to the occlusion.
Case Report
A 54-year-old female patient had previously undergone bilateral craniotomies for the clipping of three cerebral aneurysms on the right side and a middle cerebral bifurcation aneurysm on the left. A fifth aneurysm arose from the communicating segment of the left internal carotid artery. The neurosurgeon had been unable to place a clip across the neck of the fifth aneurysm and had instead placed a tissue wrap around the fundus of this aneurysm. The patient had also developed a right-sided hemiplegia after the second operation, from which she had made a slow but good recovery.
Follow-up cerebral angiography performed >1 year later showed growth of the distal aspect of the left communicating segment aneurysm, including the development of a small distal daughter dome. At that stage, the patient was complaining of intermittent ipsilateral retro-orbital pain. Because the surgeon involved was reluctant to perform another craniotomy on the same side, endovascular treatment was considered. Furthermore, the patient did not wish to undergo any further surgery, particularly considering the transient postoperative hemiplegia that had developed previously. It was apparent from the outset that complete occlusion of the aneurysm would not be possible because of the broad neck of the aneurysm with incorporation of the posterior communicating arterial origin within the proximal part of the aneurysm. The ipsilateral posterior cerebral arterial supply was dependent upon this posterior communicating artery; thus, preservation of the latter was necessary. It was decided to treat the distal aspect of the aneurysm with coils, because that was the part showing the greatest degree of growth and potential weakness. It was hoped that coiling would offer some degree of protection against rupture and would reduce the severity of the headaches.
The embolization was performed with the patient under full general anesthesia. A right common femoral access was used, and a 6F Northstar Lumax Guiding Catheter (Cook, Bloomington, IN) was placed with the aid of fluoroscopic guidance (with road mapping) into the left internal carotid artery, just below the skull base. Systemic heparin was administered with the activated clotting time kept at approximately 300 s throughout the procedure. The aneurysm was catheterized by using a steam shaped Excel-14 microcatheter (Target Therapeutics, Fremont, CA), and the distal pouch of the aneurysm was occluded by using a total of four GDC-10 GDCs (Target Therapeutics), with diameters ranging from 4 to 7 mm. After placement of the final coil and withdrawal of the Excel microcatheter from the aneurysm, a final check arteriogram showed occlusion of one of the post-trifurcation M2 branches of the ipsilateral middle cerebral artery, with absent blood flow seen in the paracentral and parietal regions (Fig. 2).
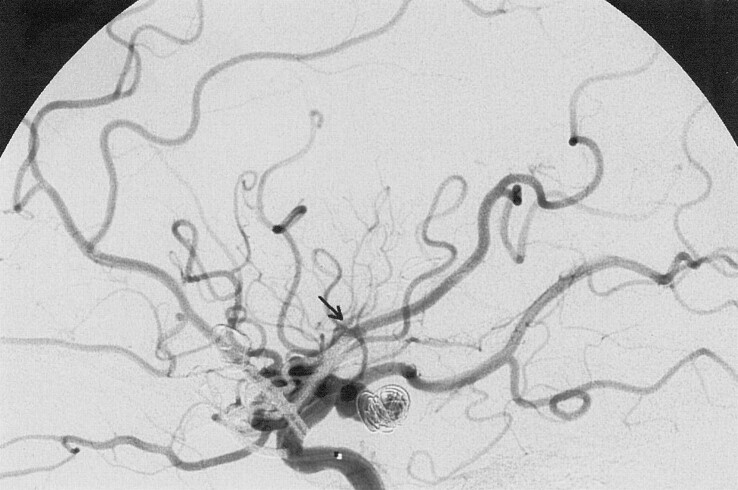
Fig 2.
Check arteriogram during coil placement shows proximal occlusion of one of the middle cerebral artery post-trifurcation trunks with absence of filling of vessels in the paracentral and parietal region. The arrow indicates the level at which the trunk is occluded before intra-arterial abciximab infusion.
Retrospective analysis of runs conducted after the placement of each coil showed that this vessel had occluded after placement of the first coil but had gone unnoticed at that stage. Based on our previous experience with acute procedure-related thrombosis, we placed the same Excel-14 microcatheter directly into the proximal aspect of the occluded vessel and injected a bolus dose of 15 mg of abciximab (Reo Pro; Eli Lilly, Alexandria, VA) through the catheter during a period of 1 min. Within 3 min, most of the clot within this artery had dissolved, leaving only one small fragment of undissolved clot bridging a small distal branch bifurcation (Fig. 3). The microcatheter tip was then advanced up against this small clot remnant, and an additional 7.5 mg of abciximab was administered at that point. The residual fragment did not respond to this second dose, and we elected to try to snare the clot. A 2-mm diameter Amplatz “Goose-Neck” microsnare (Microvena, White Bear Lake, MN) was placed through the Excel-14 microcatheter (Fig. 4). The microsnare loop was gently rotated several times in the vicinity of the residual clot fragment and then drawn back partly into the microcatheter tip. No clear resistance was felt while withdrawing the loop to indicate that the fragment had been successfully captured. The snare loop was left protruding by approximately 80% of the loop’s diameter so as not to fragment the clot, and assuming that it had been captured at that stage when the entire microcatheter was withdrawn completely through the guiding catheter. A check arteriogram showed that the small residual clot fragment had been completely removed by the snare and normal flow had been reestablished in the affected artery (Fig. 5). No spasm was encountered in any artery during the procedure. The patient achieved a normal recovery from the anesthetic, with no postprocedural neurologic deficit.
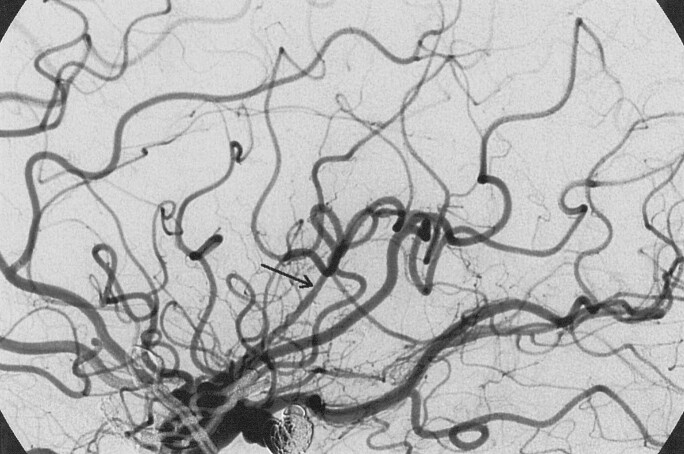
Fig 3.
After abciximab bolus administration via direct microcatheter infusion, flow has been reestablished within the occluded trunk, leaving a small fragment of resistant material within a distal branch (arrow).
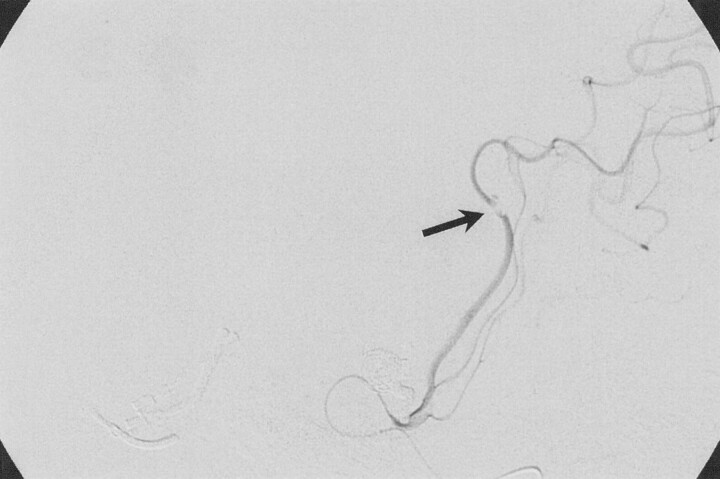
Fig 4.
Microsnare tip is seen protruding from the Excel microcatheter at the level of the fragment.
Fig 5.
Check arteriogram obtained after removal of the microsnare and microcatheter shows that the fragment has been removed and normal flow has been reestablished in the previously occluded branch (arrow).
An overnight infusion of heparin was maintained. No further abciximab was administered, and no other complications were encountered. The patient was discharged 2 days later. The clot fragment was sent for histologic analysis and was found to be an organized thrombus.
Discussion
Thrombus formation or displacement is a well-recognized complication during neurointerventional procedures (1). The formation of new thrombi within catheters or vessels can be prevented by the use of flush solutions in guiding and microcatheters and systemic heparinization, respectively. A fresh thrombus can also form within the aneurysm sac as part of the reaction to the presence of platinum coils. Organized thrombi may be present on ulcerated plaques within the internal carotid or vertebral arteries but may also be present within the aneurysm lumen and can potentially be displaced by coil manipulation within the aneurysm.
The traditional methods of treatment of thromboembolic complications during neurointerventional procedures include general systemic support therapy, which involves the administration of oxygen and IV fluids, the use of plasma volume expanders, the maintenance of blood pressure and systemic heparinization (2), and the application of more direct therapy with thrombolytic agents (3) or platelet aggregation inhibitors (4, 5). Since 1995, we have performed nearly 250 neurointerventional procedures and >1200 diagnostic cerebral angiographic procedures in our unit. Including the case under discussion, we have encountered eight documented intraprocedural thrombotic complications. Of the first seven, six were treated with microcatheter-directed intra-arterial infusions of abciximab administered directly into the occluded vessel (6). In five of these cases, we noted complete dissolution of the clot, with partial dissolution in one. Based on this experience, our initial treatment of choice in the case described herein was catheter-directed abciximab infusion, which resulted in dissolution of most of the clot within the affected artery but left a small distal fragment obviously resistant to the abciximab. Having heard anecdotally of the use of microsnares for clot removal, including the case report described by Chopko et al (7), we elected to attempt the transcatheter microsnare clot removal technique, which proved very successful in this case. The histologic report of organized thrombus confirmed our earlier suspicion that clot fragments that appear resistant to fibrinolytic or anti-platelet agents could represent organized clot or even intimal or plaque fragments. When these embolize and lodge within a vessel, the stasis produced probably initiates acute thrombosis proximal to the fragment, which will more readily respond to clot dissolution.
In this case, we had the choice between leaving the resistant clot fragment alone and awaiting the eventual clinical outcome and attempting mechanical removal of the clot. In some cases in which occlusion of the middle cerebral artery occurs at or beyond the bifurcation or trifurcation, the patients may still do well clinically, mainly because of the presence of collateral blood supply to the affected middle cerebral artery territory through pial anastomoses from the adjacent cerebral arterial territories. However, the extent and effectiveness of this collateral supply is somewhat variable and may not be sufficient to sustain adequate blood flow to the threatened area.
As seen in Figure 2, an absence of blood flow was initially seen in the paracentral and parietal region threatening the primary motor and sensory cortices. Flow was reestablished in most of this area after intra-arterial abciximab administration, leaving a small resistant clot fragment partly obstructing a vessel supplying the parietal lobe. Despite the presence of blood flow around the clot, we could not guarantee that the vessel would not re-occlude once the heparin and abciximab levels had diminished, nor could we be certain that the antegrade flow around the clot with or without assistance from pial collaterals would be adequate to ensure that no permanent neurologic deficit would result. The patient had initially been very insistent regarding endovascular management of her aneurysm, refusing surgery mainly because of the neurologic deficits that had resulted after the previous surgical procedure. Considering this fear of any further iatrogenic complications, we were determined not to leave her with any neurologic deficits and elected to attempt snare-assisted removal of the remaining clot fragment. We were aware of the potential risks of this procedure, including dissection, perforation, spasm, or clot fragmentation with distal embolization. However, working carefully and gently, we managed to pass the microcatheter up to the position of the residual clot fragment and deploy the microsnare through the microcatheter with an absolute minimum of manipulation. The clot was thus trapped and removed with no trauma to the vessel concerned.
This case has again shown the potential usefulness of mechanical clot removal for cases in which pharmacologic methods of treatment have failed. When considering this technique, the potential for vessel damage due to dissection, perforation, spasm, or clot fragmentation with more distal embolization during the snare manipulation should be weighed against the risks of infarction if the vessel concerned remains occluded.
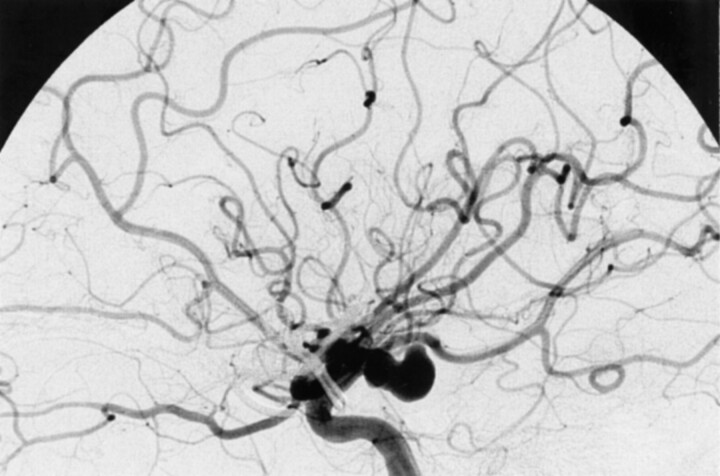
Fig 1.
Selective left internal carotid digital subtraction arteriogram shows the communicating segment aneurysm.
References
- 1.Connors JJ, Wojak JC. Other problems, complications and solutions. In: Connors JJ, Wojak JC, eds. Interventional Neuroradiology: Strategies and Practical Techniques. Philadelphia: WB Saunders Company;1999. :777–781
- 2.Connors JJ, Wojak JC. Specific stroke situations, territories and guidelines for therapy. In: Connors JJ, Wojak JC, eds. Interventional Neuroradiology: Strategies and Practical Techniques. Philadelphia: WB Saunders Company;1999. :692–751
- 3.Cronqvist M, Pierot L, Boulin A, Cognard C, Castaigns L, Moret J. Local intra-arterial fibrinolysis of thromboemboli occurring during endovascular treatment of intracerebral aneurysm: a comparison of anatomic results and clinical outcome. AJNR Am J Neuroradiol 1998;19:157–165 [PMC free article] [PubMed] [Google Scholar]
- 4.Ng PP, Phatouros CC, Khangure MS. Use of glycoprotein IIb-IIIa inhibitor for a thromboembolic complication during Guglielmi detachable coil treatment of an acutely ruptured aneurysm. AJNR Am J Neuroradiol 2001;22:1761–1763 [PMC free article] [PubMed] [Google Scholar]
- 5.Cloft HJ, Samuels OB, Tong FC, Dion JE. Use of abciximab for mediation of thromboembolic complications of endovascular therapy. AJNR Am J Neuroradiol 2001;22:1764–1767 [PMC free article] [PubMed] [Google Scholar]
- 6.Duncan IC, Fourie PA. Catheter-directed intra-arterial abciximab administration for acute thrombotic occlusions during neurointerventional procedures. Interventional Neuroradiology 2002;8:159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chopko BW, Kerber C, Wong W, Georgy B. Transcatheter snare removal of acute middle cerebral artery thromboembolism: technical case report. Neurosurgery 2000;46:1529–1531 [DOI] [PubMed] [Google Scholar]