Abstract
Summary: We present two cases of focal, tumefactive, masslike lesions of diffuse cerebral amyloid angiopathy (CAA) that presented as areas of increased signal intensity on long TR sequences without contrast enhancement or restricted diffusion. MR spectroscopy revealed normal metabolite ratios and unremarkable spectra. Pathologic tissue showed CAA and CAA with angitis of the CNS. Tumefactive CAA is a rare condition, and we describe its characteristics at MR spectroscopy and diffusion-weighted imaging.
Primary deposition of amyloid in the brain can take the form of senile plaques as in Alzheimer disease, as focal masslike deposition with sparing of the remainder of the parenchyma called amyloidoma, and as diffuse cerebral amyloid angiopathy (CAA) and its variant, CAA with angitis of the CNS (CAA/ACNS) (1, 2). There are very few reports (3–8) of CAA and CAA/ACNS presenting as tumefactive mass lesions mimicking neoplasms, and none describes the MR spectroscopic pattern of these lesions or its appearance at diffusion-weighted (DW) imaging. In this report, we present the imaging findings including those of MR spectroscopy and DW imaging of two cases of diffuse cerebral amyloid angiopathy with tumor-like masses that caused diagnostic dilemmas.
Case Reports
Patient 1
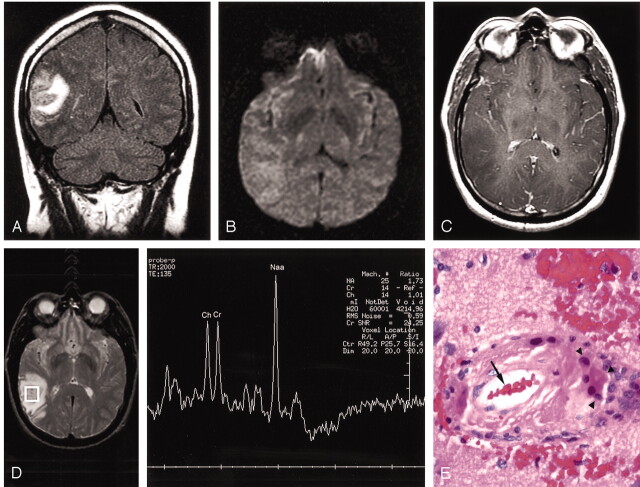
A 49-year-old man presented to our institution with a new onset of generalized seizures. Family history did not reveal any pertinent findings. After recovery from a brief postictal state, his physical examination was unremarkable. Laboratory tests at admission were all within normal limits. After initial MR imaging (Fig 1A–C) and MR spectroscopy (Fig 1D) that showed a tumefactive right occipitotemporal mass without enhancement or restricted diffusion and with unremarkable spectra, the patient was given dexamethazone and Phenytoin, and open MR imaging–directed surgical removal was contemplated for a presumed low-grade glioma. At the commencement of the planned procedure, intraoperative MR imaging showed that the lesion had reduced in size and the procedure was limited to stereotactic biopsy. Histopathologic findings were consistent with granulomatous angitis on the background of CAA/ACNS (Fig 1E and F). Coagulation testing and autoimmune assays were negative. The patient was started on Warfarin, and corticosteroids were tapered off over 6 weeks. Independent from his steroid dose, he complained of difficulty concentrating and migraine-like headaches. Repeat MR imaging performed 9 months after the initial event showed partial resolution of the right occipitotemporal tumafactive area previously seen (Fig 1G) and new T2 abnormalities in both temporal regions without mass effect or enhancement (Fig 1H).
Fig 1.
Imaging of CAA/ACNS at presentation and after 9 months of therapy with pathologic correlation.
A, FLAIR coronal image shows increased signal intensity with mass effect on the adjacent sulci and lateral ventricles in the right occipitotemporal region.
B, Diffusion-weighted axial image shows no restricted diffusion.
C, T1-weighted axial contrast-enhanced image shows decreased signal intensity without contrast enhancement.
D, Probe-press, single voxel (2 cm3 [TE = 135]) centered on the region of abnormality shows normal spectra and ratios.
E, Hematoxylin and eosin slide shows a blood vessel (arrow) with surrounding inflammation and multinucleated giant cells (arrowheads).
F, Immunohistochemistry with a beta-amyloid specific antibody demarcates the vessel wall (arrowheads). Both vessels are surrounded by a mononuclear inflammatory infiltrate (hematoxylin counter-stain).
G, FLAIR coronal image obtained 9 months after presentation shows significant resolution of previously seen tumafactive lesion in the right occipitotemporal region.
H, FLAIR axial image obtained 9 months after presentation shows increased signal intensity in the right and left temporal regions (arrows).
Patient 2
A 71-year-old woman presented in 1999 with a chronic decline in neurologic function since 1992. In 1996, MR imaging at another institution showed evidence of stroke; however, no DW imaging was performed at the time.
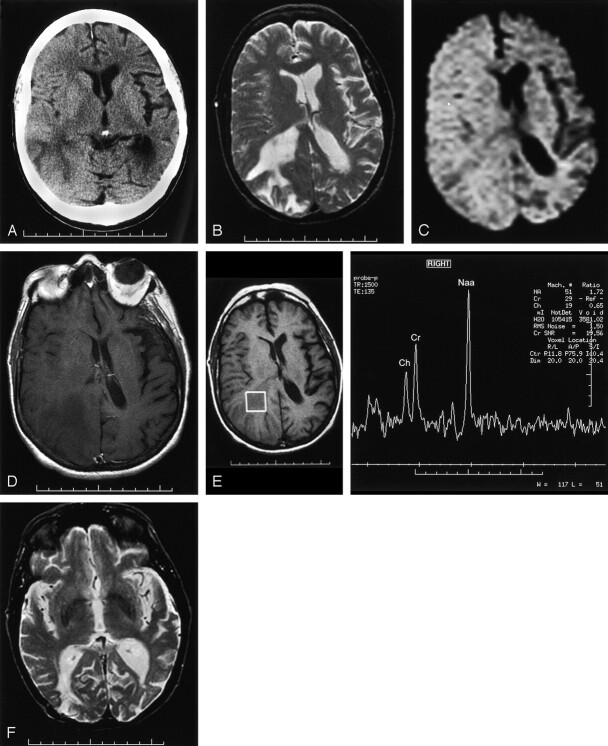
Medical history was significant only for hypercholesterolemia. Physical examination was within normal limits, but higher function testing revealed dementia. Laboratory studies revealed a positive ANA at 1:320, and negative rheumatoid titer. CT (Fig 2A) and MR imaging (Fig 2B–D) performed in 1999 showed an ill-defined, infiltrative, nonenhancing mass without restricted diffusion or calcification involving the right occipital and lower parietal lobes and with an unremarkable MR spectroscopic pattern (Fig 2E). The patient underwent a biopsy, which revealed cerebral amyloid angiopathy, and subsequently was discharged. The patient has now been followed up for 11 years since initial presentation and shows continued degeneration of higher functions. Repeat MR imaging in 2000 showed near-complete resolution of the abnormality with a small residual area of encephalomalacia at the biopsy site (Fig 2F).
Fig 2.
Multi-modality imaging of CAA at presentation and after treatment with pathologic correlation.
A, Noncontrast CT scan from 1999 shows decreased attenuation with sulcal effacement and mass effect on the occipital horn of the lateral ventricle.
B, T2-weighted axial image also from 1999 shows increased signal intensity with mass effect on the adjacent sulci and the right lateral ventricle.
C, Diffusion-weighted axial image shows no restricted diffusion.
D, T1-weighted axial contrast-enhanced image shows decreased signal intensity without enhancement.
E, Probe-press single voxel (2 cm3 [TE = 135]) centered on the region of abnormality shows normal spectra and ratios.
F, T2-weighted axial image obtained in 2000 shows near-complete resolution of right occipital abnormality with a small residual area of encephalomalacia at the biopsy site.
Discussion
CAA and its variant, CAA/ACNS, denote diffuse amyloid deposition in the brain that may occur with or without granulomatos inflammation and traditionally have not been thought to present as mass lesions. To our knowledge, there have only been three published cases each of CAA (3–5) and CAA/ACNS (6–8) detailing the MR imaging findings of a tumor-like presentation (Table 1). A review of prior cases and our cases shows that tumefactive mass lesions associated with CAA present with increased signal intensity at T2-weighted imaging and decreased signal intensity at T1-weighted imaging without postcontrast enhancement. Multiple lesions occur more frequently in the presence of CAA/ACNS rather than pure CAA, and all lesions were adjacent to either an ependymal or a dural surface. The imaging findings described make distinguishing tumefactive CAA from low-grade gliomas difficult.
Previous case reports with MR imaging of CAA and CAA/ACNS
| Series | Pathology | Contrast Enhancement | Location | Age | Sex | Antecedent Event |
|---|---|---|---|---|---|---|
| Vandermissen et al (3) | CAA | No | Supratentorial, abuts ependyma | 46 recurred @ 59 | M | ICH |
| Osumi et al (4) | CAA | No | Supratentorial, abuts ependyma | 56 recurred @ 59 | F | Seizure |
| Ortiz and Reed (5) | CAA | No | Supratentorial, abuts ependyma | 68 | F | |
| Safriel | CAA | No | Supratentorial, abuts ependyma | 71 | F | ‘Stroke’ |
| Safriel | CAA/ACNS | No | Supratentorial, abuts ependyma | 48 | M | |
| Tamargo et al (6) | CAA/ACNS | Slight | Multiple supratentorial, abuts ependyma | 80 | F | |
| Polivka et al (7) | CAA/ACNS | No | Supratentorial, abuts dura | 60 | M | |
| Polivka et al (7) | CAA/ACNS | No | Supratentorial, abuts dura | 74 | F | |
| Fountain and Eberhard (8) | CAA/ACNS | No | Multiple supratentorial, abuts ependyma | 66 | M | ICH |
Note.—Caa, cerebral amyloid angiopathy; ACNS, angitis of the central nervous system; ICH, intracranial hemorrhage.
None of the previously published reports detailed the MR spectroscopic or diffusion findings of this condition. The cases in our study suggest that MR spectroscopy and DW imaging can be used to distinguish abnormal amyloid deposition from tumors or infarcts as seen in this case series. Spectroscopic findings of low-grade gliomas may include decreased creatine and increased choline with an abnormal NAA/Cr ratio (<1). There may also be an abnormal lactate peak with gliomas. By contrast, tumefactive CAA show a normal NAA/Cr ratio (>1) and no increase in Cho/Cr ratio in keeping with the normal metabolic state of the underlying brain tissue. In addition, there is no evidence of restricted diffusion.
Amyloidomas are a form of amyloid deposition that is distinct from tumefactive CAA both histopathologically and radiographically. Whereas CAA refers to diffuse parenchymal deposition of amyloid with a focal masslike lesion, amyloidomas are a focal deposition of amyloid with sparing of the remainder of the parenchyma. On postcontrast imaging, amyloidomas invariably show postcontrast enhancement (9), whereas tumefactive CAA usually does not exhibit postcontrast enhancement.
Treatment of tumefactive CAA and CAA/ACNS involves steroids and immunosuppressants. Two prior studies (5, 10) showed success with steroid therapy, while another (8) showed success with immunosuppressants. The response rate to medical therapy in CAA/ACNS is 60%, with spontaneous recovery in only 5% (8). There is autopsy evidence to suggest that immunosuppressive treatment decreases the amyloid burden (10). Moore (11) found symptoms recurred in CAA/ACNS when therapy was not continued for prolonged periods, whereas the patients with CAA showed good response over time.
References
- 1.Calabrese LH, Mallek JA. Primary angitis of the central nervous system: report of 8 cases, review of the literature, and proposal for diagnostic criteria. Medicine (Baltimore) 1988;67:20–39 [DOI] [PubMed] [Google Scholar]
- 2.Ehsan T, Hasan S, Powers MJ, Heiserman JE. Serial magnetic resonance imaging in isolated angitis of the central nervous system. Neurology 1995;45:1462–1465 [DOI] [PubMed] [Google Scholar]
- 3.Vandermissen B, Salmon I, Hildebrand J. Recurrent nonhemorrhagic mass lesion due to cerebral amyloid angiopathy. J Neurol 2003;250:239–240 [DOI] [PubMed] [Google Scholar]
- 4.Osumi AK, Tien RD, Felsberg GJ, Rosenbloom M. Cerebral amyloid angiopathy presenting as a brain mass [Suppl]. AJNR Am J Neuroradiol 1995;16:911–915 [PMC free article] [PubMed] [Google Scholar]
- 5.Ortiz O, Reed L. Cerebral amyloid angiopathy presenting as a nonhemorrhagic, infiltrating mass. Neuroradiology 1996;38:449–452 [DOI] [PubMed] [Google Scholar]
- 6.Tamargo RJ, Connolly ES Jr, McKhann GM, et al. Clinicopathological review: primary angitis of the central nervous system in association with cerebral amyloid angiopathy. Neurosurgery 2003;53:136–143 [DOI] [PubMed] [Google Scholar]
- 7.Polivka M, Vallat AV, Woimant F, et al. Cerebral amyloid angiopathy (CAA) with presentation as a brain inflammatory pseudo-tumour. Clin Exp Pathol 1999;47:303–310 [PubMed] [Google Scholar]
- 8.Fountain NB, Eberhard DA. Primary angitis of the central nervous system associated with cerebral amyloid angiopathy. Neurology 1996;46:190–197 [DOI] [PubMed] [Google Scholar]
- 9.Ghandi D, Wee R, Goyal M. CT and MR imaging of intracerebral amyloidoma: case report and review of the literature. AJNR Am J Neuroradiol 2003;24:519–522 [PMC free article] [PubMed] [Google Scholar]
- 10.Mandybur TI, Balko G. Cerebral amyloid angiopathy with granulomatous angitis ameliorated by steroid-cytoxan treatment. Clin Neuropharmacol 1992;15:241–247 [DOI] [PubMed] [Google Scholar]
- 11.Moore PM. Diagnosis and management of isolated angiitis of the central nervous system. Neurology 1989;39:167–173 [DOI] [PubMed] [Google Scholar]