Abstract
BACKGROUND AND PURPOSE: Cerebral vasospasm remains a major problem in patients recovering after surgical treatment of cerebral aneurysms. The purpose of this study was to evaluate cerebral vasospasm at multidetector-row spiral CT angiography (MDCTA) compared with digital subtraction angiography (DSA) in patients with aneurysmal subarachnoid hemorrhages (SAHs).
METHODS: Seventeen patients suspected of having vasospasm on clinical findings underwent both postoperative MDCTA and DSA. MDCTA was analyzed by using volume-rendered images as well as axial images. A total of 251 arterial segments were analyzed for vasospasm by using a 5-point grading system. The MDCTA results were then compared with findings on the corresponding DSA images. Sensitivity, specificity, and accuracy of MDCTA for detection of hemodynamically significant spasms were also calculated, with findings at DSA used as the reference standard.
RESULTS: On DSA, 74 spasmatic segments were found among the 251 segments evaluated, and 40 segments with hemodynamically significant vasospasms were present. The overall agreement between MDCTA and DSA was 95.2%. We had 12 (4.8%) cases of disagreement between MDCTA and DSA. In 11 segments, the degree of stenosis was overestimated at MDCTA. Overall accuracy, sensitivity and specificity of MDCTA in the detection of hemodynamically significant vasospasm were 97.5%, 98.1%, and 98.0%, respectively, with positive and negative predictive values of 90.7% and 99.5%.
CONCLUSION: MDCTA appears to be a reliable alternative imaging technique to DSA in the assessment of patients with cerebral vasospasm after aneurysmal SAH.
Cerebral vasospasm following aneurysmal subarachnoid hemorrhage (SAH) is one of the main causes of morbidity and mortality after surgical treatment of cerebral aneurysms.1, 2 Vasospasm frequently occurs within 2 weeks after the SAH, and its accurate postoperative diagnosis is essential to initiate appropriate therapy that may prevent ischemic insults.3, 4
Digital subtraction angiography (DSA) has been the gold-standard diagnostic test in this setting. This procedure, however, is invasive and is not always available in critically ill patients and not without its own risk.5, 6 There has been, therefore, great demand for noninvasive alternatives for accurate depiction of cerebral vasospasm. Transcranial Doppler sonography (TCD) is a relatively simple method for detecting vasospasm.7, 8 The diagnosis of a spasm with a TCD device is based on the hemodynamic principle that the angle-corrected velocity of blood flow increases with vessel narrowing. Despite numerous published reports about this subject, however, its operator dependence, low reproducibility, lack of standard cutoff velocities, and problem in detecting vasospasm at distal arteries limit its use to a screening method only, with confirmative diagnosis made by DSA.9, 10
Since the introduction of the spiral CT, CT angiography is a noninvasive, feasible diagnostic imaging technique that has been used for the evaluation of intracranial vascular lesions.11, 12 Recent reports discuss the capacities of single-detector-row spiral CT angiography in the detection of vasospasm following SAH.13, 14 The purpose of this study was to compare multidetector-row spiral CT angiography (MDCTA) with DSA in the detection of vasospasm in patients with aneurysmal SAH.
Methods
Case Collection
From September 2003 through March 2005, 135 consecutive patients, who underwent both MDCTA and DSA to make an initial diagnosis of intracranial aneurysm, were evaluated retrospectively. Clipping of aneurysm(s) was performed in 104 of them, and the remaining 31 patients were treated nonsurgically or by detachable coil. Of the 104 patients who underwent surgery for aneurysm clipping, 17 (8 men and 9 women; mean age, 52 years; age range, 33–73 years) who underwent both MDCTA and conventional DSA to evaluate cerebral vasospasm after aneurysm clipping were included in this study. Indications for postoperative MDCTA and DSA were patients clinically suspected of having intracranial vasospasm with at least one of the following 5 items: (1) worsening headache or neck stiffness; (2) low-grade fever; (3) decline in level of consciousness or focal neurologic deficit; (4) a head CT scan excluding other causes of clinical worsening; or (5) substantially elevated mean flow velocity of intracranial artery as determined with TCD study.
Preoperative MDCTA and DSA were performed to make an initial diagnosis of intracranial aneurysm within 24 hours of each other. Seventeen patients had 10 angiographically and surgically documented saccular aneurysms with SAH confirmed by initial CT. Two patients (cases 6 and 13) each had an aneurysm that caused SAH and an unruptured aneurysm in another location that was treated during the same operation. Seven aneurysms were located in the anterior communicating artery, 6 were in the internal carotid artery-posterior communicating artery, 4 were in the middle cerebral artery, and 2 were in the posterior circulation. The aneurysm was treated by surgical clipping within the first 48 hours after hemorrhage in all patients. All aneurysm operations were performed by using titanium clips (Yasargil-Titanium Aneurysm Clip, Aesculap AG, Tuttlingen, Germany). For 18 aneurysms, a single clip was applied; for one patient (case 1), 2 clips were needed because of the size and the anatomy of the aneurysm.
The postoperative MDCTA and DSA studies were performed on average 5.7 days (range, 4–9 days) after surgery. In all patients, MDCTA was performed before DSA, with the longest interval between the 2 examinations being 12 hours.
MDCTA
MDCTA was performed with a 16-channel multidetector-row spiral CT scanner (MX8000 Infinite Detector Technology; Philips, Haifa, Israel) in all 17 patients. After obtaining a nonenhanced scout image (120 kV, 100 mAs), the scanning range was planned in a caudocranial direction from the level of the foramen magnum through a point 1 cm above the level of the lateral ventricles (mean coverage, 95 mm; range, 90–100 mm).
For optimal intraluminal contrast enhancement, the delay time between start of contrast material administration and start of scanning was determined for each patient individually by using a bolus-tracking technique (MX8000 Infinite Detector Technology; Philips). For this purpose, a single nonenhanced low-dose scan (20 mAs) at the level of the distal common carotid artery was first obtained. On the basis of this transverse image, a region of interest with an area of 3 mm2–5 mm2 was set in the lumen of the distal common carotid artery. This region of interest served as a reference for the following dynamic measurements of contrast enhancement. Subsequently, a total of 100–120 mL of iohexol (Omnipaque 300; Amersham Health, Cork, Ireland), a low-osmolar iodinated contrast material, was administered intravenously via an 18- or 20-gauge catheter positioned in an antecubital vein. The contrast medium was administered with a power injector (CT9000ADV; Liebel-Flarsheim, Cincinnati, Ohio) at a rate of 3–4 mL/s. At 10 seconds after the start of contrast material administration, repetitive low-dose monitoring scans (120 kV, 20 mAs, 0.5-second scanning time, 1-second interscan delay) were obtained. When the Hounsfield units in the preset lumen of the distal common carotid artery rose by 100, the multidetector-row CT scan was triggered automatically 3 seconds later.
Parameters for the CT angiographic acquisition were 1-mm section thickness, 6-mm table feed per rotation, 0.5-second gantry rotation time, pitch of 6, 120 kV, and 200–280 mA. The entire examination, including acquisition of image data, took 15–20 minutes.
3D Reconstruction
The volumetric data so obtained were transferred to a workstation (RAPIDIA 3D; Infinitt, Seoul, Korea) for further processing. Transverse sections were reconstructed with a section width of 1.25 mm at an interval of 0.6 mm. Matrix size was 512 × 512, and the field of view varied between 20 and 22 cm, depending on patient size. Volume-rendered images (VRIs) from the source image datasets were generated by using the commercially available software (RAPIDIA 3D) installed on the workstation.
For diagnostic purposes, VRIs after automatic segmentation of obscuring bone structures were also obtained in 10 patients. The display of only the contrast-enhanced vascular lumen was possible by using CTA software (RAPIDIA 3D), which allowed automatic segmentation of precontrast scan dataset (ie, any overlapping bony structures, calcification, and surgical clip) from the intact CTA dataset.
All standardized reconstructions were completed by an experienced neuroradiologist (D.Y.Y.) by using the same technique; image processing time ranged between 8 and 10 minutes. The radiologist was aware only of the fact that the patients were examined for postoperative assessment of vasospasm and was blinded to DSA results.
DSA Procedure
Intra-arterial DSA was performed transfemorally in all 17 patients with a 5F catheter by using a monoplane DSA unit (Integris Allura; Philips Medical Systems, Best, the Netherlands) with an image intensifier matrix of 1024 × 1024 pixels. DSA was performed with bilateral selective internal carotid artery injections and either unilateral or bilateral vertebral artery injections, as necessary. For each vessel, we injected 6–9 mL of nonionic contrast medium (320 mg of iodine per milliliter of iodixanol, Visipaque 320; Amersham Health) at a rate of 4–7 mL/s by using a power injector (Angiomat Illumena; Liebel-Flarsheim). In all patents, standard anteroposterior and lateral projections were obtained for carotid and vertebral injections. If an abnormality was suspected on the standard angiograms, additional oblique projections were also obtained.
Image Analysis
MDCT angiograms and DSA images were reviewed by separate investigators. In this study, the interpretation of images from each technique was performed in a blinded manner so that both readers were not aware of the results of interpretation of images from the other technique.
For analysis purposes, the intracranial arteries were separately evaluated as 15 anatomic segments on MDCT angiograms and DSA images: vertebrobasilar artery, right and left distal internal carotid artery (ICA), M1 and M2 segments of middle cerebral artery (MCA), A1 and A2 segments of anterior cerebral artery (ACA), and P1 and P2 segments of posterior cerebral artery (PCA). Two A1 and 2 P1 segments were excluded in this analysis because of absence or severe hypoplasia; a total of 251 arterial segments in 17 patients were analyzed.
Each segment was assigned 1 of 5 categories based on the degree of spasm: 0, no spasm; 1, mild spasm (< 25% luminal narrowing); 2, moderate spasm (25%–50% luminal narrowing); 3, severe spasm (51%–99% luminal narrowing); 4, total occlusion (Table 1). Grading of arterial stenosis was performed by using an electronic caliper. When more than one lesion was detected in the same arterial segment, the most severe change was used for grading and analysis. Stenosis grades 1 and 2 (<50% luminal narrowing) were considered hemodynamically insignificant arterial stenosis, whereas grades 3 and 4 (51%–100% luminal narrowing) were considered hemodynamically significant arterial stenosis. The preoperative MDCT angiograms and DSA images, without obvious vasospasm, were used as a reference for grade of spasm by comparing the corresponding arterial segment on the preoperative and postoperative studies.
Table 1:
Scale for grading intracranial vasospasm
| Grade | Definition |
|---|---|
| 0 | No spasm |
| 1 | Mild spasm (<25% luminal narrowing) |
| 2 | Moderate spasm (25–50% luminal narrowing) |
| 3 | Severe spasm (51–99% luminal narrowing) |
| 4 | Total occlusion |
Moreover, the following parameters were also evaluated: the occlusion of the sac and dome of the aneurysm, the presence of the residual neck of the aneurysm, and the presence of clip artifacts.
Statistical Analysis
The MDCTA and DSA examinations were compared for each arterial segment by using Spearman correlation coefficient. Sensitivity, specificity, positive and negative predictive values, and accuracy of MDCTA for determination of hemodynamically significant arterial stenosis were calculated by using DSA as standard for all 15 segments combined and were also calculated according to the anatomic district, the proximal (distal ICA, M1 segment of the MCA, A1 segment of the ACA, P1 segment of PCA, and vertebrobasilar artery), and the distal (A2, M2, and P2 segments and their branches) locations. Statistical significance was calculated by using the Fisher exact test.
Results
In 15 (88.2%) of 17 patients who were evaluated for postoperative vasospasm, the evidence for vasospasm was observed on DSA. Two patients had no signs of angiographic vasospasm despite clinical or TCD findings. Overall, DSA identified 34 segments with hemodynamically insignificant vasospasms (16 with grade 1 vasospasm and 18 with grade 2 vasospasm) and 40 segments with significant vasospasms (38 with grade 3 vasospasm, and 2 with grade 4 vasospasm). Table 2 demonstrates the breakdown of the degrees and sites of vasospasms of 251 arterial segments in all 17 patients.
Table 2:
Summary of patients and angiographic findings
| Patient No./Age (y)/Sex | Site of Aneurysm | Vasospasm on DSA |
|
|---|---|---|---|
| Site | Grade | ||
| 1/40/M | AcomA | Bil ICA, Rt A1 | 2 |
| Bil A2, M1, M2, Lt A1 | 3 | ||
| 2/72/F | Rt ICA bifurcation | Lt A1 | 1 |
| Rt M2 | 2 | ||
| Bil A2 | 3 | ||
| Rt A1 | 4 | ||
| 3/55/M | Rt MCA bifurcation | Lt A1 | 2 |
| Rt A1, Lt M2 | 3 | ||
| 4/67/F | AcomA | Bil A2, Lt M2 | 3 |
| 5/42/M | AcomA | Bil ICA | 1 |
| Bil A1, A2, Lt M1 | 3 | ||
| 6/73/F | AcomA | Lt A2 | 1 |
| Lt PcomA | Bil A1, Rt A2 | 3 | |
| 7/33/M | AcomA | Rt ICA | 1 |
| Lt M1 | 2 | ||
| Bil A1, A2, Rt M1 | 3 | ||
| 8/69/F | Lt PcomA | Lt P1 | 1 |
| 9/46/M | AcomA | Bil M1 | 1 |
| Bil A1, A2 | 3 | ||
| 10/52/M | BA tip | BA, Rt P1 | 1 |
| 11/61/M | Rt PcomA | Rt ICA | 1 |
| Rt A1, A2, M1, P1, P2 | 2 | ||
| 12/39/F | Lt PcomA | Lt ICA | 1 |
| Lt A1, M1, M2 | 2 | ||
| Lt P1, P2 | 3 | ||
| 13/67/F | AcomA | Lt M1, M2 | 1 |
| Rt MCA bifurcation | Bil ICA | 2 | |
| Bil A2, Lt A1, Rt M1, M2 | 3 | ||
| Rt A1 | 4 | ||
| 14/44/M | Rt MCA bifurcation | Rt ICA | 1 |
| Rt M1, M2 | 2 | ||
| 15/47/F | Lt PICA | Lt. VA | 1 |
| 16/47/F | Rt PcomA | None | |
| 17/65/F | Lt PcomA | None | |
Note.— Bil indicates bilateral; Lt, left; Rt, right; AComA, anterior communicating artery; MCA, middle cerebral artery; ICA, internal carotid artery; PcomA, posterior communicating artery; PICA, posterior inferior cerebellar artery; A1, first segment of anterior cerebral artery; A2, second segment of anterior cerebral artery; M1, first segment of middle cerebral artery; M2, second segment of middle cerebral artery; P1, first segment of posterior cerebral artery; P2, second segment of posterior cerebral artery; VA, vertebral artery; BA, basilar artery.
The agreement between the degree of vasospasm revealed by MDCTA and DSA in the overall, proximal, and distal segments of the cerebral arteries was 95.2%, 96.0%, and 94.1%, respectively (Table 3). We had 12 (4.8%) of 251 segments of disagreement between MDCTA and DSA. In 11 segments, the degree of spasm was overestimated at MDCTA by one grade (Fig 1); in one segment, the degree of spasm was underestimated by one grade.
Table 3:
Vasospasm (grades 0–4) of the cerebral arteries (251 segments) as determined with DSA and multidetector row CT angiography in 17 patients
| Arterial Segment (No. of Segment)* | Grade of Vasospasm |
No. of Arteries Overestimated by CTA | No. of Arteries Underestimated by CTA | r† | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 |
1 |
2 |
3 |
4 |
|||||||||
| DSA | CTA | DSA | CTA | DSA | CTA | DSA | CTA | DSA | CTA | ||||
| ICA (34) | 24 | 24 | 6 | 6 | 4 | 4 | 0 | 0 | 0 | 0 | 1.00 | ||
| A1 (32) | 14 | 14 | 1 | 0 | 4 | 5 | 11 | 9 | 2 | 4 | 4 | 1 | 0.9623 |
| A2 (34) | 17 | 17 | 1 | 1 | 1 | 0 | 15 | 14 | 0 | 2 | 3 | 0.9702 | |
| M1 (34) | 22 | 22 | 3 | 2 | 4 | 5 | 5 | 5 | 0 | 0 | 1 | 0.9978 | |
| M2 (34) | 25 | 25 | 1 | 0 | 3 | 3 | 5 | 6 | 0 | 0 | 2 | 0.9958 | |
| P1 (32) | 28 | 28 | 2 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 1.00 | ||
| P2 (34) | 32 | 32 | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 0 | 1 | 0.9995 | |
| VBA (17) | 15 | 15 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 | ||
| Total (251) | 177 | 177 | 16 | 13 | 18 | 18 | 38 | 35 | 2 | 6 | 11 | 1 | 0.9966 |
Note.— Data are number of segments. Grade 0 indicates no spasm; grade 1, <25%; grade 2, 25%–50%; grade 3, 51%–99% luminal reduction; grade 4, total occlusion. DSA indicates digital subtraction angiography; CTA, multidetector row CT angiography.
See Table 2 for definitions of segments.
r, Spearman correlation coefficient.
Fig 1.
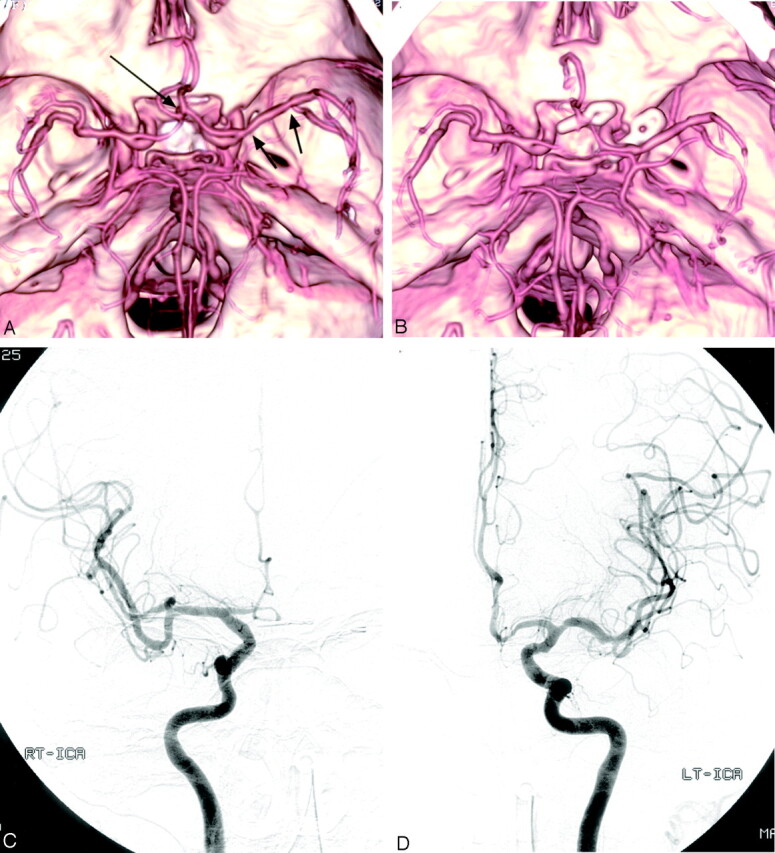
Case 6, a 73-year-old woman with SAH.
A, Preoperative MDCTA image, superior view, shows an anterior communicating artery aneurysm (long arrow). Left posterior communicating artery aneurysm was well visualized on other projections of MDCTA images (not shown). Note the multiple focal stenoses in the left M1 segment (arrows).
B, Postoperative MDCTA image, obtained 7 days after surgery, shows clipping of the aneurysms and multiple spasms of bilateral A1 and A2 segments. Note total occlusion of right A1 segment, as well as no change of stenoses in the left M1 segment consistent with preexisting atherosclerotic stenoses.
C and D, Postoperative right (C) and left (D) carotid angiograms, anteroposterior view, confirm vasospasm involving bilateral anterior cerebral arteries. Note grade 3 spasm (50%–99% luminal narrowing) in the right A1 segment, which was overestimated as grade 4 spasm (total occlusion) on MDCTA (B).
On the basis of DSA, 40 arterial segments with hemodynamically significant vasospasms (2 occlusions and 38 stenoses >50% diameter reduction) were present, and 39 of these 40 lesions were correctly detected by MDCTA (sensitivity, 97.5%; specificity, 98.1%; positive and negative predictive value, 90.7% and 99.5%; diagnostic accuracy, 98.0% [Table 4]). Sensitivity, specificity, positive and negative predictive values, and diagnostic accuracy were also calculated according to the anatomic district and the proximal and the distal locations; no statistical difference in accuracy of MDCTA in the distal arterial segment was noted with respect to accuracy in the more proximal arterial bed.
Table 4:
Diagnostic performance of multidetector row CT angiography compared with DSA in the detection of hemodynamically significant vasospasm (51%–100% of luminal narrowing) according to the anatomic district in 17 patients (251 segments)
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | DA (%) | P Value | |
|---|---|---|---|---|---|---|
| Proximal locations (ICA, M1, A1, P1, VA, and BA) | 94.7 | 99.2 | 94.7 | 99.2 | 98.7 | <.001 |
| Distal locations (A2, M2, P2, and their branches) | 100 | 96.3 | 87.5 | 100 | 97.1 | <.001 |
| Total | 97.5 | 98.1 | 90.7 | 99.5 | 98.0 | <.001 |
Note.— PPV indicates positive predictive value; NPV, negative predictive value; DA, diagnostic accuracy; ICA, internal carotid artery; M1, first segment of middle cerebral artery; M2, second segment of middle cerebral artery; A1, first segment of anterior cerebral artery; A2, second segment of anterior cerebral artery; P1, first segment of posterior cerebral artery; P2, second segment of posterior cerebral artery; VA, vertebral artery; BA, basilar artery.
Differences between proximal and distal locations are statistically nonsignificant.
Five patients with hemodynamically significant cerebral vasospasm were treated with intra-arterial and/or intravenous infusion of nimodipine. Four of 5 patients had substantial improvement in their clinical condition following treatment. The second postoperative MDCTAs were performed and revealed improved spasm in 3 patients; none of these patients had follow-up DSA.
In addition, occlusion of the dome and the sac of the aneurysm could be evaluated in all MDCTA images. Two small remnant necks of the aneurysm were identified on MDCTA, and all of them were confirmed on DSA (cases 4 and 5 [Fig 2]).
Fig 2.
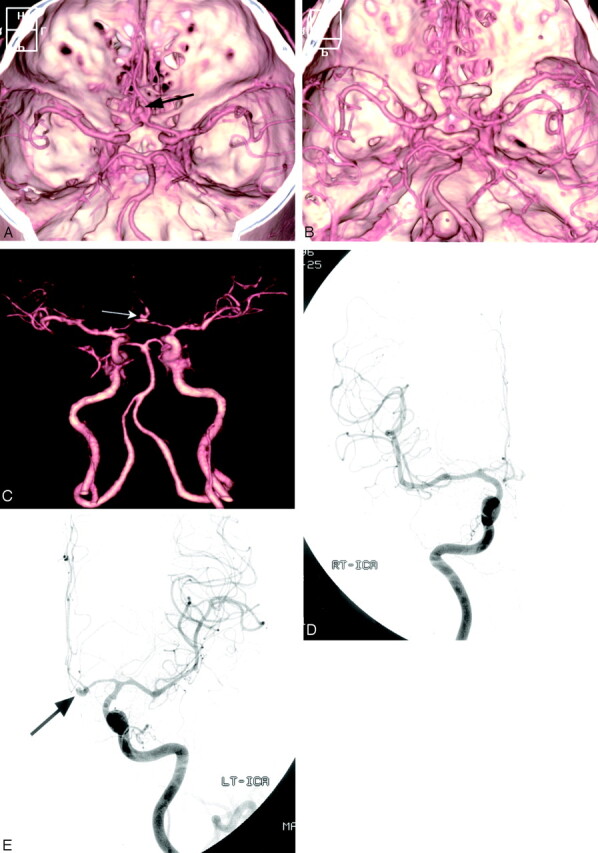
Case 5, a 42-year-old man with SAH.
A, Preoperative MDCTA image, superior view, shows an anterior communicating artery aneurysm (arrow).
B, Postoperative MDCTA image, obtained 5 days after surgery, shows clipping of the aneurysms and multiple spasms of bilateral A1, A2, left M1, and M2 segments.
C, The small remnant aneurysm (arrow) after aneurysm clipping is depicted on the anteroposterior MDCTA image with automated segmentation.
D and E, Postoperative right (D) and left (E) carotid angiograms, anteroposterior view, confirm vasospasm involving bilateral anterior cerebral and left middle cerebral arteries. The small remnant aneurysm (arrow) is also noted.
Discussion
It is well established that timely identification and adequate treatment of intracranial vasospasm can avoid the risk of cerebral ischemia or infarction. In many institutions, angioplasty and intra-arterial infusion of papaverine are the primary methods of endovascular treatment of vasospasm refractory to medical treatment.15, 16 Recent reports have also described the successful treatment of vasospasm with intra-arterial injection of nimodipine.17
Although DSA has remained the standard of reference for the evaluation of intracranial vasospasm, it has several disadvantages, including the invasive nature, a high skill level to perform the procedure, and relatively high costs. Furthermore, DSA reportedly has a 1% risk of a disabling neurologic deficit and a 0.1% risk of mortality.5 DSA cannot therefore be routinely used after intracranial aneurysm surgery.
MR angiography (MRA) is not ideal because of the relatively long acquisition time and limited access to the critically ill patient with intracranial vasospasm. The artifacts caused by the clips, metallic devices in craniotomy site, and subarachnoid blood also preclude routine use in postoperative patients.18 Therefore, it may not be suitable for investigation of vasospasm in patients with metallic intracranial aneurysm clips.
The use of TCD in the postoperative surveillance of intracranial vasospasm is well documented. Because of its noninvasive quality, low cost, and the rapid access it provides, TCD is considered to be the primary imaging technique for use in detection of vasospasm.7, 8 With DSA as the reference standard, TCD has been shown to be 84%–85% sensitive and 89%–98% specific in detecting spasm at the proximal MCA.19–20 Recent studies, however, have highlighted its limitations in making a definitive diagnosis in patients with possible cerebral vasospasm.9, 10 Lysakowski et al21 pointed out that the diagnostic accuracy of TCD for spasms of the anterior cerebral artery is low. They also found that for, all the other arteries, there is no evidence of any usefulness of TCD as a diagnostic tool for spasms. Another potential limitation of TCD is its operator dependency. The operator’s skills and experience with the TCD devices may have influenced the results.
CTA has increasingly been recognized as an efficient imaging technique that could possibly replace DSA in the evaluation of SAH.11,12 Moreover, the minimal invasiveness, rapidity, and reliability make CTA extremely useful for emergency examinations of critically ill patients. Therefore, CTA could be potentially an ideal imaging technique for assessing patients suspected of having vasospasm after SAH.
Several initial studies, limited to small case series, reported the potential usefulness of CTA in the diagnosis of intracranial vasospasm.22–24 These authors, however, focused on the state of clipped aneurysm, so no objective quantifications of vasospasm were made. Takagi et al13 compared CTA and DSA in 17 cases of intracranial vasospasm. They found severe spasm (>75% luminal reduction) in 5 cases and moderate spasm (50%–75% reduction) in 2 cases and confirmed with DSA. More recently, Anderson et al14 compared CTA and DSA in the detection and quantification of intracranial vasospasm in 7 patients with SAH. They found that agreement between CTA and DSA was greater for no spasm (92%) and severe spasm (100%) than for mild (57%) or moderate (64%) spasm.
With the recent development of multidetector-row CT scanners, MDCTA can be performed more efficiently because of shorter data acquisition times, higher longitudinal resolution, and improved quality with diminished artifacts than was possible with single-detector-row spiral CT scanners. Therefore, the advantages of MDCTA could allow the radiologists to overcome the limitations of single-detector-row spiral CT in the diagnosis of cerebral vasospasm. In the literature, we found only one article25 comparing MDCTA and DSA in the evaluation of vasospasm in patients with aneurysmal SAH. The authors concluded that MDCTA could detect angiographic vasospasm after SAH with accuracy equal to that of DSA. The agreements between the severity of vasospasm on MDCTA and DSA in the overall, proximal, and distal segments of the cerebral arteries were 91.6%, 90.8%, and 92.3%, respectively; however, they used maximum intensity projection (MIP) for 3D reconstructions of CT angiographic data and a thresholding technique and manual editing to remove bone of the cranial base.
In current publications that deal with MDCTA, most authors use a combination of MIPs, VRIs, and multiplanar reconstructions for image analysis of MDCT angiograms. MIP images have an advantage over VRIs for differentiating parietal calcifications from true lumen and determining the degree of atherosclerotic stenosis. In this series, however, there was no case with heavy calcifications in intracranial artery that may impair the quantification of vasospasm. Furthermore, postprocessing by using MIP reconstruction algorithm, requires long postprocessing times and the operator’s extensive experience to provide the most useful images.26
We used the VRI technique instead of the MIP algorithm for the comparison with DSA in this study. VRI technique is in our experience far quicker and easier to produce than MIP algorithms and can give better anatomic detail of the intracranial vasculature. The “4-D angio” software package (RAPIDIA 3D) allows rapid VRIs to be produced, which highlights the vessels over the bony landmarks. Fortunately, recently upgraded workstation software that allows automated segmentation of bony structures and surgical clips was available in this study. We believe that the clinical use of this software reduces the time required for 3D reconstructions of CT angiographic data, and avoids overlapping with unnecessary parts in the images so that an even better evaluation may be possible.
To the best of our knowledge, ours is the first report on the application of MDCTA for the evaluation of vasospasms, by using VRI algorithm and automated segmentation technique. In most patients in this series, an experienced radiologist was able to generate 3D reconstruction in less than 10 minutes.
This was a study that compared MDCTA with DSA in 17 patients with clinically suspected vasospasm. With the VRI algorithm used in this study, the agreements between the degree of vasospasm revealed by MDCTA and DSA in the overall, proximal, and distal segments of the cerebral arteries were 96.8%, 97.3%, and 96.1%, respectively. The excellent quality of MDCT angiograms in this study is reflected in the overall sensitivity and specificity of 97.5 and 98.1%, respectively, and in positive and negative predictive values of 90.7 and 99.5%, respectively, for the detection of hemodynamically significant vasospasm.
An important disadvantage of postoperative brain imaging has been the significant beam hardening artifacts caused by aneurysm clips. Several studies have demonstrated that the recent use of titanium clips showed much less artifact in CTA and MRA than alloy clips did.27 At our institution, titanium clips are routinely used for cerebral aneurysms, so no considerable signal intensity loss due to clip artifact occurred on MDCTA studies. Furthermore, with the increase in the number of detectors, MDCT scanners will provide thinner collimation and higher temporal resolution, which may reduce artifacts from aneurysm clips.
We acknowledge several limitations of our study. A major limitation of our study was the low power of statistics due to the small study population. At our institution, most patients with surgically treated aneurysm are followed-up by MDCTA and do not undergo additional DSA studies. For this reason, it is difficult to collect a large number of patients who have both MDCTA and DSA. Moreover, our small prevalence of hemodynamically significant arterial spasms (16%) may limit the calculated descriptive statistics for each of the 8 different vascular regions. Another possible problem in our study relates to the fact that cerebral vasospasm is a relatively dynamic process. Thus, even if postoperative MDCTA and DSA were performed with 12 hours of each other, the problem of the time delay between the 2 studies remains. In particular, all the MDCTA studies were conducted before the angiographic study in this series. Prospective studies with large patient populations evaluating cerebral vasospasm are needed to confirm these results and to determine whether MDCTA can replace postoperative conventional DSA.
In addition, postoperative MDCTA can also provide other valuable information concerning the occlusion of the dome, the presence of a residual sac, and the state of untreated coincidental aneurysms. The reported incidences of residual sacs are between 4% and 18%.28 A recent publication indicates that MDCTA is less hampered by clip artifact and able to demonstrate a residual aneurysm neck.29 In our study, MDCTA was also useful in demonstrating small residual sac, as described in a previous study.29
Furthermore, if DSA is still required after MDCTA, it can often be combined with endovascular treatment of vasospasm. Thus, CTA will generally provide useful information for planning of the interventional procedure. For these purposes, we believe that postoperative MDCTA can replace DSA as the screening imaging test in selected patients with aneurysmal SAH.
In conclusion, the results of this study have demonstrated that MDCTA is highly accurate in the assessment of intracranial vasospasm. Because of its less-invasive nature and substantially shorter acquisition time, MDCTA has potential to substitute for DSA in the postoperative care of patients with SAH.
References
- 1.Weir B, MacDonald L. Cerebral vasospasm. Clin Neurosurg 1993;40:40–55 [PubMed] [Google Scholar]
- 2.Bleck TP. Rebleeding and vasospasm after SAH: new strategies for improving outcome. J Crit Illness 1997;12:572–82 [Google Scholar]
- 3.Weir B, Grace M, Hansen J, et al. Time course of vasospasm in man. J Neurosurg 1978;48:173–79 [DOI] [PubMed] [Google Scholar]
- 4.Rosenwasser RH, Armonda RA, Thomas JE, et al. Therapeutic modalities for the management of cerebral vasospasm: timing of endovascular options. Neurosurgery 1999;44:975–79 [DOI] [PubMed] [Google Scholar]
- 5.Pryor JC, Setton A, Nelson PK, et al. Complications of diagnostic cerebral angiography and tips on avoidance. Neuroimaging Clin N Am 1996;6:751–58 [PubMed] [Google Scholar]
- 6.Cloft HJ, Joseph GJ, Dion JE. Risk of cerebral angiography in patients with subarachnoid hemorrhage, cerebral aneurysm, and arteriovenous malformation: a meta-analysis. Stroke 1999;30:317–20 [DOI] [PubMed] [Google Scholar]
- 7.Aaslid R, Huber P, Nornes H. Evaluation of cerebrovascular spasm with transcranial Doppler ultrasound. J Neurosurg 1984;60:37–41 [DOI] [PubMed] [Google Scholar]
- 8.Lindegaard KF, Nornes H, Bakke SJ, et al. Cerebral vasospasm diagnosis by means of angiography and blood velocity measurements. Acta Neurochir (Wien) 1989;100:12–24 [DOI] [PubMed] [Google Scholar]
- 9.Krejza J, Mariak Z, Babikian VL. Importance of angle correction in the measurement of blood flow velocity with transcranial Doppler sonography. AJNR Am J Neuroradiol 2001;22:1743–47 [PMC free article] [PubMed] [Google Scholar]
- 10.Krejza J, Mariak Z, Walecki J, et al. Transcranial color Doppler sonography of basal cerebral arteries in 182 healthy subjects: age and sex variability and normal reference values for blood flow parameters. AJR Am J Roetgenol 1999;172:213–18 [DOI] [PubMed] [Google Scholar]
- 11.Schwartz RB, Tice HM, Hooten SM, et al. Evaluation of cerebral aneurysm with helical CT: correlation with conventional angiography and MR angiography. Radiology 1994;192:717–22 [DOI] [PubMed] [Google Scholar]
- 12.Aoki S, Sasaki Y, Machida T, et al. Cerebral aneurysm: detection and delineation using 3D-CT angiography. AJNR Am J Neuroradiol 1992;13:1115–20 [PMC free article] [PubMed] [Google Scholar]
- 13.Takagi R, Hayashi H, Kobayashi H, et al. Three-dimensional CT angiography of intracranial vasospasm following subarachnoid haemorrhage. Neuroradiology 1998;40:631–35 [DOI] [PubMed] [Google Scholar]
- 14.Anderson GB, Ashforth R, Steinke DE, et al. CT angiography for the detection of cerebral vasospasm in patients with acute subarachnoid hemorrhage. AJNR Am J Neuroradiol 2000;21:1011–15 [PMC free article] [PubMed] [Google Scholar]
- 15.Song JK, Elliott JP, Eskridge JM. Neuroradiologic diagnosis and treatment of vasospasm. Neuroimaging Clin N Am 1997;7:819–35 [PubMed] [Google Scholar]
- 16.Kessel NF, Helm G, Simmins N, et al. Treatment of cerebral vasospasm with intra-arterial papaverine. J Neurosurg 1992;77:848–52 [DOI] [PubMed] [Google Scholar]
- 17.Biondi A, Ricciardi GK, Puybasset L, et al. Intra-arterial nimodipine for the treatment of symptomatic cerebral vasospasm after aneurysmal subarachnoid hemorrhage: preliminary results. AJNR Am J Neuroradiol 2004;25:1067–76 [PMC free article] [PubMed] [Google Scholar]
- 18.Klucznik R, Carrier DA, Pyka R, et al. Placement of ferromagnetic intracerebral aneurysm clip in a magnetic field with a fatal outcome. Radiology 1993;187:855–56 [DOI] [PubMed] [Google Scholar]
- 19.Sloan MA, Haley EC Jr, Kassel NF, et al. Sensitivity and specificity of transcranial Doppler ultrasonography in the diagnosis of vasospasm following subarachnoid hemorrhage. Neurology 1989;39:1514–18 [DOI] [PubMed] [Google Scholar]
- 20.Vora YY, Suarez-Almazor M, Steinke DE, et al. Role of transcranial Doppler monitoring in the diagnosis of cerebral vasospasm after subarachnoid hemorrhage. Neurosurgery 1999;44:1237–47 [PubMed] [Google Scholar]
- 21.Lysakowski C, Walder B, Costanza MC, et al. Transcranial Doppler versus angiography in patients with vasospasm due to a ruptured cerebral aneurysm: a systematic review. Stroke 2001;32:2292–98 [DOI] [PubMed] [Google Scholar]
- 22.Zouaoui A, Sahel M, Marro B, et al. Three-dimensional computed tomographic angiography in detection of cerebral aneurysms in acute subarachnoid hemorrhage. Neurosurgery 1997;41:125–30 [DOI] [PubMed] [Google Scholar]
- 23.Velthuis BK, Rinkel GJE, Ramos LMP, et al. Subarachnoid hemorrhage: aneurysm detection and preoperative evaluation with CT angiography. Radiology 1998;208:423–30 [DOI] [PubMed] [Google Scholar]
- 24.Van Loon JJL, Yousry TA, Fink U, et al. Postoperative spiral computed tomography and magnetic resonance angiography after aneurysm clipping with titanium clips. Neurosurgery 1997;41:851–57 [DOI] [PubMed] [Google Scholar]
- 25.Otawara Y, Ogasawara K, Ogawa A, et al. Evaluation of vasospasm after subarachnoid hemorrhage by use of multislice computed tomographic angiography. Neurosurgery 2002;51:939–43 [DOI] [PubMed] [Google Scholar]
- 26.Johnson PT, Halpern EJ, Kuszyk BS, et al. Renal artery stenosis: CT angiography-comparison of real-time volume-rendering and maximum intensity projection algorithms. Radiology 1999;211:337–43 [DOI] [PubMed] [Google Scholar]
- 27.Lawton MT, Heiserman JE, Prendergast VC, et al. Titanium aneurysm clips. Part III. Clinical application in 16 patients with subarachnoid hemorrhage. Neurosurgery 1996;38:1170–75 [DOI] [PubMed] [Google Scholar]
- 28.Macdonald RL, Wallace MC, Kestle JRW. Role of angiography following aneurysm surgery. J Neurosurg 1993;79:826–32 [DOI] [PubMed] [Google Scholar]
- 29.Teksam M, McKinney A, Cakir B, et al. Multi-slice computed tomography angiography in the detection of residual or recurrent cerebral aneurysms after surgical clipping. Acta Radiol 2004;45:571–76 [DOI] [PubMed] [Google Scholar]


