Abstract
Summary: Cortical venous thrombosis (CVT) without concomitant dural sinus thrombosis is an uncommon disorder. Isolated CVT usually manifests on imaging studies as focal parenchymal hemorrhage or edema. We report three cases of isolated CVT that presented with unilateral, localized subarachnoid hemorrhage without parenchymal involvement.
The clinical expression of cerebral venous thrombosis is variable, with headache as the most frequent presenting symptom. Cerebral venous thrombosis, which includes dural sinus thrombosis and the more unusual isolated cortical venous thrombosis (CVT), is an established cause of parenchymal hemorrhage (1–3). Subarachnoid hemorrhage (SAH) is a rare presentation of cerebral venous thrombosis (4). In this study, we describe three cases of isolated CVT that manifested on imaging solely as localized SAH without underlying parenchymal involvement.
Case Reports
Patient 1
A 29-year-old woman with a history of migraine headaches and petit mal seizures presented with abrupt onset of left-sided weakness and numbness. She also noted a sharp, “dagger-like” headache in her right occiput that radiated to her right eye and was associated with a “flashing white light” in her left eye, visual blurring, nausea, vomiting, and slurred speech. Despite a chronic history of migraine headaches, the patient reported that this episode was more intense and different in character from her prior migraine attacks. The patient had recently discontinued the use of oral contraceptives. Physical examination revealed mildly decreased left upper and lower extremity strength, as well as mildly decreased proprioception and sensation to light touch and vibration on the left side.
MR examination of the brain was subsequently obtained within 24 hours of her initial symptoms. Fluid-attenuated inversion recovery (FLAIR) images demonstrated abnormally increased signal intensity within the sulci at the right convexity, most compatible with SAH (Fig 1A). This abnormal signal intensity was not visualized by using any other pulse sequence, including the diffusion-weighted technique. The lack of restricted diffusion indicated that the abnormal signal intensity on FLAIR images did not represent an acute cortical infarction. The cerebral parenchyma appeared normal, and MR angiography showed no evidence of an underlying vascular lesion. A tubular, serpiginous hyperintense structure on T1- and proton density–weighted images (Fig 1B) was identified adjacent to the area of SAH. MR venography showed patency of the major dural sinuses and deep veins. These findings indicated a diagnosis of isolated cortical venous thrombosis, probably involving the vein of Trolard.
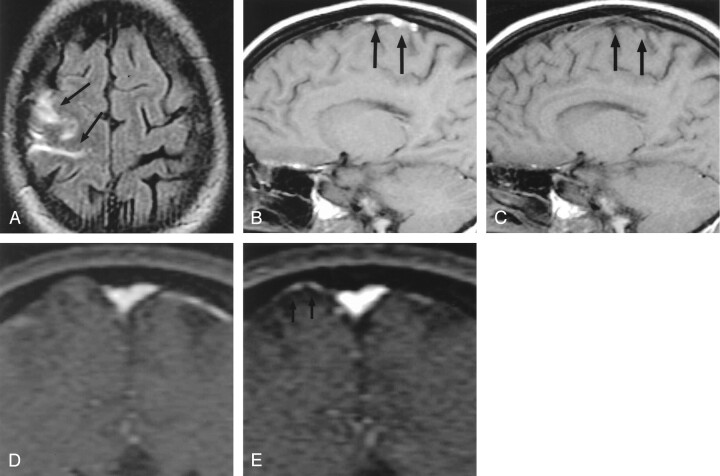
Fig 1.
Patient 1, a 29-year-old woman with headaches, seizures, and cortical venous thrombosis.
A, Axial FLAIR (10,002/158/2200) [TR/TE/TI] MR image shows focal sulcal hyperintensity at the right frontoparietal convexity (arrows).
B, Right parasagittal T1-weighted (500/14) MR image shows tubular hyperintense thrombus (arrows) in a right convexity cortical vein, probably the vein of Trolard.
C, Right parasagittal T1-weighted (500/14) MR image, obtained approximately 3 months after the FLAIR image in panel A, shows resolution of the hyperintense thrombus (arrows).
D and E, Source data from MR venograms obtained at presentation (D) and approximately 3 months later (E) show interval appearance of flow signal intensity (arrows) in the previously occluded cortical vein.
Heparin and warfarin anticoagulation were initiated. She was also maintained on dilantin for seizure prophylaxis. A comprehensive hypercoagulability workup was unrevealing. The patient improved and continued to take warfarin as an outpatient.
Follow-up MR imaging was obtained approximately 3 months after the patient’s initial presentation. This examination showed interval resolution of the clot in the cortical vein (Fig 1C). In addition, partial recanalization of the thrombosed cortical vein was noted on MR venography (Figs 1D and E).
Patient 2
A 46-year-old woman with a history of hypertension presented with 3 weeks of worsening headaches. The patient described a “viselike” grip on her head, a stabbing sensation behind her left eye, and transient episodes of vision loss in her left eye. She was taking atenolol for treatment of hypertension. She was a nonsmoker and avid athlete, regularly participating in triathlons. An MR examination of the brain was performed on an outpatient basis approximately 3 weeks after the onset of her initial symptoms.
FLAIR images from the MR examination showed abnormally high signal intensity in the right central sulcus at the convexity, most compatible with SAH (Fig 2A). This abnormal signal intensity was not visualized by using any other pulse sequence, including diffusion-weighted imaging. The parenchyma appeared normal, and MR angiography showed no evidence of an underlying vascular lesion. T1- and proton density–weighted images showed a tubular structure of high signal intensity in the region of the vein of Trolard, adjacent to the area of SAH (Fig 2B and C). MR venography demonstrated patency of the major dural sinuses. These findings indicated a diagnosis of isolated cortical venous thrombosis, probably involving the vein of Trolard.
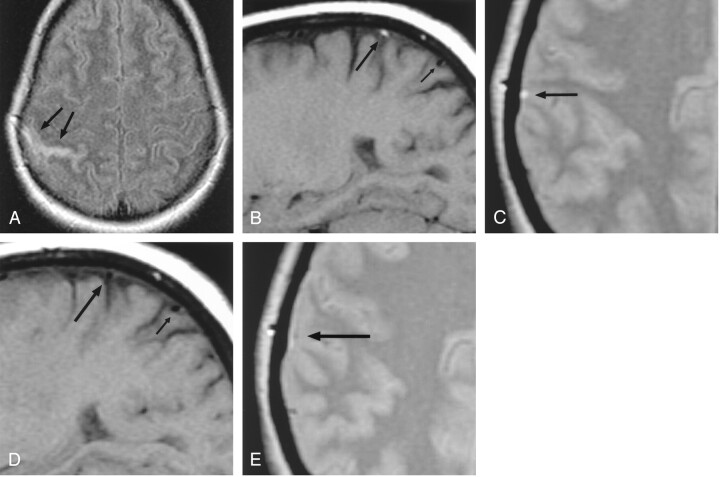
Fig 2.
Patient 2, a 46-year-old woman with headaches and cortical venous thrombosis.
A, Axial FLAIR (10,327/158/2200) MR image shows focal sulcal hyperintensity (arrows), representing SAH in the right central sulcus. T2-weighted and diffusion-weighted images (not shown) were normal.
B, Right parasagittal T1-weighted (367/14) MR image shows luminal hyperintensity (large arrow), representing thrombus, in a right convexity cortical vein, probably the vein of Trolard. This abnormality was also identified on multiple adjacent sections. A flow void (small arrow) is seen in a more posterior, normal cortical vein.
C, Axial proton density–weighted (2267/30) MR image shows luminal hyperintensity (arrow) in a right convexity cortical vein; this was identified on multiple adjacent sections.
D, Right parasagittal T1-weighted (367/14) MR image, obtained approximately 3 months after the image in panel B, shows restoration of the normal signal intensity void (large arrow), representing recanalization in the previously occluded cortical vein. The more posterior cortical vein (small arrow) is unchanged.
E, Axial proton density–weighted (2450/30) MR image, obtained approximated 3 months after the image in panel C, shows a small signal intensity void (arrow) in the previously thrombosed cortical vein.
The patient was admitted to the hospital by the neurology service and placed on heparin and warfarin anticoagulation. She developed intermittent jerking of her left arm and was placed on antiepileptic medication for empiric treatment of simple partial seizures. Findings at a comprehensive hypercoagulability workup were normal. The patient improved and continued to take warfarin as an outpatient.
Follow-up MR imaging was obtained approximately 3 months after the patient’s initial presentation. This showed resolution of the tubular structure of high signal intensity previously seen on the T1- and proton density–weighted images (Fig 2D and E).
Patient 3
A 64-year-old woman with a history of rheumatoid arthritis and vertebral compression fractures was admitted to the hospital for posterior lumbar decompression and fusion. She did well and was discharged to a rehabilitation facility. The patient was soon readmitted to the hospital with sepsis, requiring irrigation and debridement of her lumbar wound on two separate occasions. During her hospital course, she complained of headaches and neck pain. An MR examination of the brain was performed within 24 hours of her initial symptoms.
FLAIR images showed localized SAH at the right convexity (Fig 3A). This was confirmed by noncontrast CT performed the following day (Fig 3B). MR angiography showed no evidence of an underlying vascular lesion. A tubular structure of high signal intensity on T1-weighted images was identified adjacent to the SAH (Fig 3C). These findings were most compatible with isolated cortical venous thrombosis, probably involving the vein of Trolard. MR venography showed patency of the major dural sinuses.
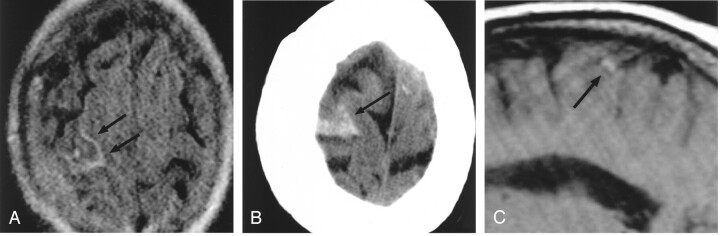
Fig 3.
Patient 3, a 64-year-old woman with headaches and cortical venous thrombosis.
A, Axial FLAIR (10,002/158/2200) MR image shows focal sulcal hyperintensity (arrows), representing SAH, at the right frontoparietal convexity.
B, Axial CT image at the same level as the FLAIR image in panel A, obtained the following day, confirms the presence of SAH (arrow).
C, Right parasagittal T1-weighted (500/14) MR image shows luminal hyperintensity (arrow) in a right convexity cortical vein, probably the vein of Trolard; this was also identified on multiple adjacent sections.
The patient’s clinical status continued to decline. She remained febrile and septic and eventually died.
Discussion
Thrombosis of the cerebral veins is a relatively uncommon but potentially life-threatening condition, accounting for 1–2% of strokes in young adults. Reported death rates range between 5% and 30%, but one study of 49 patients showed a 48% mortality rate in untreated patients (1, 3, 5). Thus, early diagnosis and treatment are crucial.
The terminology of cerebral venous thrombosis is often applied liberally and is usually used to refer to thrombosis of one of the major dural sinuses. Isolated involvement of a cortical vein is unusual and predominantly described in case reports (6, 7). Cortical vein thrombosis is usually secondary to dural sinus thrombosis, with thrombus propagating in a retrograde fashion from the occluded sinus (8).
There are myriad predisposing factors for cerebral venous thrombotic disease. These include pregnancy, dehydration, medications, hereditary coagulopathies, systemic disease, trauma, infection, and idiopathic causes (1, 6). In our study, patient 1 had recently been taking oral contraceptives. Patient 3 was predisposed to venous thrombosis from sepsis and a perioperative state. A predisposing cause could not be found in patient 2.
The prospective clinical diagnosis of cerebral venous thrombosis is difficult because of a wide spectrum of clinical manifestations; the diagnosis is typically made on the basis of imaging studies. The most commonly described symptoms are headache, seizures, mental obtundation, and focal motor or sensory deficits (9). The clinical manifestations of isolated cortical vein thrombosis are less well known, but review of the literature suggests that there is similarity with dural sinus thrombosis (6, 8). In our series, all three patients presented with headache. In addition, one patient developed unilateral weakness and parasthesias, whereas a second patient developed a partial seizure. These manifestations are likely related to the irritative effects of SAH around the central sulcus and localized venous hypertension that is radiographically unapparent. The varied clinical presentations may be explained by greater variability in size and location of the cerebral veins in comparison to the cerebral arteries (10).
In the past, conventional angiography was regarded as the criterion standard diagnostic examination for cerebral venous thrombosis. With the advent of MR imaging, however, conventional angiography is now rarely required for diagnosis. Because the accuracy of angiography depends on the lack of opacification of the cerebral veins, there are known limitations. The diagnosis of isolated CVT is particularly difficult on the basis of angiographic findings, because the pattern of cortical venous drainage is variable and often asymmetric. The MR appearance of a thrombosed cerebral vein is dependent on the age of the clot. Initially, when blood products are in the deoxyhemoglobin state, thrombus is isointense relative to parenchyma on T1-weighted images and hypointense on T2-weighted images. As the blood products in the thrombus evolve to the state of methemoglobin, the clot appears hyperintense on T1- and T2-weighted sequences (11). A similar time course of signal intensity changes has been described in isolated CVT (6, 8).
In addition to avoiding intravenous contrast material administration and ionizing radiation, MR imaging is far more sensitive than CT or conventional angiography in detection of the parenchymal changes associated with venous thrombosis. Cerebral venous thrombosis often results in localized cerebral edema or infarction that does not conform to an arterial vascular territory. Hemorrhagic venous infarction is common. In contradistinction to arterial infarction, these parenchymal changes are usually subcortical (11).
All three patients in this report showed isolated thrombosis of a cortical vein, probably the vein of Trolard. All three patients had MR imaging findings that demonstrated localized, unilateral SAH at the convexity immediately adjacent to the thrombosed cortical vein. No associated parenchymal abnormalities were identified by using any other imaging sequences, including diffusion-weighted imaging. Although cerebral hemorrhage may produce diffusion abnormalities by itself, the lack of diffusion abnormalities in our patients may be related to the small size, extraparenchymal location, and complexity of blood products within the SAH. SAH has rarely been reported in association with cerebral venous thrombosis. Sztajzel et al (4) described one patient who presented with right cerebellar SAH associated with thrombosis of the right transverse or sigmoid sinus. This patient subsequently developed hemorrhagic infarction of the cerebellum. The mechanism behind the development of SAH in isolated CVT is not certain. To the best of our knowledge, this association has not been previously reported in the literature. Other possible etiologies of unilateral, localized, nontraumatic SAH at the convexity are relatively few. Arterial infarction and dural arteriovenous fistula would be two additional considerations. Berry aneurysms and nonaneurysmal perimesencephalic SAHs typically present with SAHs at the skull base. Mycotic aneurysms and parenchymal arteriovenous malformations usually present with a component of parenchymal hemorrhage.
In cases of suspected CVT, spin-echo T1-weighted sagittal, double-echo T2-weighted axial, and axial FLAIR images are often adequate for diagnosis. In addition, spin-echo T1-weighted axial or coronal images can be useful in following the course of the thrombosed cortical vein, as well as for excluding entry and exit phenomena as the cause for luminal hyperintensity. Diffusion-weighted images can verify that the sulcal hyperintensity on FLAIR images is not related to an acute cortical infarction. Finally, 2D time-of-flight MR venography (acquired in the coronal plane) can establish the presence or absence of flow signal intensity in a cortical vein that is visualized on the anatomic images.
Conclusion
We observed that isolated CVT can present solely as unilateral, localized SAH at the convexity without underlying parenchymal involvement. Because other causes of nontraumatic SAH do not ordinarily present in this manner, identification of this unusual imaging appearance should prompt strong consideration of the diagnosis of cortical venous thrombosis.
References
- 1.van Gijn J. Cerebral venous thrombosis: pathogenesis, presentation and prognosis. J R Soc Med 2002;93:230–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Provenzale JM, Joseph GJ, Barboriak DP. Dural sinus thrombosis: findings on CT and MR imaging and diagnostic pitfalls. Am J Radiol 1998;170:777–783 [DOI] [PubMed] [Google Scholar]
- 3.Lafitte F, Boukobza M, Guichard JP, et al. Deep cerebral venous thrombosis: imaging in eight cases. Neuroradiology 1999;41:410–418 [DOI] [PubMed] [Google Scholar]
- 4.Sztajzel R, Coeytaux A, Dehdashti AR, et al. Subarachnoid hemorrhage: a rare presentation of cerebral venous thrombosis. Headache 2001;41:889–891 [PubMed] [Google Scholar]
- 5.Yuh WT, Simonson TM, Wang AM, et al. Venous sinus occlusive disease: MR findings. AJNR Am J Neuroradiol 1994;15:309–316 [PMC free article] [PubMed] [Google Scholar]
- 6.Derdeyn CP, Powers WJ. Isolated cortical venous thrombosis and ulcerative colitis. AJNR Am J Neuroradiol 1998;19:488–490 [PMC free article] [PubMed] [Google Scholar]
- 7.Dorndorf D, Wessel K, Kessler C, Kömpf D. Thrombosis of the right vein of Labbé: radiological and clinical findings. Neuroradiology 1993;35:202–203 [DOI] [PubMed] [Google Scholar]
- 8.Jacobs K, Moulin T, Bogousslavsky J, et al. The stroke syndrome of cortical vein thrombosis. Neurology 1996;47:376–382 [DOI] [PubMed] [Google Scholar]
- 9.Connor SEJ, Jarosz JM. Magnetic resonance imaging of cerebral venous sinus thrombosis. Clin Radiol 2002;57:449–461 [DOI] [PubMed] [Google Scholar]
- 10.Curé JK, Van Tassel P, Smith MT. Normal and variant anatomy of the dural venous sinuses. Semin Ultrasound CT MRI 1994;15:499–519 [DOI] [PubMed] [Google Scholar]
- 11.Keiper MD, Ng SE, Atlas SW, Grossman RI. Subcortical hemorrhage: marker for radiographically occult cerebral vein thrombosis on CT. J Comput Assist Tomogr 1995;19:527–531 [PubMed] [Google Scholar]