Abstract
SUMMARY: We describe the case of a 26-year-old man who presented with symptoms compatible with Lhermitte sign that occurred during yawning. It was associated with congenital partial aplasia of the posterior arch of the atlas. Cervical multisection-detector CT myelography during yawning showed compression of the upper cervical cord due to the inward mobility of the isolated posterior tubercle. The symptoms completely disappeared following removal of the isolated posterior tubercle.
Congenital partial aplasia of the posterior arch of the atlas (C1), a developmental failure of chondrogenesis, is a rare anomaly.1–10 It is clinically important because it causes acute neurologic deficits, which are closely associated with neck extension.5,7,9,10 We report a case of congenital partial aplasia of the posterior arch of the C1 with a transient Lhermitte sign caused by yawning, which worsened after minor head trauma. We discuss the etiologic factors of the myelopathy on the basis of our radiologic and operative findings.
Case Report
A 26-year-old man with other medical history had experienced years of an electric-shock-like pain running from his spine to all 4 extremities during yawning. He also noted the strong electric-shock-like pain and slight weakness in all extremities and a giddy feeling immediately after a minor frontal head trauma 1.5 years before admission. His motor functions spontaneously improved after 18 months with a conservative treatment, but he noticed that the electric-shock-like pain during yawning was worsening. He was admitted to our hospital for investigation and treatment. He was alert, and no abnormalities were found on cranial nerve and motor examination. Deep tendon reflexes were slightly increased bilaterally. Pinprick, temperature, light touch, and vibratory sensations were intact. A Romberg test was positive, when closing his eyes. The electric-shock-like pain was transiently induced by yawning.
Cervical radiography (Fig 1A), CT, and bone 3D-CT of the upper cervical spine showed bilateral bony defects of the posterior arch of the C1 with an isolated posterior bone (Fig 1B), diagnosed as a type D of congenital partial aplasia of the posterior arch of the C1.8 There was no cervical canal stenosis or fractures. Dynamic cervical radiography during neck extension and during neck extension with the mouth opening to simulate a posture during yawning showed slight inward mobility of the posterior bone fragment, though Lhermitte sign was not induced by these examinations. Cervical MR imaging showed a small intramedullary T2-weighted hyperintensity with slight contrast enhancement in the posterior column at a level slightly below the posterior tubercle of the C1 (Fig 1C). Sagittal MR imaging during neck extension showed inward mobility of the posterior tubercle, affecting the posterior column of the cord (Fig 1D). Dynamic cervical multisection-detector CT (MDCT) myelography with sagittal reconstruction was obtained during neck extension with opening of the mouth and during yawning. When the patient was in a neutral position, no abnormalities were evident (Fig 1E). During maximal neck extension with the mouth open, reduction in distance between the occiput and the spinous process of the axis and inward mobility of the posterior bone fragment were shown. Although the dorsal dural sac was slightly depressed by the posterior bone fragment, the spinal cord was intact (Fig 1F). MDCT myelography during yawning clearly showed strong compression of the cord due to the inward mobility of the posterior bone fragment (Fig 1G), and Lhermitte sign was transiently induced. Follow-up MR imaging after 6 months showed persistent contrast enhancement and slight reduction of the intramedullary hyperintensity, which indicated serial changes of a cord injury.
Fig 1.
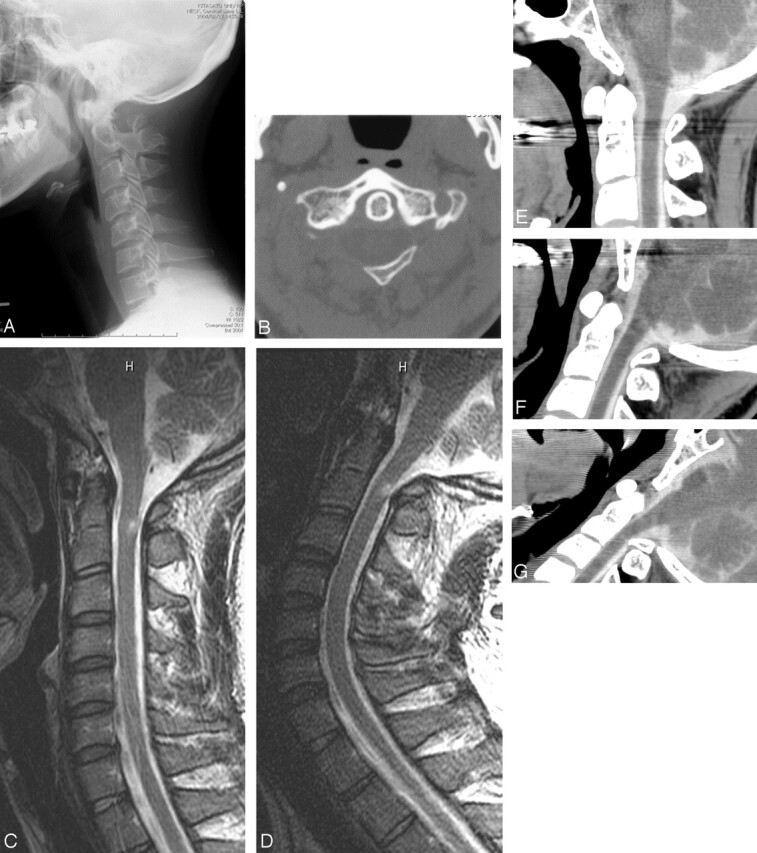
A, Lateral cervical radiograph shows bilateral bony defects of the posterior arch of the atlas and an isolated posterior tubercle.
B, CT of C1 shows bilateral bony defects of the posterior arch and an isolated posterior tubercle.
C, Sagittal T2-weighted MR image in neutral position shows an intramedullary hyperintensity lesion in the posterior column of the spinal cord slightly below the posterior tubercle of C1.
D, Sagittal T2-weighted MR image in neck extension shows the inward displacement of the posterior tubercle, corresponding to the T2-weighted hyperintensity.
E, MDCT myelography in neutral position shows no abnormality.
F, MDCT myelography in maximal neck extension with the mouth open shows slight ventral displacement of the posterior bone fragment, without cord compression.
G, MDCT myelography during yawning shows cord compression due to the ventral displacement of the posterior bone fragment.
Total removal of the isolated posterior bone fragment was performed in the prone position. During surgery, the bone fragment was confirmed to be a part of the posterior tubercle of the C1. It was covered with loose and fibrous connective tissue, and attachment of the rectus capitus posterior minor was observed in the center. The isolated bone fragment was gently removed by resection of 2 elastic connective tissue bands extending from the lateral mass to the 2 ends. Thus, the final diagnosis was type D of congenital partial aplasia of the posterior arch of the C1.8 Immediately after the surgery, the patient’s symptoms during yawning completely disappeared, and he was discharged without any complications on the seventh postoperative day.
Discussion
Three primary ossification centers of the C1—one at the anterior center and forming the anterior tubercle and the other 2 at lateral centers and forming the posterior arch and lateral mass—appear during the embryonic period. The 2 lateral centers form the posterior arches with chondrogenesis, extending dorsally at 3–5 years of age, and the anterior center unites these at 5–9 years of age. It is speculated that the cause of congenital aplasia of the posterior arch is a failure of chondrogenesis.7,9,10 Currarino et al classified the anomalous C1 arch into 5 types on the basis of CT and radiographs.8 In this classification, type C and D, manifesting as absence of the bilateral posterior arches and an isolated posterior tubercle, are clinically very important because these anomalies often cause acute neurologic deficits such as transient quadriparesis,2,5–10 paraparesis,6 Lhermitte sign,5,10 chronic neck pain,8,9 and headache.6 To the best of our knowledge, 18 case reports have been published in the literature.2–10 Patient backgrounds, including ours, were 9 women and 10 men ranging in age from 8.5 to 57 years (mean, 32.7 years). Regarding manifestation of these neurologic deficits, Richardson et al5 and Sharma et al9 suggested the cause was the secondary cord compression caused by inward mobility of the isolated posterior tubercle and surrounding fragile soft tissue due to reduction in distance between the occiput and the spinous process of the axis during neck extension. In the diagnosis, cervical lateral radiograph and CT are very useful and important tools for the initial diagnosis. Dynamic cervical radiographs are helpful for evaluating mobility of the isolated posterior tubercle.9
Lhermitte sign in 3 patients, including ours, was related to neck extension.5,10 Lhermitte sign caused by yawning is a rare phenomenon. Although our dynamic studies on cervical radiography, MR imaging, and MDCT myelography clearly identified inward mobility of the isolated posterior tubercle related to the voluntary neck extension. These radiologic findings precisely corresponded to the theories of Richardson et al5 and Sharma et al,9 but Lhermitte sign was not induced by these dynamic studies because the voluntary neck extension with or without the mouth open could not obtain mobility of the posterior tubercle enough to press the cord. On the other hand, only dynamic MDCT myelography obtained during yawning clearly demonstrated direct evidence of cord compression occurring transiently. It provided very useful information for explaining manifestation of Lhermitte sign. Although the significant influence of yawning on the neck movement is unclear, it was confirmed in our studies that yawning as an involuntary movement induced maximal neck extension. This movement was greater than the neck extension with voluntary mouth opening. Neck extension due to yawning invited maximal inward mobility of the posterior tubercle enough to press the cord, leading to transient cord compression. In addition, we consider that the aggravation of the neurologic symptoms after minor frontal head trauma was caused by spinal cord injury. It is assumed that sudden neck extension following head movement due to injury and associated involuntary mouth opening (as a defensive reaction) leads to transient and maximal inward mobility of the posterior tubercle. As a result, the spinal cord is strongly compressed and injured from the dorsal side. In 5—including ours—of 6 patients investigated by MR imaging,7,9,10 the intramedullary T2-weighted hyperintensity was observed at the level slightly below the posterior tubercle. This change might reflect cord contusion,7,10 myelomalacia, cord edema, or a presyrinx state.9 In our case, the affected cord might have been injured every time the patient yawned.
The treatment is removal of isolated posterior tubercle, after which a good outcome can be expected.7,10 We especially consider that patients with type C or D should undergo surgery at an early stage to prevent cumulative damage to the cord.
References
- 1.Brown CE. Complete absence of the posterior arch of the atlas. Anat Rec 1941;81:499–503 [Google Scholar]
- 2.Hamblen DL. Occipito-cervical fusion: indications, technique and results. J Bone Joint Surg (Br) 1967;49:33–44 [PubMed] [Google Scholar]
- 3.Holsten DR. Eine besondere Form von Defektbildung in hinteren Atlasbogen. Fortschr Rontgenstr 1968;108:541–43 [PubMed] [Google Scholar]
- 4.Dalinka MK, Rosenbaum AE, van Houten F. Congenital absence of the posterior arch of the atlas. Radiology 1972;103:581–83 [DOI] [PubMed] [Google Scholar]
- 5.Richardson EG, Boone SC, Reid RL. Intermittent quadriparesis associated with a congenital anomaly of the posterior arch of the atlas. J Bone Joint Surg (Am) 1975;57:853–54 [PubMed] [Google Scholar]
- 6.Spadaro A, Rotondo M, Conforti R, et al. Aplasia of the posterior arch of the atlas associated with isolated posterior tubercle. Acta Neurol Napoli 1987;9:19–25 [PubMed] [Google Scholar]
- 7.Torreman M, Verhagen ITHJ, Sluzewski M, et al. Recurrent transient quadriparesis after minor cervical trauma associated with bilateral partial agenesis of the posterior arch of the atlas. J Neurosurg 1996;84:663–65 [DOI] [PubMed] [Google Scholar]
- 8.Currarino G, Rollins N, Diehl JT. Congenital defects of the posterior arch of the atlas: a report of seven cases including an affected mother and son. AJNR Am J Neuroradiol 1997;15:249–54 [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma A, Gaikwad SB, Deol PS, et al. Partial aplasia of the posterior arch of the atlas remnant: findings in three cases. AJNR Am J Neuroradiol 2000;21:1167–71 [PMC free article] [PubMed] [Google Scholar]
- 10.Klimo P Jr, Blumenthal DT, Couldwell WT. Congenital partial aplasia of the posterior arch of the atlas causing myelopathy: case report and review of the literature. Spine 2003;28:E224–28 [DOI] [PubMed] [Google Scholar]


