Abstract
Summary: Currently, most carotid artery pathologic abnormalities resulting in pseudoaneurysm formation or stenosis are repaired by surgical intervention. Because surgical intervention requires proximal and distal control of the artery, pseudoaneurysms near the skull base may be very difficult to repair and pose greater risk to the patient. As a result, endovascular techniques have evolved in an effort to reduce morbidity associated with surgical techniques. Parent vessel occlusion and coil placement are the most frequently used endovascular techniques for carotid artery repair of pseudoaneurysms. Intimal hyperplasia is generally treated with balloon angioplasty, often in conjunction with uncovered stent placement. Parent vessel occlusion may be impractical if the patient is unable to tolerate occlusion of that artery. We report our experience in treating three patients with carotid artery stent grafts.
Because of recent advances in medical technology, covered stent grafts are becoming a common alternative in the treatment of disease processes that were once treated exclusively by surgical intervention. Covered stent grafts currently are used for the successful treatment of aneurysms and pseudoaneurysms in the aorta, iliac arteries, and femoral arteries. Covered stent grafts have had limited use in the carotid artery because of the risk of cerebral infarction resulting from acute dissection, occlusion, or embolic sequelae. Presumed risk of latent occlusion from thrombus formation or intimal hyperplasia additionally is a concern. The following case reports show extracranial carotid artery repair by using covered stent grafts for the treatment of neoplastic and traumatic pseudoaneurysms and intractable intimal hyperplasia. Because commercially manufactured stent grafts for the carotid artery were not available, the covered stent grafts used in the cases reported herein were individually constructed for all three patients.
Case Reports
The covered stent grafts were built as follows. A segment of thin walled 3-mm polytetrafluoroethylene (PTFE) graft was predilated to 6 mm with a balloon dilation catheter. A stent was partially inflated. The PTFE was then meticulously sewn to the outside of the stent at the leading and trailing edges with 6–0 Prolene suture on a noncutting needle. Two sutures were placed at each end at the 12 and 6 o’clock positions. A 6 × 4 mm balloon dilation catheter was then back-loaded through a 10F guiding catheter. The stent graft was crimped on the balloon portion of the catheter and withdrawn into the guiding catheter. A van Andel dilator was “filleted” and placed behind the covered stent graft to ensure that it was not pushed off the balloon as the stent graft was passed through the guiding catheter. Next, a 12F short sheath was placed in the groin. Heparin was IV administered. The 10F guiding catheter containing the covered stent graft was placed through the 12F short sheath in the groin and advanced to the appropriate position within the internal carotid artery over an exchange length Rosen wire. Arteriography was then performed to verify the location of the covered stent graft. After correctly positioning the stent graft, the 10F guiding catheter was retracted and the balloon dilation catheter was dilated to the size of the internal carotid artery. Difficult placement of the system was encountered because of its inherent stiffness and size. All patients were placed on antiplatelet therapy with aspirin or a combination of aspirin and Plavix.
Patient 1
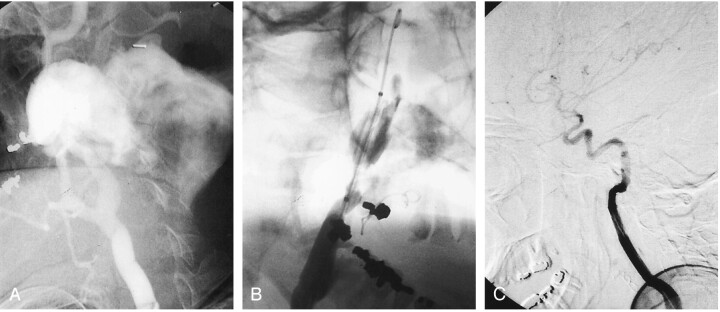
A 31-year-old woman with a known history of neurofibromatosis type 1 presented with an acutely enlarging left neck mass. On the basis of clinical findings and the acute nature of the mass, a ruptured pseudoaneurysm from tumor invasion was suspected. Diagnostic arteriography was performed, and a large pseudoaneurysm was noted (Fig 1A). The internal carotid artery and the proximal internal maxillary artery were found to supply the pseudoaneurysm.
Fig 1.
Images obtained in patient 1, a 31-year-old woman who presented with an acutely enlarging left neck mass.
A, Selective arteriogram of the left common carotid artery shows a large pseudoaneurysm of the left internal carotid artery with contrast material extravasation.
B, Covered stent graft is placed across the neck of the pseudoaneurysm.
C, Arteriogram obtained after stent graft placement shows occlusion of the pseudoaneurysm from the internal carotid artery.
Intervention
On the day of presentation, the proximal internal maxillary artery was selectively catheterized and coils were deployed to occlude the artery. The supply to the pseudoaneurysm from the internal carotid artery was occluded with two covered stent grafts (Fig 1B). Stagnation of contrast material in the internal carotid artery was related to vasospasm (Fig 1B). Two stent grafts were required to completely occlude the pseudoaneurysm, because the initial graft did not completely cover the neck (Fig 1C). Follow-up of this patient’s covered stent graft included carotid sonography, and sonography was performed annually thereafter. The last sonogram obtained 27 months later showed a patent left carotid artery with no stenosis.
Patient 2
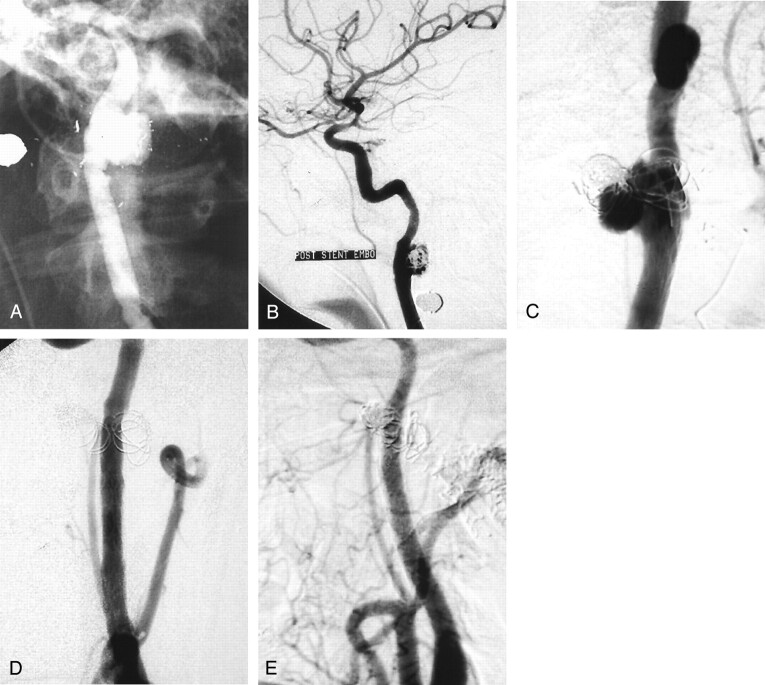
A 23-year-old man presented initially after receiving gunshot wounds to the skull and neck region. Because of the location of the bullet fragments, arterial injury was suspected and arteriography was performed. A 2.0-cm-diameter multi-lobulated skull base level pseudoaneurysm was noted from a large defect in the posterolateral wall of the left internal carotid artery (Fig 2A). A high flow left external carotid artery to left external jugular vein AVF also was noted. The AVF was occluded with coils. Because of the location of the pseudoaneurysm, the patient was referred to the interventional neuroradiology department for endovascular repair of the pseudoaneurysm.
Fig 2.
Images obtained in patient 2, a 23-year-old man who presented with gunshot wounds to the skull and neck.
A, Selective left internal carotid arteriogram shows a posttraumatic pseudoaneurysm near the skull base.
B, Selective left internal carotid artery injection shows a stent across the pseudoaneurysm, with multiple coils within the pseudoaneurysm. Minimal residual flow is noted in the pseudoaneurysm.
C, Selective injection of the internal carotid artery shows a recurrent pseudoaneurysm.
D, Selective common carotid artery injection shows the covered stent grafts in place with occlusion of the pseudoaneurysm.
E, Injection of the common carotid artery performed 8 months after stent graft placement shows continued occlusion of the pseudoaneurysm.
Intervention
Thirteen days after presentation, pseudoaneurysm occlusion was attempted by placing a wall stent across the neck of the pseudoaneurysm and filling the pseudoaneurysm with multiple 4-mm coils (Fig 2B). We elected to initially treat the patient with a stent and coils because he was clinically stable and we were uncertain the 10-French guiding catheter required for stent graft placement could be successfully navigated into position within the internal carotid artery at the level of the skull base. At the conclusion of the procedure, there was minimal flow with near-complete stasis within the pseudoaneurysm. Four weeks after initial presentation, repeat arteriography confirmed the clinical suspicion of recurrent AVF. Further enlargement of the left internal carotid artery pseudoaneurysm was also noted (Fig 2C). Twelve days later, a covered stent graft was fashioned and placed as described above to close the known internal carotid artery pseudoaneurysm (Fig 2D). Two stent grafts were required to completely occlude the pseudoaneurysm, as the first graft was deployed slightly proximal to its desired location. With the two stent grafts in place, the pseudoaneurysm was successfully occluded. The AVF was closed 10 days later by using a direct puncture transvenous approach. A follow-up arteriogram was performed 8 months after stent graft placement. The left internal carotid artery was normal in appearance without evidence of residual pseudoaneurysm or stenosis (Fig 2E). The fistula remained closed.
Patient 3
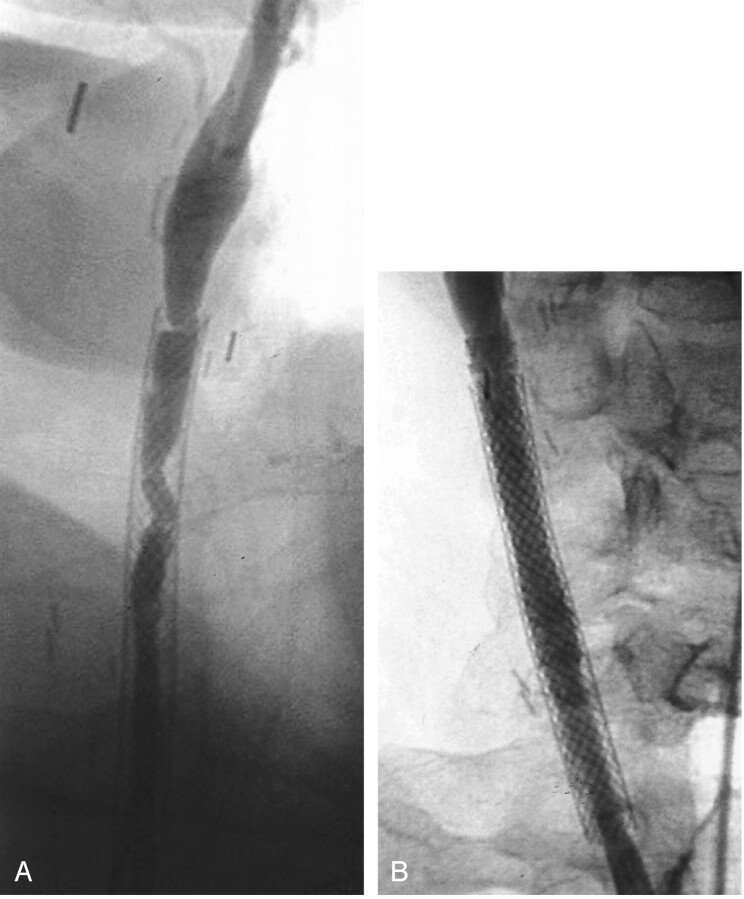
A 63-year-old woman with a history of atherosclerotic carotid artery disease had undergone three carotid endarterectomies. Follow-up arteriography showed recurrent stenosis secondary to intimal hyperplasia. The stenosis was initially treated with a wall stent in the right common carotid artery and a Palmaz stent in the right internal carotid artery. A Palmaz stent was used in the internal carotid artery; it was placed behind the mandible in a position where it was not easily compressible. Surveillance sonography detected recurrent stenosis in the common carotid artery. Recurrent intimal hyperplasia causing moderate stenosis was noted within the mid-aspect of the common carotid artery within the previously deployed wall stent (Fig 3A).
Fig 3.
Images obtained in patient 3, a 63-year-old woman who had undergone three carotid endarterectomies.
A, Selective arteriogram of the right common carotid artery shows moderate stenosis at the midportion of the previously deployed wall stent.
B, Covered stent graft is placed overlying the midportion of the wall stent. Angiogram obtained after stent graft shows no evidence of stenosis.
Intervention
Covered stent graft placement was performed because of recurrent intimal hyperplasia within the wall stent despite multiple treatments with conventional angioplasty (Fig 3B). Using the technique described above, a stent graft was placed across the focal stenosis within the distal aspect of the wall stent. During multiple follow-up examinations, sonography revealed recurrent high grade stenosis within the portion of the wall stent that was not covered with the stent graft. The recurrent intimal hyperplasia within the wall stent was treated with conventional angioplasty on five separate occasions during the next 2 years. The portion of the common carotid artery containing the stent graft never developed intimal hyperplasia. Because of the intractable nature of the disease, intra-arterial brachytherapy at the site of recurrent high grade stenosis was performed after angioplasty. Iridium 192 was placed at the site of angioplasty and left in place for 30 minutes. A total estimated dose of 14 Gy was administered to a depth of 3 mm. One additional treatment with angioplasty was required 4 months after brachytherapy. The last carotid sonography, performed more than 3 years after stent graft placement, showed no high grade stenosis within either the stent graft or proximal wall stent.
Discussion
The initial results of these case reports suggest that the use of covered stent grafts in the carotid artery can be a safe and effective alternative to conventional surgery and other endovascular techniques. Currently, most carotid artery pathologic abnormalities resulting in pseudoaneurysm formation or stenosis are repaired by surgical intervention (1). Parent vessel occlusion may be impractical if the patient is unable to tolerate occlusion of that artery (2). As seen in the above described intimal hyperplasia case report, recurrence is a possibility after angioplasty and uncovered wall stent placement; however, intimal hyperplasia did not recur in the portion of the carotid artery treated with a covered stent graft (3). More cases with long-term follow-up are needed to determine the potential role for this technique as a routine treatment for the repair of carotid artery pseudoaneurysms and recurrent intimal hyperplasia.
References
- 1.Ruebben A, Merlo M, Verri A, et al. Exclusion of an internal carotid aneurysm by a covered stent. J Cardiovasc Surg 1997;38:301–303 [PubMed] [Google Scholar]
- 2.Fox AJ, Viñuela F, Pelz DM, et al. Use of detachable balloons for proximal artery occlusion in the treatment of unclippable cerebral aneurysms. J Neurosurg 1987;66:40–46 [DOI] [PubMed] [Google Scholar]
- 3.Nicholson A, Cook AM, Dyet JF, et al. Case report: treatment of a carotid artery pseudoaneurism with a polyester covered nitinol stent. Clin Radiol 1995;50:872–873 [DOI] [PubMed] [Google Scholar]