Abstract
BACKGROUND AND PURPOSE: The purpose of this study was to evaluate the degree of organization and fibrocellular tissue development in aneurysms treated with bare platinum or biologically active microcoils.
METHODS: Twelve aneurysms were removed at autopsy between 1–18 days and another 2 between 2–3 months posttreatment. Four aneurysms were surgically removed between 6 months and 3 years following treatment. One aneurysm removed at 8 days and another at 6 months were treated with bioactive (Matrix) coils; the other 16 with bare platinum (Guglielmi detachable coils; GDCs). All specimens were embedded in plastic, stained with hematoxilin-eosin and elastin and examined by light microscopy.
RESULTS: All specimens removed within 3 weeks demonstrated intra-aneurysmal thrombus, without signs of organization or fibrotic tissue formation over the neck regardless of the type of coils used. In the GDC-treated aneurysms, evidence of early thrombus organization was observed within 2–3 months, and completed yet imperfect fibrocellular reaction together with residual thrombus at 2–3 years. In the Matrix-treated specimens, the aneurysm cavity was completely filled with granulation tissue corresponding to still ongoing fibrocellular reaction at 6 months, including newly formed blood vessels, smooth muscle cells, and collagen deposition without signs of residual thrombus.
CONCLUSIONS: Our results indicate that in aneurysms treated with bare platinum coils thrombus organization does not occur until late after treatment and may remain imperfect for years. In one aneurysm studied 8 days following treatment with Matrix coils, no difference was noted compared to aneurysms treated with bare platinum coils. In another aneurysm examined 6 months following packing with Matrix coils, the histologic changes support the hypothesis that the biologically active polymer may accelerate aneurysm healing.
Recurrence and rebleeding of cerebral aneurysms after coiling remains a problem. Several histologic studies have demonstrated that platinum coils induce thrombus formation within the aneurysm sac shortly after treatment.1–8 Fresh thrombus, however, is unstable and subject to thrombolysis. Organization of thrombus into granulation tissue is thought to be necessary for healing to be completed. In many cases, however, thrombus organization occurs very late and may remain incomplete in the long term.4, 7 To develop better devices for embolization that would induce a healing reaction, different materials have been applied experimentally either inside or on the surface of platinum microcoils, including collagen,9–12 tissue plasminogen activator inhibitor and tissue thromboplastin,13 and vitronectin, laminin, fibrinogen, and fibronectin.11 The results of experimental studies with these materials led to the development of a new bioactive microcoil known as the Matrix Detachable Coil (Boston Scientific Neurovascular, Fremont, Calif), which is constructed of platinum and coated with a combination of a polyglycolic acid/lactid copolymer known to be a bioabsorbable material that induces a tissue reaction. This device has demonstrated accelerated thrombus organization and fibrocellular tissue formation in experimental swine aneurysms.14
More than 7000 patients have already been treated with Matrix Detachable Coils. An observational safety and feasibility study (“The Matrix ACTIVE Study”) reportedly demonstrated less aneurysm recanalization, compared with historical reports involving endovascular treatment with bare platinum coils. The effect of the bioactive material in the coils has not yet been investigated by histologic methods in humans.
In this study, we analyzed the tissue reaction in human ruptured aneurysms after treatment with Matrix or Guglielmi detachable coils (GDCs; Boston Scientific Neurovascular).
Patients and Techniques
Between 1996 and 2003, a total of 18 aneurysm specimens were collected from 18 patients treated for subarachnoid hemorrhage (SAH) with endovascular coil embolization. Standard endovascular technique was used to access and pack the aneurysms with detachable coils as tightly as possible. Size of the aneurysms and the number and length of coils used in each case are detailed in Table 1. Microcatheters were routinely flushed with heparinized saline. Fifty units/kg body weight heparin were given to each patient as an intravenous bolus once the first and second coils were deposited inside the aneurysm cavity. Activated clotting time (ACT) was measured and additional doses of heparin were injected as needed to keep ACT at twice the baseline value. Heparin administration was terminated upon completion of the procedure except in one case treated with Matrix coils (case 18, Table 1) in which heparin infusion was prolonged for 24 hours following the procedure because of sluggish flow within distal branches of the anterior cerebral artery (ACA). Aspirin (200 mg) and clopidogrel (300 mg) were administered before aneurysm treatment in one case (case 15, Table 1) in which an intracranial stent was used to assist packing with bare platinum coils. No antiplatelet medication was applied in the other 17 cases. Angiographic results were assessed by using the Jean Raymond Scale15 and defined as a complete occlusion, a dog ear, a residual neck, or as a residual aneurysm and are listed in Table 1. Aneurysms were located at the origin of the posterior communicating artery (PComA, 3), at the bifurcation of the internal carotid artery (ICA, 1), on the anterior communicating artery (AComA, 6), at the bifurcation of the middle cerebral artery (MCA, 4), at the origin of the pericallosal artery (Peric, 1), on the intradural vertebral artery (VA, 1), or at the origin of the posterior inferior cerebellar artery (PICA, 2).
Clinical, morphologic, and histologic characteristics of aneurysms
| Patient No./Age (y)/Sex | Indication | H&H | Location | Size (mm) | Type of Coils Used | No. of Coils Used | Length (cm) of Coils Used | Angiographic Results | Coil Compaction | Reason for Death | Implant Time |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/38/M | SAH | 4 | ICA | 8 | Matrix, GDC | 6 | 60 | Compl | N/A | Vasospasm | 8 d |
| 2/44/F | SAH | 4 | AComA | 6 | GDC | 3 | 30 | Compl | Yes | N/A | 36 mo |
| 3/43/F | SAH | 4 | AComA | 8 | GDC | 3 | 24 | RN | N/A | Vasospasm | 8 d |
| 4/39/M | SAH | 4 | PComA | 5 | GDC | 5 | 30 | RN | Yes | N/A | 36 mo |
| 5/39/F | SAH | 4 | Peric | 5 | GDC | 4 | 22 | Compl | N/A | SAH | 18 d |
| 6/56/M | SAH | 3 | MCA | 5 | GDC | 2 | 6 | Compl | N/A | io rupt | 5 d |
| 7/16/M | SAH | 5 | PICA | 4 | GDC | 3 | 8 | Compl | N/A | SAH | 3 d |
| 8/59/F | SAH | 4 | AComA | 4 | GDC | 3 | 14 | Compl | N/A | SAH | 5 d |
| 9/43/M | SAH | 3 | MCA | 5 | GDC | 5 | 36 | RN | Yes | N/A | 35 mo |
| 10/39/F | SAH | 5 | PComA | 6 | GDC | 3 | 22 | RN | N/A | SAH | 2 mo |
| 11/17/M | SAH | 5 | PComA | 10 | GDC | 5 | 61 | RA | N/A | SAH | 3d |
| 12/33/M | ICH | 5 | AComA | 3 | GDC | 2 | 7 | RN | N/A | SAH | 11 d |
| 13/27/F | SAH | 2 | AComA | 5 | GDC | 4 | 14 | Compl | N/A | Vasospasm | 9 d |
| 14/52/F | SAH | 3 | MCA | 4 | GDC | 4 | 13 | Compl | N/A | io rupt | 2 d |
| 15/49/F | SAH | 3 | VA | 4 | GDC | 3 | 12 | RN | N/A | io rupt | 12 d |
| 16/65/F | SAH | 2 | PICA | 3 | GDC | 2 | 6 | RN | N/A | io rupt | 1 d |
| 17/67/F | SAH | 3 | MCA | 3 | GDC | 1 | 4 | Compl | N/A | SAH | 3 mo |
| 18/44/F | SAH | 1 | AComA | 8 | Matrix, GDC | 6 | 55 | RN | Yes | N/A | 6 mo |
Note.—HH indicates Hunt and Hess grade; SAH, subarachnoid hemorrhage; ICH, intracerebral hemorrhage; ICA, internal carotid artery; AComA, anterior communicating arter; PComA, posterior communicating artery; Peric, pericallosal artery; MCA, middle cerebral artery; PICA, posterior inferior cerebellar artery; VA, vertebral artery; Compl, complete occlusion; RN, residual neck; RA, residual aneurysm; io rupt, intraoperative aneurysm rupture.
Of the 18 aneurysms studied, 14 were removed at autopsy. The patients died from intraoperative aneurysm rupture (n = 4), vasospasm (n = 3), and complications from the original SAH (n = 7). Twelve of the 14 aneurysms removed at autopsy were removed 1–18 days after treatment, and 2 aneurysms were removed at 2–3 months after treatment. In addition, 4 aneurysms were removed surgically because of a persistent or growing neck remnant, one aneurysm at 6 months following treatment, and 3 aneurysms at 3 years following treatment. One of those patients had an additional unruptured MCA aneurysm that required surgical clipping. Because of a growing neck remnant found on the 6-month follow-up angiogram, the endovascularly treated aneurysm was also clipped and removed during surgery of the MCA aneurysm. This surgically removed specimen (case 18) and another aneurysm removed 8 days following treatment during autopsy (case 1) were embolized with Matrix bioactive and some bare platinum coils. In both cases, 4 Matrix and 2 platinum coils were used. All other aneurysms were treated with GDC coils only (Table 1).
The aneurysms were inspected grossly upon removal. The specimens were fixed in buffered formaldehyde and embedded in plastic. Serial sections were made by using a diamond saw and hand polishing. Slides were stained with hematoxilin-eosin (H&E) and elastin, and all were independently studied by 2 histopathologists (P.S. and Z.H.) by using light microscopy. An attempt was made to identify the aneurysm neck and to define whether coils at the neck were covered by any kind of a tissue layer. Signs of incomplete thrombosis (fresh blood, unorganized thrombus, void spaces), tissue reaction (foreign body giant cells, leukocyte invasion, macrophages), and thrombus organization (fibrocellular reaction, collagen formation, neovascularization) were recorded in each case.
Results
Angiographic Findings
Angiographic follow-up was performed in all aneurysms that were removed surgically later than 3 months. All of those aneurysms (cases 2, 4, 9, 18; Table 1) demonstrated angiographic evidence of coil compaction and either a growing neck remnant or aneurysm recanalization.
Macroscopic Observations
All 14 aneurysms removed at autopsy were characterized by a very thin, transparent wall, through which the coils were easily observed. The sac itself was filled with coils and thrombus with coils protruding through the thin aneurysm wall. Inside the neck, free coil loops could be observed, partially covered by a thin layer of fibrin. The coil mass inside the neck was not completely covered by a tissue layer even 2 months after treatment (Fig 1). No gross difference was evident regardless of coil type used.
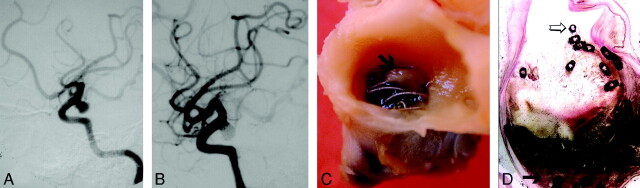
Fig 1.
Ruptured aneurysm of the PComA removed 2 months after treatment with GDCs (case 10, Tables 1 and 2).
A, Digital subtraction angiography (DSA) before treatment demonstrates PComA aneurysm.
B, DSA immediately following treatment demonstrates minimal neck remnant.
C, Gross pathology, demonstrating free coils covered by an incomplete fibrin layer (arrow).
D, Microscopic section (H&E stain, low-power magnification, 2×) demonstrates unorganized thrombus in the aneurysm sac (arrow) and exposed coils within the neck (open arrow).
In the 4 aneurysms removed surgically, a very thin wall was observed, which was particularly transparent at the aneurysm dome. Macroscopically, thrombus was not seen. The cavity of the neck remnant (for which the aneurysm was removed) was well visualized in each case and was bordered by fairly thick tissue as compared with the dome. Coil loops were seen within the neck covered by a continuous layer of thin tissue in 3 of 4 cases. Partially uncovered coil loops were still seen in the neck in one case (Fig 2). The aneurysm packed with Matrix coils and removed at 6 months following treatment demonstrated a yellowish color that was different from all other specimens. In the Matrix-treated aneurysm, the wall was extremely thin, through which coil loops were seen embedded in an attenuated, compact tissue. Macroscopically, the neck was covered by a thick, continuous tissue layer (Fig 3).
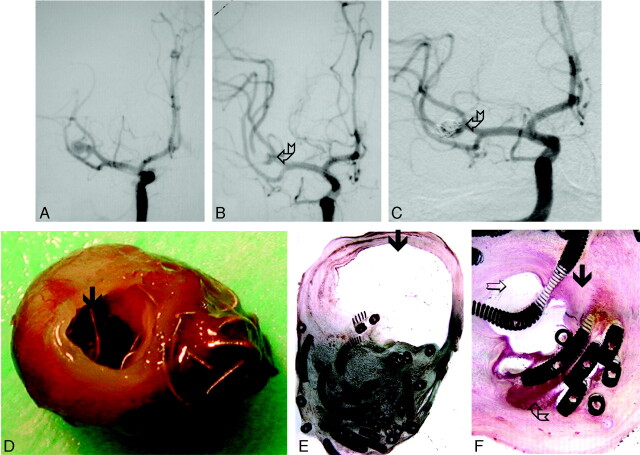
Fig 2.
Ruptured aneurysm of the MCA removed surgically 3 years after treatment with GDCs (case 9, Tables 1 and 2).
A, DSA before treatment.
B, DSA immediately following treatment with standard GDCs, demonstrating neck remnant (broken arrow).
C, DSA 3 years after treatment demonstrates aneurysm recanalization (broken arrow).
D, Gross pathology demonstrating partially exposed coils within the neck (arrow) and coils protruding through the thin wall of the aneurysm dome.
E, Histologic section (H&E stain, low-power magnification, 2×) of the same specimen. Most of the aneurysm sac is filled with organized thrombus, but a large empty space is also seen (arrow).
F, Higher power magnification (20×) demonstrates attenuated fibrocellular tissue (arrow), an empty space (open arrow), and residual unorganized thrombus (broken arrow) within the same aneurysm.
Fig 3.
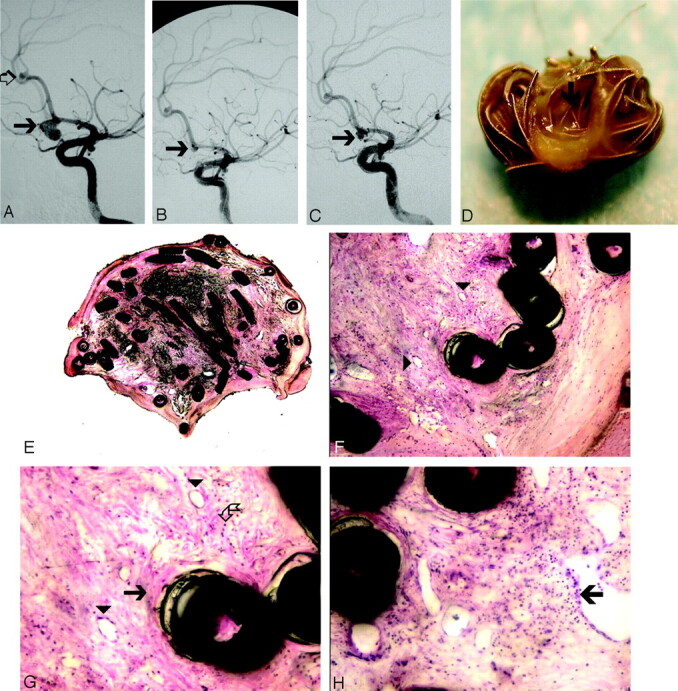
Ruptured aneurysm of the AComA, treated with Matrix coils and removed during surgery 6 months later (case 18, Tables 1 and 2).
A, DSA before treatment demonstrates ruptured AComA aneurysm (arrow) and a small incidental aneurysm at the pericallosal artery (open arrow).
B, DSA immediately after treatment demonstrating a small neck remnant (arrow).
C, DSA, 6 months later, demonstrates growing neck remnant (arrow). Two incidental aneurysms (one on the left MCA and the other on the left pericallosal artery) were clipped, and the AComA aneurysm was clipped and removed during surgery.
D, Gross pathology of the surgical specimen. The coils within the neck (arrow) are covered by a thick tissue layer. The wall of the aneurysm is very thin.
E, Microscopic section of the specimen (H&E stain, low magnification, 2×). The aneurysm cavity is filled with fibrocellular tissue without any residual blood clot or empty spaces.
F, Higher magnification (10×) H&E stain demonstrates coils embedded in fibrocellular granulation tissue with multiple neocapillaries (arrowheads).
G, Higher power view (20×) demonstrates collagen deposition (arrow), smooth muscle cells (broken arrow), and small blood vessels (arrowheads).
H, Leukocyte invasion (arrow) represents granulation tissue (20×, H&E stain).
Histologic Findings
All specimens were prepared with the goal of creating sections parallel to the aneurysm orifice so that the neck could be visualized. This, however, could not be achieved in all cases, because of complex anatomy and difficulties of the histologic technique. Two to 4 slides were made from each aneurysm, depending on the diameter of the sack. All slides were studied in a similar fashion and the most representative ones selected for demonstration. Details of the histologic findings are listed in Table 2.
Clinical, morphologic, and histologic characteristics of aneurysms (cont.)
| Patient No. | Neck Identified | Layer Covering Coils at Neck | Content of the Aneurysm Sack Blood | Unorganized Thrombus | Void Spaces | Foreign Body Giant Cells | Leukocyte Infiltration | Macrophages | Fibrocellular Reaction | Neocapillaries | Collagen Formation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | N/A | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| 2 | 1 | Fibrotic | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 |
| 3 | 1 | Fibrin | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| 4 | 1 | Fibrotic | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| 5 | 1 | Fibrin | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 0 | N/A | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| 7 | 0 | N/A | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| 8 | 1 | None | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 |
| 9 | 1 | Fibrotic | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| 10 | 1 | Fibrin | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 |
| 11 | 1 | Fibrin | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| 12 | 1 | Fibrin | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| 13 | 0 | N/A | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 14 | 0 | N/A | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| 15 | 0 | N/A | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| 16 | 0 | N/A | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| 17 | 1 | Fibrotic | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 |
| 18 | 0 | N/A | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
The aneurysm neck was histologically recognized in 10 cases. In those specimens the coil loops were either uncovered or covered by some fibrin in all cases with an implant time <3 months (Fig 1C,-D). Some fibrocellular tissue was found covering the coils at the neck in all cases that were removed surgically (Fig 2D).
In those specimens removed within 3 months following coil packing, fresh blood and unorganized thrombus were found regardless of whether the aneurysm was packed with Matrix or standard GDC coils (Fig 1D).
In the 3 aneurysms removed 3 years following GDC coil implantation, the aneurysm sacs were mostly filled with organized thrombus but remnants of unorganized thrombus and fresh blood were observed together with void spaces (Fig 2F). The aneurysm removed 6 months after treatment with Matrix coils was completely filled with granulation tissue representing active fibrocellular reaction without residual thrombus (Fig 3E). It showed some leukocyte invasion, collagen formation, and smooth muscle cells demonstrating maturing granulation tissue without any residual unorganized thrombus or void spaces. The neck of this aneurysm could not be identified on the histologic sections (Figs 2F and 3F–H).
Macrophages were seen in almost every specimen, even in the very early stages. Foreign body giant cells were found in all cases older than 2 months and in some older than 10 days. Significant leukocyte infiltration was found in all specimens.
Discussion
Incomplete aneurysm occlusion, persistent neck remnant, regrowth, and recanalization are frequently associated with endosaccular packing of both ruptured and unruptured aneurysms. Roy et al16 found a 47% initial rate of complete occlusion with a neck remnant in 43%, residual aneurysm in 5%, and failure in 5%. After at least 30 months, they found complete occlusion in 49%, residual neck in 38%, and residual aneurysm in 13%.16 Although the risk of aneurysm rupture/rerupture posed by a small neck remnant is probably very low, the long-term natural history of a partially packed aneurysm is simply unknown and maybe concerning. This frequently leads to late surgical clipping of a previously coil-packed aneurysm which can be complicated.17
The aim of coil embolization is to pack the aneurysm cavity as tightly as possible with coils. This may be difficult, however, especially if the neck is wide or the sac is large. In a study analyzing the volumetric packing attenuation of coil-treated aneurysms, Sluzewski et al18 found that, in general, not more than 20%–25% of the aneurysm’s cavity is filled with coils. Coil compaction may occur if the attenuation of packing is less than 24% in aneurysms 200–600 mm3.18 This means that even in a “tightly packed” aneurysm, 75% of the aneurysm sac is filled with thrombus. Fresh, unorganized thrombus is unstable, because it is subject to fibrinolysis. Fibrotic transformation of the thrombus is necessary for the intra-aneurysmal mass to become a permanent barrier that resists both the mechanical effect of pulsatile blood flow and the fibrinolytic mechanisms.
Several histologic case reports have demonstrated incomplete aneurysm thrombosis in coil-packed aneurysms. In giant aneurysms, thrombus remains largely unorganized and the neck uncovered for several months.1, 6, 8, 17 Other studies have demonstrated that in small aneurysms the neck is covered by a fibrin layer 36 hours after coil packing2 and by endothelium as early as 4 weeks after treatment.19 Serial studies have demonstrated a progressive healing process in coil packed aneurysms. Bavinzski et al.4 found unorganized thrombus within 1 week, incomplete fibrous transformation of the thrombus after 2–3 weeks, and collagen-rich vascularized tissue 4 years after treatment. In a single case, they found endothelium covering the aneurysm neck 6 weeks after coil packing.4 Groden7 reported only unorganized thrombus at 5 days posttreatment. Thirteen days later, macrophages appeared in the clot. Vascularized connective tissue filled the aneurysm cavity 10 months after treatment.7
These experiences inspired the development of a new concept that uses the coils not only as obstructive devices but also as carriers of a bioactive material. “Biologically active” coils are intended to deliver a biochemical agent within the aneurysm cavity, which is supposed to accelerate thrombus organization into fibrocellular granulation tissue.
As a bioactive material, collagen was first used in several experiments and was found to induce some tissue reaction.9, 10, 12 Further experiments confirmed the beneficial effects of either collagen, fibronectin, vitronectin, laminin, or fibrinogen when applied to the coil surface by ion implantation technology.11 Bavinzski tried to use tissue thromboplastin to enhance thrombus formation and plasminogen activator inhibitor to reduce thrombolysis but could not prove significant advantages of either material as compared with bare platinum GDCs.13 Finally, polyglycolic acid/lactide copolymer was tested on surgically created swine sidewall aneurysms. This polymer has been used previously for surgical suture lines and is known to induce granulation tissue. Instead of ion implantation or coating, this bioabsorbable material is applied in the form of a double coil. The inner coil is made of platinum, which is covered by an outer coil made of the copolymer. In experimental animal models, these coils accelerated thrombus organization at 2 weeks and produced a thicker fibrocellular tissue covering the neck at 3 months as compared with standard GDCs. These bioactive coils were approved for clinical use in 2002.
In our study, a total of 18 human aneurysms were endovascularly treated and histologically analyzed. From these, 2 aneurysms were treated with Matrix coils and removed at 8 days and 6 months after treatment. All of the other aneurysms were treated with GDC. All specimens were studied in a similar fashion, except that the aneurysm neck could not be visualized in all cases. The specimen removed 8 days treatment with Matrix coils did not exhibit any features different from those treated with standard GDCs within the same timeframe; however, the specimen treated with Matrix coils and removed at 6 months was completely filled with maturing fibrocellular granulation tissue, characterized by collagen fibers and numerous neovessels. Unfortunately, the neck of this aneurysm could not be microscopically studied as it was removed without the parent artery and histologic sections were not made in a plane parallel to the neck. We also did not have a 6-month GDC specimen to compare it to directly. Nevertheless, this aneurysm was completely filled with granulation tissue and, unlike the 3-year GDC specimen, did not contain any residual thrombus or void spaces.
Conclusion
Our results confirm that significant organization of the thrombus does not occur within a timeframe of less than 3 months following packing with platinum coils as previously reported. Intra-aneurysmal thrombus converts into fibrous tissue on a scale of years, but may remain imperfect for as long as 3 years after treatment. In one case, the Matrix biologically active coils did not alter this process within the 8 days after implantation. In another case, the aneurysm cavity was completely filled with granulation tissue corresponding to active fibrocellular reaction 6 months after treatment. These findings suggest that the biologically active polymer may accelerate aneurysm healing. Additional data are required to confirm these findings.
Footnotes
This study was supported in part by a grant from the Hungarian Science and Research Fund (Grant T32770).
References
- 1.Molyneux AJ, Ellison DW, Morris J, et al. Histological findings in giant aneurysms treated with Guglielmi detachable coils: report of two cases with autopsy correlation. J Neurosurg 1995;83:129–32 [DOI] [PubMed] [Google Scholar]
- 2.Stiver SI, Porter PJ, Willinsky RA, et al. Acute human histopathology of an intracranial aneurysm treated using Guglielmi detachable coils: case report and review of the literature. Neurosurgery 1998;43:1203–08 [DOI] [PubMed] [Google Scholar]
- 3.Strother CM, Berenstein A, Vinuela F. A pitfall in the surgery of a recurrent aneurysm after coil embolization and its histological observation: technical case report. Neurosurgery 1998;42:1199–200 [DOI] [PubMed] [Google Scholar]
- 4.Bavinzski G, Talazoglu V, Killer M, et al. Gross and microscopic histopathological findings in aneurysms of the human brain treated with Guglielmi detachable coils. J Neurosurg 1999;91:284–93 [DOI] [PubMed] [Google Scholar]
- 5.Shimizu S, Kurata A, Takano M, et al. Tissue response of a small saccular aneurysm after incomplete occlusion with a Guglielmi detachable coil. AJNR Am J Neuroradiol 1999;20:546–48 [PMC free article] [PubMed] [Google Scholar]
- 6.Asai J, Suzuki R, Fujimoto T, et al. Correlation of magnetic resonance imaging and histological findings in a large basilar tip aneurysm after coil embolization: case report. Neurol Med Chir (Tokyo)2000;40:519–23 [DOI] [PubMed] [Google Scholar]
- 7.Groden C, Hagel C, Delling G, et al. Histological findings in ruptured aneurysms treated with GDCs: six examples at varying times after treatment. AJNR Am J Neuroradiol 2003;24:579–84 [PMC free article] [PubMed] [Google Scholar]
- 8.Mori K, Nakao Y, Horinaka N, et al. Cerebral aneurysm regrowth and coil unraveling after incomplete Guglielmi detachable coil embolization: serial angiographical and histological findings. Neurol Med Chir (Tokyo)2003;43:293–97 [DOI] [PubMed] [Google Scholar]
- 9.Dawson RC, Krisht AF, Barrow DL, et al. Treatment of experimental aneurysms using collagen-coated microcoils. Neurosurgery 1995;36:133–39; discussion 139–40 [DOI] [PubMed] [Google Scholar]
- 10.Dawson RC 3rd, Shengelaia GG, Krisht AF, et al. Histologic effects of collagen-filled interlocking detachable coils in the ablation of experimental aneurysms in swine. AJNR Am J Neuroradiol 1996;17:853–58 [PMC free article] [PubMed] [Google Scholar]
- 11.Murayama Y, Vinuela F, Suzuki Y, et al. Development of the biologically active Guglielmi detachable coil for the treatment of cerebral aneurysms. Part II. An experimental study in a swine aneurysm model. AJNR Am J Neuroradiol 1999;20:1992–99 [PMC free article] [PubMed] [Google Scholar]
- 12.Szikora I, Wakhloo AK, Guterman LR, et al. Initial experience with collagen-filled Guglielmi detachable coils for endovascular treatment of experimental aneurysms. AJNR Am J Neuroradiol 1997;18:667–72 [PMC free article] [PubMed] [Google Scholar]
- 13.Bavinzski G, Richling B, Binder BR, et al. Histopathological findings in experimental aneurysms embolized with conventional and thrombogenic/antithrombolytic Guglielmi coils. Minim Invasive Neurosurg 1999;42:167–74 [DOI] [PubMed] [Google Scholar]
- 14.Murayama Y, Tateshima S, Gonzalez NR, et al. Matrix and bioabsorbable polymeric coils accelerate healing of intracranial aneurysms: long-term experimental study. Stroke 2003;34:2031–37 [DOI] [PubMed] [Google Scholar]
- 15.Raymond J, Roy D. Safety and efficacy of endovascular treatment of acutely ruptured aneurysms. Neurosurgery 1997;41:1235–45; discussion 1245–46 [DOI] [PubMed] [Google Scholar]
- 16.Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke 2001;32:1998–2004 [DOI] [PubMed] [Google Scholar]
- 17.Mizoi K, Yoshimoto T, Takahashi A, et al. A pitfall in the surgery of a recurrent aneurysm after coil embolization and its histological observation: technical case report. Neurosurgery 1996;39:165–68; discussion 168–69 [DOI] [PubMed] [Google Scholar]
- 18.Sluzewski M, van Rooij WJ, Slob MJ, et al. Relation between aneurysm volume, packing, and compaction in 145 cerebral aneurysms treated with coils. Radiology 2004;231:653–58 [DOI] [PubMed] [Google Scholar]
- 19.Koizumi T, Kawano T, Kazekawa K, et al. [Histological findings in aneurysm treated with IDC: scanning electron microscopical study]. No Shinkei Geka 1997;25:1027–31 [PubMed] [Google Scholar]