Abstract
PURPOSE: Visual acuity (VA) disturbance other than field defect is important in evaluating patients with pituitary macroadenoma. The purpose of this study was to evaluate MR imaging appearances of optic nerves in patients with pituitary macroadenoma and to ascertain whether visual impairment was correlated with abnormality in optic nerve signal intensity.
PATIENTS AND METHODS: Twenty-seven patients with pituitary macroadenoma were examined. Optic nerves were evaluated on T2-weighted images and correlations of signal intensity abnormality with VA disturbance, visual field disturbance, degree of optic chiasm compression, pathologic findings of surgical specimen, and disease duration were statistically analyzed. Correlations between recovery of VA after treatment and the above-mentioned factors were also determined.
RESULTS: Coronal T2-weighted images demonstrated unilateral optic nerve hyperintensity lesions in 9 patients. Bilateral signal intensity abnormality of the optic nerve was seen in 5 patients. Signal intensity abnormality of the optic nerve was seen at the site of compression and in the ventral side of the tumor. These patients did not demonstrate signal intensity abnormality posterior to the tumor. Presence of such signal intensity abnormalities was correlated with the degree of optic chiasmal compression and with VA disturbance. Recovery of VA after treatment was correlated with disease duration.
CONCLUSION: Hyperintensity of the optic nerves ventral to the pituitary macroadenoma was associated with VA impairment. Recovery of VA after treatment was correlated with disease duration. MR imaging of the optic nerves can provide valuable information for management of pituitary macroadenoma.
In some patients with pituitary macroadenoma, visual acuity (VA) diminishes despite the existence of no field defect other than bitemporal hemianopia. This clinical information is very important in patient management; however, no published reports have analyzed the relationship between VA disturbance and MR imaging findings of the optic nerves of the patients with the pituitary macroadenoma. In this study, the authors used T2-weighted MR imaging to investigate signal intensity abnormality of the optic nerves and statistically analyzed the relationship between this and factors including the degree of optic chiasm compression, disease duration, degree of VA disturbance, and tumor histopathology.
Hyperintensity of the optic nerves was frequently detected among patients with diminished VA. Among those with long disease duration and prolonged signal intensity abnormality, improvements in VA disturbance tended to be poor, which suggests the possibility of degeneration of optic nerves caused not only by compression-induced edema, but also by compression of the arteries, veins, and capillary networks in the optic chiasm.
Patients and Methods
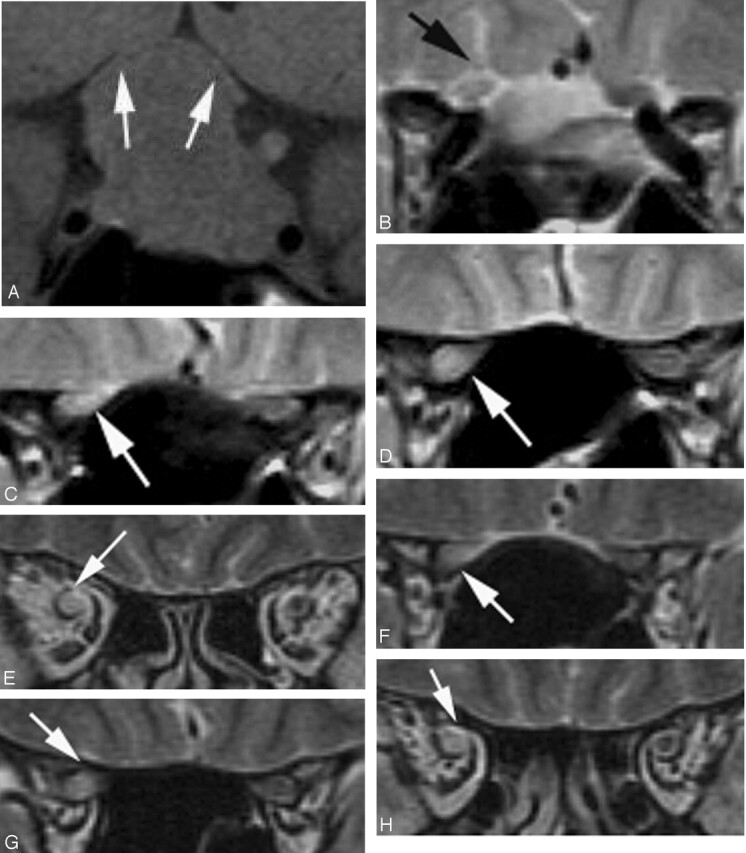
We reviewed pre- and postoperative MR images from 27 consecutive patients with pituitary macroadenoma, 24 of whom were treated surgically at the National Defense Medical College between April 1999 and November 2003. All MR examinations were performed by using a 1.5T system. We used fast spin-echo sequences for T2-weighted (3500/110/2) and T1-weighted (500/15/1) images. Three-millimeter-thick sections were obtained in the coronal and sagittal planes with a 256 × 512 acquisition matrix. All images were blindly reviewed by 2 radiologists, focusing on the presence of abnormal signal intensity in the optic nerves ventral to the tumor, at the site of compression, and dorsal to the tumor. We also classified degree of optic chiasm compression into 3 grades as follows: (−) no compression; (+) compression of less than half of the optic chiasm; and (++) compression with severe thinning. Signal intensity abnormality of the optic nerve was defined as abnormality involving not only the area around the optic nerve, but also the septum (+). In patients with (+) or (++) compression, we also evaluated the location of the tumor in relation to the optic nerve and optic chiasm. Cephalocaudal diameter of each tumor was also measured
Visual signs (visual field defect and VA) were evaluated by an ophthalmologist. Twenty-four patients underwent surgical procedures, and histologic confirmation of the diagnosis was obtained in each case.
A Mann-Whitney U test was used for statistical analysis of the relationships between signal intensity abnormality and tumor size, degree of chiasm compression, histology, VA disturbance, and disease duration. Correlations between recovery of VA after treatment and the above-mentioned factors were also ascertained.
Results
Patient clinical data are summarized in Table 1. The results are shown in Figs 1–4.
Optic tract hyperintensity on T2-weighted images among patients with pituitary macroadenoma: correlation with visual impairment
| Patient No./Age (y)/Sex | Duration | Size (mm) | OC | AS | VAD | VFD | Pathology | IAT |
|---|---|---|---|---|---|---|---|---|
| 1/36/M | 2 mo | 18 | − | − | − | + | ||
| 2/61/M | 9 y | 23.7 | + | − | + (rt 0.7, lt blind) | + | PA | − |
| 3/42/F | 16 mo | 26.5 | ++ | + | + (rt 0.02, lt 1.5) | + | PA (corticotro) | − |
| 4/38/F | 10 mo | 14 | − | − | − | + | ||
| 5/40/M | 6 mo | 35 | ++ | + | + (rt 0.07, lt 0.05) | + | PA (null) | − |
| 6/55/F | 4 y | 46 | ++ | + | + (rt 0.06, lt 0.06) | + | PA (chromophobe) | − |
| 7/65/F | 2 mo | 13 | − | − | − | + | PA (null) | |
| 8/74/M | 18 mo | 16.4 | + | + | + (rt 0.7, lt 0.4) | + | PA (null) | − |
| 9/54/F | 3 mo | 27 | + | + | + (rt 0.06, lt 0.1) | + | PA (nonfunctioning) | + (rt 1.2, lt 0.8) |
| 10/65/M | 2 mo | 13 | − | + | + (rt light sense) | + | Pituitary carcinoma | − |
| 11/59/F | 14 mo | 35 | ++ | + | + (rt 0.3, lt 0.9) | + | PA (null) | − |
| 12/55/M | 3 mo | 22 | ++ | + | + (rt 0.5, lt 0.1) | + | PA | + (rt 1.0, lt 1.0) |
| 13/41/F | 19 mo | 25 | ++ | + | + (hand sense) | + | PA (null) | − |
| 14/37/F | 3 mo | 16 | − | − | − | + | ||
| 15/70/F | 6 mo | 18 | + | + | + (rt 0.1, lt blind) | − | ||
| 16/27/M | 6 mo | 15 | − | + | + (finger sense) | + | PA (chromophobe) | + (rt 0.7, lt 0.9) |
| 17/58/M | 26 mo | 24 | ++ | + | + (rt 1.5, lt 0.4) | − | PA (null) | − |
| 18/21/F | 8 mo | 30 | + | − | − | + | PA (GH) | − |
| 19/50/F | 10 y | 51 | ++ | + | + (rt 0.03, lt 0.1) | + | PA(FSH-LH) | − |
| 20/74/F | 1.5 mo | 18 | − | + | + (lt light sense) | − | PA (prolactinoma) | + (rt 0.8, lt 0.4) |
| 21/66/F | 2 mo | 15 | − | − | − | − | PA (null) | |
| 22/63/M | 2 mo | 23.7 | ++ | + | + (rt 0.1, lt 0.7) | + | PA (chromophobe) | + (rt 0.7, lt 0.8) |
| 23/54/F | 3 mo | 13 | − | − | − | − | PA | |
| 24/56/F | 3 mo | 20 | − | − | − | − | PA | |
| 25/45/M | 2 mo | 16 | − | − | − | + | PA (chromophobe) | |
| 26/36/M | 2 mo | 12.2 | − | − | − | − | ||
| 27/50/M | 3 mo | 16 | ++ | + | + (rt 0.9, lt 0.3) | + | PA (GH) | + (rt 0.9, lt 0.7) |
Note.—OC indicates optic chiasm compression; AS, abnormal signal in optic nerve; VAD, visual acuity disturbance; VFD, visual field disturbance; IAT, improvement of visual acuity after treatment; PA, pituitary adenoma; FSH-LH, follicle stimulating hormone-luteinizing hormone; GH, growth hormone.
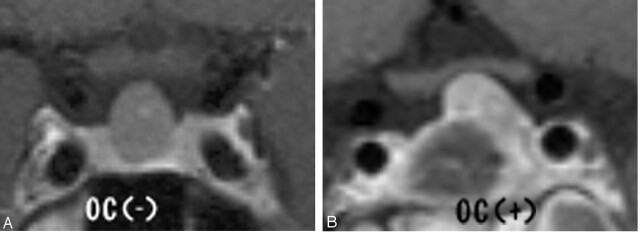
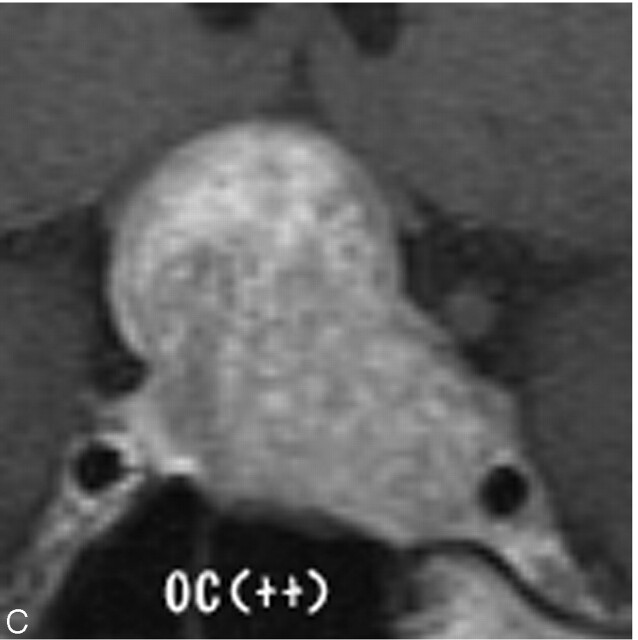
Fig 1.
Degree of optic chiasmal compression. A, No compression to optic chiasma (−). B, Compression of less than half of the optic chiasm (+). C, Compression with marked thinning (++).
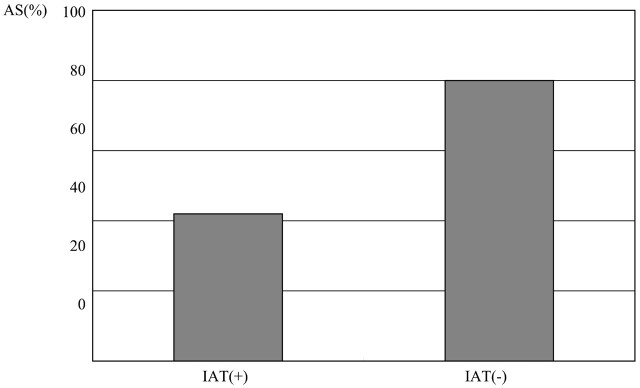
Fig 4.
Correlation between abnormal signal intensity of optic nerve and improvement after treatment of VA.
Coronal T2-weighted images demonstrated unilateral optic nerve hyperintensity lesions in 9 patients. Bilateral signal intensity abnormality of the optic nerve was seen in 5 patients. Signal intensity abnormality of optic nerve was seen at the compression site and at the ventral side of the tumor. No patients demonstrated signal intensity abnormality posterior to the tumor.
Signal intensity normalized in 9 of 21 patients after surgery or other treatment.
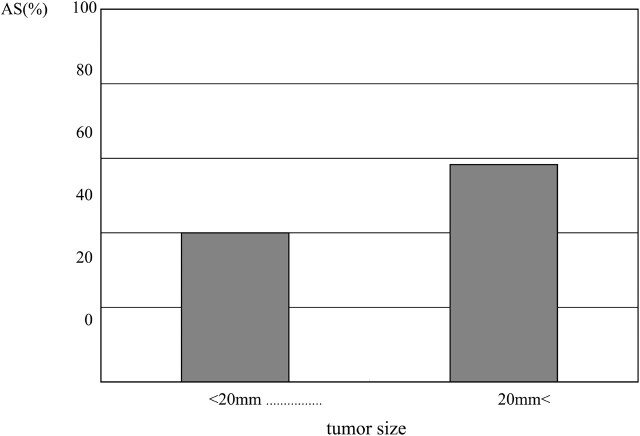
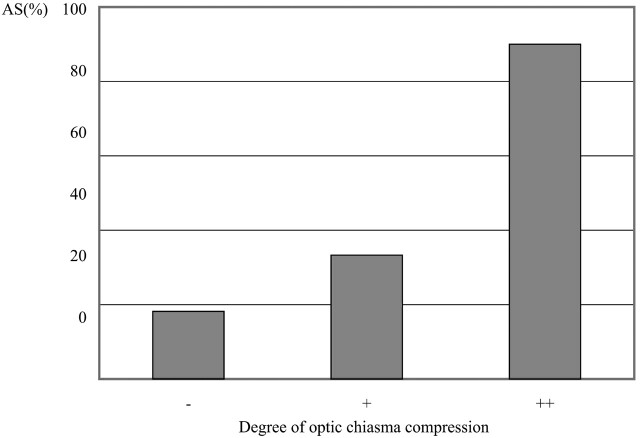
Presence of optic nerve hyperintensity lesions was correlated to the degree of optic chiasm compression and to the presence of diminished VA (P < .01). In all patients with (++) optic chiasm compression, the optic nerve was compressed beyond 1 cm anterior and posterior to the optic chiasm, and tumor location exhibited no significant correlation to the presence or absence of optic nerve signal intensity abnormality; however, no correlation between hyperintensity and tumor size was demonstrated in this study. Other than in patients with a tumor size <2 cm, the results suggested the need to consider type II error. Hence, the relationship to tumor size might be clarified by studying greater numbers of patients.
After surgery and other forms of treatment, size of the tumor diminished and compression of the optic chiasm resolved. Of patients with improved VA, signal intensity abnormality improved in 4 patients but persisted in 5 cases (Fig. 5). Atrophy of optic nerves was seen on MR imaging in 3 patients in whom VA abnormalities persisted on ophthalmologic examination (Fig. 6). In one case with improved VA, although the degree of compression to the optic chiasm was mild, steroids were administered from an early stage as a result of signal intensity abnormality in optic nerves on MR imaging and papillary edema, leading to an improvement in VA. Degree of improvement in VA was significantly correlated with disease duration but not with any other factors in this study.
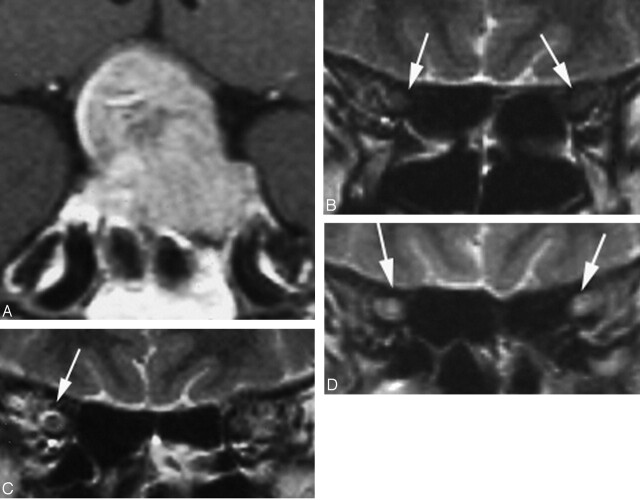
Fig 5.
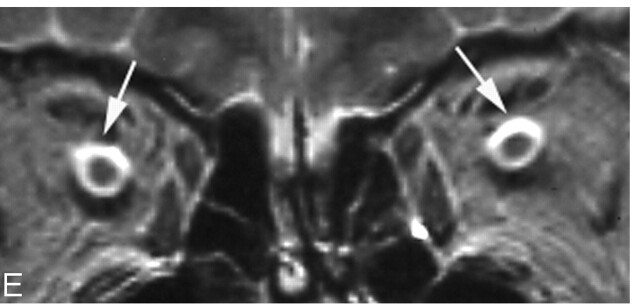
Case 3, a 42-year-old woman whose disease duration was 6 months. Right VA disturbance was recognized (right VA = 0.02; left VA = 1.5). A, Pituitary macroadenoma markedly compressed the optic chiasm especially the right side (white arrows). B–E, Hyperintensity was recognized in the right optic nerve on T2-weighted image (arrows). F–H, Hyperintensiy in the right optic nerve lasted for 2 years after the tumor reduction (arrows).
Fig 6.
Case 17, a 58-year-old man, whose disease duration from the initial examination to the operation was 26 months. VA was not stable, though fixed VA disturbance was not recognized on the initial examination. A, Pituitary adenoma compressed the optic chiasm. B, Hyperintensiy of the optic nerve was not shown (arrows) on the initial examination. C, Right-side perioptic subarachnoid space dilated slightly on the initial examination (arrow). D and E, Tumor reduction was performed 26 months after the initial examination. Hyperintensity was shown in the bilateral optic nerve ventral to the optic chiasm (D, white arrows) and bilateral perioptic subarachnoid space dilated markedly (E, white arrows), probably representing atrophic change of the optic nerves.
Discussion
The literature contains several reports concerning edema-like change along the optic pathway in association with suprasellar tumors such as craniopharyngioma, pituitary adenoma, and meningioma.1–2 Hyperintensity in the optic nerve ventral to pituitary macroadenoma, however, has not been reported. As far as the management of pituitary macroadenoma is concerned, VA disturbance is an important factor, and, as a result, related diagnostic imaging findings are also of consequence.7 In the present study, the relationship between optic nerve signal intensity abnormality, degree of optic chiasm compression, and the presence of VA disturbance were statistically analyzed. The results suggested that prolonged compression caused signal intensity abnormality of the optic nerve and VA disturbance. Consequently, decompression should be performed promptly in patients demonstrating compression of the optic chiasm or signal intensity abnormality of the optic nerve.
Signal intensity abnormality persists after decompression, can lead to atrophy, and is associated with a high frequency of VA disturbance and thus appears to represent changes that extend beyond edema. The mechanism by which pituitary macroadenoma appears to damage the optic nerve and impair VA is unclear. VA disturbance, however, cannot be explained solely in terms of mechanical damage due to compression. Because the axons for the entire superior visual field course through the inferior aspect of the optic nerves and chiasm, compression of these structures from below would be expected to produce a defect in the entire upper field. Such defects, however, are extremely rare.3 As reported by Hoyt, however, compression disrupts the arterial supply, and long-term compression of the arteries, veins, and capillary networks in the optic chiasm leads to stagnant anoxia, thus resulting in a characteristic bitemporal visual field defect.4 Signal intensity abnormality in the optic nerves was considered to represent damage by compression and stagnant anoxia at the optic chiasm and Wallerian degeneration in the ventral side of the optic chiasm.
With regard to VA disturbance caused by compression near the optic chiasm, several studies have reported that this was caused by optic chiasm compression from the internal carotid artery,5,6 but few studies have compared imaging findings.5–7 The present study on tumor-induced compression demonstrated a statistically significant correlation between disease duration and improvement in VA. We also showed that it is meaningful to be able to visually assess compression of the optic chiasm and optic nerves and accurately examine the optic nerves.
In the present study, signal intensity abnormality did not advance to the optic pathway posterior to the tumor, even in patients in whom compression advanced posterior to the optic chiasm. Moreover, tumor location in relation to the optic chiasm and optic nerves exhibited no correlation to signal intensity abnormality. In craniopharyngioma arising in the suprachiasmal region, though, edema has been known to occur in the optic pathway posterior to a tumor, and, as a result, we believe that it will be necessary to further investigate the relationship of signal intensity abnormality to tumor location.
In case 17 (Fig. 6), only signal intensity abnormality of the perioptic subarachnoid space was initially observed, and, though VA was not stable, surgery was delayed for various reasons and optic atrophy eventually developed. In other words, signal intensity abnormality can lead to severe atrophy, which suggests the importance of performing surgery at an appropriate stage. Because VA improvement is closely correlated to disease stage, it is important to recommend expedient decompression.
VA disturbance was marked from the beginning in some patients despite mild compression. In one such patient (case 20), signal intensity abnormality was localized to the left prechiasmatic region and the perioptic subarachnoid space was enlarged, resulting in symptoms resembling acute optic neuritis. In this patient, pituitary apoplexy was suspected, and microhemorrhage could have caused chemical inflammation. Hence, optic nerve degeneration due to long-term compression was not the sole factor in VA impairment. In other words, it is necessary to assess etiology in each patient. Furthermore, in this patient, as a result of imaging findings and papillary edema, steroids were administered from an early stage, leading to an improvement in VA. This case emphasized the importance of carefully analyzing imaging findings.
Fig 2.
Correlation between abnormal signal intensity of optic nerve and tumor size.
Fig 3.
Correlation between abnormal signal intensity of optic nerve and optic chiasm compression.
References
- 1.Nagahata M, Hosoya T, Kayama T, et al. Edema along the optic tract: useful MR findings for the diagnosis of craniopharyngiomas. AJNR Am J Neuroradiol 1998;19:1753–57 [PMC free article] [PubMed] [Google Scholar]
- 2.Saeki N, Uchino Y, Murai H, et al. MR imaging study of edema-like change along the optic tract in patients with pituitary region tumors. AJNR Am J Neuroradiol 2003;24:336–42 [PMC free article] [PubMed] [Google Scholar]
- 3.Miller NR. Tumors of the pituitary gland. In: Walsh and Hoyt’s clinical neuro-opthalmology. 4th ed. Philadelphia: Willliams and Wilkins; 1424–86
- 4.Hoyt WF. Correlative function anatomy of the optic chiasm. Clin Neurosurg 1970;17:189–208 [DOI] [PubMed] [Google Scholar]
- 5.Ogata N, Imaizumi M, Kurokawa H, et al. Optic nerve compression by normal carotid artery in patients with normal tension glaucoma. Br J Ophthalmol 2005;89:174–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawasaki A, Puruvin VA. Photophobia as a the presenting visual symptom of chiasmal compression. J Neuroophthalmol 2002;22:3–8 [DOI] [PubMed] [Google Scholar]
- 7.Forooan R. Chiasmal syndrome. Curr Opin Ophthalmol 2003;14:325–31 [DOI] [PubMed] [Google Scholar]