Abstract
BACKGROUND AND PURPOSE: We present our preliminary experience, including mid-term angiographic and clinical follow-up results, with an alternative technique for the endovascular treatment of intracranial aneurysms in a series of patients. This new method, previously described in anecdotal case reports, consists of endovascular deployment of an artificial vessel graft (stent graft or covered stent) in the parent vessel to exclude the intracranial aneurysm sac from circulation.
METHODS: Twenty-five internal carotid artery (ICA) aneurysms in 24 patients were successfully treated by using a Jostent coronary stent graft deployed in the parent artery across the aneurysm neck. All except four aneurysms were extradural, located in the petrous or cavernous portion of the ICA. The four intradural aneurysms were located in the carotico-ophthalmic region. Seventeen aneurysms in 16 patients occurred posttraumatically, secondary to motor vehicle accidents or surgical injury.
RESULTS: Twenty-three aneurysms were immediately excluded from circulation after stent graft placement. In two aneurysms, a slow contrast material filling (endoleak) into the aneurysm cavity was observed immediately after treatment. One was thrombosed, as shown by late control angiography; in the other one, a second larger bare stent was used to appose the stent graft’s distal end to the ICA wall, thus sealing the endoleak into the distal graft. No technical adverse event, including vessel dissection, vessel perforation, or thromboembolism, occurred with or without clinical consequence. No mortality or morbidity developed during or after the procedure, including the follow-up period. Two-year control angiography in one patient, 1.5-year control angiography in two patients, 1-year control angiography in six patients, and 6-month control angiography in 12 patients were performed, revealing reconstruction of the ICA with no aneurysm recanalization. All symptoms resolved after treatment in the patients who had initially presented with mass effect.
CONCLUSION: Initial anatomic, clinical and mid-term follow-up results in this small series of patients are encouraging. This technique has been proved to have potential in the reconstructive treatment of intracranial aneurysms. Further research and development are needed to optimize the stent graft technology for the cerebrovascular system.
Endovascular treatment of cerebral aneurysms with detachable coils has now been proven to be a superior alternative to open microsurgery in terms of survival free of disability at 1 year, according to the recently published large randomized International Subarachnoid Aneurysm Trial (ISAT), which studied patients with ruptured aneurysms (1). Despite this, recanalization of the aneurysms with recurrences in 10–20% of patients after coil placement is still the main drawback of the endovascular technique. The recanalization rate is even higher in complicated, wide necked, large, or giant aneurysms treated with detachable coils (2). Such aneurysms also represent serious difficulties for the surgeon—in particular, aneurysms that are large or giant sized and located in the internal carotid artery (ICA)—because of bony obstacles and difficulty in proximal control (3, 4). Because of these drawbacks, interventional neuroradiologists and medical technology have been urged to create more innovative techniques for better endovascular reconstructive treatment options (5, 6). One of these recent advances is the use of stents deployed across the aneurysm neck in the parent artery for the reconstructive endovascular treatment of challenging aneurysms in combination with intra-aneurysmal filler materials, such as metallic coils or liquid polymer Onyx (6–8), leading to favorable outcomes.
We present an advanced reconstruction technique with which a stent graft is deployed in the parent artery to exclude the cerebral aneurysms from circulation. Although the use of stent grafts in the endovascular aneurysm treatment has been previously described as anecdotal experiences in various case reports, this article, for the first time in the English literature, presents data from a series of patients in whom this technique has been used, including mid-term angiographic and clinical follow-up.
Patients and Techniques
This study included 25 ICA aneurysms in 24 patients, successfully treated at a single institution by using the Jostent coronary stent graft (Jomed international, Helsingborg, Sweden) placed in the parent artery across the aneurysm neck during the period from December 2001 through December 2003. During that time, 418 intracranial aneurysms were treated by endovascular methods. These 24 patients represented all the patients whose aneurysms were treated with this technique (i.e., no technical failures had occurred, as this group consisted of highly selected cases, especially in terms of proximal vascular tortuosity). Patient age ranged from 17 to 59 years; 16 were male and eight were female patients. All except four aneurysms were extradural, being located in the petrous and/or cavernous portion of the ICA. The remaining four aneurysms were located in the carotico-ophthalmic portion of the ICA. In one patient, two posttraumatic ICA aneurysms in the same carotid artery were excluded from circulation with a single graft. Seventeen aneurysms (in 16 patients) were posttraumatic, secondary to motor vehicle accidents (13 patients) or surgical injury (three patients). Six of the 16 patients had severe nasal bleeding at presentation. Several patients had mass effect symptoms (e.g., severe headache in five and varied cranial nerve palsies in nine patients). Two patients were treated after subarachnoid hemorrhage, and both recovered well without any neurologic deficit. Detailed clinical information is provided in Table 1.
Patient demographics, aneurysm location and size, clinical data, post-procedural clinical and angiographic follow-up
| Patient No. | Age (yr) | Sex | Aneurysm Location | Size (mm) | Mass Effect | Trauma | Control Angiography | Mass Effect Early Outcome* | Mass Effect Late Outcome* |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 55 | M | R cav | 33 × 28 | 6n Palsy | + | 6 month | Full recovery | Stable |
| 2 | 22 | M | R cav | 34 × 29 | Headache | + | 1 year | Improved | Full recovery |
| 3 | 48 | F | R petrocav | 27 × 31 | Headache | + | NA | Improved | NA |
| 4 | 49 | M | L cav† | 15 × 20 | 6n and 3n Palsy | − | NA | Full recovery | NA |
| 5α | 18 | M | L cav | 16 × 24 | None | + | 6 month | NA | NA |
| 6α | 23 | M | L cav† | 39 × 33 | 2n Palsy | +(β) | 2 year | Full recovery | Stable |
| L cav | 6 × 6 | NA | NA | ||||||
| 7 | 54 | M | R cav | 20 × 17 | Headache | − | 6 month | Improved | Full recovery |
| 8α | 43 | F | R cav† | 22 × 15 | 6n Palsy | + | 6 month | No change | Full recovery 6 months |
| 9 | 32 | M | L cav | 14 × 21 | None | +(β) | 6 month | NA | NA |
| 10 | 25 | M | L petrocav† | 5 × 7 | None | +(β) | 1 year | NA | NA |
| 11 | 35 | M | R cav | 21 × 17 | Headache | + | 1 year | Improved | Full recovery |
| 12α | 34 | M | R caroticooph | 37 × 25 | Total ophthalmoplegia | + | 18 month | Full recovery | Stable |
| 13α | 59 | F | L caroticooph | 14 × 17 | None (SAH) | − | 6 month | NA | NA |
| 14α | 43 | M | L cav† | 27 × 20 | Total ophthalmoplegia | + | 18 month | Partial recovery | Full recovery 6 months |
| 15 | 18 | M | L petrocav | 12 × 14 | None | +(β) | 6 month | NA | NA |
| 16 | 22 | M | R petrocav | 9 × 6 | None | +(β) | 6 month | NA | NA |
| 17α | 34 | M | L cav | 28 × 34 | 3n Palsy | − | 1 year | Partial recovery | Full recovery 6 months |
| 18 | 17 | F | L cav | 18 × 14 | 6n Palsy | + | 1 year | Full recovery | Stable |
| 19 | 23 | M | L cav | 16 × 11 | None | − | NA | NA | NA |
| 20α | 27 | F | L caroticooph | 9 × 7 | None | − | 6 month | NA | NA |
| 21 | 29 | M | L cav | 4 × 3 | None | +(β) | 6 month | NA | NA |
| 22 | 36 | F | L petrous | 24 × 28 | Headache | + | 6 month | Full recovery | Stable |
| 23 | 24 | F | R caroticooph | 3 × 4 | None (SAH) | − | 1 year | NA | NA |
| 24 | 34 | F | R cavernous | 22 × 27 | 3n Palsy | + | 6 month | Partial recovery | Full recovery |
Note.—α indicates patients in whom ophthalmic artery was covered with the stent graft; M, male; F, female; R, right; L, left; cav, cavernous internal carotid artery; petrocav, petrous and cavernous portion of the internal carotid artery; caroticooph, carotico-ophthalmic segment of the internal carotid artery; 6n, sixth nerve; 3n, third nerve; 2n, second nerve; SAH, subarachnoid hemorrhage; β, patients who had nasal bleeding; NA, not applicable because the time interval since treatment was <6 months and no control angiography had yet been performed.
Control Angiography, latest angiography performed; Mass Effect Early Outcome, evaluation of mass effect symptom at end of second week; Mass Effect Late Outcome, evaluation during clinical follow-up (during third month unless otherwise indicated).
Aneurysms were partially thrombosed.
This treatment was considered for the aneurysm group in which mass effect was the principal symptom; for very wide or fusiform neck aneurysms for which conventional modalities were likely to result in recanalization, except for the stent and Onyx combination, which is more complicated than single stent deployment; and for bizarrely shaped or dissecting aneurysms in which endosaccular occlusion is not seemingly feasible. In this subset of aneurysms, two additional criteria were taken into consideration before attempting this treatment option: 1) the relevant carotid artery should not be tortuous with deep curves (e.g., for the ophthalmic aneurysms, the cavernous segment of the ICA should be C-shaped rather than U-shaped); and 2) the aneurysms should be definitely proximal to the origin of the anterior choroidal artery. The posterior communicating artery origin can be covered with the stent in selected cases, if necessary, in cases in which the posterior communicating artery is not of fetal type with a patent ipsilateral P1. Except for patient 20, in whom the seal test for Onyx failed, no other previous treatment had been attempted in any of the patients.
After placement of a 6-French Envoy guiding catheter (Cordis-Johnson & Johnson, Miami Lakes, FL) in the ICA, the aneurysm was bypassed with a microcatheter distal to the aneurysm neck. A 300-cm 0.014-in Choice extra support coronary exchange wire (Scimed, Boston Scientific, Maple Grove, MN) was placed through the microcatheter. A Jomed coronary stent graft, the size of which was determined before the procedure, was then navigated over the exchange wire and placed across the aneurysm neck. Multiple control angiograms were obtained to confirm full coverage of the aneurysm neck by the graft. The balloon-expandable stent graft was then deployed across the aneurysm neck. The balloon of the stent graft was always inflated very slowly up to the nominal pressure of 12 atm. If the size of the ICA was bigger than 4 mm, the balloon was slowly inflated up to 16 atm, at which the stent graft diameter increased to 4.4 mm, especially in the petrous portion of the ICA.
Eighty-centimeter 7-French reinforced long Arrow introducer sheaths (Arrow International, Inc., Reading, PA) were used for all patients, regardless of the aortic arch tortuosity, to ensure enough support for the 6-French guiding catheter. In some instances, Arrow sheaths were placed even in the ICA to avoid “kickbacking” of the guiding catheter when advancing further into the petrous ICA and to obtain the necessary push force for the stent graft to the desired location (Fig 1). When distal positioning of the Arrow sheath and guiding catheter for further support was necessary, they were advanced over the shaft of the stent graft and the extra support wire after the stent graft reached into the proximal cavernous portion. During navigation of the stent graft, vasospasm of the vessel, caused by a rigid coronary exchange wire, long reinforced sheath or stent graft, was treated by intra-arterial injections of 100 to 200 μg of nitroglycerine, repeated when necessary. Before deployment of the graft, extreme care was taken by viewing multiple angiograms to confirm positioning and to not cover any important side branch, such as the anterior choroidal artery, especially in the intradural segment of the ICA (Fig 1). The ophthalmic artery origin was covered with the graft, if necessary (Fig 2). After deployment of the stent graft, control angiographies were obtained, confirming the exclusion of the aneurysm sac from the circulation. If contrast material endoleak was present in the aneurysm sac, the balloon of the deployed stent was reinflated in the proximal and distal ends of the graft to better appose it to the vessel wall for the full exclusion of the aneurysm from circulation.
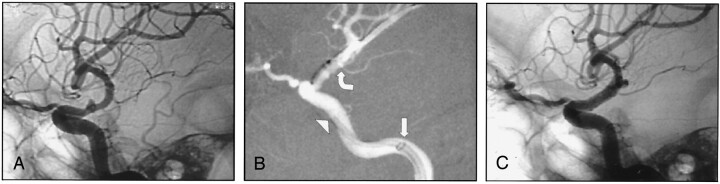
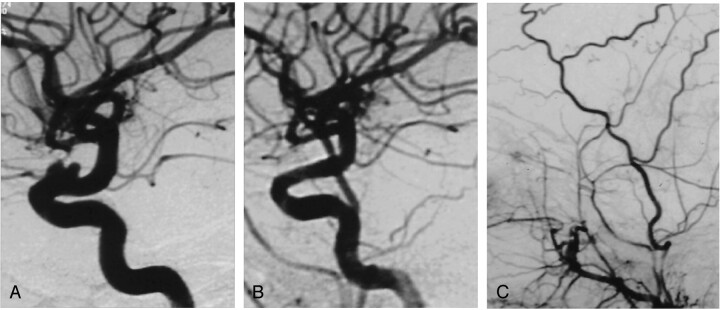
Fig 1.
A, Lateral angiogram obtained before treatment shows a right ruptured dissecting ICA aneurysm.
B, 4 × 9 mm Jomed covered stent placed across the aneurysm neck with the support of a long reinforced Arrow sheath in the proximal petrous (straight arrow) and a 6-French Envoy guiding catheter in the distal petrous ICA (arrowhead). Extreme care was taken not to cover the anterior choroidal artery origin with the graft (curved arrow).
C, Post-treatment lateral view shows exclusion of the aneurysm and the reconstructed internal carotid artery.
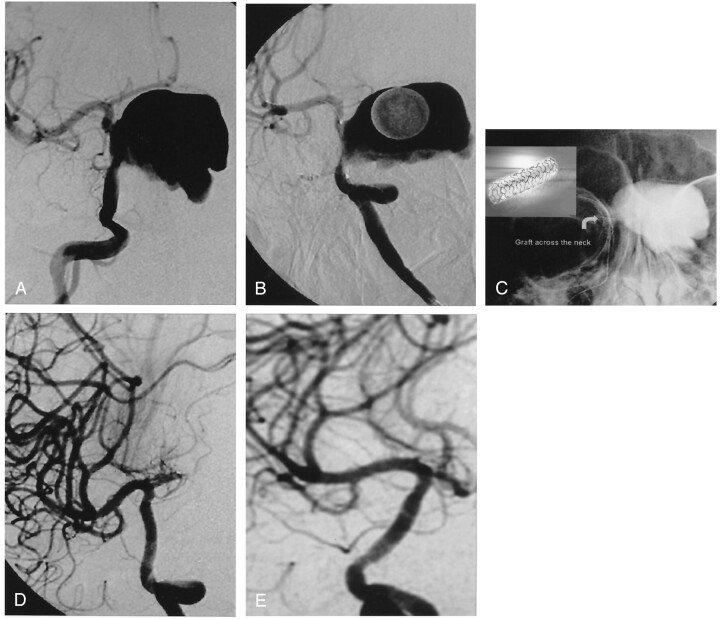
Fig 2.
A, Giant right carotico-ophthalmic aneurysm.
B, Oblique image of stent graft placed across the aneurysm neck before its deployment. Note that the ophthalmic artery is covered with the graft but not the anterior choroidal artery.
C, Non-subtracted view of the deployed stent graft (curved arrow). Note the contrast material trapped in the aneurysm sac because of the immediate exclusion.
D, Oblique angiogram obtained after treatment reveals the reconstruction of the ICA. Note that the ophthalmic artery is not filling but the anterior choroidal artery origin is preserved.
E, Oblique control angiogram obtained 18 months later shows no stenosis.
All the procedures were performed with the patients under general anesthesia, with IV administered heparinization maintaining the activated clotting time at 2.5 times the basal level. Our management protocol after intracranial stent placement included immediate post-treatment cranial CT, which was performed in all cases to exclude any intracranial hemorrhage due to stiff coronary exchange wire. Clopidogrel (300 mg) was then administered to the patients through the nasogastric tube, immediately after CT confirmed normal findings. In addition to clopidogrel, the IV administered heparinization was continued for 24 hours. The patients were discharged with daily doses of 75 mg of clopidogrel, 300 mg of aspirin, and 2 × 0.6 mg of low molecular weight heparin. The heparin was discontinued after 1 week, but the patients were to continue receiving 75 mg of clopidogrel and 300 mg of aspirin per day until control angiography was performed at 1 year. After the 1-year control angiography was performed, the patients were to receive only 300 mg of aspirin daily.
Detailed neurologic examinations were performed before treatment, immediately after treatment, 2 weeks after treatment, and later during follow-up. The patient who had second nerve palsy at presentation (patient 6) underwent ophthalmologic examinations during the pre- and post-treatment evaluations. CT and MR imaging were performed for all patients.
Results
Twenty-five intracranial aneurysms were treated by using the Jomed covered stent graft. In eight patients, transient endoleaks into the aneurysm sac were observed immediately after the deployment and were easily avoided by balloon re-inflation in the proximal and distal ends of the graft to appose it to the vessel wall for the full exclusion of the aneurysm from the circulation in six aneurysms. In two aneurysms, a slow contrast material endoleak into the aneurysm persisted despite balloon re-inflation. In one of the two, a second larger (4.5 mm) bare stent (Boa stent, Balt Montmorency, France) was used to appose the distal end of the stent graft to the ICA wall, which was larger than the maximum diameter of the stent graft, sealing the endoleak into the distal graft. The remaining aneurysm with initial endoleak after stent deployment was shown by late control angiography to have complete thrombosis (Fig 3) despite absence of further intervention.
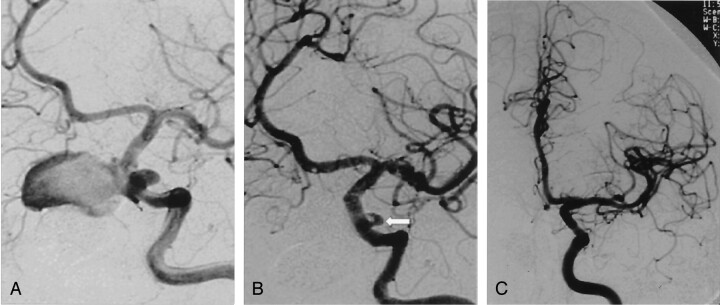
Fig 3.
A, Oblique angiogram of the left carotid artery reveals two posttraumatic aneurysms. One is giant, extending medially, and the other is small, extending laterally. Both originate from the cavernous portion of the ICA.
B, Oblique post-treatment angiogram obtained after deployment of the stent graft reveals exclusion of the giant aneurysm but persistent endoleak into the small aneurysm (arrow).
C, Control angiogram obtained 2 years later reveals spontaneous occlusion of the endoleak and excellent reconstruction of the ICA, with no stenosis.
No morbidity or mortality occurred in any of the patients during or after the treatment, including the follow-up period. No technical adverse event, including vessel perforation or thromboembolism, occurred. Despite very distal positioning of the guiding catheter and the Arrow sheath in several patients, vessel dissection did not occur in any of the patients, possibly because these were navigated over the shaft of the stent graft, which had already been placed in the proximal cavernous ICA over the extra support wire. Post-treatment CT confirmed the uneventful procedure, with no evidence of bleeding. None of the post-treatment MR images revealed any significant ischemic lesions, except for a few tiny foci of diffusion abnormality, not necessarily in the relevant vascular territory.
Control angiography performed at 2 years in one patient (Fig 3), at 1.5 years in two (Fig 2), at 1 year in six, and at 6 months in 12 confirmed complete reconstruction of the ICA, with no recurrent aneurysmal filling. No hemodynamically significant stenosis of the ICA was revealed by the control angiography. In one patient, some intimal hyperplasia that did not cause significant stenosis was noted at the proximal edge of the stent graft (Fig 4).
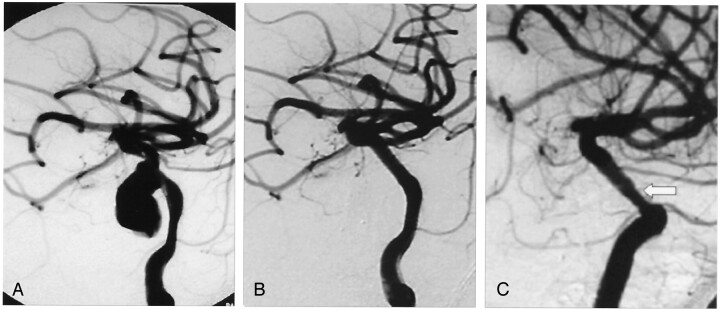
Fig 4.
A, Oblique posttraumatic angiogram of a left cavernous ICA aneurysm.
B, Oblique angiogram obtained after stent graft deployment shows reconstruction of the ICA with no residual aneurysm filling.
C, Oblique control angiogram obtained 6 months later reveals intimal hyperplasia (arrow) with no hemodynamic significance.
Cranial nerve palsies (nine patients) showed complete resolution in five patients, partial resolution in three patients, and no change in one patient during the 2-week postoperative period. In the four patients with partial resolution and no change of cranial nerve palsies, the symptoms were shown to be fully resolved by the 3- or 6-month clinical control angiography. In eight patients in whom the ophthalmic artery origin was covered with the stent graft, no clinical problem occurred because of the reconstruction of the ophthalmic artery via external carotid artery collaterals (Fig 5). All patients, including those who had no pain at presentation, suffered from headache during the early post-treatment days, presumably because of fast thrombosis of the aneurysm sac. However, all patients were subsequently relieved of headache (detailed post-treatment data are presented in Table 1).
Fig 5.
A, Lateral angiogram shows a broad based, bilobulated carotico-ophthalmic ICA aneurysm for which a previous seal test for Onyx treatment failed.
B, Lateral angiogram obtained after treatment shows reconstruction of the ICA with the stent graft deployed across the aneurysm neck. Note the occlusion of the ophthalmic artery origin covered by the graft.
C, Retrograde filling of the ophthalmic artery via external carotid artery.
Discussion
Varying endovascular treatment alternatives currently are available for the management of cerebral aneurysms. 3D coil technology and balloon remodeling techniques have been very important advances (5, 9). During recent years, new treatment materials and techniques offering a different concept in endovascular reconstruction have been described for the more complicated broad necked, fusiform, and large and giant aneurysms. One of these is the use of liquid embolic Onyx (MTI-tv3 Inc., Irvine, CA) for cerebral aneurysm treatment, both with or without stent, which has recently been reported in the literature (6, 10). These studies have shown that Onyx can produce durable aneurysm occlusion in patients with difficult large and giant wide necked intracranial aneurysms in whom other endovascular techniques are likely to fail and for whom surgery carries substantial morbidity risk. The clinical results and complication rates seem comparable to those of other endovascular techniques for similar patient populations, but the final complete occlusion rate of 79% seems significantly better than those reported for this type of aneurysm with coil treatment (10). To obtain more stable aneurysm occlusion, the combination of stents and detachable coils has been suggested for both extradural and intradural aneurysms (7, 8, 11, 12). However, a considerable rate of incomplete occlusion is associated with the stent and coil combination, resulting in recanalization of 20% in the follow-up studies after treatment of complicated aneurysms (8). In the above noted studies, the combination of stents with either Onyx or coils was suggested as an alternative treatment to balloon remodeling or parent vessel occlusion (6–8, 11, 12). However, the clinical use of stent for aneurysm treatment has certain limitations because of difficult navigation of stents within the cerebral vasculature. Improvements in technology providing better navigation of the stents have enabled us to use them more frequently in our routine practice, transforming this technique into a very effective endovascular therapeutic alternative for the treatment of wide necked or complicated aneurysms (giant, fusiform, pseudoaneurysms).
The use of artificial endoluminal vessel grafts (stent grafts) has emerged as a very effective therapeutic alternative to surgical reconstruction techniques during the last decade, and their use in the treatment of aortic and peripheric aneurysms, acute coronary and peripheric artery ruptures, and peripheric AVF (13–17) has already been reported in the literature. A few reported cases describe stent graft use in the head and neck arteries (18–20). Anecdotal cases have shown that stent graft treatment would be very effective for the endovascular management of skull base aneurysms and fistulae, carotid blow-out syndrome in the neck, and iatrogenic injury of the cavernous ICA leading to massive epistaxis and caroticocavernous fistula (21, 22). Chiaradio et al (23) reported the use of stent graft for the treatment of a ruptured fusiform dissecting aneurysm of the intracranial vertebral artery. Islak et al (24) reported two cases in which they successfully excluded a giant fusiform vertebrobasilar junction and an ophthalmic aneurysm from the circulation by using a stent graft. They achieved stable short-term follow-up results. It is very clear that closure of the side branches coming from the treated segment of the artery where the graft is deployed is the main concern in the use of a covered stent for cerebral aneurysm treatment. This report limited the use of the device to certain anatomic locations from which perforating or side branches originate, such as the posterior communicating or anterior choroidal artery.
The Jomed coronary stent graft originally had a sandwich-like design to fix a thin polytetrafluoroethylene (PTFE) membrane between two stainless steel stents. Later, the stent graft design was refined to extend the membrane up to the stent edges because restenosis was noticed to be developing at the proximal and distal ends, which were not covered with the membrane (25). PTFE membrane is the best available covering material for the stent grafts in terms of patency rates. Restenosis rates between 24% and 38% have been reported in studies using Jomed coronary stent grafts in cases of coronary atherosclerosis, which is not different from the restenosis rates of regular bare stent placement. Although the design of the stent graft was refined, restenosis still occurred, primarily at the stent edges rather than within the stent (26–28). In eight reported cases, the Jomed coronary stent graft was used to exclude nonatherosclerotic coronary artery aneurysms from circulation. Follow-up angiography performed up to 18 months later showed excellent results, with no restenosis (27, 29, 30). Moreover, very favorable results with low rates of neointimal hyperplasia indicating the antiproliferative effect of sealing the vessel wall have been shown in the reports documenting the vascular wall response in not only the coronary but also peripheral arteries with the use of PTFE-covered stents (21). Laboratory investigations on PTFE-covered stents also supported these clinical experiences, whereas Dacron- and silicone-covered stents have poor patency rates secondary to acute inflammation and extreme fibrous connective tissue ingrowth (21).
Encouraged by these favorable results in the literature, we decided to use stent grafts for the endovascular treatment of cerebral aneurysms. Twenty-five ICA aneurysms located on the petrous, cavernous, and ophthalmic segments were successfully excluded from the circulation with the placement of grafts. The treatment resulted in occlusion of the ophthalmic artery in eight patients. This was accepted before the treatment, and reconstruction of the ophthalmic artery from the external carotid artery collaterals was seen in all patients, as anticipated (Fig 5). Balloon occlusions of the ICA, for the treatment of ICA aneurysms with the intentional occlusion of the ophthalmic artery to avoid the retrograde flow into the aneurysm sac, have been performed during the last 2 decades, resulting in no ocular symptoms. However, extreme caution has been undertaken not to cover the anterior choroidal artery origin during graft placement across the neck of the ophthalmic aneurysms (Fig 1).
This technique has associated difficulties and risks because we do not have stent grafts especially designed for use in cerebral vasculature; we use coronary stent grafts. The limited flexibility is the main technical limitation; it is difficult to adapt a rigid stent designed for coronary use to the curves of the neurovascular anatomy associated with the poor navigation of the stent graft in the cerebral arteries. Possible complications that may result from this rigidity are dissection and vasospasm of the cerebral arteries. However, if necessary precautions are taken both in case selection and during the procedure by using intra-arterial nitroglycerine injections and necessary interventional adjunctive tools as described, technical success can be achieved with no harm to the patient (21). Nevertheless, we emphasize that the presence of significant vessel tortuosity hindered us from attempting this treatment technique in some cases at the case selection stage.
Stent patency rates have not been of great concern in the treatment of complicated cerebral aneurysms because the stents are placed in the nonatherosclerotic vessels. No hemodynamically significant vessel stenosis was reported in the series that combined stents with detachable coils or liquid embolic Onyx for cerebral aneurysm treatment (6, 8, 10). However, treating aneurysms with covered stents is considered to be more logical without deployment of any embolizing materials in the aneurysm sac, which may complicate the procedure and enhance the risk associated with the treatment. We think that this technique offers not only a solution to the issue of mass effect of the aneurysms but also a much more definitive reconstructive treatment than that provided by endosaccular aneurysm treatment combined with stents, which continues to have associated recanalization problems (8). Moreover, this technique is a very valuable treatment alternative for dissecting aneurysms for which neither endosaccular embolization nor surgical reconstruction is possible (Fig 1).
The follow-up data in our series are very encouraging. No hemodynamically significant stenosis was shown by the late control angiography, performed up to 2 years later, similar to the combined stent and coil/Onyx treatment series (6, 8). Although the long-term patency rate of stent grafts in the cerebral aneurysms is still unknown, with this experience, we expect a positive long-term outcome in terms of parent artery patency because the intimal hyperplasia, a consequence of the foreign body reaction in the vascular wall, usually occurs within the first few months of the treatment and usually becomes stable with time. However, we need to confirm this with long-term follow-up angiography in this series.
Our report, to our knowledge, is the first to show the use of stent grafts in the cerebral aneurysm treatment in a series of patients with mid-term follow-up results. The exclusion of cerebral aneurysms from circulation with the use of stent grafts placed across the aneurysm neck proved to be effective. However, an appropriate case selection is crucial for successful results. This technique would be a very effective alternative treatment for the intracranial aneurysms located at the ICA below the level of the anterior choroidal and posterior communicating artery or for any location that is free of side wall branches or perforating arteries. This treatment warrants more research and development to create better stent graft designs dedicated to neurovascular use to solve the present limitations.
References
- 1.International Subarachnoid Aneurysm Trial (ISAT) Colloborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. Lancet 2002;360:1267–1274 [DOI] [PubMed] [Google Scholar]
- 2.Gruber A, Killer M, Bavinzski G, Richling B. Clinical and angiographic results of endovascular coiling treatment of giant and very large intracranial aneurysms: a 7-year, single-center experience. Neurosurgery 1999;45:793–804 [DOI] [PubMed] [Google Scholar]
- 3.Kattner KA, Bailes J, Fukushima T. Direct surgical management of large bulbous and giant aneurysms involving the paraclinoid segment of the internal carotid artery: report of 29 cases. Surg Neurol 1998;49:471–480 [DOI] [PubMed] [Google Scholar]
- 4.Roy D, Raymond J, Bouthillier A, Bojanowski MW, Moumdjian R, L’Esperance G. Endovascular treatment of ophthalmic segment aneurysms with Guglielmi detachable coils. AJNR Am J Neuroradiol 1997;45:793–803 [PMC free article] [PubMed] [Google Scholar]
- 5.Moret J, Cognard C, Weill A, Castaings L, Rey A. Reconstruction technique in the treatment of wide-neck intracranial aneurysms: long-term angiographic and clinical results: apropos of 56 cases [in French]. J Neuroradiol 1997;24:30–44 [PubMed] [Google Scholar]
- 6.Mawad ME, Cekirge S, Ciceri E, Saatci I. Endovascular treatment of giant and large intracranial aneurysms by using a combination of stent placement and liquid polymer injection. J Neurosurg 2002;96:474–482 [DOI] [PubMed] [Google Scholar]
- 7.Phatouros CC, Sasaki TY, Higashida RT, et al. Stent-supported coil embolization: the treatment of fusiform and wide-neck aneurysms and pseudoaneurysms. Neurosurgery 2000;47:107–113 [DOI] [PubMed] [Google Scholar]
- 8.Lylyk P, Cohen JE, Ceratto R, Ferrario A, Miranda C. Endovascular reconstruction of intracranial arteries by stent placement and combined techniques. J Neurosurg 2002;97:1306–1313 [DOI] [PubMed] [Google Scholar]
- 9.Cloft HJ, Joseph GJ, Tong FC, Goldstein JH, Dion JE. Use of three dimensional Guglielmi detachable coils in the treatment of wide neck cerebral aneurysms AJNR Am J Neuroradiol 2000;21:1312–1314 [PMC free article] [PubMed] [Google Scholar]
- 10.Molyneux AJ, Cekirge S, Saatci I, Gal G. Onyx liquid embolic system in the treatment of cerebral aneurysms: results of a prospective observational study in 20 European centers: CAMEO study. AJNR Am J Neuroradiol 2004;25:39–51 [PMC free article] [PubMed] [Google Scholar]
- 11.Higashida RT, Smith W, Gress D, et al. Intravascular stent and endovascular coil placement for a ruptured fusiform aneurysm of the basilar artery: case report and review of the literature. J Neurosurg 1997;87:944–949 [DOI] [PubMed] [Google Scholar]
- 12.Fessler RD, Ringer AJ, Qureshi AI, Guterman LR, Hopkins LN. Intracranial stent placement to trap an extruded coil during endovascular aneurysm treatment: technical note. Neurosurgery 2000;46:248–251 [PubMed] [Google Scholar]
- 13.Mcdonald S, Gan J, Mckay AJ, Edwards RD. Endovascular treatment of acute carotid blow-out syndrome. J Vasc Interv Radiol 2000;11:1184–1188 [DOI] [PubMed] [Google Scholar]
- 14.Parodi JC. Endovascular repair of abdominal aortic aneurysms and other arterial lesions. J Vasc Surg 1995;21:549–555 [DOI] [PubMed] [Google Scholar]
- 15.Elsner M, Auch-Schwelk W, Britten M, Walter DH, Schachinger V, Zeiher AM. Coronary stent grafts covered by a PTFE membrane. Am J Cardiol 1999;84:335–338 [DOI] [PubMed] [Google Scholar]
- 16.Roongsritong C, Laothavorn P, Sanguanwong S. Stent grafting for coronary arteriovenous fistula with adjacent atherosclerotic plaque in a patient with myocardial infarction. J Invasive Cardiol 2000;12:283–285 [PubMed] [Google Scholar]
- 17.Baltacioglu F, Cimcedil NC, Cil B, Cekirge S, Ispir S. Endovascular stent-graft applications in iatrogenic vascular injuries. Cardiovasc Intervent Radiol 2003;26:434–439 [DOI] [PubMed] [Google Scholar]
- 18.Marotta TR, Buller C, Taylor D, Morris C, Zwimpfer T. Autologous vein covered stent repair of a cervical internal carotid pseudoaneurysm: technical case report. Neurosurgery 1998;42:408–412 [DOI] [PubMed] [Google Scholar]
- 19.Van Nieuwenhove Y, Van den Brande P, Van Tussenbroek F, Debing E, von Kemp K. Iatrogenic carotid artery pseudoaneurysm treated by an autologous vein-covered stent. Eur J Vasc Endovasc Surg 1998;16:262–265 [DOI] [PubMed] [Google Scholar]
- 20.Martin JB, Bednarkiewichz M, Christenson JT, Rufenacht DA. Endovascular repair using vein-covered stents in the carotid artery bifurcation. Cardiovasc Surg 2000;8:499–502 [DOI] [PubMed] [Google Scholar]
- 21.Redekop G, Marotta T, Weill A. Treatment of traumatic aneurysms and arteriovenous fistulas of the skull base by using endovascular stents. J Neurosurg 2001;95:412–419 [DOI] [PubMed] [Google Scholar]
- 22.Kocer N, Kizilkilic O, Albayram S, Adaletli S, Kantarci F, Islak C. Treatment of iatrogenic internal carotid artery laceration and carotid cavernous fistula with endovascular stent graft placement. AJNR Am J Neuroradiol 2002;23:442–446 [PMC free article] [PubMed] [Google Scholar]
- 23.Chiaradio JC, Guzman L, Padilla L, Chiaradio MP. Intravascular graft stent treatment of a ruptured fusiform dissecting aneurysm of the intracranial vertebral artery: technical case report. Neurosurgery 2002;50:213–217 [DOI] [PubMed] [Google Scholar]
- 24.Islak C, Kocer N, Albayram S, Kizilkiliç O, Uzma O, Cokyuksel O. Bare stent graft technique: a new method of endoluminal vascular reconstruction for the treatment of giant and fusiform aneurysms. AJNR Am J Neuroradiol 2002;23:1589–1595 [PMC free article] [PubMed] [Google Scholar]
- 25.Lukito G, Vandergoten P, Jaspers L, DendaleP, Benit E. Six months clinical, angiographic and IVUS follow-up after PTFE graft stent implantation in native coronary arteries. Acta Cardiol 2000;55:255–260 [DOI] [PubMed] [Google Scholar]
- 26.Sovik E, Klow N-E, Brekke M, Stavnes S. Elective placement of covered stents in native coronary arteries. Acta Radiologica 2003;44:294–301 [DOI] [PubMed] [Google Scholar]
- 27.Gercken U, Lansky AJ, Buellesfeld L, et al. Results of the Jostent coronary stent graft implantation in various clinical settings: procedural and follow-up results. Catheter Cardiovasc Interv 2002;56:353–360 [DOI] [PubMed] [Google Scholar]
- 28.Schachinger V, Zeiher AM. Covered stent grafts: role in intervention of coronary arteries and degenerated vein grafts [Suppl]. Z Kardiol 2002;91:58–63 [DOI] [PubMed] [Google Scholar]
- 29.Rubartelli P, Terzi G, Borgo L, Giachero C. Coronary artery aneurysm after stent implantation: acute and long-term results after percutaneous treatment with a stent-graft. Ital Heart J 2002;3:202–205 [PubMed] [Google Scholar]
- 30.Strozzi M, Ernst A, Banfic L. Obliteration of a left main coronary artery aneurysm with a PTFE-coated stent. J Invasive Cardiol 2002;14:280–281 [PubMed] [Google Scholar]