Abstract
BACKGROUND AND PURPOSE: The factors that predict favorable outcome after local intra-arterial thrombolysis (LIT) remain unknown. We aimed to clarify these factors in patients with middle cerebral artery occlusion treated by LIT.
METHODS: We performed LIT in 26 consecutive patients who had middle cerebral artery occlusion with a modified Rankin scale (mRS) score ≤2 before stroke onset. We assessed background characteristics, angiographic findings, and mRS score at discharge. We compared these factors between patients with good outcome (mRS score, ≤2) and those with poor outcome (mRS score, ≥3).
RESULTS: The duration from symptom onset to hospital admission was 0.96 ± 0.87 (mean ± SD) hour and from onset of stroke to LIT was 3.78 ± 1.17 hours. No patients developed symptomatic intracerebral hemorrhage or died. Thirteen patients achieved good outcomes. No significant differences existed between the two groups in baseline National Institutes of Health Stroke Scale (NIHSS) scores, time from stroke onset to LIT, blood pressure, early CT signs, or subsequent hemorrhagic transformation shown by CT. However, univariate analysis showed that patients with good outcomes were younger, more often had absence of hypertension history, had better collaterals shown by angiography, and had better recanalization rates than those with poor outcomes. NIHSS scores after LIT were lower in patients with good outcomes than in patients with poor outcomes. Logistic regression analysis indicated improvement of the NIHSS scores by ≥2 immediately after LIT was independently associated with good outcome.
CONCLUSION: Improvement of the NIHSS score by ≥2 immediately after LIT is a useful predictor of patient outcome at discharge.
In 1995, the National Institute of Neurologic Disorders and Stroke rt-PA Stroke Study Group reported that IV thrombolytic therapy using recombinant tissue plasminogen activator (rt-PA) within 3 hours of ischemic stroke onset improved patient outcomes (1). However, a randomized trial that used rt-PA within 6 hours of onset failed to show the therapeutic efficacy (2). No benefits were shown in the Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke study (known as the ATLANTIS Trial), a randomized controlled trial with patients receiving rt-PA from 3 to 5 hours after onset (3). Therefore, the American Heart Association guidelines for acute ischemic stroke recommend that the IV administration of rt-PA has to begin within 3 hours of onset (4). On the other hand, local intra-arterial thrombolysis (LIT) was reported to be efficacious in patients with middle cerebral artery (MCA) occlusion if performed within 6 hours of onset. In the Prolyse in Acute Cerebral Thromboembolism II study, LIT improved the prognosis of stroke within 6 hours of onset (5).
In 6% to 20% of patients who undergo thrombolysis, a serious side effect of symptomatic intracerebral hemorrhage (SICH) develops (1–6). SICH in patients receiving rt-PA was found to be related to tissue plasminogen activator dose, National Institute of Health Stroke Scale (NIHSS) score, and diastolic blood pressure (7, 8).
Early ischemic changes revealed by CT, such as cortical effacement (9), were also involved in the occurrence of SICH. The American Heart Association guidelines warn about the administration of rt-PA in patients with high blood pressure and early signs revealed by CT (4). We performed LIT in patients with acute MCA occlusion during the past 4 years in a prospective study design. We strictly adhered to predefined inclusion and exclusion criteria, and no patient developed SICH. Our purpose was to determine which clinical factors are predictors of favorable outcome.
Methods
Inclusion and Exclusion Criteria
The clinical inclusion criteria for LIT were acute embolic stroke within 6 hours of symptom onset, patient age from 20 to 85 years, and NIHSS score from 5 to 29. The exclusion criteria included the following: 1) neurologic symptoms caused by subarachnoid hemorrhage, neoplasm, septic embolism, moyamoya disease, or vasculitis; 2) seizure at onset; 3) rapidly improving neurologic signs at any point before LIT; 4) history of stroke within the previous 4 weeks (except transient ischemic attacks); 5) surgery, biopsy, or trauma with internal injuries within 2 weeks; 6) active or recent hemorrhage within 2 weeks; 7) baseline international normalized ratio of prothrombin time >1.7 or baseline platelet count <10 × 104/L; 8) uncontrolled hypertension defined by systolic blood pressure >185 mmHg or diastolic blood pressure >100 mmHg; 9) pregnancy or puerperal period. CT of the brain exclusion criteria included intracranial tumor, hemorrhage, and an early sign revealed by CT (acute hypoattenuated parenchymal lesion) in more than one-third of the MCA territory.
In patients who satisfied all the clinical and CT inclusion criteria, diagnostic cerebral angiography was performed. The angiographic exclusion criteria were occlusion of the internal carotid artery or long segment basilar artery occlusion and aneurysms or arterial dissections.
From April 1997 to December 2001, we screened 364 patients by using cerebral angiography. We found internal carotid artery occlusion in 51 patients, MCA occlusion in 135, basilar artery occlusion in 13, cerebral artery dissections in eight, cerebral aneurysms in eight, and other arterial occlusion or no occlusion in the other 149. Forty-seven patients (mean age, 66.4 ± 11.6 years; men, 35; women, 12) who were diagnosed as having MCA occlusion based on cerebral angiography findings within 6 hours of symptom onset were admitted to our Stroke Care Unit. Excluded were 18 patients: one with a malignant tumor, one with rapid improvement of symptoms before cerebral angiography, three who were older than 85 years, two with high blood pressure, and 11 with early CT signs involving more than one-third of the MCA territory. The remaining 29 patients underwent LIT. None of the 29 patients developed SICH or died during the hospital stay. Three of the 29 patients were not independent (modified Rankin scale score, ≥3) before the index stroke. We analyzed the remaining 26 patients who had modified Rankin scale (mRS) scores <2 before stroke onset.
Thrombolytic Procedure and Post-LIT Treatment
We performed cerebral angiography via a femoral approach. The LIT was performed when clinical, CT, and angiographic criteria were completely met and written informed consent was obtained from the patient or a family member. An infusion microcatheter with a single end hole was placed on the distal portion of the MCA thrombus by using a steerable microguidewire through a 6-French guiding catheter. Maximum dose of 420,000IU of urokinase (Urokinase 60,000IU, Mitsubishi Pharma Corporation) was infused manually at the rate of 35 mL/hr through the microcatheter. Simultaneously, mechanical disruption of the clot was performed by using a flexible microguidewire with a tip angle of 90 degrees (GT wire TERMO, 0.016 or 0.012 in). Penetration and fragmentation of the thrombus were achieved by gently advancing and rotating the tip of microguidewire. The LIT was stopped when the infusion time from the start of LIT exceeded 1 hour or when the operator confirmed that the artery was recanalized by >50% of the area perfused by the initial occluded artery during capillary, as seen in the lateral view.
The protocol required that no anticoagulants or antiplatelet agents were administered for 24 hours after treatment and that arterial blood pressure was monitored during the first 24 hours. When systolic blood pressure increased to >180 mmHg after LIT, IV antihypertensive drugs were continuously infused for at least 48 hours.
Clinical Variables and Outcome Measures
We assessed a history of hypertension (previous systolic ≥160 mmHg or diastolic ≥95 mmHg or receiving antihypertensive drugs), diabetes mellitus (fasting blood sugar >7.7 mmol/L, random blood sugar >11.1 mmol/L, or receiving insulin or oral hypoglycemic agents), hypercholesterolemia (total cholesterol ≥220 mg/dL or receiving antihyperlipidemic agents), smoking or alcohol habit, and anticoagulant and antiplatelet therapy before stroke onset. Grades of collateral flows and vessel recanalization were assessed by using cerebral angiography. All the angiograms were analyzed by a stroke neurologist without knowing the final result. The grades of collaterals were simply classified as poor (<50% of the occluded vascular territory was caused by collaterals) and good (collateral flows via leptomeningeal anastomoses allowed >50% filling of the territory distal to the occlusion). The achieved vessel recanalization was classified as described by Mori et al (10) and Yamaguchi et al (11). Vessel recanalization was classified into two groups. The lesion was categorized to be low grade if no or slight recanalization was perfusing <50% of the ischemic area. The grade of vessel recanalization was considered to be high grade when reperfusion covering >50% of the ischemic area was achieved.
Early signs revealed by initial CT were defined on the basis of loss of insular ribbon and cortical ribbon effacement. The presence or absence of hemorrhagic transformation was evaluated by follow-up CT performed >24 hours after LIT. SICH was diagnosed when hemorrhagic transformation was confirmed by CT and NIHSS score increased by ≥4 or when level of consciousness deteriorated by 1 point compared with before LIT.
Neurologic severity was assessed immediately, at 24 hours, and at 1 month after LIT by using NIHSS scores. Outcome at discharge was assessed by using the mRS and the Barthel index scores. Number of deaths during the hospital stay was monitored.
Statistical Analysis
All values are expressed as mean ± SD for parametric values or as a median (range) for nonparametric values. Comparisons among groups were made by analysis of variance and then Mann-Whitney U test. The χ2 test was conducted for the analysis of discrete variables. P < 0.05 was considered to be statistically significant. Logistic regression analysis was used to evaluate the contribution of the various factors to the outcome.
Results
Demographics and Clinical Data
The baseline characteristics of the 26 patients who underwent LIT are shown in Table 1. LIT was commenced 3.78 ± 1.17 hours after stroke onset. Hemorrhagic transformation in 11 patients (42.4%) was revealed by CT. However, no patients developed SICH or died. The median of the baseline NIHSS score was 18 (range, score of 6–23) at admission and became 6 (range, score of 0–16) 1 month later. Stroke recurred in two patients during their hospital stay. The median mRS score was 2 (range, score of 0–5), and the median Barthel index score was 95 (range, score of 0–100) at the time of discharge.
Table 1:
Characteristics of patients undergoing local intra-arterial thrombolysis
| Age, mean (SD), yr | 65 (13) |
| Men, % | 80.8 |
| Cardioembolic stroke, % | 92.3 |
| Atrial fibrillation, % | 76.9 |
| Occlusion of M1 segment, % | 42.3 |
| History of risk factors, % | |
| Hypertension | 57.7 |
| Diabetes mellitus | 19.2 |
| Hypercholesterolemia | 19.2 |
| Smoking | 46.2 |
| Alcohol | 42.3 |
| Time to admission, mean (SD), hr | 0.96 (0.87) |
| Time to starting LIT, mean (SD), hr | 3.78 (1.17) |
| Early CT sign, % | 46.2 |
| Previous anticoagulant therapy, % | 26.9 |
| Previous antiplatelet therapy, % | 15.4 |
| Symptomatic hemorrhage, % | 0 |
| Baseline NIHSS score, median (range) | 18 (6–23) |
| NIHSS score after LIT, median (range) | |
| Immediately | 15 (3–22) |
| 24 hr later | 12 (2–22) |
| 1 month later | 6 (0–16) |
| BI score at discharge, median (range) | 95 (0–100) |
| mRS score at discharge, median (range) | 2 (0–5) |
| Deaths, % | 0 |
| Hospital stay, mean (SD), days | 50 (23) |
Note.—LIT indicates local intra-arterial thrombolysis; NIHSS, National Institutes of Health Stroke Scale; BI, Barthel index; mRS, modified Rankin scale.
Outcome Data
Thirteen of 26 patients achieved good outcomes (mRS score, ≤2), and the other 13 had poor outcomes (mRS score, ≥3). The median baseline NIHSS scores were not different between the two groups (median score of 16 versus median score of 18). Patients with poor outcomes experienced no recurrence of stroke and no major complication, such as pneumonia or heart failure, during their hospital stay. The length of hospital stay was longer for patients with poor outcomes than for those with good outcomes (61 versus 38 days). Patients with good outcomes were younger (age 57.3 years ± 13.1 for good outcome group versus age 72.5 years ± 6.2 for poor outcome group), had hypertension less frequently (38.5% versus 76.9%), had an alcohol habit more frequently (61.5% versus 23.0%), M1 occlusion (61.5% versus 23.1%), good collaterals (61.5% versus 15.4%), and good recanalization (69.2% versus 30.7%). No differences were observed based on sex, baseline NIHSS scores, time to admission, time to LIT commencement, frequency of early signs revealed by CT, antiplatelet or anticoagulant therapy before stroke, hemorrhagic transformation on CT, blood pressure levels, or dose of urokinase or heparin between the two groups (Table 2). The antihypertensive agents were IV infused in four patients with good outcomes (31%) and in six patients with poor outcomes (46%). No differences were noted in arterial blood pressure after LIT between the two groups (Table 2). No patient received anticoagulants or antiplatelet agents for 24 hours after treatment.
Table 2:
Demographic and clinical variables for patients undergoing local intra-arterial thrombolysis
| Variable | mRS Score ≤2 (n = 13) | mRS Score ≥3 (n = 13) | P Value |
|---|---|---|---|
| Age, mean (SD), yr | 57 (13) | 73 (6) | .0003 |
| Men, % | 84.6 | 76.9 | >.9999 |
| History of risk factors, % | |||
| Hypertension | 38.5 | 76.9 | .047 |
| Diabetes mellitus | 15.4 | 23.0 | >.9999 |
| Hypercholesterolemia | 23.1 | 15.4 | >.9999 |
| Smoking | 61.5 | 30.8 | .116 |
| Alcohol | 61.5 | 23.0 | .047 |
| Previous anticoagulant therapy, % | 38.5 | 15.4 | .378 |
| Previous antiplatelet therapy, % | 23.0 | 7.6 | .593 |
| Time to admission, mean (SD), hr | 1.06 (0.95) | 0.85 (0.81) | .519 |
| Time to starting LIT, mean (SD), hr | 3.81 (1.23) | 3.75 (1.15) | .858 |
| Time to end LIT, mean (SD), hr | 4.86 (1.11) | 4.85 (1.22) | .739 |
| Baseline NIHSS score, median (range) | 16 (6–22) | 18 (9–23) | .757 |
| Blood pressure at admission, mean (SD), mmHg | |||
| Systolic | 149 (32) | 151 (23) | .741 |
| Diastolic | 86 (15) | 83 (9) | .739 |
| Early CT sign, % | 53.8 | 38.5 | .431 |
| Occlusion of M1 segment, % | 61.5 | 23.1 | .047 |
| Collateral flow (≥50%), % | 61.5 | 15.4 | .016 |
| Dose of urokinase, mean (SD), ×104 IU | 40.6 (11.6) | 36 (14.9) | .527 |
| Dose of heparin, mean (SD), ×103 IU | 3.8 (2.8) | 3.3 (2.1) | .723 |
| Recanalization (≥50%), % | 69.2 | 30.7 | .049 |
| Hemorrhagic transformation on CT, % | 38.5 | 46.2 | .691 |
| Use of antihypertensive drug, % | 31 | 46 | .973 |
| Blood pressure after LIT (with/without antihypertensive drug), mean (SD), mmHg | |||
| Systolic | 138 (10) | 132 (23) | .275 |
| Diastolic | 73 (10) | 76 (8) | .572 |
| Hospital stay, mean (SD), days | 38 (9) | 61 (26) | .014 |
Note.—mRS indicates modified Rankin scale; LIT, local intra-arterial thrombolysis; NIHSS, National Institutes of Health Stroke Scale.
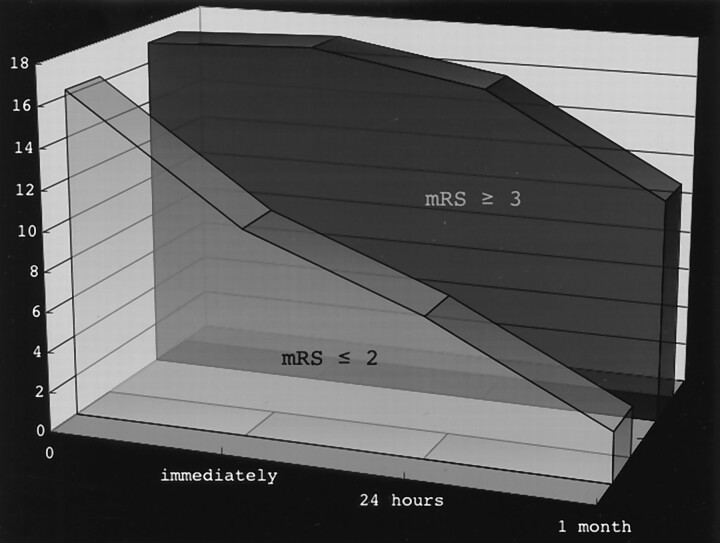
Figure 1 shows the evolution of the NIHSS scores after LIT in patients with good and poor outcomes. The NIHSS scores of patients with good outcomes improved immediately after LIT. On the other hand, this immediate improvement was not observed for those with poor outcomes. The median NIHSS score was 9 (range, score of 3–19) for the good outcome group and 18 (range, score of 13–22) for the poor outcome group immediately after LIT (P = .001). The NIHSS scores of 11 patients (85%) with good outcomes improved immediately after LIT by ≥2. On the other hand, such an improvement was noted for only one patient with poor outcome. The differences in the median NIHSS scores between the two groups gradually increased with time. Thus, the NIHSS scores were 7 (range, score of 2–19) for the patients with good outcomes and 18 (range, score of 10–22) for the patients with poor outcomes after 24 hours (P = .001). One month later, the scores were 2 (range, score of 0–9) versus 10 (range, score of 6–18) (P < .0001). The Barthel index score in the good outcome group was 100 for all patients at discharge, whereas in the poor outcome group, the median Barthel index score was 40 (range, score of 0–90) (P < .0001). The median mRS score at discharge was 1 (range, score of 0–2) in the patients with good outcomes and 4 (range, score of 3–5) in the patients with poor outcomes (P < .0001).
Fig 1.
Progression of NIHSS scores of patients with good outcomes and those with poor outcomes.
Logistic Regression Analysis
Univariate analysis showed that the variables associated with good outcome (mRS score, ≤2) at discharge were age, good collaterals, occlusion of M1 segment, alcohol habit, no history of hypertension, high grade recanalization, and improvement of NIHSS score by ≥2 immediately after LIT (ΔNIHSS score, ≥2) (Tables 3 and 4). We selected factors in baseline characteristics, such as age, good collaterals, occlusion of M1 segment, alcohol habit, and nonhypertension, as independent factors for multivariate analysis with a logistic regression model for good outcome (Table 3, Model 1). The analyses showed no factors to be significant.
Table 3:
Logistic regression analysis for pretreatment predictors of favorable outcome in patients undergoing local intra-arterial thrombolysis (Model 1)
| Variable | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95%CI | P Value | |
| Age <60 yr | 5.1 | 1.02–30.06 | .027 | 7.8 | 0.49–308.36 | .182 |
| Good collaterals | 8.8 | 1.55–74.68 | .016 | 6.1 | 0.53–102.44 | .166 |
| Occlusion of M1 segment | 5.3 | 1.04–33.91 | .047 | 5.4 | 0.41–139.85 | .218 |
| Alcohol habit (+) | 5.3 | 1.04–33.91 | .047 | 3.5 | 0.22–95.49 | .377 |
| Hypertension (−) | 5.3 | 1.04–33.91 | .047 | 2.3 | 0.19–29.67 | .483 |
Note.—OR indicates odds ratio; CI, confidence interval.
Table 4:
Logistic regression analysis for predictors of favorable outcome for patients undergoing local intra-arterial thrombolysis (Model 2)
| Variable | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| ΔNIHSS score ≥2 | 65.9 | 7.46–1656.23 | .001 | 38.8 | 2.40–2112.46 | .024 |
| High grade recanalization | 10.3 | 1.37–216.38 | .049 | 18.0 | 0.59–1538.90 | .126 |
| Age <60 yr | 5.1 | 1.02–30.06 | .027 | 7.7 | 0.32–296.83 | .265 |
| Good collaterals | 8.8 | 1.55–74.68 | .016 | 6.0 | 0.18–522.90 | .261 |
Note.—OR indicates odds ratio; CI, confidence interval; NIHSS, National Institutes of Health Stroke Scale.
ΔNIHSS score was calculated by subtracting immediate NIHSS score from baseline NIHSS score.
In a Model 2 analysis, we added ΔNIHSS score ≥ 2 and high grade recanalization to factors of age and good collaterals that showed P < .2 in the Model 1 multivariate analysis as an independent factors (for multivariate analysis with a logistic regression model) for good outcome (Table 4). As a result, ΔNIHSS ≥ 2 was the only significant predictor for favorable outcome.
Discussion
The National Institute of Neurologic Disorders and Stroke rt-PA trial revealed that SICH occurred in the rt-PA group 10 times more often than in the placebo group (1). Other randomized controlled trials reported that SICH occurred in 8% to 20% of patients (2–6). In our study, in which LIT was performed only in patients with embolic MCA occlusion, no cases of SICH (95% confidence interval, 0.871–1) or death occurred. Levy et al (7) reported that a predictor of SICH was a high mean blood pressure before IV tissue plasminogen activator treatment. Kidwell et al (8) found that in patients who had undergone LIT, the NIHSS score in the SICH group was higher than in the non-SICH group (mean NIHSS scores, 20 in SICH versus 15 in non-SICH). Other predictors of hemorrhagic transformation were longer time to recanalization, lower platelet count, and higher glucose level. The inclusion and exclusion criteria for LIT in our study were similar to those of the National Institute of Neurologic Disorders and Stroke rt-PA trial except for the time window, and we strictly followed the American Heart Association guidelines (3). As a result, patients with excessively high baseline NIHSS scores (scores > 29) were excluded from the study. In our study, 50% of the patients had mRS scores ≤2 at discharge, whereas 40% of the patients in the Prolyse in Acute Cerebral Thromboembolism II study had mRS scores ≤2 at day 90. Therefore, strict application of the criteria for the management of patients with LIT seems to be essential to obtain favorable outcomes and to avoid SICH. Additionally, antihypertensive medicine was aggressively infused through an IV route in patients who had high systolic blood pressure >180 mmHg after LIT. None of our patients had blood pressure >180 mmHg after antihypertensive treatment. It was suggested that control of blood pressure after LIT was also an important factor against SICH.
Bendszus et al (12) reported artery factors related to good outcomes in patients who had undergone LIT of MCA and internal carotid artery occlusion. They noted that presence of leptomeningeal collaterals and successful recanalization after LIT correlated with good outcome, whereas the interval from stroke onset to LIT was not related to it. However, their series had a 10% incidence of SICH. Arnold et al (13) reported that a low NIHSS score at admission and good vessel recanalization were independently associated with good outcome, and SICH occurred in 7% of patients. In the Prolyse in Acute Cerebral Thromboembolism II study, it was reported that outcome is associated with history of cerebrovascular disease, hypertension, and diabetes (14).
In our study, as in previous reports, the good outcome group was younger and had a higher rate of good collaterals and higher grade recanalization than the poor outcome group. In addition, the good outcome group had few cases of hypertension, as in the Prolyse in Acute Cerebral Thromboembolism II study, and alcohol habit and M1 occlusion were frequent. Aronowski et al (15) reported that a low dose of ethanol plus caffeine reduced infarct volume in a rat model of transient MCA occlusion. Although the optimal dose of alcohol was unknown, our result suggested that alcohol was a neuroprotective factor in the thrombolytic therapy. It seems very interesting that in our study, 62% of the good outcome group had M1 occlusion, compared with only 23% of the group with mRS ≥3. Gönner et al (16) indicated that good outcomes were achieved in 57% of patients with occlusion of M1 and perforators. On the other hand, all patients who had M1 occlusion without involving perforators or an M2 occlusion achieved good outcomes (100%). All our patients had M1 distal occlusions without perforator involvement. Although the rate of recanalization was not significantly different between patients with M1 occlusion and those with M2 occlusion, the patients with M1 occlusion had good collaterals (90%) compared with the patients with M2 occlusion (6%). The grade of collaterals may be more important for later outcome than the site of arterial occlusion.
No study has reported in detail on the evolution of NIHSS scores after LIT. In the good outcome group, the NIHSS score improved immediately after LIT and then continued to improve gradually. On the other hand, in the poor outcome group, the NIHSS score remained stable for nearly 24 hours (Fig 1). Multiple logistic regression analysis revealed that ΔNIHSS immediately after LIT was the only independent factor for good outcome. Thus, later outcome can be predicted by a change in NIHSS score (ΔNIHSS) immediately after LIT.
The present study had some limitations. First, it was an observational study. We cannot compare our results with those of patients who did not undergo LIT. Therefore, the effect of LIT for MCA occlusion is unclear. Second, our study size was small, so care must be taken in the interpretation of the analyses.
Conclusion
Strict criteria should be applied in selecting patients with MCA occlusion for LIT to avoid SICH. Favorable outcome at a later time or discharge may be predicted by improvement in NIHSS score immediately after LIT. These observations should be confirmed in large prospective randomized controlled trials.
Footnotes
This study was supported in part by Research Grants (15C-1 for Cardiovascular Disease, H14-shinkin-007) from the Ministry of Health, Labor and Welfare of Japan.
References
- 1.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587 [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke: The European Cooperative Acute Stroke Study (ECASS). JAMA 1995;274:1017–1025 [PubMed] [Google Scholar]
- 3.Albers GW, Clark WM, Madden KP, et al. ATLANTIS trial: results for patients treated within 3 hours of stroke onset: alteplase thrombolysis for acute noninterventional therapy in ischemic Stroke. Stroke 2002;33:493–495 [DOI] [PubMed] [Google Scholar]
- 4.Adams HP Jr, Brott TG, Furlan AJ, et al. Guidelines for thrombolytic therapy for acute Stroke: a supplement to the guidelines for the management of patients with acute ischemic stroke: a statement for healthcare professionals from a Special Writing Group of the Stroke Council, American Heart Association. Stroke 1996;27:1711–1718 [PubMed] [Google Scholar]
- 5.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke: The PROACT II study: a randomized controlled trial: prolyse in acute cerebral thromboembolism. JAMA 1999;282:2003–2011 [DOI] [PubMed] [Google Scholar]
- 6.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II): Second European-Australasian Acute Stroke Study Investigators. Lancet 1998;352:1245–1251 [DOI] [PubMed] [Google Scholar]
- 7.Levy DE, Brott TG, Haley EC Jr, et al. Factors related to intracranial hematoma formation in patients receiving tissue-type plasminogen activator for acute ischemic stroke. Stroke 1994;25:291–297 [DOI] [PubMed] [Google Scholar]
- 8.Kidwell CS, Saver JL, Carneado J, et al. Predictors of hemorrhagic transformation in patients receiving intra-arterial thrombolysis. Stroke 2002;33:717–724 [DOI] [PubMed] [Google Scholar]
- 9.Moulin T, Cattin F, Crepin-Leblond T, et al. Early CT signs in acute middle cerebral artery infarction: predictive value for subsequent infarct locations and outcome. Neurology 1996;47:366–375 [DOI] [PubMed] [Google Scholar]
- 10.Mori E, Yoneda Y, Tabuchi M, et al. Intravenous recombinant tissue plasminogen activator in acute carotid artery territory stroke. Neurology 1992;42:976–982 [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi T, Hayakawa T, Kikuchi H, et al. Intravenous tissue plasminogen activator ameliorates the outcome of hyperacute embolic stroke. Cerebrovasc Dis 1993;3:269–272 [Google Scholar]
- 12.Bendszus M, Urbach H, Ries F, et al. Outcome after local intra-arterial fibrinolysis compared with the natural course of patients with a dense middle cerebral artery on early CT. Neuroradiology 1998;40:54–58 [DOI] [PubMed] [Google Scholar]
- 13.Arnold M, Schroth G, Nedeltchev K, et al. Intra-arterial thrombolysis in 100 patients with acute stroke due to middle cerebral artery occlusion. Stroke. 2002;33:1828–1833 [DOI] [PubMed] [Google Scholar]
- 14.Wechsler LR, Roberts R, Furlan AJ, et al. Factors influencing outcome and treatment effect in PROACT II. Stroke 2003;34:1224–1229 [DOI] [PubMed] [Google Scholar]
- 15.Aronowski J, Strong R, Shirzadi A, et al. Ethanol plus caffeine (caffeinol) for treatment of ischemic stroke: preclinical experience. Stroke 2003;34:1246–1251 [DOI] [PubMed] [Google Scholar]
- 16.Gönner F, Remonda L, Mattle H, et al. Local intra-arterial thrombolysis in acute ischemic stroke. Stroke 1998;29:1894–1900 [DOI] [PubMed] [Google Scholar]