Abstract
BACKGROUND AND PURPOSE: Bilateral engorged superior ophthalmic veins (SOV) have been reported in patients with diffuse brain swelling. We investigated the relationship between the diameter of the SOV on brain MR images and the intracranial pressure (ICP).
METHODS: We reviewed the medical records of neurologic inpatients who had undergone both MR imaging of the brain and lumbar puncture. MR imaging had to have been performed before lumbar puncture, and the two studies had to have occurred within 2 days. The diameters of the SOV were measured on coronal contrast-enhanced fat-saturated T1-weighted MR images. For this, the image nearest the rear of the globe of the eye was chosen.
RESULTS: Sixty-nine patients (32 male, 37 female; mean age, 46 years ± 19) were included. The average diameters of the SOV and the ICP were positively correlated (r = 0.58, P < .001), if an SOV diameter of <1 mm was treated as 0.5 mm for calculations. In patients with increased ICP (CSF pressure >200 mm H2O), SOV diameters were larger than those of patients with a normal CSF pressure (3.0 vs 1.6 mm, P < .001). Frequencies of increased ICP were 3% among patients with an average SOV diameter of 0.5–1 mm, 15% for 1.5–2 mm, and 58% for 2.5–5 mm (P < .001).
CONCLUSION: This study showed that the SOV diameter, determined on the basis on MR imaging, was positively correlated with ICP. Dilatation of the SOV should alert physicians to the possibility of increased ICP.
Unilateral engorged superior ophthalmic veins (SOVs) have been noted to occur with carotid-cavernous fistulas (1), Graves disease (2), orbital pseudotumors, and retrocavernous meningiomas (3). Recently, a relationship between the diameter of the SOV and intracranial pressure (ICP) has also been reported (4). The investigators discussed a review of 11 patients with bilateral SOV enlargement associated with diffuse cerebral swelling, as detected with CT (4). The SOV carries most the venous drainage from the orbit and the eye, it is usually fused with the thinner inferior ophthalmic vein, and it empties the combined venous blood into the cavernous sinus (5). Anatomically, the two cavernous sinuses are not completely isolated in the dura, and thus, they are easily influenced by alteration in intracranial hydrodynamics (5). According to the Monro-Kellie doctrine, intracranial blood volumes vary reciprocally with changes in the CSF volume, when the volume of the closed cranial cavity is constant (6, 7). Therefore, a decrease or increase in CSF volume directly or indirectly influences the intracranial venous sinuses, including the cavernous sinuses, and thus, influences the bilateral SOVs.
The SOV is located just under the superior rectus muscle. It can be easily identified and measured on coronal contrast-enhanced T1-weighted images obtained with fat-saturation pulse sequencing. This simple technique provided us with an opportunity to investigate the relationship between the diameter of the SOV and the ICP among neurology inpatients.
Methods
We retrospectively reviewed the data of the inpatients at our neurology service during the period from February 2000 to February 2002. Inclusion was restricted to patients who had undergone both MR imaging of the brain with contrast enhancement and lumbar puncture for the diagnosis of their illness during their hospitalization. MR imaging had to have been performed before lumbar puncture, and the two studies had to have occurred within a 2-day interval. Patients with Graves disease, carotid-cavernous fistula, or cavernous sinus lesions were excluded.
CSF- opening pressures were measured by using the standard lumbar puncture method; that is, with the patients fully extending their knees. The clinical data, including patient demographic information, relevant neurologic disorders, and CSF results were collected. In this study, increased ICP was defined as CSF pressure >200 mm H2O (8), and normal ICP was defined as CSF pressure of 60–200 mm H2O.
Coronal 5-mm-thick sections with 2.5-mm intersection gaps were obtained by using 1.5-T MR units (Signa CV/i, GE Medical Systems, Milwaukee, WI; Magnetom Vision, Siemens, Erlangen, Germany). The matrix was 256 × 256, corresponding to a field of view of 24 cm. The diameters of the SOVs were measured on the contrast-enhanced coronal T1-weighted images (TR/TE/NEX, 450/30/2; Magnevist, Schering, Berlin, Germany) obtained with fat-saturation pulse sequencing. This technique, which began as a brain MR imaging routine at our hospital in February 2000, suppresses the signal intensity of the fat tissue in the retrobulbar area of the orbital cavities.
We measured the diameter of the SOV on the images closest to the most posterior aspect of the globe of the eye. The measurement was performed on a workstation with the input of the patient imaging data transmitted through optical disks or an intra-network. A neuroradiologist (J.-F.L.) who was blinded to the patients’ clinical conditions and the CSF study results independently measured the diameter of the SOVs. Because the sensitivity of the length measurement was 1 mm, the diameters were recorded in millimeters as <1, 1, 2, 3, 4, 5, and so on. With SOV diameters of <1 mm, we used a value of 0.5 mm for all analyses. In a pilot study, the neuroradiologist measured the SOV diameters twice in 11 patients (22 orbits) without knowing the patient demographics and clinical data. The test-retest reliability was good (r = 0.901, P < .001).
For statistical analysis, the diameters of the SOVs were treated as continuous variables. The average diameters of both SOVs in all patients were calculated for further analysis because the values in the left and right SOVs were highly correlated. The Student t, paired t, and χ2 tests were used for comparisons, as appropriate. Pearson coefficients were used to assess the correlation between two variables. Two-tailed P values of less than .05 were considered to indicate a statistically significant difference.
Results
After a chart review, we identified 69 patients who met the inclusion criteria during the study period. Their demographic information and diseases or reasons for lumbar puncture are shown in the Table. The mean ICP was 156 mm H2O ± 81 (range, 30–400 mm H2O), and 18 patients (26%) were identified as having increased ICP. Three patients had CSF pressures <60 mm H2O, and in two, spontaneous intracranial hypotension was diagnosed.
Clinical characteristics of the study patients
| Characteristic | Value |
|---|---|
| Age, y | |
| Mean ± SD | 46 ± 19 |
| Range | 17–90 |
| Male-to-female ratio | 32:37 |
| Body height, cm | |
| Mean ± SD | 163 ± 8 |
| Range | 141–180 |
| Body weight, kg | |
| Mean ± SD | 62 ± 12 |
| Range | 40–99 |
| Body mass index | |
| Mean ± SD | 23.3 ± 3.3 |
| Range | 17.3–31.6 |
| Diseases or reasons for lumbar puncture* | |
| Acute headache or headache exacerbation | 28 (41) |
| Meningitis, CNS infection, or pachymeningitis | 15 (22) |
| Brain tumor or metastasis | 10 (14) |
| Change in consciousness | 4 (6) |
| Spontaneous intracranial hypotension, stroke, pseudotumor cerebri, cranial nerve lesions | 2 each (3) |
| Multiple sclerosis, Guillain-Barré syndrome, normal pressure hydrocephalus, cranial nerve lesions | 1 each (1) |
| Duration between MRI studies and lumbar puncture* | |
| <1 d | 34 (49) |
| 1–2 d | 35 (51) |
Data are the number of patients. Data in parentheses are percentages.
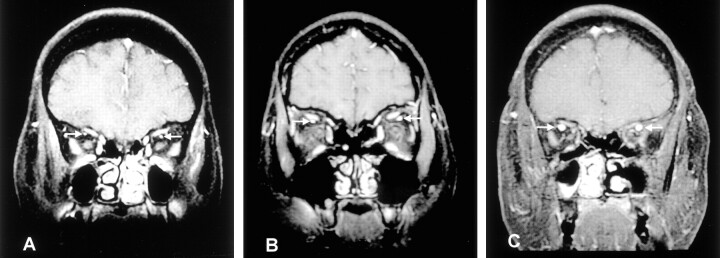
The SOVs were easily identified in the lateral regions under the superior rectus muscles (Fig 1). The diameters of the SOV on both sides were similar, without side-to-side differences (t = 0.82; df = 68; P = .4, paired t test). In fact, the diameters of the SOVs in both orbits were the same in 47 patients (68%). They were 1 mm different in 20 (29%) patients; they were 2 and 3 mm in each patient. In addition, the Pearson correlation coefficient was 0.85 between the diameters of the SOV on the two sides (P < .001). Because the diameters of the SOVs on both sides were similar and highly correlated, we calculated the average diameters of the bilateral SOVs for each patient for further analysis.
Fig 1.
The SOVs (arrows) are located under the superior rectus muscles. Three examples of the diameters of the SOVs are illustrated.
A, SOV 1 mm in diameter in a 57-year-old woman with a CSF pressure of 108 mm H2O.
B, SOV 3 mm in diameter over the left side and SOV 2 mm in diameter over the right side in a 32-year-old women with a CSF pressure of 230 mm H2O.
C, SOV 5 mm in diameter in a 34-year-old man with a CSF pressure of 245 mm H2O.
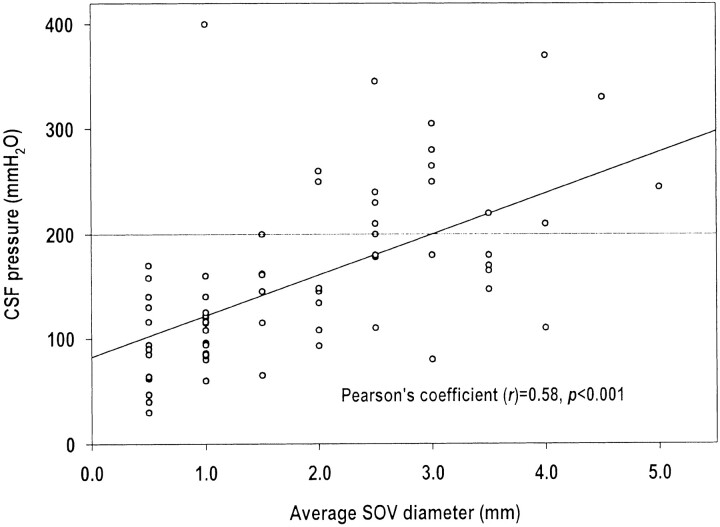
The scatter plot of the average SOV diameters and CSF pressures is shown in Figure 2. The average SOV diameters and the CSF pressure were positively correlated (Pearson correlation coefficient r = 0.58, P < .001). In the 48 patients whose CSF pressures were 60–200 mm H2O (normal ICP), the mean average SOV diameter was 1.6 mm ± 1.0. In the 18 patients with increased ICP (> 200 mm H2O), the mean average SOV diameter was 3.0 mm ± 1.0 mm. The difference in mean average SOV diameters was significant between these two patient groups (P < .001, t test). The percentage of patients with increased ICP was 3% (one of 30) among patients with an average SOV diameter ≤1 mm, 15% (two of 13) among patients with an average SOV diameter of 1.5–2 mm, and 58% (15 of 26) among patients with an average SOV diameter of 2.5–5 mm (χ2 for trend analysis, P < .001). The average SOV diameter was <1 mm in all three patients with low CSF pressure (< 60 mm H2O), including the two patients with spontaneous intracranial hypotension.
Fig 2.
Scatter plot of CSF pressures in relationship to average SOV diameters.
Except for CSF pressure, we did not find further significant correlations between the average SOV diameter and the patient’s age, sex, or body mass index (data not shown).
Discussion
This study showed that the diameters of the SOVs, as measured by using coronal MR images sections, were positively correlated with CSF pressure (r = 0.58). Patients with larger diameter SOVs (especially those ≥2.5 mm) were more likely to have increased ICP than were those with SOVs with smaller diameters.
In our patients with increased ICP, the mean average diameters of the SOV were significantly larger than those in patients with normal CSF pressure. Among the patients with SOV diameters ≤1 mm, their likelihood of having increased ICP was small (3%). In contrast, the risk of increased ICP markedly increased (58%) if the diameter of the SOV was ≥2.5 mm. Our findings appear to be compatible with those of one brain CT study in which the authors found bilateral SOV engorgement in 11 patients with diffuse cerebral swelling (4). The diameters reversed to normal after the cerebral swelling resolved. They considered SOV diameters of ≥2 mm on axial CT scans to be possible indicators of enlargement, diameters ≥ 3 mm to be likely indicators, and diameters ≥4 mm to be definite indicators. Of 40 patients with an SOV ≥2 mm, 21 (53%) were found to have had increased ICP (≥20 mm Hg) at some point; this observation is in line with our study results.
In our study, the average SOV diameters were <1 mm in two patients with spontaneous intracranial hypotension. Nonetheless, these two cases are too few to support a conclusion. Notably, not all patients with enlarged SOVs had increased ICP. Therefore, the diameter of the SOV was also influenced by other unknown factors. Nevertheless, except for CSF pressure, no further variables were related to the diameter of the SOV in our study. Correlation analyses did not reveal a causal relationship between the ICP and the diameter of the SOV. However, from a clinical point of view, it is more plausible that the change in ICP leads to the change in the size of the lumen in the SOV (4).
The SOV is valveless (9) and directly connected to the cavernous sinus. Therefore, it is easily influenced by hydrodynamic changes in the intracranial CSF. Nonetheless, the exact mechanism for the positive correlation between the diameter of the SOV and the ICP is uncertain. We hypothesize that an increased ICP impairs the pressure gradient for venous return from the extracranial SOV to the intracranial cavernous sinus. Hence, blood stagnation dilates the SOV. On the contrary, low ICP increases this pressure gradient and facilitates venous return from the SOV. The increased flow velocity in the SOV causes the transmural pressure to decrease, as demonstrated with the Bernoulli-Poiseuille equation (10). The lumen of the SOV thus collapses. Khanna et al (4) proposed another theory. They suggested that the engorged SOVs in their patients were due to a mechanical obstruction of the cavernous sinus caused by a compression due to brain swelling.
Because this retrospective study was conducted at a single institution and because we recruited patients with a broad spectrum of neurologic disorders, our findings require validation. Future studies might recruit more patients with only CSF disturbances, such as pseudotumor cerebri or spontaneous intracranial hypotension. Our study had other limitations. Since the MR imaging performed in these patients was not intended for a study of the SOV, the sensitivity for measuring the diameter of the SOV cannot be compared with those MR imaging studies focusing on the orbital structure. Nonetheless, the mean average SOV diameter in our patients with normal CSF pressure (1.6 mm) was similar to that of a high-resolution orbital MR imaging study (1.9 mm; range, 1.0–2.9 mm) (11). Also, in this study, the opening pressure determined by means of lumbar puncture was used as the surrogate for the ICP, and this approach could have potential limitations. However, the criterion standard of ICP measurement—use of intraventricular catheter—was, in fact, not our routine practice for measuring CSF pressure.
Conclusion
This study shows that the SOV diameter, determined on the basis of the MR images, was positively correlated with ICP. Dilatation of the SOV (especially ≥2.5 mm) should alert physicians to the possibility of increased ICP.
Acknowledgments
The authors wish to thank Ms Shin-Jung Lee for her invaluable help in reviewing the statistics in this article.
Footnotes
Supported in part by grants (VGH331) from the Taipei Veterans General Hospital, Taiwan.
References
- 1.Bacon KT, Duchesneau PM, Weinstein MA. Demonstration of the superior ophthalmic vein by high resolution computed tomography. Radiology 1977;124:129–131 [DOI] [PubMed] [Google Scholar]
- 2.Nugent RA, Belkin RI, Neigel JM, et al. Graves’ orbitopathy: correlation of CT and clinical findings. Radiology 1990;177:675–682 [DOI] [PubMed] [Google Scholar]
- 3.Peyster RG, Savino PJ, Hoover ED, Schatz NJ. Differential diagnosis of the enlarged superior ophthalmic vein. J Comput Assist Tomogr 1984;8:103–107 [DOI] [PubMed] [Google Scholar]
- 4.Khanna RK, Pham CJ, Malik GM, Spickler EM, Metha B, Rosenblum ML. Bilateral superior ophthalmic vein enlargement associated with diffuse cerebral swelling: report of 11 cases. J Neurosurg 1997;86:893–897 [DOI] [PubMed] [Google Scholar]
- 5.Spektor S, Piontek E, Umansky F. Orbital venous drainage into the anterior cavernous sinus space: microanatomic relationships. Neurosurgery 1997;40:532–540 [PubMed] [Google Scholar]
- 6.Mokri B. The Monro-Kellie hypothesis: application in CSF volume depletion. Neurology 2001;56:1746–1748 [DOI] [PubMed] [Google Scholar]
- 7.Fishman RA. Cerebrospinal Fluid in Diseases of the Nervous System. 2nd ed. Philadelphia: WB Saunders;1992
- 8.Headache Classification Committee of the International Headache Society. Classification and diagnosis criteria for headache disorders, cranial neuralgias and facial pains. Cephalalgia 1988;8(Suppl 7):1–96 [PubMed] [Google Scholar]
- 9.Murakami K, Murakami G, Komatsu A, Sato T, Tane S. Gross anatomical study of veins in the orbit. Acta Soc Ophthalmol Jpn 1991;95:31–38 [PubMed] [Google Scholar]
- 10.Badeer HS. Hemodynamics for medical students. Adv Physiol Educ 2001;25:44–52 [DOI] [PubMed] [Google Scholar]
- 11.Ozgen Ali, Aydingoz U. Normative measurements of orbital structures using MRI. J Comput Assist Tomogr 2000;24:493–496 [DOI] [PubMed] [Google Scholar]