Abstract
BACKGROUND AND PURPOSE: Intra-arterial therapies for ischemic stroke offer promise as a means to extend the time window for acute treatment. The purpose of this study was to identify the percentage of the US population with potential access to interventional neuroradiologic expertise within 6 hours of the onset of stroke symptoms.
METHODS: Hospital locations of interventional neuroradiologists were identified from the 2002 roster of the American Society of Interventional and Therapeutic Neuroradiology. Data for populations in surrounding regions were extracted from US Census 2001 data by zip code. Standard transport speeds for emergency medical services were used in our estimates of the population living within a 5-hour transport time, which was a 6-hour treatment window less a 1-hour door-to-needle time, resulting in a 200-mile radius. A 2-hour transport time, or 3-hour treatment window, reflected a 65-mile radius.
RESULTS: A total of 385 interventional neuroradiologists were identified, practicing in 45 states. With a 200-mile radius, 99% of the total US population had access to neurointerventional treatment within 6 hours of symptom onset. With a 65-mile radius, 82% of the population had access within 3 hours of symptom onset. Alaska and the Mid-Northwest region covering Idaho, Wyoming, North Dakota, and South Dakota had limited coverage.
CONCLUSION: Most of the US population has access to interventional neuroradiologic expertise for acute stroke therapy. These data suggest that interventional therapies that extend the time window for treating acute ischemic stroke could have a major effect on public health and merit further research development and investment.
Therapeutic strategies to reverse or minimize the effects of acute ischemic stroke are generally designed to salvage tissue in the ischemic penumbral region. The most effective therapies demonstrated in animal models and now in patients are recanalization approaches intended to restore blood flow to the penumbral region (1–4). Experimental animal models have shown that the penumbral zone can be salvaged if prompt reperfusion occurs within 3–4 hours (5, 6). However, in humans, findings from positron emission tomography (PET), single photon emission CT (SPECT), and multimodal MR imaging have suggested that the time window for beneficial recanalization therapies may be longer, frequently up to 6 hours, and sometimes as long as 12–24 hours from symptom onset in selected patients (7–9).
The US Food and Drug Administration’s approval of intravenous tissue plasminogen activator (t-PA) in 1996 as the first drug therapy indicated for acute ischemic stroke was a landmark event, changing the course of stroke treatment throughout the US and other parts of the world. The National Institute of Neurologic Disorders and Stroke (NINDS) t-PA trials showed that intravenous thrombolysis improves functional outcome when administered within 3 hours of symptom onset (1). However, only 1–3% of acute stroke patients in the US currently receives this therapy (10–12). Patient presentation beyond the narrow 3-hour therapeutic time window is the leading reason for treatment disqualification and indicates an urgent need to extend the time window for treatment beyond 3 hours. In addition, a substantial proportion of patients presenting within 3 hours of onset have contraindications to systemic fibrinolysis (although not endovascular recanalization therapies), such as current anticoagulation or recent surgery. Additionally, six phase III trials of intravenous thrombolytics administered within 3–6 hours of symptom onset have failed to show a definitive treatment benefit, suggesting the need to explore alternative late recanalization approaches (12–17).
Endovascular interventions offer promise for the treatment of acute ischemic stroke for patients presenting within 3 hours in whom conventional intravenous thrombolysis is contraindicated and for patients presenting beyond the 3-hour window. The Prolyse in Acute Cerebral Thromboembolism II (PROACT II) large-scale, randomized clinical trial demonstrated a beneficial effect of intra-arterial thrombolysis up to 6 hours after onset in 180 patients with occlusions of the middle cerebral artery (2). The intra-arterial approach has the advantages of delivering a high concentration of drug directly to the clot (reducing systemic exposure to the agent) and the opportunity to carry out gentle mechanical disruption of the clot by using the delivery catheter and guidewire (18). More recently, interest in the use of mechanical devices for acute stroke treatment has grown. Such treatments include the use of clot-retrieval devices, lasers, and microsnare catheters; angioplasty; waterjet thrombectomy; and sonography (3, 19). These approaches have the potential to lower rates of hemorrhagic complications. A variety of combined intravenous and endovascular approaches are currently under investigation.
Although neurointerventional therapies for ischemic stroke offer promise as a means to extend the time window available for acute treatment, their widespread application may be constrained by the limited availability of skilled neurointerventionalists and sophisticated endovascular suites. Because neurointerventional expertise has been steadily expanding throughout the US, we sought to determine the current proportion of the US population with potential access to interventional neuroradiologic expertise within 6 hours of stroke symptom onset. For this, we developed models using Geographic Information System (GIS) analysis that allows digital population data to be combined with geographic information.
Methods
Interventional neuroradiologists and their affiliated US hospital or institutional locations were identified from the 2002 roster of the American Society of Interventional and Therapeutic Neuroradiology (ASITN) and the society’s Web site (available at www.asitn.org). If a member’s practice location was unclearly specified in these listings, the information was obtained by means of individual contact via e-mail or telephone.
Several assumptions were made for the analyses. First, we assumed that at least 1 hour is required after the patient’s arrival at the hospital for initial assessment and the initiation of interventional procedures before acute endovascular therapy is begun (door-to-needle time) (20, 21). The remaining time allowed for patient transport (onset-to-door time) is therefore 2 hours for therapies with a 3-hour treatment window or 5 hours for therapies with a 6-hour treatment window. Second, we assumed that patients arriving by ground ambulance are transported at EMS transport speeds of 50 mph, covering 20 miles in hour 1 (allowing for on-scene evaluation and loading time) and 50 mph in subsequent hours. We assumed that patients arriving by air ambulance patients are transported at EMS air-medical transport speeds of 130 mph, covering 50 miles during the hour of pick-up and 130 mph in full travel hours (21, 22).
For land-ambulance transport, one-way transport times were modeled as follows: Ambulances are generally dispatched from locations near the patient. The estimated land-ambulance transport distance of 20 miles in the first 60 minutes includes time for dispatching the ambulance and for on-site evaluation. Accordingly, in this model, ground transport times of 1, 2, 3, 4, and 5 hours correspond to land transport distances of 40, 65, 115, 165, and 200 miles, respectively (21).
Because helicopters are generally dispatched from the tertiary hospital or central heliport to the field or primary facilities to retrieve patients, round-trip transport times were included in our air transport analyses. Although helicopters can fly a direct route at about 120–130 mph, (23–25) this advantage is attenuated by the need to travel long distances to the pick-up site, to find an appropriate landing site, to obtain authorization for lift-off and landing, to complete mechanical preparations, and to account for greater vulnerability to inclement weather (21, 26). By including the round-trip time, transport times and distances therefore became identical for helicopter versus ambulance group transport.
Population data for the regions surrounding each interventional neuroradiologist’s target hospital was extracted from US Census 2001 data by zip code for all 50 states. The zip code-based population data provided small, subdivided area information compared with the broader county-based data. These data permitted more precise, radial estimates of the population in the surrounding area of individual hospitals. GIS software (ArcGIS) developed by the Environmental System Research Institute (Redland, CA) was used to perform the zip code–based population analyses. The facility addresses of the neurointerventional radiologists, along with ArcGIS Street Map U.S.A. (Environmental System Research Institute, Redland, CA), were used to pinpoint the location of facilities on a standard US map. Circular boundaries with 65- and 200-mile radii surrounding the center facilities were overlaid on the facility location map for buffering. A US zip code polygon map was superimposed to the location map, and the area of each zip code polygon within each buffering zone was calculated. Using the code based population, we calculated the population residing in each zip code polygon within the buffering zone on the basis of the proportion of the coverage area by buffering zone.
State-by-state analysis was performed to compare the number of interventional neuroradiologists, the number of hospitals capable of performing neuroendovascular procedures, and the population per hospital or interventional neuroradiologist. States were divided into two groups by the number of interventional neuroradiologists: ≥10 and <10. The two groups were compared in terms of population, population per neuroradiologists, and population per capable institute. The Student t test was used for statistical analysis.
For analyses of access to care by age subgroup, county-level Census 2000 age-group population data provided by the Environmental System Research Institute were used in a similar GIS analysis. Subgroups were stratified by age: 20–44, 45–64, and ≥65 years.
Results
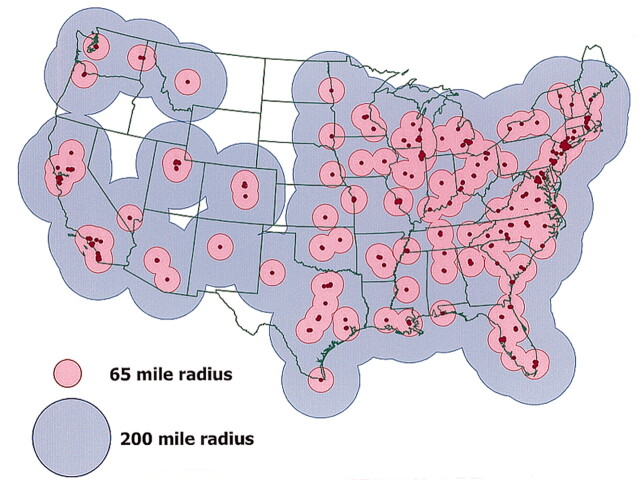
A total of 385 interventional neuroradiologists were identified in the US, practicing in 238 hospitals covering 45 states. Five states did not have an interventional neuroradiologist; however, a portion of the population in three of these states had access to those in neighboring states. Table 1 shows the percentage of the US population residing in the accessible area surrounding each neurointerventionalist’s primary hospital. With a 200-mile radius (6-hour treatment window), 99% of the total US population (282 million people) has access to an interventional neuroradiologist for treatment within 6 hours of the onset of stroke symptoms (Fig). With a 65-mile radius (6-hour treatment window), 82% of the US population (236 million people) has access to possible interventional treatment within 3 hours of symptom onset. Within the 20-mile radius (2-hour treatment window), 54% of the population (153 million people) has access to possible interventional treatment.
TABLE 1:
Percentage of the population with access to endovascular treatment in 3- and 6-hour treatment windows
| Treatment Window (h) | Transport Time (h) | Distance (mi) | Population |
|---|---|---|---|
| 3 | 2 | 65 | 82% |
| 6 | 5 | 200 | 99% |
Fig 1.
US map displays geographic zones in which population dwells within 2-hour (pink) or 5-hour (blue) transport time to a hospital with acute interventional neuroradiologic services (red dots)
As illustrated in Figure 1, potential endovascular access coverage includes the preponderance of the continental US, with extensive coverage of high-population urban areas. In addition to Alaska, the Mid-Northwest region of Montana, Idaho, Wyoming, North Dakota, and South Dakota had limited coverage. Although Delaware and West Virginia did not have an interventional neuroradiologist listed on the ASITN roster, these areas were well covered because of their proximity to neurointerventional centers in neighboring states. State-by-state analysis revealed that California, New York, Florida, and Texas have the greatest number of interventional neuroradiologists (44, 32, 23, and 22 respectively), as well as the greatest number of hospitals or facilities capable of neurointerventional procedures (25, 18, 15, and 14, respectively). In 16 states, 10 or more interventional neuroradiologists are practicing. In 29 states, <10 interventional neuroradiologists practice (and five states have no interventional neuroradiologist). The statewide population was substantially larger in states with ≥10 interventional neuroradiologists than states with <10 (Table 2). However, the population per interventional neuroradiologists and per capable institute did not differ. Age-subgroup analysis demonstrated no difference in access by age, indicating widespread access to intra-arterial therapy in the stroke-prone population 65 years or older (Table 3).
TABLE 2:
Comparison of states with high and low numbers of interventional neuroradiologists
| No. of Interventional Neuroradiologist |
P Value | ||
|---|---|---|---|
| ≥10 | <10 | ||
| No. of states | 16 | 29 | |
| Population* | 11,616,000 ± 7,738,000 | 2,730,550 ± 1,991,000 | <.001 |
| Population per interventional neuroradiologist | 662,000 ± 166,000 | 837,000 ± 413,000 | .05 |
| Population per institute | 1,164,000 ± 312,000 | 1,121,000 ± 461,000 | .7 |
Note.—Data are the mean ± standard deviation unless otherwise specified.
Population is significantly greater in the group of states where ≥10 or more interventional neuroradiologists practice than the group of states where ≤10 practice (P < .001, Student t test).
TABLE 3:
Proportion of population in age subgroups with access to intra-arterial treatments in the 3- and 6-hour treatment windows
| Treatment Window (h) | Age Subgroup |
All Adults >18 Years | ||
|---|---|---|---|---|
| 20–44 Years | 45–64 Years | ≥65 Years | ||
| 3 | 82% | 81% | 80% | 81% |
| 6 | 99% | 99% | 99% | 99% |
Discussion
Neurointerventional endovascular therapies offer increasing promise for the treatment of acute ischemic stroke (2, 3, 18, 27). The PROACT II trial demonstrated that intra-arterial thrombolysis commencing as long as 6 hours after the onset of stroke symptom is associated with higher recanalization rates and better clinical outcomes (2). Numerous mechanical devices are currently undergoing investigation, as are intravenous and intra-arterial combination strategies. These approaches offer a means to extend the time window for acute therapies to 6 hours or longer and to improve the efficacy and benefits of recanalization therapy for stroke. However, the potential benefit of neuroendovascular therapies can be realized only if patients have access to the required facilities and neurointerventionalist expertise.
Our findings suggest that 99% of the US population has access to interventional neuroradiologic expertise for acute stroke therapy that is initiated within 6 hours of symptom onset. Moreover, more than four of five Americans currently have access to neurointerventional expertise for treatments within a 3-hour window. Compared with previous observations, (22), this finding represents a substantial increase in the proportion of the US population with access to acute neuroendovascular interventions between 1994 and 2002, with a change from 62% to 82% in the 3-hour window and 96% to 99% in the 6-hour window. This increase reflects the continued growth of interventional neuroradiology as a specialty, as neuroendovascular treatments for cerebrovascular disease continue to advance.
Delivering acute neuroendovascular care to stroke patients within the geographic distributions we analyzed requires substantial changes in the current systems for prehospital stroke care and for interhospital transfers of these patients. To transport large numbers of patients to neuroendovascular centers in clinical practice, comprehensive stroke centers with neurointerventional expertise must be formally designated, and EMS personnel must be able to accurately diagnose stroke and then divert patients to the stroke centers. Fortunately, progress is being made in both of these areas.
Designated stroke-center systems have been implemented in Canada and Germany, with dramatic improvements in the proportion of acute stroke patients treated with effective interventions (28, 29). In the US, leading organizations involved in the case of acute stroke, including the American College of Emergency Physicians, the American Academy of Neurology, the ASITN, the American Heart Association, the National Stroke Association and the Brain Attack Coalition (30) have endorsed the designation of stroke centers. Most neurologists, neurosurgeons, and emergency physicians also support this approach (31). A demonstration project for designated stroke centers in Houston has proved the feasibility, effectiveness, and desirability of establishing such systems in the US (25, 28).
Prehospital stroke recognition instruments are routinely used in most EMS systems in the US (32, 33). High accuracy rates in the paramedic diversion of patients to designated stroke centers when these instruments are used supports the feasibility of diverting stroke patients to comprehensive stroke centers with neurointerventional capability (28, 34).
Our findings have important implications on future stroke care. At a research level, our data support further investment in and the development of endovascular approaches in acute stroke therapy. At a systems and organizational level, our findings suggest the need for a coordinated EMS response for stroke patients. We found that interventional neuroradiologists are unevenly distributed geographically in the US, with about one-third found in California, New York, Florida, and Texas. We found substantial overlaps in the service areas surrounding urban and suburban hospitals with interventional neuroradiologists, especially in states on the East Coast. On the other hand, rural regions of Mid-Northwest states have limited availability of interventional neuroradiologists. This geographic distribution suggests that, in metropolitan areas, a prehospital ground-transport system could be used to adequately transport patients to designated endovascular stroke centers, whereas in rural areas, a helicopter-based transport system may be necessary (25). A rendezvous system combining ground-ambulance and helicopter transport can efficiently increase the speed of air transport (23). In addition, coordinated EMS efforts across county and state borders may be necessary to provide optimal access to neuroendovascular care for populations living in areas with widely separated interventionalists.
There are several limitations to our study. Our analyses assumed standard ground and air speeds for transport times across the country. However, in reality, there is some variation in these times related to a variety of factors, including rural versus urban settings. In addition, our model likely underestimated the proportion of the population with access to neurointerventional expertise because of its sole reliance on the ASITN roster to identify physicians trained in neuroendovascular procedures. An increasing number of neurosurgeons and neurologists are receiving neuroendovascular training, and a large group of general interventional radiologists may be available to provide endovascular therapy for acute stroke. These subspecialty groups were not fully represented in the current analysis, which therefore might have underestimated the availability of acute stroke endovascular therapy. Conversely, the presence of a single physician trained in endovascular neuroradiology at one site should not suggest that 24-hour coverage is available; such an assumption would lead to overestimation of service availability.
In addition, our model might have overestimated access by assuming that only 1 hour is required for patient examination, brain imaging, and diagnostic angiography between the patient’s arrival at the emergency department and the start of intra-arterial therapy. Data from intravenous t-PA studies have suggested that this goal for door-to-needle time is often difficult to achieve in clinical practice (30). Also, we assumed that all hospitals with an interventional neuroradiologists had the capability (stroke units, neurologic expertise, etc) to provide appropriate periprocedural stroke care for patients treated with intra-arterial therapy; this might not have been the case in all institutions.
Conclusion
Our findings suggest that most of the US population has access to neurointerventional expertise within a 6-hour window for the treatment of acute ischemic stroke. Interventional therapies that extend the time window for the treatment of acute ischemic stroke could therefore have a major effect on public health and merit further research development and investment. Geographic analysis of the distribution of neuroendovascular centers may be used in the future to organize and optimize stroke-care delivery systems.
References
- 1.NINDS rt-PA Stroke Group: Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587 [DOI] [PubMed] [Google Scholar]
- 2.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke: the PROACT II study—a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA 1999;282:2003–2011 [DOI] [PubMed] [Google Scholar]
- 3.Leary MC, Saver JL, Gobin YP, et al. Beyond tissue plasminogen activator: mechanical intervention in acute stroke. Ann Emerg Med 2003;41:838–846 [DOI] [PubMed] [Google Scholar]
- 4.del Zoppo GJ. Thrombolytic therapy in the treatment of stroke. Drugs 1997;54:90–98 [DOI] [PubMed] [Google Scholar]
- 5.Ginsberg MD. The validity of rodent brain-ischemia models is self-evident. Arch Neurol 1996;53:1065–1067 [DOI] [PubMed] [Google Scholar]
- 6.Memezawa H, Minamisawa H, Smith ML, Siesjo BK. Ischemic penumbra in a model of reversible middle cerebral artery occlusion in the rat. Exp Brain Res 1992;89:67–78 [DOI] [PubMed] [Google Scholar]
- 7.Furlan M, Marchal G, Viader F, et al. Spontaneous neurological recovery after stroke and the fate of the ischemic penumbra. Ann Neurol 1996;40:216–226 [DOI] [PubMed] [Google Scholar]
- 8.Barber PA, Darby DG, Desmond PM, et al. Prediction of stroke outcome with echoplanar perfusion- and diffusion-weighted MRI. Neurology 1998;51:418–426 [DOI] [PubMed] [Google Scholar]
- 9.Darby DG, Barber PA, Gerraty RP, et al. Pathophysiological topography of acute ischemia by combined diffusion-weighted and perfusion MRI. Stroke 1999;30:2043–2052 [DOI] [PubMed] [Google Scholar]
- 10.Nilasena DS, Kresowik TF, Wiblin RT, et al. Assessing patterns of tPA use in acute stroke [Abstract]. Stroke 2002;33:354 [Google Scholar]
- 11.Chiu D, Krieger D, Villar-Cordova C, et al. Intravenous tissue plasminogen activator for acute ischemic stroke: feasibility, safety, and efficacy in the first year of clinical practice. Stroke 1998;29:18–22 [DOI] [PubMed] [Google Scholar]
- 12.Clark WM, Wissman S, Albers GW, et al. Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset: the ATLANTIS Study—a randomized controlled trial. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA 1999;282:2019–2026 [DOI] [PubMed] [Google Scholar]
- 13.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II): Second European-Australasian Acute Stroke Study Investigators. Lancet 1998;352:1245–1251 [DOI] [PubMed] [Google Scholar]
- 14.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke: the European Cooperative Acute Stroke Study (ECASS). JAMA 1995;274:1017–1025 [PubMed] [Google Scholar]
- 15.Donnan GA, Davis SM, Chambers BR, et al. Streptokinase for acute ischemic stroke with relationship to time of administration: Australian Streptokinase (ASK) Trial Study Group. JAMA 1996;276:961–966 [PubMed] [Google Scholar]
- 16.Thrombolytic therapy with streptokinase in acute ischemic stroke: the Multicenter Acute Stroke Trial–Europe Study Group. N Engl J Med 1996;335:145–150 [DOI] [PubMed] [Google Scholar]
- 17.Randomised controlled trial of streptokinase, aspirin, and combination of both in treatment of acute ischaemic stroke: Multicentre Acute Stroke Trial–Italy (MAST-I) Group. Lancet 1995;346:1509–1514 [PubMed] [Google Scholar]
- 18.Saver JL. Intra-arterial thrombolysis [Suppl]. Neurology 2001;57:S58–S60 [DOI] [PubMed] [Google Scholar]
- 19.Furlan AJ. Acute stroke therapy: beyond i.v. tPA. Cleve Clin J Med 2002;69:730–734 [DOI] [PubMed] [Google Scholar]
- 20.Marler JR, Winters Jones P, Emr M. The National Institute of Neurological Disorders and Stroke: Proceedings of National Symposium on Rapid Identification and Treatment of Acute Stroke. Bethesda: National Institute of Neurological Disorders and Stroke,1997
- 21.Scott P, Temovsky CJ, Lawrence K, et al. Analysis of Canadian population with geographic access to intravenous thrombolysis for acute ischemic stroke [Abstract]. Stroke 1998;29:289. [DOI] [PubMed] [Google Scholar]
- 22.Scott P, Lowell MJ, Longstreth K. Analysis of US population with geographic access to interventional neuroradiology and intra-arterial thrombolysis for acute ischemic stroke [Abstract]. Stroke 1997;28:266 [Google Scholar]
- 23.Smith JS Jr, Smith BJ, Pletcher SE, et al. When is air medical service faster than ground transportation? Air Med J 1993;12:258–261 [DOI] [PubMed] [Google Scholar]
- 24.Kasner SE, Chalela JA, Luciano JM, et al. Reliability and validity of estimating the NIH Stroke Scale from medical records [Abstract]. Neurology 1999;52:A149. [DOI] [PubMed] [Google Scholar]
- 25.Silliman SL, Quinn B, Huggett V, Merino JG. Use of a field-to-stroke center helicopter transport program to extend thrombolytic therapy to rural residents. Stroke 2003;34:729–733 [DOI] [PubMed] [Google Scholar]
- 26.Nicholl JP, Beeby NR, Brazier JE. A comparison of the costs and performance of an emergency helicopter and land ambulances in a rural area. Injury 1994;25:145–153 [DOI] [PubMed] [Google Scholar]
- 27.Intraarterial thrombolysis: ready for prime time? Executive Committee of the ASITN: American Society of Interventional and Therapeutic Neuroradiology. AJNR Am J Neuroradiol 2001;22:55–58 [PMC free article] [PubMed] [Google Scholar]
- 28.Wojner AW, Persse D, Alexandrov AV, Grotta J. Weaving a web for stroke treatment: the Houston Paramedic/Emergency Stroke Treatment Outcomes Study (HoPSTO) [Abstract]. Stroke 2003;34:248A [Google Scholar]
- 29.Merino JG, Silver B, Wong E, et al. Extending tissue plasminogen activator use to community and rural stroke patients. Stroke 2002;33:141–146 [DOI] [PubMed] [Google Scholar]
- 30.Alberts MJ, Hademenos G, Latchaw RE, et al. Recommendations for the establishment of primary stroke centers: Brain Attack Coalition. JAMA 2000;283:3102–3109 [DOI] [PubMed] [Google Scholar]
- 31.Kidwell CS, Shephard T, Tonn S, et al. Establishment of primary stroke centers: a survey of physician attitudes and hospital resources. Neurology 2003;60:1452–1456 [DOI] [PubMed] [Google Scholar]
- 32.Kidwell CS, Starkman S, Eckstein M, et al. Identifying stroke in the field: prospective validation of the Los Angeles Prehospital Stroke Screen (LAPSS). Stroke 2000;31:71–76 [DOI] [PubMed] [Google Scholar]
- 33.Kothari R, Hall K, Brott T, Broderick J. Early stroke recognition: developing an out-of-hospital NIH stroke scale. Acad Emerg Med 1997;4:986–990 [DOI] [PubMed] [Google Scholar]
- 34.Harbison J, Hossain O, Jenkinson D, et al. Diagnostic accuracy of stroke referrals from primary care, emergency room physicians, and ambulance staff using the face arm speech test. Stroke 2003;34:71–76 [DOI] [PubMed] [Google Scholar]