Abstract
BACKGROUND AND PURPOSE: Early parenchymal gadolinium enhancement on T1-weighted MR images is predictive of hemorrhagic transformation (HT) in rodent focal ischemia models, but its value in humans is unknown. We sought to investigate gadolinium enhancement in acute ischemic stroke patients to determine their association with subsequent HT.
METHODS: We retrospectively examined 22 patients with ischemic stroke who underwent MR imaging within 4.9 hours (±1.4) of symptom onset. Patients receiving intravenous tissue plasminogen activator (tPA) (n = 6) were included. Twenty-one patients underwent repeat MR studies at 48 hours, 13 underwent additional MR imaging at 1 week, and one underwent follow-up head CT at 24 hours. Initial images were analyzed for enhancement patterns (vascular, meningeal, parenchymal). Follow-up T2- and T2*-weighted images were evaluated for hemorrhage.
RESULTS: In all patients, initial MR images showed vascular enhancement in the vascular territory of the stroke lesion: 19 with vascular enhancement alone and three with vascular and parenchymal enhancement. All three patients with both enhancement patterns had HT: two large and symptomatic, and one asymptomatic (petechial hemorrhage). They received tPA before MR imaging. None of the patients without early parenchymal enhancement developed symptomatic hemorrhage. Six (32%) patients with vascular enhancement alone had petechial hemorrhage at follow-up imaging. In this limited sample, initial mean volumes on diffusion-weighted images, National Institute of Health Stroke Scale scores, and intervals from stroke onset to imaging did not differ between patients with vascular and parenchymal enhancement versus those with vascular enhancement alone.
CONCLUSION: Early parenchymal enhancement of stroke lesions may be a good predictor of subsequent symptomatic HT may help identify patients at risk, especially after thrombolytic therapy.
Early identification of risk factors for hemorrhagic transformation (HT) in patients with acute ischemic stroke may aid in the selection of patients for thrombolytic therapy. While IV tissue plasminogen activator (tPA) substantially improves clinical outcome in patients treated within 3 hours of symptom onset, the risk of life-threatening HT increases 10-fold (0.6% vs 6.4%) (1). Indeed, concerns about this complication may prevent physicians from treating appropriate patients with recombinant tPA (rt-PA) (2).
The mechanism of HT after cerebral ischemia is not clearly understood. However, several clinical risk factors have been associated with HT, including thrombolytic drugs (eg, rt-PA and streptokinase), severe baseline neurologic deficits, congestive heart failure, increasing age, elevated baseline systolic blood pressure, aspirin use (1, 3–6), cardioembolic stroke (7), delayed administration of thrombolytic therapy (8), delayed reperfusion (9, 10), and diabetes mellitus (3, 11). Early ischemic changes visible on pretreatment CT scans—blurring of gray matter-white matter distinction, blurring of the putaminal border, and sulcal effacement—have been reported to provide radiologic predictors of subsequent HT (3, 4), and these findings have been routinely used in many centers to withhold thrombolytic therapy. However, the identification of early signs of infarct is difficult, even among expert readers (12, 13), and a systematic, quantitative approach to analyzing early CT changes may be more reliable (14).
Other imaging modalities (eg, MR imaging) that may provide pathophysiologic information relevant to hemorrhagic risk are beginning to be explored. Diffusion-weighted imaging has been used to depict acute stroke lesions with high sensitivity and specificity; however, its use in predicting HT is not known. Several recent studies have shown that ischemic brain regions destined for HT have notably lower apparent diffusion coefficient values (15–17) and persistently delayed perfusion (16) compared with ischemic regions not destined for hemorrhage. In addition, the presence of silent microbleeds (detected by using T2*-weighted sequences) is a possible indicator of pre-existing severe microangiopathy, and may be a marker for an increased risk of HT after thrombolytic treatment (18, 19). Contrast enhancement, which is indicative of a leak in the blood-brain barrier (BBB), may be another important tool in assessing the risk of HT. Early parenchymal enhancement with gadopentetate dimeglumine was observed in rats subjected to transient middle cerebral artery (MCA) occlusion that subsequently developed petechial hemorrhage (20–22). In patients with MCA ischemic stroke treated with intra-arterial thrombolysis, parenchymal enhancement observed on immediate post-treatment CT scans is well correlated with subsequent HT (23). To our knowledge, the predictive value of MR contrast enhancement in patients with acute ischemic stroke has not been evaluated. In this study, we investigated the MR patterns of contrast enhancement in the setting of hyperacute ischemic stroke to determine if these patterns are associated with subsequent HT. We hypothesized that early parenchymal enhancement on MR images is associated with higher risk of subsequent HT in patients with acute ischemic stroke.
Methods
Patients
We retrospectively reviewed the findings in all patients with ischemic stroke who were admitted through the stroke service from August 1999 to December 2000; we evaluated patients who underwent MR imaging within the first 7 hours of symptom onset. Patients enrolled in randomized clinical trials involving neuroprotective agents (n = 20), and those who had received IV r-tPA (n = 6) were included. Administration of putative neuroprotective agents (if any) occurred after the baseline MR imaging session to preclude any effect of study medications on the likelihood of contrast enhancement. The inclusion criteria for the study included the following: 1) baseline CT scan at presentation that did not show an intracranial hemorrhage, 2) initial MR imaging performed within 7 hours of symptom onset (diffusion-weighted imaging to confirm a hemispheric ischemic stroke) with T2-, T2*, and T1-weighted images before and after gadolinium enhancement, and 3)follow-up MR imaging at day 3 (n = 21) and day 7 (n = 13) if available (to assess for HT). No further MR imaging was needed if the patient developed HT, as detected on CT scans before that time (n = 1). Any urgent CT scans (n = 7) performed within 1 week of the initial imaging study were also reviewed. National Institute of Health Stroke Scale (NIHSS) scores were obtained in all patients at presentation. The research was approved by our institutional review board, and written informed consent was obtained from all patients.
Imaging Protocol
All MR studies were performed by using a 1.5-T whole-body unit with echo-planar imaging (EPI) capabilities (Vision; Siemens Medical Systems, Erlangen, Germany) by using a conventional CP head coil. The protocol included the following: conventional turbo spin-echo T2-weighted imaging (TR/TE/NEX, 4000/99/1; echo train length, 11; FOV, 22 × 22 cm2; matrix, 242 × 256), T2*-weighted EPI (8000/47; matrix, 242 × 256; FOV, 22 × 22 cm2), and diffusion-weighted EPI (6000/100; matrix, 96 × 200; FOV, 22 × 22 cm2; diffusion gradients with a b value of 1000 s/mm2 applied separately in three orthogonal directions to generate trace images). IV gadopentetate dimeglumine 0.1–0.2 mmol/kg was injected, and patients were imaged by using conventional spin-echo T1-weighted imaging (500/12/1; matrix, 106 × 256; FOV, 16.5 × 22 cm2) 10 minutes later. All images were acquired in the axial plane with 6-mm-thick sections and a 1-mm intersection gap.
Aside from the baseline head CT study, additional CT examinations were performed only if the patients had acute changes in their neurologic status. CT studies were performed by using Somatom Plus scanners (140 kV, 206–223 mA; Siemens Medical Systems) by using 8-mm contiguous sections with 5-mm-thick sections through the skull base without contrast enhancement.
Image Analysis
A board-certified neuroradiologist (K.D.V.) analyzed the initial gadolinium-enhanced T1-weighted MR images for the presence of enhancement within the stroke lesion and compared the findings with those in the contralateral hemisphere. This reader was blinded to the clinical data and follow-up images. Lesions with enhancement were classified into three groups according to the enhancement pattern. The following standard criteria (24) were used: 1) vascular enhancement, which was defined as tubular enhancing vessels located in the sulci supplying the ischemic territory; 2) meningeal enhancement, which was enhancement of the meninges and/or dura within the sulci adjacent to the ischemic area; and 3) parenchymal enhancement, which was enhancement within the ischemic brain parenchyma.
The presence of HT was assessed on initial and follow-up T2-weighted (conventional or b = 0 s/mm2) and T2*-weighted images by using the standard MR imaging criteria for hemorrhage, and HT was also assessed on any unscheduled CT scans obtained as a results of the patient’s clinical deterioration. On both T2- and T2*-weighted images, low signal intensity within the ischemic area indicated hemorrhage. On CT scans, a hyperattenuating lesion indicated hemorrhage. HT was divided into two categories: asymptomatic hemorrhage (AH), which was not associated with neurologic deterioration, and symptomatic hemorrhage (SH), which was associated with clinical deterioration.
On the trace diffusion-weighted images, the acute stroke lesion was identified as a hyperintense lesion that was not detectable on the T2-weighted images. The volumes of the lesions on initial diffusion-weighted images were measured offline on a workstation (Sun Microsystems, Santa Clara, CA). For each initial MR study, the diffusion-weighted image of the entire unaffected cerebral hemisphere was used to generate average signal intensity and the SD for normal brain tissue. Lesion volume on the diffusion-weighted image was determined by calculating the sum of pixels with signal intensity greater than the mean + 2SD in the affected hemisphere multiplied by the voxel size.
Statistical Analysis
The Fisher exact test was used to compare the incidence of SH in patients with parenchymal enhancement and in those without such enhancement. This test was also used and to compare the incidence of SH in patients treated with tPA and in those without tPA treatment. A P value of less than .05 was considered to indicate a significant difference. The age, NIHSS score, time interval from stroke onset to MR imaging, and lesion volume on diffusion-weighted images was determined for two subgroups: those with vascular enhancement alone and those with vascular and parenchymal enhancement. The data were expressed as the mean ± SD. Data derived from the two groups were compared by using the Student t test.
Results
Between August 1998 and December 2000, 22 patients (12 men, 10 women; age range, 46–84 years; mean age ± SD, 67.8 years ± 10.2) fulfilled criteria to be included in this study (Table 1). The mean NIHSS score was 12.9 (range, 5–21). Six patients received IV tPA treatment within 3 hours of symptom onset. All 22 patients had baseline CT scans without hemorrhage and an MR image obtained within 7 hours from symptom onset (4.9 hours ± 1.4). Lesions visible on diffusion-weighted images were located within the MCA territory in all patients except one (patient 11), who had a lesion in the posterior cerebral artery territory. Twenty-one patients underwent follow-up MR imaging at 48 hours. Thirteen of the 21 patients underwent additional MR imaging at 7 days, while six underwent CT at 24 hours. One patient developed a large symptomatic intraparenchymal hemorrhage 18 hours after symptom onset; this prompted emergent CT scanning without any follow-up MR imaging (Table 1).
TABLE 1:
Demographic, clinical, treatment, and imaging data
| Pt/Age, y/Sex | IV tPA | Initial NIHSS score | Time to First MR Study, h:min | Initial DWI Volume, cm3 | Enhancement Pattern | Contrast Agent Dose† | Follow-Up Studies* | Hemorrhage |
|---|---|---|---|---|---|---|---|---|
| 1/57/F | Yes | 14 | 5:00 | 44.73 | Vascular | 1× | MR3, CT | AH |
| 2/71/M | Yes | 12 | 4:00 | 62.73 | Parenchymal and vascular | 1× | CT | SH |
| 3/66/M | Yes | 19 | 3:30 | 117.58 | Vascular | 1× | MR3, CT | None |
| 4/72/F | Yes | 16 | 4:00 | 17.65 | Vascular | 1× | MR3 | None |
| 5/56/M | Yes | 10 | 3:00 | 18.59 | Parenchymal and vascular | 1× | MR3, CT | AH |
| 6/68/M | Yes | 15 | 5:30 | 28.09 | Parenchymal and vascular | 1× | MR3, CT | SH |
| 7/63/F | No | 14 | 6:00 | 33.66 | Vascular | 2× | MR3, MR7 | AH |
| 8/70/M | No | 16 | 6:30 | 58.07 | Vascular | 2× | MR3, MR7 | None |
| 9/67/F | No | 7 | 5:00 | 16.59 | Vascular | 2× | MR3, MR7 | None |
| 10/67/F | No | 5 | 6:00 | 34.6 | Vascular | 2× | MR3, MR7 | None |
| 11/84/F | No | 13 | 5:00 | 8.47 | Vascular | 2× | MR3, MR7 | None |
| 12/75/M | No | 5 | 6:30 | 10.79 | Vascular | 2× | MR3, MR7 | None |
| 13/74/F | No | 15 | 3:30 | 13.88 | Vascular | 2× | MR3, MR7, CT | None |
| 14/52/M | No | 10 | 5:00 | 35.6 | Vascular | 2× | MR3 | None |
| 15/77/M | No | 16 | 2:00 | 12.92 | Vascular | 2× | MR3, MR7, CT | None |
| 16/81/F | No | 17 | 3:00 | 84.81 | Vascular | 1× | MR3, MR7 | None |
| 17/78/F | No | 5 | 5:00 | 16.52 | Vascular | 2× | MR3, MR7 | None |
| 18/55/M | No | 21 | 6:00 | 65.07 | Vascular | 2× | MR3, MR7 | AH |
| 19/77/M | No | 7 | 6:30 | 2.3 | Vascular | 2× | MR3, MR7 | AH |
| 20/46/F | No | 14 | 6:30 | 12.41 | Vascular | 2× | MR3, MR7 | None |
| 21/57/M | No | 16 | 7:00 | 72.65 | Vascular | 2× | MR3, MR7 | AH |
| 22/78/M | No | 20 | 3:30 | 90.43 | Vascular | 2× | MR3, MR7 | AH |
MR3 indicates MR imaging at day 3; MR7, MR imaging at day 7.
Gadopentetate dimeglumine dose, 0.1 mmol/kg (= 1×) or 0.2 mmol/kg (= 2×).
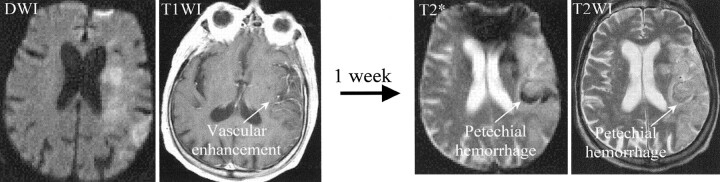
Of the 22 eligible patients, all had vascular enhancement on their initial MR images (Table 1). Nineteen had vascular enhancement alone, three had parenchymal and vascular enhancement, and none had meningeal enhancement. In all patients, initial CT or MR images (including T2- and T2*-weighted images) did not demonstrate evidence of hemorrhage. All three patients with both parenchymal and vascular enhancement developed subsequent HT: two had a large SH, and one had a small petechial AH (Table 1, Fig 1). Among the remaining 19 patients with vascular but not parenchymal enhancement, none developed SH. Thus, early parenchymal (and vascular) enhancement on MR images was significantly associated with subsequent SH (P = .013). Six of the 19 patients with initial vascular enhancement alone had a subsequent small petechial AH, as shown on follow-up images (Fig 2), whereas one of the three patients with vascular and parenchymal enhancement had AH.
Fig 1.

In three patients, initial MR images demonstrate parenchymal enhancement within the area of ischemia. All three patients subsequently developed HT: Patients 6 and 2 developed large SHs (detected with CT), while patient 5 developed a small AH (detected with T2*-weighted MR imaging). F/u indicates follow-up; T1WI, T1-weighted image.
Fig 2.
Initial MR images show vascular enhancement in the territory of the right MCA (left). This patient developed a petechial AH, which was detected on T2*- and T2-weighted images (right) obtained 1 week later. T1WI indicates T1-weighted image; T2WI, T2-weighted image
In patients with acute stroke, the administration of IV tPA is a known risk factor for HT (1, 3). In our study population, two of six patients treated with tPA just before MR imaging developed SH. Of the 16 who were not treated, none had SH. Although we observed a trend for an association between tPA administration and SH (P = .065), statistical significance was not attained. All three patients in whom initial MR images demonstrated parenchymal enhancement were treated with tPA; this finding suggested that tPA induced the parenchymal enhancement (ie, BBB leak), followed by HT. The mean time between tPA administration and MR imaging was 2 hours 18 minutes.
In our group of patients, HT was common, occurring in nine (41%) of 22 patients. However, only two patients (9%) developed a large SH. In three of the seven patients with asymptomatic petechial hemorrhage, hemorrhages were detected earlier with T2*-weighted images than with conventional T2-weighted images. Stroke severity and large infarct volumes have been associated with an increased risk of HT (25, 26), raising the possibility that these and other variables could have confounded the findings in our study. To assess this possibility, the mean ages, NIHSS scores, time intervals from symptom onset to MR imaging, and diffusion-weighted imaging lesion volumes were determined for patients with vascular enhancement and compared with those of patients with both vascular and parenchymal enhancement. These measures did not differ between the two groups of patients (Table 2).
TABLE 2:
Relationship between the type of enhancement and age, baseline NIHSS, time to baseline MR study, or DWI volume
| Measure | Enhancement |
P Value | |
|---|---|---|---|
| Vascular (n = 19) | Vascular and Parenchymal (n = 3) | ||
| Age, y | 68.3 ± 10.7 | 65.0 ± 7.94 | .62 |
| Initial NIHSS score | 13.2 ± 5.2 | 12.3 ± 2.5 | .79 |
| Time to first MR study, h | 5.0 ± 1.4 | 4.2 ± 1.3 | .34 |
| Initial DWI volume, cm3 | 39.4 ± 33.1 | 36.5 ± 23.2 | .89 |
Discussion
In this study, we demonstrated that early parenchymal enhancement, as demonstrated on MR images obtained during the hyperacute stage (within 7 hours of ischemic stroke onset), was significantly associated with subsequent symptomatic hemorrhagic conversion. Gadolinium enhancement was a common finding on MR images of ischemic stroke lesions obtained at the hyperacute stage, occurring in all of the patients in our study. Two patterns of enhancement were identified during this hyperacute phase: vascular enhancement alone and both vascular and parenchymal enhancement. Vascular enhancement, present in all of our patients, was not predictive of SH, though AH was commonly found in patients with vascular enhancement alone (32%). However, early parenchymal enhancement (in addition to vascular enhancement) was a strong predictor of SH: Two of three parenchymally enhancing lesions developed SH. Conversely, the absence of parenchymal enhancement was predictive of no SH; none of 19 patients without parenchymal enhancement developed SH. Previous studies demonstrated early parenchymal enhancement after gadopentetate dimeglumine administration before the development of petechial hemorrhage in a transient MCA occlusion model in rats (20–22) and rabbits (27). Enhancement was observed immediately after reperfusion and was accurately predictive of subsequent petechial hemorrhage in 100% of the rats (20). In addition, a clinical case report demonstrated parenchymal enhancement on MR images (18 hours after initial CT scanning), before the development of petechial hemorrhage 3.5 hours later (24). In contrast to the published reports in which all of the hemorrhages were of the petechial type, two of our three patients with parenchymal enhancement developed large SHs.
A prospective study of MR contrast enhancement patterns after cerebral ischemia demonstrated that parenchymal enhancement is a late finding, occurring at 7–14 days after infarction and that intravascular enhancement is the earliest finding at 1–3 days post ictus, followed by meningeal enhancement at days 3–6 (28). Parenchymal enhancement is noted to be rare early after the onset of stroke. Indeed, in our study, hyperacute parenchymal enhancement was not observed, except in patients treated with tPA. Enhancement of the brain parenchyma may be related to damage to the BBB, which allows the extravasation of small contrast medium molecules into the extravascular space. Animal studies show that the areas of HT and parenchymal enhancement are histologically characterized by a substantial loss of basal lamina in the cerebral microvessels (29). Some have proposed that the loss of vessel-wall integrity after ischemia results from plasmin-activated laminin degradation, the activation of matrix metalloproteases (MMPs), and the transmigration of leukocytes, all of which lead to petechial hemorrhage (29). Moreover, results of a recent study in an embolic focal ischemia model in rats suggest that tPA-induced MMP activity results in HT, whereas the inhibition of MMP in tPA-treated rats reduces the extent of HT (30). Thus, in patients treated with rt-PA, early MR parenchymal enhancement (indicative of BBB breakdown) may be an early predictor of subsequent symptomatic HT.
In addition to MR imaging-based techniques, other imaging modalities have been investigated for their usefulness in predicting HT before thrombolysis. As discussed above, early signs of ischemia on CT, while difficult even for expert readers to discern, may be predictive of symptomatic intracerebral hemorrhage, especially when using a systematic-quantitative approach, such as the Alberta Stroke Program Early CT Score method (14). Others suggest that the volume of early infarct abnormalities on baseline CT scans may be predictive of HT after intra-arterial thrombolysis (31). More advanced CT-based technologies, such as CT angiography or xenon-enhanced CT-measured cerebral blood flow CBF, have been postulated to aid in making decisions about acute thrombolysis based on predicted clinical outcome and site of arterial occlusion (32). In addition, in patients treated with intra-arterial thrombolysis, ischemic brain tissue with a CBF less than 35% of the cerebellar flow (as measured with single photon emission CT) may be at risk for hemorrhage (33).
Our study had several limitations. A major limitation was the small sample size, with potential bias. Although our results are suggestive, a prospective trial with larger numbers of patients is required to reinforce our preliminary findings about the clinical utility of early MR parenchymal enhancement patterns in the prediction of HT. The sensitivity of measuring a BBB leak by using parenchymal enhancement is substantially limited by the contrast agent used in this study. Subtle BBB injury may be better detected by using contrast agents with a smaller molecular size. Nevertheless, to allow greater extravasation of the contrast agent from the intravascular compartments to the extravascular compartments while maintaining contrast enhancement, we acquired the contrast-enhanced T1-weighted images 10 minutes after the injection. Finally, differences in the doses of the contrast agents used (0.1–0.2 mmol/kg) could have altered the extent of contrast enhancement. However, both patients with parenchymal enhancement received the lower 0.1-mmol/kg dose of contrast agent.
Conclusion
With or without tPA treatment, vascular enhancement on MR images is a common finding in acute ischemia and is not associated with symptomatic HT. The combination of early parenchymal and vascular enhancement of the stroke lesion is predictive of symptomatic HT, especially after tPA administration. This hypothesis needs further evaluation in a larger prospective study.
Footnotes
Presented at the symposium and 39th annual meeting of the American Society of Neuroradiology, Boston, MA, April, 2001.
References
- 1.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587 [DOI] [PubMed] [Google Scholar]
- 2.Alberts MJ. tPA in acute ischemic stroke: United States experience and issues for the future. Neurology 1998;51:S53–S55 [DOI] [PubMed] [Google Scholar]
- 3.Jaillard A, Cornu C, Durieux A, et al. Hemorrhagic transformation in acute ischemic stroke: The MAST-E study—MAST-E group. Stroke 1999;30:1326–1332 [DOI] [PubMed] [Google Scholar]
- 4.Larrue V, von Kummer RR, Muller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke 2001;32:438–441 [DOI] [PubMed] [Google Scholar]
- 5.Bowes MP, Zivin JA, Thomas GR, Thibodeaux H, Fagan SC. Acute hypertension, but not thrombolysis, increases the incidence and severity of hemorrhagic transformation following experimental stroke in rabbits. Exp Neurol 1996;141:40–46 [DOI] [PubMed] [Google Scholar]
- 6.Fisher M. Aspirin, anticoagulants, and hemorrhagic conversion of ischemic infarction: hypothesis and implications. Bull Clin Neurosci 1986;51:68–72 [PubMed] [Google Scholar]
- 7.Okada Y, Yamaguchi T, Minematsu K, et al. Hemorrhagic transformation in cerebral embolism. Stroke 1989;20:598–603 [DOI] [PubMed] [Google Scholar]
- 8.Kano T, Katayama Y, Tejima E, Lo EH. Hemorrhagic transformation after fibrinolytic therapy with tissue plasminogen activator in a rat thromboembolic model of stroke. Brain Res 2000;854:245–248 [DOI] [PubMed] [Google Scholar]
- 9.Fagan SC, Garcia JH. Hemorrhagic transformation in focal cerebral ischemia: influence of time to artery reopening and tissue plasminogen activator. Pharmacotherapy 1999;19:139–142 [DOI] [PubMed] [Google Scholar]
- 10.Molina CA, Montaner J, Abilleira S, et al. Timing of spontaneous recanalization and risk of hemorrhagic transformation in acute cardioembolic stroke. Stroke 2001;32:1079–1084 [DOI] [PubMed] [Google Scholar]
- 11.Broderick JP, Hagen T, Brott T, Tomsick T. Hyperglycemia and hemorrhagic transformation of cerebral infarcts. Stroke 1995;26:484–487 [DOI] [PubMed] [Google Scholar]
- 12.Vo KD, Lee JM, Kido DK, Littenberg B. Agreement among the experts in interpreting CT scans in stroke patients eligible for iv r-tPA. Paper presented at: Annual Meeting of the Association of University Radiologists;2001; Toronto, Ontario, Canada.
- 13.Grotta JC, Chiu D, Lu M, et al. Agreement and variability in the interpretation of early CT changes in stroke patients qualifying for intravenous rtPA therapy. Stroke 1999;30:1528–1533 [DOI] [PubMed] [Google Scholar]
- 14.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy: ASPECTS Study Group—Alberta Stroke Programme Early CT Score. Lancet 2000;355:1670–1674 [DOI] [PubMed] [Google Scholar]
- 15.Tong DC, Adami A, Moseley ME, Marks MP. Relationship between apparent diffusion coefficient and subsequent hemorrhagic transformation following acute ischemic stroke. Stroke 2000;31:2378–2384 [DOI] [PubMed] [Google Scholar]
- 16.Tong DC, Adami A, Moseley ME, Marks MP. Prediction of hemorrhagic transformation following acute stroke: role of diffusion- and perfusion-weighted magnetic resonance imaging. Arch Neurol 2001;58:587–593 [DOI] [PubMed] [Google Scholar]
- 17.Selim M, Fink JN, Kumar S, et al. Predictors of hemorrhagic transformation after intravenous recombinant tissue plasminogen activator: prognostic value of the initial apparent diffusion coefficient and diffusion-weighted lesion volume. Stroke 2002;33:2047–2052 [DOI] [PubMed] [Google Scholar]
- 18.Kidwell CS, Saver JL, Villablanca JP, et al. Magnetic resonance imaging detection of microbleeds before thrombolysis: an emerging application. Stroke 2002;33:95–98 [DOI] [PubMed] [Google Scholar]
- 19.Nighoghossian N, Hermier M, Adeleine P, et al. Old microbleeds are a potential risk factor for cerebral bleeding after ischemic stroke: a gradient-echo T2*-weighted brain MRI study. Stroke 2002;33:735–742 [DOI] [PubMed] [Google Scholar]
- 20.Knight RA, Barker PB, Fagan SC, Li Y, Jacobs MA, Welch KM. Prediction of impending hemorrhagic transformation in ischemic stroke using magnetic resonance imaging in rats. Stroke 1998;29:144–151 [DOI] [PubMed] [Google Scholar]
- 21.Dijkhuizen RM, Asahi M, Wu O, Rosen BR, Lo EH. Delayed rt-PA treatment in a rat embolic stroke model: diagnosis and prognosis of ischemic injury and hemorrhagic transformation with magnetic resonance imaging. J Cereb Blood Flow Metab 2001;21:964–971 [DOI] [PubMed] [Google Scholar]
- 22.Neumann-Haefelin C, Brinker G, Uhlenkuken U, Pillekamp F, Hossmann KA, Hoehn M. Prediction of hemorrhagic transformation after thrombolytic therapy of clot embolism: an MRI investigation in rat brain. Stroke 2002;33:1392–1398 [DOI] [PubMed] [Google Scholar]
- 23.Yokogami K, Nakano S, Ohta H, Goya T, Wakisaka S. Prediction of hemorrhagic complications after thrombolytic therapy for middle cerebral artery occlusion: value of pre- and post-therapeutic computed tomographic findings and angiographic occlusive site. Neurosurgery 1996;39:1102–1107 [DOI] [PubMed] [Google Scholar]
- 24.Koenigsberg RA, Gul N, Faro S, Elfont R, Baker K, Tsai F. Hyperacute cerebral enhancement: the earliest predictor of hemorrhage by MR imaging? J Neuroimaging 1999;9:235–236 [DOI] [PubMed] [Google Scholar]
- 25.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke 1997;28:2109–2118 [DOI] [PubMed] [Google Scholar]
- 26.Mayer TE, Schulte-Altedorneburg G, Droste DW, Bruckmann H. Serial CT and MRI of ischaemic cerebral infarcts: frequency and clinical impact of haemorrhagic transformation. Neuroradiology 2000;42:233–239 [DOI] [PubMed] [Google Scholar]
- 27.Yenari MA, Beaulieu C, Steinberg GK, Moseley ME. Diffusion-weighted magnetic resonance imaging characteristics of hemorrhagic transformation in experimental embolic stroke. J Neuroimaging 1997;7:227–231 [DOI] [PubMed] [Google Scholar]
- 28.Elster AD, Moody DM. Early cerebral infarction: gadopentetate dimeglumine enhancement. Radiology 1990;177:627–632 [DOI] [PubMed] [Google Scholar]
- 29.Hamann GF, del Zoppo GJ, von Kummer R. Hemorrhagic transformation of cerebral infarction-possible mechanisms. Thromb Haemost 1999;82(Suppl 1):92–94 [PubMed] [Google Scholar]
- 30.Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke 2002;33:831–836 [DOI] [PubMed] [Google Scholar]
- 31.Roberts HC, Dillon WP, Furlan AJ, et al. Computed tomographic findings in patients undergoing intra-arterial thrombolysis for acute ischemic stroke due to middle cerebral artery occlusion: results from the PROACT II trial. Stroke 2002;33:1557–1565 [DOI] [PubMed] [Google Scholar]
- 32.Kilpatrick MM, Yonas H, Goldstein S, et al. CT-based assessment of acute stroke: CT, CT angiography, and xenon-enhanced CT cerebral blood flow. Stroke 2001;32:2543–2549 [DOI] [PubMed] [Google Scholar]
- 33.Ueda T, Sakaki S, Yuh WT, Nochide I, Ohta S. Outcome in acute stroke with successful intra-arterial thrombolysis and predictive value of initial single-photon emission-computed tomography. J Cereb Blood Flow Metab 1999;19:99–108 [DOI] [PubMed] [Google Scholar]